Abstract
The neuropeptide galanin and its receptors have been found to have protective effects on neurons. However, the role of galanin on astrocytes is still unclear. The present study is aimed at investigating the effects of galanin on the viability of cultured rat cortical astrocytes after oxidative stress induced by H2O2 and possible receptor and signaling mechanisms involved. Treatment of galanin had significant protective effects against H2O2-induced toxicity in the cultured cortical astrocytes. H2O2 induced an upregulation of phosphorylated extracellular signal-related kinase1/2 (pERK1/2) in astrocytes, which was suppressed by coapplication of galanin, suggesting an involvement of the pERK1/2 signal pathway in the protective effects of galanin. GalR2 has higher expression levels than GalR1 and GalR3 in the cultured cortical astrocytes, and GalR2 agonist AR-M1896 mimicked galanin effects on the astrocytes, implying that galanin protective effects mainly mediated by GalR2. Meanwhile, galanin had no effect on the A1-type transformation of rat cortical astrocytes. All those results suggest that galanin protects rat cortical astrocytes from oxidative stress by suppressing H2O2-induced upregulation of pERK1/2, mainly through GalR2.
1. Introduction
In the central nervous system (CNS), astrocytes contribute to maintain the homeostasis of the CNS [1]. As a component of blood-brain barrier, astrocytes serve as functional barriers that attract and restrict CNS inflammation [2]. Astrocytes keep the balance between their opposing functions of glutamate uptake and release [3, 4], providing glial cell involvement in the pathophysiology of epilepsy [5]. Astrocytes could drive seizure generation in mitochondrial epilepsy [6], even atypical astrocytes contributed to spontaneous recurrent seizures after diffuse traumatic brain injury [7]. Also, astrocytes contribute to synaptic plasticity, neuronal network oscillations, and cognitive processes, playing a role in Alzheimer's disease [8]. Astrocytes respond to all forms of CNS insults through a process referred to as reactive astrogliosis. This process is a highly heterogeneous state; the function of astrocytes may be harmful or beneficial [9]. Thus, two different types of reactive astrocytes were termed “A1” (harmful) and “A2” (beneficial), respectively, according to neuroinflammation and ischemia [10].
Galanin, a 29-30 amino acid neuropeptide [11], is widely expressed in the CNS [12]. It is involved in many physiological and pathological functions, such as memory, epilepsy, Alzheimer's disease, and depression [13]. So far, three galanin receptors have been cloned, termed as GalR1, GalR2, and GalR3 [13]. Galanin is found coexisting with many classic neurotransmitters, such as 5-hydroxytryptamine in the dorsal raphe nucleus (DR), norepinephrine in the locus coeruleus (LC), and acetylcholine in the medial septal nuclei (MS) [12, 14]. Thus, galanin plays a cotransmission role in the CNS [15]. Many studies have showed that galanin may also have neurotrophic/neuroprotective effects in addition to its neurotransmission role. Its neuron protection functions have been proved from in vitro primary cultured hippocampal neurons to in vivo animal models and transgenic models [16–18]. The neuronal protections of galanin are mediated mostly by GalR2 [17, 18]. However, GalR1 has recently been found to have a protective effect on neurons in the rat hippocampus and ischemic mouse brain [19, 20]. However, little research has been performed to investigate the protective role of galanin on astrocytes. Priller and colleagues found that galanin is able to induce c-fos mRNA in cultured rat astrocytes, providing evidence for the presence of functional galanin receptors on glial cells [21]. In the present study, the effects of galanin on the viability of cultured rat cortical astrocytes after H2O2-induced oxidative stress as well as the receptor and signaling mechanisms involved were investigated.
2. Materials and Methods
2.1. Culture of Rat Cortical Astrocytes
Astrocyte cultures were prepared from the cerebral cortex of 1-day-old neonatal Sprague Dawley rats. After decapitation, the brainstem, cerebellum, and diencephalons were removed in cold dissection buffer, the meninges were peeled off, then the brain were minced by scissors, incubated with 0.25% trypsin–EDTA at 37°C for 5 min, filtered through a 200 mesh. Cells were incubated at 37°C in a 5% CO2 for 1 hour in DMEM/F12 supplemented with 10% fetal bovine serum. The culture media were collected, and cells were resuspended in DMEM/F12 supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin and plated on poly-L-lysine-coated 75 cm2 flasks at 37°C in a 5% CO2 incubator. After about 2 weeks, cultures reached confluence and were shaken at 250 rpm for 18 hr at 37°C to dislodge cells adhering to the astrocyte layer, mainly oligodendrocytes. Secondary astrocyte cultures were established by trypsinizing confluent cultures and subplating onto dishes. In the present study, astrocytes were used at passage 3. When astrocytes were transformed, TNFα (30 ng/ml, MCE), IL-1α (3 ng/ml, MCE), and C1q (400 ng/ml, BioVision) were added in the medium.
2.2. Cytotoxicity Assay
Sensitivities of astrocytes to various chemicals were examined using the Cell-Counting Kit (CCK, Sigma, St. Louis, MO, USA) technique. Astrocytes were plated at a density of 5000 cells per well in 96-well plates. After 24 hr incubation at 37°C in a 5% CO2 incubator, culture medium was replaced with new medium and drugs, incubated for an additional 24 hr. 10 μl CCK reagent was added into each well and incubated for 2 hr before reading at a wavelength of 450 nm. The drugs added into each well included several groups, vehicle, H2O2, H2O2+galanin, galanin, H2O2+AR-M1896, and AR-M1896. Absorbances were converted to percentages for comparison with the vehicle group.
2.3. Immunocytochemistry Staining
Cells on 25 mm poly-L lysine-coated glass coverslips were rinsed twice with PBS, pH 7.2-7.4, fixed with 4% paraformaldehyde in PBS for 15 min at room temperature, rinsed three times with PBS, incubated with PBS containing 0.3% Triton X-100 for 30 min, blocked in 10% goat serum in PBST for 1 hr, incubated with primary monoclonal anti-GFAP mouse antibody (Sigma, St. Louis, MO, USA) overnight at 4°C, rinsed three times with PBS, incubated with secondary goat anti-mouse Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) for 2 hr at RT, rinsed three times with PBS, mounted with glycerin, and examined under confocal microscope (Leica, USA) or inverted fluorescence microscope IX51 (Olympus, Japan).
2.4. Western Blot
Astrocytes were washed with PBS, lysed in RIPA lysate containing protease inhibitor cocktail (Applygen, China) and phosphatase inhibitor cocktail (Sigma, USA), and sonicated for 2 minutes. Cell lysates were centrifuged for 20 min at 13000 g at 4°C. Supernatant proteins were separated by SDS-PAGE on 12% gels and transferred onto PVDF membranes. After blocking with 5% nonfat milk in Tris-buffered saline, pH 7.5, containing 0.1% Tween 20 (TBS-T) for 2 hours at room temperature, blots were incubated with primary antibodies in TBS-T overnight at 4°C. The primary antibodies included rabbit anti-pERK1/2 antibody (1 : 1000, Cell Signaling Technology) and mouse anti-Gapdh antibody (1 : 10000, Sigma). Then, blots were washed with TBS-T three times and incubated with horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit or goat anti-mouse) (1 : 5000, China) at RT for 2 hours. Finally, the blots were rinsed and visualized using the enhanced chemiluminescence system (Thermo Fisher Scientific, Rockford, USA) according to the manufacturer's instructions. Optical densities of individual blot were quantified using the ImageJ software. Ratios of pERK1/2 to Gapdh were calculated for each sample, and fold changes were shown compared to the control group.
2.5. Reverse Transcription of mRNA
Total mRNA was isolated from culture of rat cortical astrocytes using the Qiagen RNeasy Mini Kits (Qiagen, Hilden, Germany), and mRNA was reverse transcribed using the SuperScript™ III RT reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's manual.
2.6. Polymerase Chain Reaction (PCR)
PCR reaction was carried out using the PrimeSTAR® HS DNA Polymerase (Takara, Tokyo, Japan) under the following conditions: 2 min 98°C, 30 cycles of 10 s 98°C and 1 min 68°C, then 10 min 68°C. The primers were listed in Table 1. The identities of the PCR products were confirmed by sequencing.
Table 1.
PCR primers.
| Primer name | Primer sequence |
|---|---|
| Rat galanin | Forward: CACATGCCATTGACAACCAC |
| Reverse: AACTCCATTATAGTGCGGACG | |
|
| |
| Rat GalR1 | Forward: TCGGGACAGCAACCAAAC |
| Reverse: TGCAGATGATTGAGAACCTTGG | |
|
| |
| Rat GalR2 | Forward: GCCGCCATCGGGCTCATCTG |
| Reverse: GTCGAGGTGCGCTCCATGCT | |
|
| |
| Rat GalR3 | Forward: ACAGATCTCTTCATCCTCAACTT |
| Reverse: GTGAGGTAGATGAGCAGATGTAC | |
2.7. Real-Time Quantitative PCR (qPCR)
Real-time Quantitative PCR was carried out with SYBR Green (ABI). The total reaction system was 20 μl, 50°C 2 min, 95°C 10 min, 40 cycles for 95°C 15 sec, and 60°C 1 min. Gapdh was set as the internal parameter and the relative mRNA levels were calculated with the 2−ΔΔCt method. The primers were listed in Table 2.
Table 2.
qPCR primers.
| Primer name | Primer sequence |
|---|---|
| Rat iNOS | Forward: TGGAGCGAGTTGTGGATTG |
| Reverse: GTGATGTCCAGGAAGTAGGTG | |
|
| |
| Rat TNFα | Forward: CTTCTGTCTACTGAACTTCGGG |
| Reverse: CTACGGGCTTGTCACTCG | |
|
| |
| Rat IL-1β | Forward: GCAGGCTTCGAGATGAAC |
| Reverse: GGGATTTTGTCGTTGCTTGTC | |
|
| |
| Rat Gapdh | Forward: GACCACCCAGCCCAGCAAGG |
| Reverse: TCCCCAGGCCCCTCCTGTTG | |
2.8. Statistical Analysis
Results were presented as means ± SEM or median (interquartile range). Data were evaluated by one-way ANOVA or nonparametric tests. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. H2O2-Induced Toxicity in Cultured Rat Cortical Astrocytes
Using CCK assay, we found that the toxic effect of H2O2 on astrocyte viability was dependent on the concentration of H2O2 applied (Figure 1). The maximum effect of H2O2 was the astrocyte viability down to below 20%. Since the astrocyte viability was about 60% when 150 μM of H2O2 was applied, we chose this concentration to test the effects of galanin in the present study.
Figure 1.
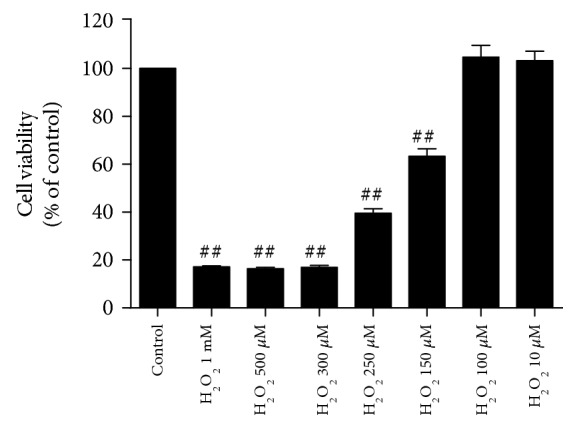
H2O2-induced toxicity in cultured rat cortical astrocytes. Cell viability was determined by CCK assay. H2O2 caused a dose-dependent effect on astrocyte viability. Data were presented as mean ± SE. #p < 0.05, ##p < 0.01 vs. control group.
3.2. The Protective Effects of Galanin against H2O2-induced Toxicity in Cultured Rat Cortical Astrocytes
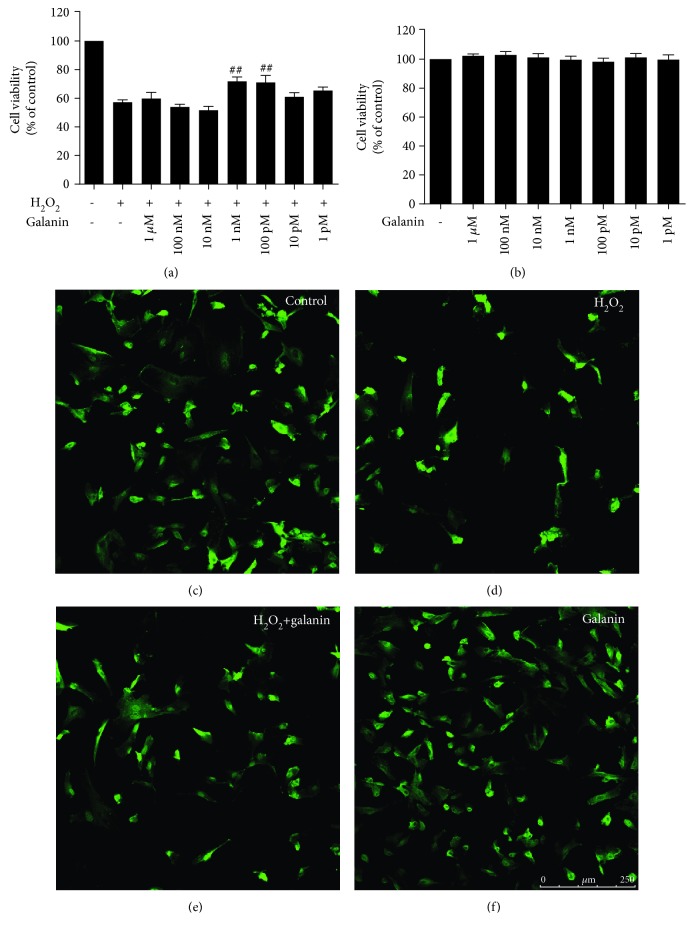
In order to investigate the protective effects of galanin against H2O2-induced toxicity, cultured rat cortical astrocytes were treated with vehicle, 150 μM H2O2, 150 μM H2O2+ galanin with various concentrations (1 μM, 100 nM, 10 nM, 1 nM, 100 pM, 10 pM, and 1 pM), respectively. As shown in Figure 2(a), when the astrocytes were treated with the coadministration of galanin at 1 nM and 100 pM, the cell viabilities were higher as compared to that of the H2O2 treatment group significantly (p < 0.01). Galanin at lower (<10 pM) or higher (>10 nM) concentrations had no significant effects on cell loss induced by H2O2. Meanwhile, treatment with galanin alone, with various concentrations (1 μM, 100 nM, 10 nM, 1 nM, 100 pM, 10 pM, and 1 pM) did not have any significant effects on the astrocyte viability (Figure 2(b)). After immunocytochemistry staining of GFAP, we found that the numbers of astrocyte were much less when treated with H2O2 compared to the control group (Figures 2(c) and 2(d)), while galanin (1 nM) rescued partly the loss of astrocytes (Figure 2(e)). Galanin alone did not change the number of astrocytes significantly (Figure 2(f)).
Figure 2.
The protective effects of galanin against H2O2-induced toxicity in cultured rat cortical astrocytes. (a) Using CCK assay, treatment with 1 nM and 100 pM of galanin showed significantly protective effects against H2O2-induced toxicity; (b) treatment with galanin alone did not have any significant effects on the astrocyte viability compared to the control group; (c–f) galanin reversed the cell death induced by H2O2. Immunocytochemistry staining of GFAP in different groups: (c) control group, (d) 150 μM H2O2 group, (e) 150 μM H2O2+1 nM galanin group, and (f) 1 nM galanin group. Data were presented as mean ± SE. #p < 0.05, ##p < 0.01 vs. H2O2 group. Bar = 250 μm.
3.3. Involvement of pERK1/2 in the Protective Effect of Galanin against H2O2-Induced Toxicity
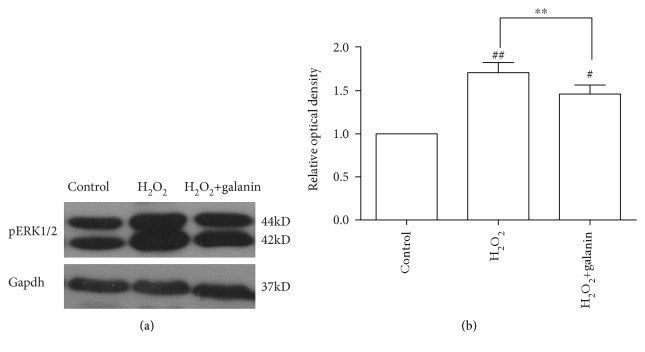
To determine the involvement of pERK1/2 in the protective effect of galanin against H2O2-induced toxicity, we performed the western blot experiment. The results showed that the pERK1/2 protein levels were significantly increased in the 150 μM H2O2 group compared to the control group (p < 0.01). Coapplication of 1 nM galanin significantly suppressed the H2O2-induced upregulation of the pERK1/2 level (p < 0.01). However, the pERK1/2 protein level was still higher in the H2O2+galanin group compared to the control group (p < 0.05) (Figure 3).
Figure 3.
Galanin suppressed the H2O2-induced upregulation of the pERK1/2 protein level. (a) Representative blots of pERK1/2 in control, 150 μM H2O2, and 150 μM H2O2+1 nM galanin groups; (b) ratios of pERK1/2 to Gapdh were calculated and compared. Data were presented as mean ± SE. #p < 0.05, ##p < 0.01 compared with the control group; ∗p < 0.05, ∗∗p < 0.01 compared with the H2O2 group.
3.4. Involvement of GalR2 in the Protective Effect of Galanin against H2O2-Induced Toxicity
The mRNA expression of galanin, GalR1, GalR2, and GalR3 was detected in the cultured rat cortical astrocytes, respectively. As shown in Figure 4, expression levels of GalR2 were moderate while levels of GalR1 and GalR3 were very weak, suggesting mainly GalR2 existed in rat cortical astrocytes.
Figure 4.

Moderate expression of GalR2 and weak expression of GalR1 and GalR3 in cultured rat cortical astrocytes. The expression of galanin, GalR1, GalR2, and GalR3 was detected by RT-PCR.
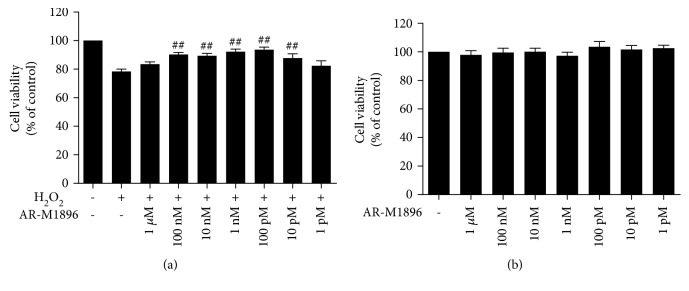
To investigate which subtype(s) of galanin receptor mediated the galanin-induced protective effects against H2O2 toxicity in cultured rat cortical astrocytes, GalR2 agonist AR-M1896 [22] was used. The cultured astrocytes were treated with vehicle, 150 μM H2O2 and H2O2+AR-M1896 with various concentrations (1 μM, 100 nM, 10 nM, 1 nM, 100 pM, 10 pM, and 1 pM), respectively. As shown in Figure 5(a), coadministration with AR-M1896 at concentrations from 100 nM to 10 pM (p < 0.01) showed significantly higher astrocyte viability compared with H2O2 alone. But treatment with AR-M1896 alone at various concentrations tested above did not change astrocyte viability measured with CCK assay (Figure 5(b)). These results suggest that mainly GalR2 mediates the protective effects of galanin in cultured rat cortical astrocytes.
Figure 5.
The protective effects of AR-M1896 against H2O2-induced toxicity in cultured cortical astrocytes of rats. (a) Using the CCK technique, treatment with 100 nM-10 pM of AR-M1896 showed significant protective effects against H2O2-induced toxicity; (b) treatment with AR-M1896 alone did not have any significant effect on the astrocyte viability compared with the control group. Data were presented as mean ± SE. #p < 0.05, ##p < 0.01 vs. the H2O2 group.
3.5. No Modulation Effects of Galanin on Levels of A1 transcripts in Cultured Cortical Astrocytes
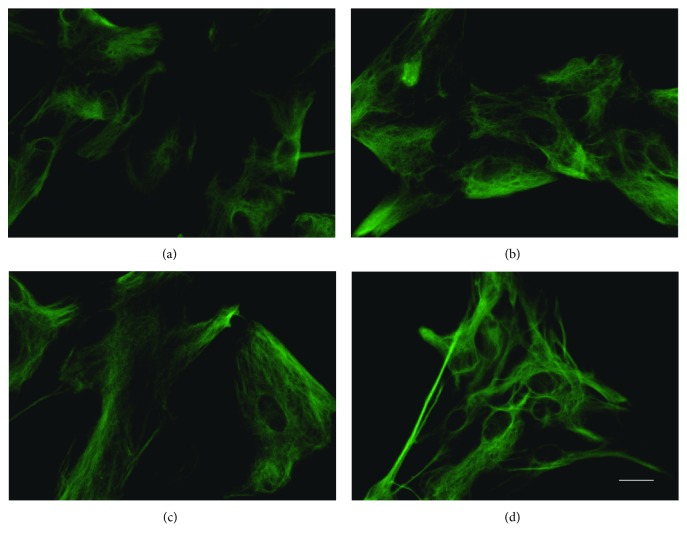
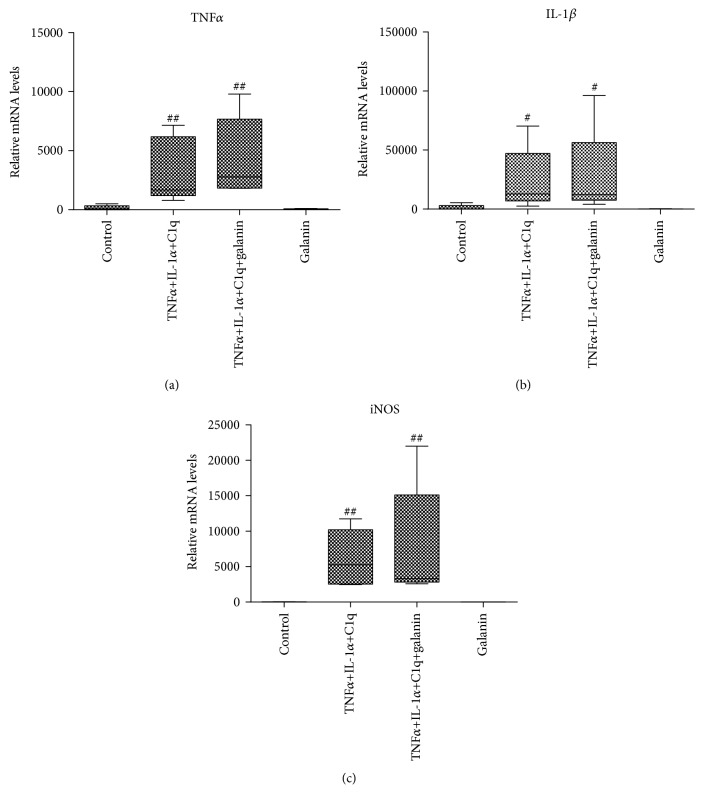
It has been reported that astrocytes could be transformed to A1-type reactive astrocytes, which contribute to neuron death [10]. In order to investigate if galanin is able to revert A1 reactive astrocytes back to resting astrocytes, IL-1α, TNFα, and C1q were applied to induce A1 reactive astrocytes [10]. As shown in Figure 6, after the treatment, the body of astrocytes turned hypertrophy. Meanwhile, A1 transcripts such as TNFα, IL-1β, and iNOS in cultured rat cortical astrocytes were robustly elevated (Figure 7 and Table 3). However, coapplication of galanin had no significant effects on the upregulation of A1 transcripts (Figure 7, Table 3).
Figure 6.
Cultured cortical astrocytes activated by combined application of TNFα, IL-1α, and C1q. The cell body turned hypertrophy. Immunocytochemistry staining of GFAP was detected in astrocytes of different groups: (a) control group, (b) TNFα+IL-1α+C1q group, (c) TNFα+IL-1α+C1q+galanin group, and (d) galanin group. Bar = 20 μm.
Figure 7.
TNFα, IL-1β, and iNOS mRNA of cultured cortical astrocytes elevated by combined application of TNFα, IL-1α, and C1q. Application of galanin did not change A1 phenotype. The relative transcriptional levels of TNFα, IL-1β, and iNOS genes were detected with qPCR. Data were presented as median (interquartile range). #p < 0.05, ##p < 0.01 vs. the control group.
Table 3.
The relative transcriptional levels of TNFα, IL-1β, and iNOS genes in astrocytes.
| Control | TNFα+IL-1α+ C1q |
TNFα+IL-1α+C1q+galanin | Galanin | |
|---|---|---|---|---|
| TNFα | 85.14 (321.11) | 1672.21 (4983.80) | 2781.99 (5841.54) | 81.13 |
| IL-1β | 165.75 (2852.47) | 12848.98 (40156.28) | 12153.73 (48747.71) | 156.90 |
| iNOS | 2.56 (28.90) | 5250.60 (7667.45) | 3292.36 (12294.74) | 1.78 |
4. Discussion
Galanin has been considered as a neurotransmitter/neuromodulator in the nervous system [23]. Meanwhile, accumulated evidences indicate that galanin also plays a neurotrophic/neuroprotective effect to subsets of neurons in the peripheral and central nervous systems [16]. For example, in both in vivo and in vitro models of injury, more hippocampal neuronal cell death was observed in the galanin knockout mice and less hippocampal neuronal cell death was observed in the galanin-overexpressing transgenic mice, compared with the WT controls [24]. Galanin also inhibits the neurotoxicity induced by amyloid-beta in primary cultured hippocampal neurons from human, rat, and transgenic animal [25, 26].
In the present study, for the first time, we demonstrated that galanin, at concentrations ranging from 100 pM to 1 nM, had significant protective effects on H2O2-induced cell death of cultured rat cortical astrocytes. Exogenous H2O2 treatment induces reactive oxygen species (ROS) generated intracellularly. When ROS accumulation exceeds cellular capacity, the resulting oxidative stress leads to astrocyte death [27]. H2O2 had been shown to enhance the phosphorylation of protein kinase ERK in astrocytes [28]. Although the activation of ERK1/2-MAPK has been considered as a signaling for cell survival, growth, and proliferation, it has also been shown that inhibiting the pERK signal pathway reduced astrocyte death induced by oxidative stress [29]. In the present study, the upregulation of pERK1/2 induced by H2O2 was partly reversed by galanin, suggesting that galanin suppressed the H2O2-induced toxicity in astrocytes of rats through suppressing the activation of pERK1/2. It should be noted that galanin has also been reported to activate ERK signal in neurons [30]. Thus, the signaling mechanisms of galanin might be different in different cells.
GalR1 and GalR2 are widely expressed in the central nervous system while the expression of GalR3 is limited [31, 32]. It has been demonstrated that the neuroprotective effects of galanin are mediated through its receptors. Thus, galanin has its neuron protection functions through GalR2 [17, 18, 24] or GalR1 [19, 20]. Our study here showed that the GalR2 mRNA expression was moderate while the GalR1 and GalR3 mRNA expression were very low in the cultured rat cortical astrocytes. Moreover, GalR2 agonist AR-M1896 mimicked the protective effects of galanin in the astrocytes. All those results suggest that GalR2 is involved in the protection of cortical astrocytes. This is consistent with previous studies in neurons that GalR2 mediates the protective effects of galanin [18, 33]. Interestingly, galanin had its effect only at concentrations ranging from 100 pM to 1 nM, and AR-M1896 had its effect only at concentrations ranging from 10 pM to 100 nM. The bell-shaped dose responses of galanin has been reported as a critical concentration window in earlier studies [26]. Moreover, we also seen AR-M1896 had broader effective concentration than galanin in the current study. The mechanisms underlying the bell-shaped dose response are still unclear. One possible explanation is galanin might act on different subtype receptors with different binding affinity. However, it needed to be further investigated.
A recent study shows that astrocytes are transformed to A1 reactive type when treated with a combination of IL-1α, TNFα, and C1q in vitro [10]. A1-type reactive astrocytes are considered to be neurotoxic and contribute to neuron death after acute CNS injury. For example, A1-type astrocytes have been proved taken a role in the neuroinflammation of traumatic spinal cord injury [34]. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Alzheimer's disease or Parkinson's disease [35, 36]. Therefore, modulating the harmful effects of A1-type astrocytes could be an important stratagem for the CNS disease treatment. However, galanin had no effect on A1-type transformation of the cultured rat cortical astrocytes in the present study.
5. Conclusion
Galanin protected rat cortical astrocytes from H2O2-induced cell death by suppressing the upregulation of pERK1/2, mainly mediated by GalR2.
Acknowledgments
This work was supported by grants from Beijing Municipal Science & Technology Commission (Z181100001518001), Capital Medical University scientific research funds (PYZ2017065), the National Natural Science Foundation of China (81671345, 81571281, and 81401119), the Municipal Science & Technology Commission (key project, KZ201610025026), and Beijing Natural Science Foundation (KM/KZ7172018).
Contributor Information
Liang Li, Email: liliang@ccmu.edu.cn.
Zhi-Qing David Xu, Email: zhiqingx@ccmu.edu.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- 1.Sofroniew M. V., Vinters H. V. Astrocytes: biology and pathology. Acta Neuropathologica. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sofroniew M. V. Astrocyte barriers to neurotoxic inflammation. Nature Reviews Neuroscience. 2015;16(5):249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy-Royal C., Dupuis J., Groc L., Oliet S. H. R. Astroglial glutamate transporters in the brain: regulating neurotransmitter homeostasis and synaptic transmission. Journal of Neuroscience Research. 2017;95(11):2140–2151. doi: 10.1002/jnr.24029. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoud S., Gharagozloo M., Simard C., Gris D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells. 2019;8(2):p. 184. doi: 10.3390/cells8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel D. C., Tewari B. P., Chaunsali L., Sontheimer H. Neuron–glia interactions in the pathophysiology of epilepsy. Nature Reviews Neuroscience. 2019;20(5):282–297. doi: 10.1038/s41583-019-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan F., Lax N. Z., Voss C. M., et al. The role of astrocytes in seizure generation: insights from a novel in vitro seizure model based on mitochondrial dysfunction. Brain. 2019;142(2):391–411. doi: 10.1093/brain/awy320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shandra O., Winemiller A. R., Heithoff B. P., et al. Repetitive diffuse mild traumatic brain injury causes an atypical astrocyte response and spontaneous recurrent seizures. The Journal of Neuroscience. 2019;39(10):1944–1963. doi: 10.1523/JNEUROSCI.1067-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santello M., Toni N., Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nature Neuroscience. 2019;22(2):154–166. doi: 10.1038/s41593-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 9.Zamanian J. L., Xu L., Foo L. C., et al. Genomic analysis of reactive astrogliosis. The Journal of Neuroscience. 2012;32(18):6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liddelow S. A., Guttenplan K. A., Clarke L. E., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatemoto K., Rökaeus A., Jörnvall H., McDonald T. J., Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Letters. 1983;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 12.Melander T., Hokfelt T., Rokaeus A. Distribution of galanin like immunoreactivity in the rat central nervous system. Journal of Comparative Neurology. 1986;248(4):475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- 13.Šípková J., Kramáriková I., Hynie S., Klenerová V. The galanin and galanin receptor subtypes, its regulatory role in the biological and pathological functions. Physiological Research. 2017;66(5):729–740. doi: 10.33549/physiolres.933576. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z. Q., Shi T. J., Hokfelt T. Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. The Journal of Comparative Neurology. 1998;392(2):227–251. doi: 10.1002/(SICI)1096-9861(19980309)392:2<227::AID-CNE6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Hökfelt T., Barde S., Xu Z. Q. D., et al. Neuropeptide and small transmitter coexistence: fundamental studies and relevance to mental illness. Frontiers in Neural Circuits. 2018;12:p. 106. doi: 10.3389/fncir.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobson S. A., Bacon A., Elliot-Hunt C. R., et al. Galanin. Vol. 102. Basel, Switzerland: Springer; 2010. Galanin acts as a trophic factor to the central and peripheral nervous systems; pp. 25–38. (Experientia supplementum). [DOI] [PubMed] [Google Scholar]
- 17.Elliott-Hunt C. R., Pope R. J. P., Vanderplank P., Wynick D. Activation of the galanin receptor 2 (GalR2) protects the hippocampus from neuronal damage. Journal of Neurochemistry. 2007;100(3):780–789. doi: 10.1111/j.1471-4159.2006.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott-Hunt C. R., Holmes F. E., Hartley D. M., Perez S., Mufson E. J., Wynick D. Endogenous galanin protects mouse hippocampal neurons against amyloid toxicity in vitro via activation of galanin receptor-2. Journal of Alzheimer's Disease. 2011;25(3):455–462. doi: 10.3233/JAD-2011-110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webling K., Groves-Chapman J. L., Runesson J., et al. Pharmacological stimulation of GAL1R but not GAL2R attenuates kainic acid-induced neuronal cell death in the rat hippocampus. Neuropeptides. 2016;58:83–92. doi: 10.1016/j.npep.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Li Y., Mei Z., Liu S., et al. Galanin protects from caspase-8/12-initiated neuronal apoptosis in the ischemic mouse brain via GalR1. Aging and Disease. 2017;8(1):85–100. doi: 10.14336/AD.2016.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priller J., Haas C. A., Reddington M., Kreutzberg G. W. Cultured astrocytes express functional receptors for galanin. Glia. 1998;24(3):323–328. doi: 10.1002/(SICI)1098-1136(199811)24:3<323::AID-GLIA6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Liu H.-X., Brumovsky P., Schmidt R., et al. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: selective actions via GalR1 and GalR2 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(17):9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang R., Gundlach A. L., Holmes F. E., et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharmacological Reviews. 2015;67(1):118–175. doi: 10.1124/pr.112.006536. [DOI] [PubMed] [Google Scholar]
- 24.Elliott-Hunt C. R., Marsh B., Bacon A., Pope R., Vanderplank P., Wynick D. Galanin acts as a neuroprotective factor to the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):5105–5110. doi: 10.1073/pnas.0304823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y., Yu L. C. Galanin protects amyloid-β-induced neurotoxicity on primary cultured hippocampal neurons of rats. Journal of Alzheimer's Disease. 2010;20(4):1143–1157. doi: 10.3233/JAD-2010-091234. [DOI] [PubMed] [Google Scholar]
- 26.Cui J., Chen Q., Yue X., et al. Galanin protects against intracellular amyloid toxicity in human primary neurons. Journal of Alzheimer's Disease. 2010;19(2):529–544. doi: 10.3233/JAD-2010-1246. [DOI] [PubMed] [Google Scholar]
- 27.Dowell J. A., Johnson J. A. Mechanisms of Nrf2 protection in astrocytes as identified by quantitative proteomics and siRNA screening. PLoS One. 2013;8(7, article e70163) doi: 10.1371/journal.pone.0070163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito J. I., Nagayasu Y., Ogawa T., Okihara H., Michikawa M. Biochemical properties in membrane of rat astrocytes under oxidative stress. Brain Research. 2015;1615:1–11. doi: 10.1016/j.brainres.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Elmann A., Telerman A., Mordechay S., et al. 3,5,4′-Trihydroxy-6,7,3′-trimethoxyflavone protects astrocytes against oxidative stress via interference with cell signaling and by reducing the levels of intracellular reactive oxygen species. Neurochemistry International. 2014;78:67–75. doi: 10.1016/j.neuint.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Hawes J. J., Narasimhaiah R., Picciotto M. R. Galanin and galanin-like peptide modulate neurite outgrowth via protein kinase C-mediated activation of extracellular signal-related kinase. European Journal of Neuroscience. 2006;23(11):2937–2946. doi: 10.1111/j.1460-9568.2006.04828.x. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell D., Ahmad S., Wahlestedt C., Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. Journal of Comparative Neurology. 1999;409(3):469–481. doi: 10.1002/(SICI)1096-9861(19990705)409:3<469::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Waters S. M., Krause J. E. Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience. 2000;95(1):265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- 33.Pirondi S., Fernandez M., Schmidt R., Hokfelt T., Giardino L., Calza L. The galanin-R2 agonist AR-M1896 reduces glutamate toxicity in primary neural hippocampal cells. Journal of Neurochemistry. 2005;95(3):821–833. doi: 10.1111/j.1471-4159.2005.03437.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu W., Wang Y., Gong F., et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. Journal of Neurotrauma. 2019;36(3):469–484. doi: 10.1089/neu.2018.5835. [DOI] [PubMed] [Google Scholar]
- 35.Xu X., Zhang A., Zhu Y., et al. MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κB and PI3K-Akt pathways. Journal of Cellular physiology. 2018;234(1):904–914. doi: 10.1002/jcp.26918. [DOI] [PubMed] [Google Scholar]
- 36.Yun S. P., Kam T. I., Panicker N., et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nature Medicine. 2018;24(7):931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.