Abstract
Background: The purpose of this study was to evaluate the relationship between preoperative inflammatory markers (neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR)) and different American Joint Committee on Cancer (AJCC) T stages in patients with hilar cholangiocarcinoma.
Methods: A total of 101 patients who underwent surgical treatment for hilar cholangiocarcinoma between 2003 and 2014 in Peking Union Medical College Hospital were retrospectively analyzed. Receiver-operating curves were used to calculate optimal cutoff values for the NLR and the PLR. Univariate and multivariate analyses were used to identify whether the NLR and PLR can independently predict different AJCC T stages.
Results: Multivariate analysis showed that higher NLR and PLR independently predicted advanced AJCC T stages (OR 3.74, 95% CI 1.09–12.83, P=0.036; and OR 7.86, 95% CI 2.25–27.43, P=0.001, respectively). At a threshold of 2.75, the NLR was 75.9% sensitive and 66.7% specific for different AJCC T stages; at a threshold of 172.25, the PLR was 65.5% sensitive and 80.6% specific.
Conclusion: Preoperative NLR and PLR can be used as independent predictors of different AJCC T stages in patients with hilar cholangiocarcinoma.
Keywords: neutrophil–lymphocyte ratio, platelet–lymphocyte ratio, hilar cholangiocarcinoma
Introduction
Hilar cholangiocarcinoma (HC), also known as Klatskin tumor, is a malignancy that affects the hepatic duct confluence, accounting for 40–60% of all bile duct cancers.1–3 Complete surgical resection is the best option for curing HC, and a negative surgical margin (R0) is an important prognostic variable.4 However, as the tumor is encircled by the hepatic artery, portal vein, and liver parenchyma, it has a strong tendency to extensively invade these structures, resulting in high operative risks and increased postoperative complications.5–8 In addition, HC always presents as malignant biliary obstruction and cholestatic hepatitis. Thus, major hepatic resection tends to be associated with an increased risk of postoperative hepatic insufficiency. A positive resection margin was associated with decreased survival.9 The American Joint Committee on Cancer (AJCC) T stage is independently associated with the tumor-free margin,10 and mainly depends on postoperative pathology to confirm.
Preoperative imaging evaluation of the AJCC T stage is important for surgery, and such imaging techniques include Doppler ultrasound, computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiography (ERCP), and percutaneous transhepatic cholangiography (PTC).11 Color Doppler ultrasonography can be used to detect hepatic parenchymal involvement and the hepatic artery or portal vein invasion, but frequent interference of bowel gas means ultrasound examination may not always be successful. CT is helpful in the staging; however, in some cases of HC, visualization of the neoplasms is not definitive because they are too small to be detected, and evaluation of the intraductal spread and the detection of lymph nodes and peripheral metastasis by CT is a suboptimal radiological investigation technique. MRCP is a noninvasive imaging technique for biliary duct carcinoma and allows for observation of HC extension to the biliary tree and vessels, the involvement of adjacent liver parenchyma, local lymphadenopathy, and distant metastasis,12,13 but this examination is expensive. ERCP and PTC are often used for biliary decompression before surgical resection and can relieve jaundice for palliative therapy, which have the advantage of providing brush cytology and biopsy specimens that can confirm the diagnosis of HC, but both of these examinations are invasive. Therefore, a readily available and noninvasive test is needed to identify different AJCC T stages in HC patients.
Inflammation has been shown to play an important role in cancer formation and progression.14 The prognostic and predictive value of inflammatory markers has been reported in many different cancers.15 The neutrophil–lymphocyte ratio (NLR) is one of these inflammatory markers and has been shown to be a reliable prognostic indicator in various cancer patients.15 The platelet–lymphocyte ratio (PLR) is another significant prognostic factor in many kinds of cancers.16
Currently, the predictive value of inflammatory markers for different AJCC T stages in HC is not reported. In our study, we intend to identify the correlation between preoperative inflammatory markers and different AJCC T stages in HC.
Materials and methods
We retrospectively reviewed patients who underwent surgical resections for HC between January 2004 and April 2013 at Peking Union Medical College Hospital. The eligibility criterion for this study was histologically confirmed HC. Patients with inflammatory disease, hematonosis, or missing complete blood count results 2 weeks before surgery were excluded.
HC is staged using the seventh AJCC staging system according to postoperative routine paraffin section pathology. The complete blood count, age, sex, AJCC T stage, tumor grade, lymph node metastasis, and tumor location were collected from patients’ clinical data.
According to previous literature reports, we define the NLR as the absolute neutrophil count divided by the absolute lymphocyte count; similarly, the PLR is defined as the absolute platelet count divided by the absolute lymphocyte count.
Statistical analyses
Continuous variables are expressed as the mean±SD. Categorical variables are described using frequency distributions. An independent-sample t-test was used to detect differences in the means of continuous variables and the χ2-test was used in cases with low expected frequencies. The receiver-operating curve was used to calculate different NLR and PLR cutoff points and obtain optimal cutoff values by maximizing the sum of sensitivity and specificity. All statistical analyses were performed using Statistical Package for Social Sciences version 22.0 software (IBM Corporation, Armonk, NY, USA). P<0.05 was considered significant.
Patient identities were anonymized before analysis. Because this is a retrospective study, the requirement for informed consent was waived. The study protocol was approved by the Peking Union Medical College Hospital Research Ethics Committee.
Results
A total of 101 patients with HC were included in the study. The distribution of surgical AJCC T stages was 29 patients in stage T2b, 65 patients in stage T2a, and seven patients in stage 1.
Table 1 shows the clinicopathological features of the patients in different T stages. In the T2b group, positive lymph node metastasis and tumor invading above the hepatic duct junction (P=0.001, and P<0.001, respectively) were more common. After logistic regression analysis, the NLR, PLR, positive lymph node metastasis, and tumor location were identified as independent risk factors for the AJCC T stage (OR 3.74, 95% CI 1.09–12.83, P=0.036; OR 7.86, 95% CI 2.25–27.43, P=0.001; OR 4.97, 95% CI 1.42–17.43, P=0.012; and OR 14.46, 95% CI 2.94–71.14, P=0.001, respectively; Table 2).
Table 1.
Patient characteristics and pathological findings in patients with different T stages (≧T2b or <T2b)
| Characteristic | T stage≧T2b (n=29) | T stage<T2b (n=72) | P |
|---|---|---|---|
| Age, mean±SD | 58.24±9.98 | 60.93±9.85 | 0.219 |
| Grade | 0.68 | ||
| High | 10 (34.5%) | 28 (38.9%) | |
| Moderate | 17 (58.6%) | 36 (50%) | |
| Low | 2 (6.9%) | 8 (11.1%) | |
| Lymph node | 0.001 | ||
| Positive | 16 (55.2%) | 15 (20.8%) | |
| Negative | 13 (44.8%) | 57 (79.2%) | |
| Tumor location | <0.001 | ||
| Above junction | 26 (89.7%) | 32 (44.4%) | |
| Below junction | 3 (10.3%) | 40 (55.6%) |
Table 2.
Effect of univariate and multivariate variables on the T stage (≧T2b or <T2b)
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| ≧CO versus<CO | ||||||
| NLR | 5.91 | 2.22–15.73 | <0.001 | 3.74 | 1.09–12.83 | 0.036 |
| PLR | 8.62 | 3.26–22.82 | <0.001 | 7.86 | 2.25–27.43 | 0.001 |
| Lymph node | 4.68 | 1.85–11.82 | 0.001 | 4.97 | 1.42–17.43 | 0.012 |
| Grade | ||||||
| High | Reference | |||||
| Moderate | 2.23 | 0.23–21.37 | 0.49 | |||
| Low | 2.63 | 0.29–23.64 | 0.39 | |||
| Tumor location | 10.8 | 3.01–39.05 | <0.001 | 14.46 | 2.94–71.14 | 0.001 |
Note: The CO for NLR is 2.75; the CO for PLR is 172.25.
Abbreviations: CO, cutoff value; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio.
Mean counts of the NLR, PLR, white blood cells, neutrophils, lymphocytes, and platelets are shown in Table 3. Mean values for the NLR, PLR, neutrophils, and platelets were increased in the T stage≧T2b group (P=0.002, P<0.001, P<0.001, and P=0.003, respectively). The mean value for lymphocytes was not significantly different between the two groups (P=0.749).
Table 3.
Mean white blood cell subtype counts, neutrophil–lymphocyte ratio, and platelet–lymphocyte ratio in study subjects
| Characteristic | T stage≧T2b | T stage<T2b | P |
|---|---|---|---|
| WBC | 7.15±2.66 | 6.41±1.87 | 0.114 |
| Neutrophils | 6.18±2.59 | 4.02±1.51 | <0.001 |
| Lymphocytes | 1.76±0.55 | 1.72±0.64 | 0.794 |
| Platelets | 303.95±130.33 | 222.07±72.69 | 0.003 |
| NLR | 3.61±1.32 | 2.66±1.41 | 0.002 |
| PLR | 181.56±44.98 | 141.32±43.79 | <0.001 |
Note: Values are expressed as the mean±SD.
Abbreviations: NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; WBC, white blood count.
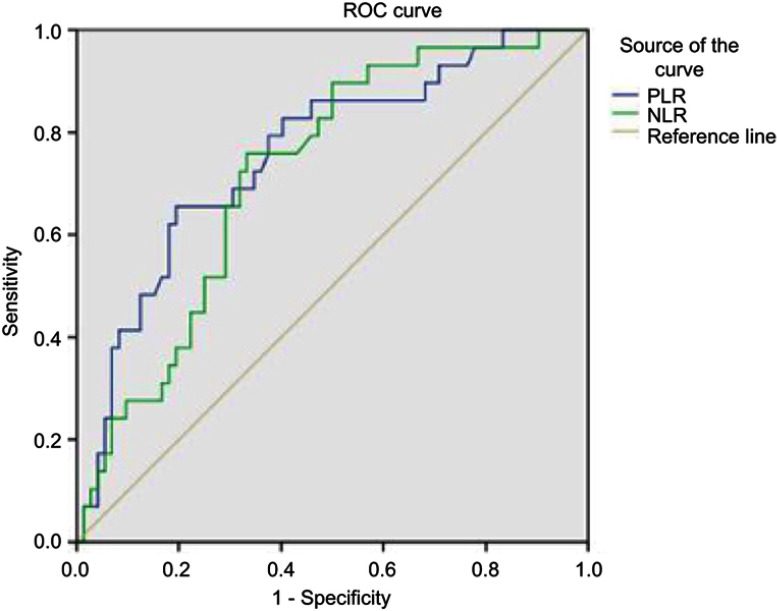
The preoperative NLR and PLR at different cutoff values predicting the AJCC T stage are shown as receiver-operating curves (Figure 1). The best cutoff value for NLR was 2.75, which was 75.90% sensitive and 66.7% specific for the different AJCC T stages. The best cutoff value for PLR was 172.25, which was 65.5% sensitive and 80.6% specific.
Figure 1.
Receiver-operating curve for the relationship between the NLR and PLR and the T stage (≧T2b or <T2b).
Notes: Areas under the curve for the NLR and PLR are 0.758 (95% CI 0.619–0.825, P=0.001) and 0.722 (95% CI 0.654–0.862, P<0.001), respectively.
Abbreviations: NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; ROC, receiver-operating characteristic.
Discussion
Our study identified the relationship between preoperative inflammatory markers (NLR and PLR) and AJCC T stages in HC.
HC is a highly malignant tumor, with an increased surgical-related risk due to its anatomical location. Recent studies have proposed that aggressive surgery, including major hepatic resection combined with extrahepatic bile duct resection and lymphadenectomy, is associated with long-term patient survival.17 The majority of reports indicate that a positive resection margin strongly affects prognosis and emphasize the importance of achieving a tumor-free resection margin. Actually, hepatectomy combined with bile duct resection is mainly performed to increase the rate of negative resection margins. The AJCC T stage was independently associated with a tumor-free margin.10 Therefore, preoperative accurate assessment of the AJCC T stage can help with determining a suitable surgical strategy.
Different preoperative examinations, including Doppler ultrasound, CT, MRCP, ERCP, and PTC, are commonly used to evaluate the AJCC T stage in HC patients. Although these examinations have different advantages in preoperative staging, the disadvantages of each one are also obvious. Doppler ultrasound can be influenced by bowel gas, so it may not be successful. CT is not sensitive enough to detect small lesions. MRCP is sensitive and specific in preoperative staging, but it is costly. Both ERCP and PTC are invasive examinations. Despite the reasonable imaging diagnosis in the preoperative examination of tumor stages, there still seems to be a gap between the preoperative evaluation and intraoperative findings for patients with HC.
Inflammation-based prognostic markers have been widely used in the prediction of prognosis and in the diagnosis of various cancer patients.18,19 The mechanism between preoperative inflammation factors and cancer is still under investigation.14,20 However, the inflammatory response may stimulate the release of cytokines and inflammatory mediators, which can promote tumor metastasis and recurrence. This response is mediated by the promotion of angiogenesis, damage to DNA, and inhibition of apoptosis.20–22 The inflammatory response can also release inhibitory mediators such as IL-10 and TGF-ß, leading to suppression of the immune system and reduced lymphocyte function. Cancer also could produce myeloid growth factors, which may induce elevation of tumor-related white blood cells and neutrophils.15 Some studies indicated that systemic inflammatory responses predict poor prognosis in various cancers.23 Thus, higher systematic inflammatory factor values may associate with advanced tumor stage.
The PLR is a potential prognostic factor as reported in other cancers, such as colorectal cancer, breast cancer, and ovarian cancer.15,16,24,25 Platelets can regulate other inflammation cells such as neutrophils and facilitate their adhesion to lymphocytes in the inflammatory response, and also can promote the spread and growth of cancer cells.26,27 This index is only based on laboratory data, and platelet counts and leukocytes are routinely tested before surgery. Therefore, the PLR can act as simple and complementary prognostic factor in HC. Recently, PLR was reported for evaluation of resectability of HC.28
Lots of emerging evidence supports that an elevated preoperative NLR is correlated with poor survival outcome in various solid tumors.29–34 Halazun et al35 were the first to report that the NLR is associated with hepatic malignancy. Furthermore, the preoperative NLR has shown a significant correlation with poor outcome in hepatocellular carcinoma patients,36,37 and a high NLR also showed correlation with early recurrence and poor overall survival in intrahepatic cholangiocarcinoma patients. It has been shown that a high NLR, which is associated with the presence of systemic inflammation, indicates the relative depletion of lymphocytes, which impairs the host immune reactions against malignancy.34,38
Our results show that a higher PLR was positively correlated with advanced AJCC T stages (OR 7.86, 95% CI 2.25–27.43, P=0.001), which was noted after multivariate analysis. Lymph node metastasis was also an independent risk factor for advanced AJCC T stages (OR 7.25, 95% CI 1.72–30.58, P=0.007). Univariate and multivariate analysis shows that the preoperative NLR can independently predict advanced AJCC T stages (OR 5.91, 95% CI 2.22–15.73, P<0.001; and OR 3.74, 95% CI 1.09–12.83, P=0.036).
There are some limitations to this study. Firstly, this is a retrospective study, and the data were collected from a single institution, which was susceptible to bias in data selection and analysis. Secondly, other inflammatory markers like C-reactive protein are not analyzed in our study, as our center does not routinely measure these. Finally, there are many other staging systems for HC, eg, the Memorial Sloan-Kettering Cancer Center system, which includes portal vein involvement and liver atrophy, and is a common and valuable evaluation method in clinical practice. However, because of the lack of adequate clinical and pathological data in our database, we did not analyze this staging system.
Conclusion
This report shows a relationship between preoperative inflammatory markers and different AJCC T stages in HC patients. Higher preoperative NLR and PLR independently predict advanced AJCC T stages.
Acknowledgments
This work was supported by CAMS Innovation Fund for Medical Science (CIFMS) (2017-I2M-4-003), International Science and Technology Cooperation Projects (2015DFA30650 and 2016YFE0107100), and Capital Special Research Project for Health Development (2014-2-4012).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Weiss MJ, Cosgrove D, Herman JM, Rastegar N, Kamel I, Pawlik TM. Multimodal treatment strategies for advanced hilar cholangiocarcinoma. Langenbeck’s Archiv Surg. 2014;399(6):679–692. doi: 10.1007/s00423-014-1219-1 [DOI] [PubMed] [Google Scholar]
- 2.Razumilava N, Gores GJ. Cholangiocarcinoma. The Lancet. 2014;383(9935):2168–2179. doi: 10.1016/S0140-6736(13)61903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463–73; discussion 73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB. 2005;7(4):259–262. doi: 10.1080/13651820500373010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SG, Lee YJ, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7(2):135–141. doi: 10.1007/s005340000070135.534 [DOI] [PubMed] [Google Scholar]
- 6.Gazzaniga GM, Filauro M, Bagarolo C, Mori L. Surgery for hilar cholangiocarcinoma: an Italian experience. J Hepatobiliary Pancreat Surg. 2000;7(2):122–127. doi: 10.1007/s005340000070122.534 [DOI] [PubMed] [Google Scholar]
- 7.Yi B, Xu AM, Lai EC, et al. Preoperative portal vein embolization for hilar cholangiocarcinoma–a comparative study. Hepato-gastroenterology. 2010;57(104):1341–1346. [PubMed] [Google Scholar]
- 8.Tsao JI, Nimura Y, Kamiya J, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen KT, Steel J, Vanounou T, et al. Initial presentation and management of hilar and peripheral cholangiocarcinoma: is a node-positive status or potential margin-positive result a contraindication to resection? Ann Surg Oncol. 2009;16(12):3308–3315. doi: 10.1245/s10434-009-0701-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu HJ, Mao H, Shrestha A, et al. Prognostic factors and long-term outcomes of hilar cholangiocarcinoma: a single-institution experience in China. World J Gastroenterol. 2016;22(8):2601–2610. doi: 10.3748/wjg.v22.i8.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugiura T, Nishio H, Nagino M, et al. Value of multidetector-row computed tomography in diagnosis of portal vein invasion by perihilar cholangiocarcinoma. World J Surg. 2008;32(7):1478–1484. doi: 10.1007/s00268-008-9547-3 [DOI] [PubMed] [Google Scholar]
- 12.Yeh TS, Jan YY, Tseng JH, et al. Malignant perihilar biliary obstruction: magnetic resonance cholangiopancreatographic findings. Am J Gastroenterol. 2000;95(2):432–440. doi: 10.1111/j.1572-0241.2000.01763.x [DOI] [PubMed] [Google Scholar]
- 13.Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):155–164. doi: 10.1055/s-2004-828892 [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 15.Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17(3):216–222. doi: 10.3109/1354750X.2012.656705 [DOI] [PubMed] [Google Scholar]
- 16.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200(2):197–203. doi: 10.1016/j.amjsurg.2009.08.041 [DOI] [PubMed] [Google Scholar]
- 17.Xiang S, Lau WY, Chen XP. Hilar cholangiocarcinoma: controversies on the extent of surgical resection aiming at cure. Int J Colorectal Dis. 2015;30(2):159–171. doi: 10.1007/s00384-014-2063-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyer SB, Powell AG, McSorley ST, et al. The pretreatment systemic inflammatory response is an important determinant of poor pathologic response for patients undergoing neoadjuvant therapy for rectal cancer. Ann Surg Oncol. 2017;24(5):1295–1303. doi: 10.1245/s10434-016-5684-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamura Y, Ashida R, Ito T, Sugiura T, Mori K, Uesaka K. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 2015;39(6):1501–1509. doi: 10.1007/s00268-015-2982-z [DOI] [PubMed] [Google Scholar]
- 20.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? The Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60(1):184–190. [PubMed] [Google Scholar]
- 22.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–810. doi: 10.1245/s10434-014-4048-0 [DOI] [PubMed] [Google Scholar]
- 24.Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13(7):499–503. doi: 10.1007/s12094-011-0687-9 [DOI] [PubMed] [Google Scholar]
- 25.Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524–2530. doi: 10.1038/bjc.2014.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. 2013;35(3):254–261. doi: 10.1111/ijlh.12084 [DOI] [PubMed] [Google Scholar]
- 27.Egan K, Crowley D, Smyth P, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. 2011;6(10):e26125. doi: 10.1371/journal.pone.0026125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu HJ, Jin YW, Zhou RX, et al. Clinical value of inflammation-based prognostic scores to predict the resectability of hyperbilirubinemia patients with potentially resectable hilar cholangiocarcinoma. J Gastrointest Surg. 2019;23(3):510–517. doi: 10.1007/s11605-018-3892-9 [DOI] [PubMed] [Google Scholar]
- 29.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104(8):1288–1295. doi: 10.1038/bjc.2011.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He C, Mao Y, Lao X, Li S, Lin X. Neutrophil-to-lymphocyte ratio predicts overall survival of patients with combined hepatocellular cholangiocarcinoma. Oncol Lett. 2018;15(4):4262–4268. doi: 10.3892/ol.2018.7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16(1):55–59. doi: 10.4048/jbc.2013.16.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416–421. doi: 10.1038/bjc.2013.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258(2):301–305. doi: 10.1097/SLA.0b013e318297ad6b [DOI] [PubMed] [Google Scholar]
- 34.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97(6):513–518. doi: 10.1002/jso.21001 [DOI] [PubMed] [Google Scholar]
- 35.Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34(1):55–60. doi: 10.1016/j.ejso.2007.02.014 [DOI] [PubMed] [Google Scholar]
- 36.de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–3145. doi: 10.1200/JCO.2011.35.6519 [DOI] [PubMed] [Google Scholar]
- 37.Spolverato G, Ejaz A, Kim Y, et al. Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2014;18(7):1284–1291. doi: 10.1007/s11605-014-2533-1 [DOI] [PubMed] [Google Scholar]
- 38.Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250(1):141–151. doi: 10.1097/SLA.0b013e3181a77e59 [DOI] [PubMed] [Google Scholar]