Abstract
Chloride (Cl−) homeostasis is an essential process involved in neuronal signalling and cell survival. Inadequate regulation of intracellular Cl− interferes with synaptic signalling and is implicated in several neurological diseases. The main inhibitory neurotransmitter of the central nervous system is γ-aminobutyric acid (GABA). GABA hyperpolarises the membrane potential by activating Cl− permeable GABAA receptor channels (GABAAR). This process is reliant on Cl− extruder K+-Cl− cotransporter 2 (KCC2), which generates the neuron's inward, hyperpolarising Cl− gradient. KCC2 is encoded by the fifth member of the solute carrier 12 family (SLC12A5) and has remained a poorly understood component in the development and severity of many neurological diseases for many years. Recent advancements in next-generation sequencing and specific gene targeting, however, have indicated that loss of KCC2 activity is involved in a number of diseases including epilepsy and schizophrenia. It has also been implicated in neuropathic pain following spinal cord injury. Any variant of SLC12A5 that negatively regulates the transporter's expression may, therefore, be implicated in neurological disease. A recent whole exome study has discovered several causative mutations in patients with epilepsy. Here, we discuss the implications of KCC2 in neurological disease and consider the evolving evidence for KCC2's potential as a therapeutic target.
1. Introduction
Chloride (Cl−) is an abundant anion involved in a variety of physiological processes including gene regulation [1, 2], pH maintenance [3], and control of cell volume [4]. Primarily important in the neuron, Cl− plays a crucial role in signalling within the central nervous system (CNS). Healthy brain function requires the correct balance of neuronal excitation and inhibition to determine the firing of action potentials. Action potentials enable rapid propagation of signals. Imbalance of inhibitory and excitatory signals can lead to the development of neurological insults [5–7].
The main inhibitory neurotransmitter, γ-aminobutyric acid (GABA), binds to the ionotropic receptor GABA type A channel (GABAAR) [8–10]. GABA's role in signalling depends on the intracellular Cl− ([Cl−]i) concentration, which determines the reversal potential for GABAA currents (EGABA). EGABA lies close to the resting membrane potential (RMP) [11, 12]. Both EGABA and RMP vary between cell types and compartments. The depolarising or hyperpolarising effect of GABAergic signalling is dependent on the relative RMP and EGABA. When [Cl−]i is high, EGABA is less negative and GABA stimulation results in depolarisation; when it is low, EGABA is more negative and GABA stimulation is hyperpolarising [13, 14]. In healthy adult neuron's [Cl−]i is usually maintained at a low concentration, enabling inhibitory, hyperpolarising GABAergic signalling [15]. This constitutes the main role of GABA in CNS neurotransmission; its potential dysfunction in neurological disease due to dysregulated cellular Cl− levels is, therefore, of significant interest. Depolarising GABA potentials, in contrast, are commonly observed in immature and peripheral neurons [16]. Finally, in addition to GABA's role in hyperpolarisation, it is able to act in a further inhibitory capacity via the mechanism of “shunting inhibition.” This process involves increased membrane conductance as a result of GABA stimulation “short circuiting” nearby excitatory potentials without producing a significant change in membrane potential.
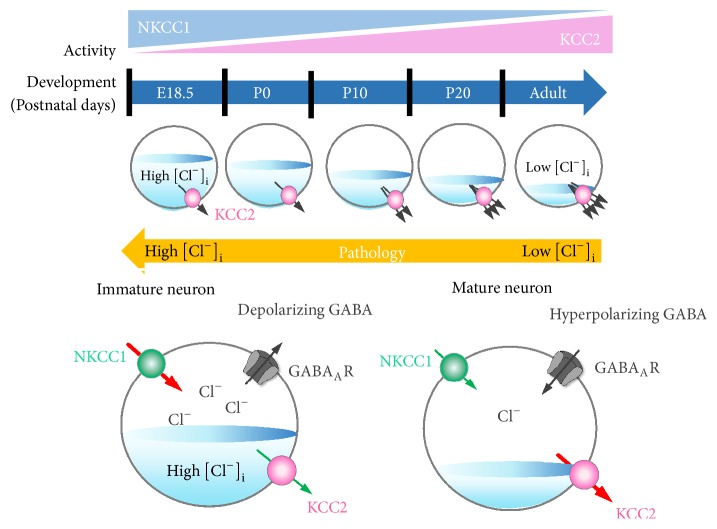
Neuronal [Cl−]i is regulated by the Na+-K+-2Cl− cotransporter 1 (NKCC1) and the K+-Cl− cotransporter 2 (KCC2) [17]. Using the Na+ gradient generated by Na/K/ATPase, NKCC1 drives Cl− into the cell; KCC2, in contrast, is the main Cl− extruder in mature neurons [18]. During development, NKCC1 and KCC2 expression patterns change. In the immature CNS, NKCC1 dominates resulting in high [Cl−]i. As maturation proceeds, KCC2 expression increases whilst NKCC1 levels fall (Figure 1) [19, 20]. Mature neurons, therefore, have low [Cl−]i causing a shift in EGABA from depolarising to hyperpolarising [19, 20]. Thus, KCC2 is a crucial regulator of GABA-mediated hyperpolarisation: an essential component of synaptic inhibition within the adult brain (Figure 2).
Figure 1.
G A B A A signalling shifts from depolarizing to hyperpolarising responses during development. In immature pyramidal cells, GABAAR-mediated Cl− currents are outward and depolarising because the relative ratio of NKCC1 to KCC2 activity is high. In mature neurons, increased KCC2 activity gives rise to inward GABAA-mediated Cl− currents that hyperpolarize the membrane potential.
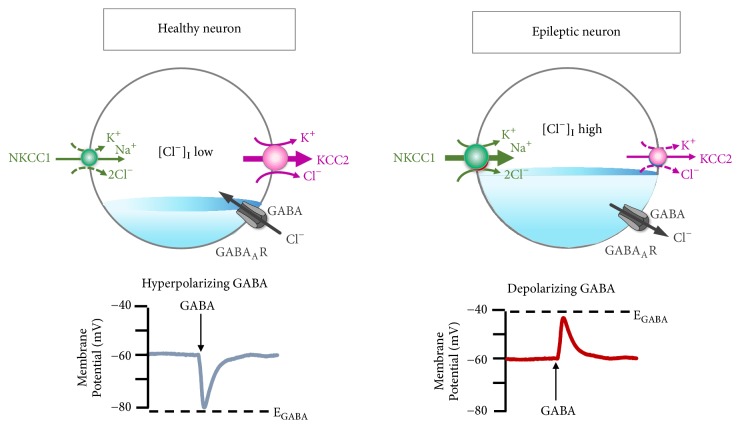
Figure 2.
Neuronal Cl − volume is reciprocally regulated via NKCC1 and KCC2. In healthy mature neurons, [Cl−]i is low due to the opposing activity profiles of NKCC1 and KCC2. This promotes GABAAR-mediated hyperpolarization, which is critical for the proper balance of excitation-inhibition in neuronal circuits (left panel). In neurons implicated in multiple neuropsychiatric conditions driven by hyperexcitable circuits (e.g., seizures, neuropathic pain, spasticity, schizophrenia, and others), [Cl−]i are elevated due to increased NKCC1 activity, and/or decreased KCC2 activity, promoting GABAAR-mediated membrane depolarization and excitation (right panel).
Loss of KCC2 activity orchestrates a depolarising shift in EGABA and is implicated in cortical development systems such as neuro- and synaptogenesis [12, 21]. The fundamental role that KCC2 downregulation plays in these processes suggests a causal link between Cl− homeostasis and the pathogenesis of neurodevelopmental disorders [22, 23]. Although categorised differently, neurodevelopmental disorders including autism and schizophrenia display phenotypic similarities, most notably high copy number variation [24]. These attributes suggest a genetic link between these diseases.
Excitatory and inhibitory imbalance is implicated in the onset of epilepsy. Biopsies of epileptic tissue have identified excitatory GABA activity in response to loss of KCC2 expression and subsequent [Cl−]i increase [25–27]. Similarly, in Huntington's disease positive rat models, upregulation of NKCC1 and loss of KCC2 caused GABA mediated stimulation to switch from an inhibitory to excitatory response [28]. Collectively, these studies suggest that researching expression patterns of KCC2 may further our understanding of the aetiology of these diseases.
The aim of this review is to evaluate the role of KCC2 in various pathological conditions. Consideration will first be given to the structure of KCC2; how this affects its function and expression is a key component to understanding its role in disease. Attention will also be given to specific diseases in which KCC2 dysfunction is implicated. Finally, KCC2 will be discussed as a pharmaceutical target for neurological diseases.
2. Structure and Diversity of the Cl− Cotransporter KCC2
The KCC2 Cl− cotransporter is transcribed from the fifth member of the solute carrier 12 (SLC12A5) gene family. During alternative splicing, SLC12A5 produces two isoforms: KCC2a and KCC2b [29]. The KCC2a transcript is commonly expressed in the spinal cord between embryonic day (E) 14 and postnatal day (P) 60, whilst KCC2b is greatly upregulated in the hippocampus and the neocortex between E17-P14 [29]. As development progresses, KCC2a expression falls whilst KCC2b is upregulated in the mature CNS. KCC2a is, therefore, the favoured isoform in the immature brain but is eventually dominated by KCC2b in adulthood [30]. Structural differences between these isoforms are localised to the N-terminus where they possess a unique 40 amino acid structure. Despite this, their ion transport activity is almost identical [31]. For the purposes of this review, KCC2 denotes KCC2b.
Although KCC2 is one of the most heavily researched transporters within the CNS, limitations in X-ray analysis have led to poor understanding of its structure and functional mechanisms. Hydropathy blot analysis suggests that KCC2 contains 12 transmembrane domains anchored by intracellular N- and C-termini [32]. Precisely half of the protein is intracellular and is the target for a number of kinases and a single phosphatase (Figure 3). Studies have begun to uncover an integral role of the C-terminus in KCC2 activity [33]. For example, posttranslational modifications - phosphorylation and/or glycosylation have been associated with the extrusive qualities displayed by KCC2 [34–36]. During development, KCC2 assembly becomes more complex, with immature brains displaying a higher monomeric count whilst oligomerisation correlates with maturation [37]. More recently, Agez and colleagues showed that KCC2 exists in a monomeric and dimeric state in solution [38]. The same group also noted that peptide C-terminal tagging of KCC2 caused detrimental functional changes and inactivation when expressed in HEK293 cells [38]. Their findings suggest a crucial role of the KCC2 C-terminus in its activity.
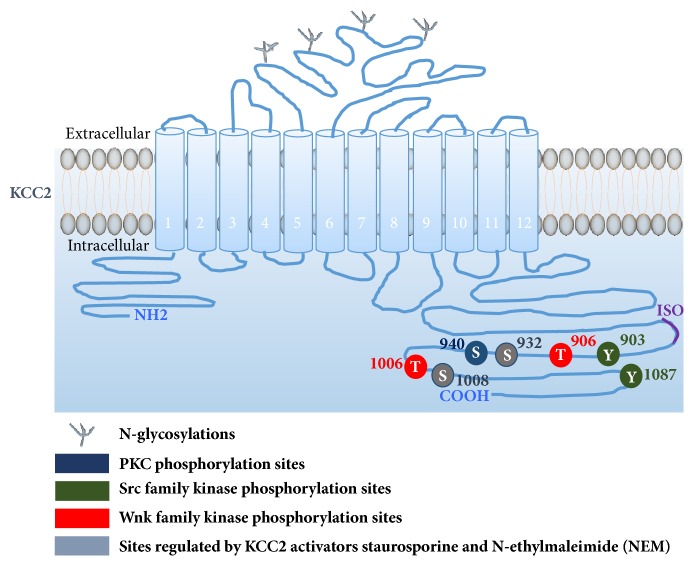
Figure 3.
Schematic representation of important regulatory phosphoresidues of the neuron-specific K–Cl cotransporter KCC2. The mouse KCC2 co-transporter consists of 12 membrane spanning predicted segments, an N-linked glycosylated extracellular domain between transmembrane domains 5 and 6. This is flanked by two cytoplasmic carboxy- and amino-terminal domains of 104 and 481 amino acids, respectively. Positions of phosphoresidues that are critical for functional regulation of KCC2, including tyrosine 903 (Y903), threonine 906 (T906), serine 940 (S940), threonine 1006 (T1006, this site is corresponding to Rat T1007), tyrosine 1087 (Y1087), and S932 and T1008 (regulated by KCC2 activators staurosporine and N-ethylmalemide (NEM)), are indicated. The Purple region denotes the KCC2 ‘ISO' domain, required for hyperpolarizing GABAergic transmission.
Whilst these findings provide insight into the functional significance of KCC2 structure, they fail to show this effect in a neuronal setting. HEK293 are an embryonic kidney cell line commonly used in the analysis of ion homeostasis. Both KCC2 isoforms are predominantly expressed in neurons of the brain and spinal cord, organs with several physiological and functional differences to the kidney. These differences are evident in the findings of Uravov and colleagues who noted that inhibition of KCC2 mRNA expression differs between neuronal and nonneuronal cells. KCC2 mRNA expression is mediated by RE-1 silencing transcription factor in nonneuronal cells, which represses the SLC12A5 gene [39]. In neurons, however, the transcription factor early growth response 4 (Erg4) is developmentally upregulated, stimulating an increase in KCC2. This indicates fundamental differences in KCC2 expression between cell types [40]. Further research in CNS specific cell types (e.g. neuroblastoma or primary neurons) is required to determine the therapeutic implications of KCC2 expression.
In animal models of traumatic and ischaemic brain injury, KCC2 is reportedly downregulated at both the protein and mRNA levels [41–43]. Six hours after transient forebrain ischaemia, the KCC2 peptide became more abundant in the dendritic regions of pyramidal cells in the cornu Ammonis 1 (CA1) region of the hippocampus, which displayed no evidence of damage. Over an extended time period (48 h after stroke induction), the same cells began to degenerate in a manner that correlated with downregulation of KCC2 and heat-shock protein 72 (HS72). HS72 can exacerbate or attenuate hypothalamic neuronal death depending on its peptide expression levels and is not expressed in the mature brain under standard conditions [44]. Parvalbumin positive interneurons, which exhibit high SLC12A5 gene expression and glutamatergic input, often survive these events even in regions of complete pyramidal cell loss [45]. This suggests that KCC2 expression is also mediated by brain health; upregulation of the cotransporter may indicate onset or previous infliction of neurological insult.
3. Neuronal Expression of KCC2
KCC2 is heavily expressed in the mature CNS and is rarely found in peripheral neurons and nonneuronal cells [46–48]. Upregulation of KCC2 is correlated with neuronal differentiation which occurs caudally to rostrally in the CNS [49]. In the rodent CNS, the caudal section, i.e., spinal cord and brain stem, shows little difference in KCC2 expression compared to that observed in the more mature neuron [49–51]. Conversely, rostral regions such as the hippocampus and neocortex display upregulation of SLC12A5 mRNA from birth [49, 52].
Whilst KCC2 clearly displays region specificity within the body, these studies fail to consider variation in the cotransporter's expression between species. In rats and mice, for example, KCC2 levels remain low resulting in greater EGABA [12]. Data collected by Dzhala et al. (2005) showed that a similar expression pattern was present in neonatal humans. Human parietal lobe autopsy specimens displayed high neuronal expression of NKCC1 and low expression of KCC2 but only before the end of the first year of life [11]. Conversely, work conducted by Sedmak et al. (2016) noted KCC2 expression begins much earlier in humans, during the mid-foetal period and increases to levels resembling adult maturity 6 months after birth [53]. Such inconsistencies may be explained by the use of only a single brain region in Dzhala's study. Alternatively, differences in maturation between humans and rodents may be responsible. Neonatal rat and mice cortices, for example, achieve a developmental stage which equates to the beginning of the third trimester of gestation in the human foetus [54, 55]. Together, these data indicate that KCC2 expression may be considered both species- and age-dependant.
KCC2 protein expression has also been associated with Ca2+-dependent mechanisms following neuronal damage [56–58]. Various studies have shown that KCC2 activity is heavily reduced after cleavage at the C-terminal domain by calpain proteases. Hypoxic-ischaemic encephalopathy is considered a major contributor to long-term neuronal damage with an apparent relationship between increased intracellular Ca2+ and neuronal damage under hypoxic conditions [59]. Calpains are Ca2+-dependent proteases. Perinatal mammals exhibit a high calpain/calpastatin (the inhibitor of calpain) ratio. Calpain overexpression or excessive activity has been associated with the symptoms of several neurological conditions including hypoxic ischaemia [60, 61], seizures [57], and epilepsy [62]. KCC2 upregulation is required during neuronal maturation to enhance the inhibitory properties of GABAAR [12, 21]. This process is, therefore, highly sensitive to excessive calpain activity causing a paucity of active KCC2. Thus, calpains may play a fundamental role in the aetiology of these diseases.
4. Regulation of KCC2 Activity
4.1. Phosphorylation
The activity and expression of KCC2 at the plasma membrane is regulated by phosphorylation. KCC2's carboxyl-domain is the target for several known kinases and is regularly phosphorylated at the serine 940 (S940) residue. Phosphorylation of S940 decreases KCC2 internalisation maintaining high KCC2 membrane expression [63]. This process is regulated by protein kinase C (PKC) which directly phosphorylates S940 resulting in greater transporter activity [63]. In contrast, dephosphorylation causes a fall in KCC2 activity mediated by a reduction in transporter stability [64]. The S940 residue and PKC activity are, therefore, key components in KCC2 regulation. Modulation of PKC activity by separate pathways, therefore, also indirectly regulates KCC2 activity and Cl− homeostasis. Of note is the neuropeptide oxytocin which was found to increase KCC2 activity and support GABAergic signalling by Leonzino et al. (2016) [65]. Using PKC-inhibitors, Leonzino and colleagues prevented oxytocin mediated KCC2 upregulation suggesting a regulatory role of the neuropeptide in this process [65].
The neurotransmitter serotonin has also been reported to influence KCC2 activity. Serotonin binds and activates the receptor 5-hydroxytryptamine type 2A (5-HT2A) in a process that increases cell membrane KCC2 levels and subsequently restores endogenous synaptic inhibitory mechanisms in mouse models displaying injury to the spinal cord [66]. This serotonin-mediated activity is believed to be PKC-dependent given that PKC inhibitors reduced KCC2 activity [66]. Together, these results suggest S940 phosphorylation is influenced by several pathways. Given the crucial regulatory role of this residue, we can infer that the transporter's expression oscillates according to a variety of paracrine stimuli. Given the increased cell-surface density of KCC2 during S940 phosphorylation, this may be a particularly promising area of therapeutic study. Therapeutic enhancers of S940 phosphorylation may prove effective in this field especially given the recent finding that KCC2 potentiation can limit onset and severity of neuropathic seizures [67].
The dependence on C-terminal domain integrity displayed by KCC2 makes this domain a potential target for therapeutic treatments. For example, KCC2 membrane stability is heavily reduced when tyrosine residues 903 and 1087 are phosphorylated causing its subsequent trafficking to the lysosome [64]. In addition, the threonine906 (Thr906) and threonine1007 (Thr1007) residues display inhibitory characteristics when phosphorylated [68, 69]. During the neonatal period, brain localised Thr906 and Thr1007 are often phosphorylated, thereby preventing premature KCC2 activity [68, 69]. Mutants of KCC2, however, commonly show variation at these phosphorylation residues. Mutations at S932 to aspartate (S932D, mimicking phosphorylation) or T1008 to alanine (T1008A, mimicking dephosphorylation) significantly enhance KCC2 activity (up to 1.5-2-fold increase) in HEK293 cells [70]. Mutation at S940 to alanine (S940A, mimicking dephosphorylation) in vivo reduces KCC2 activity and enhances the effects of kainate-induced status epilepticus [36]. In contrast, Thr906A/Thr1007A double-point alanine substitution enhances KCC2 function in cell culture [67, 68, 71, 72]. Interestingly, Thr1007A mutations do not impact KCC2 surface expression. Preventing phosphorylation of Thr906 and Thr1007 is, however, sufficient to enhance the Cl− extrusive properties of KCC2 in vivo [67]. Such findings suggest that this is not just the result of increased KCC2 protein but rather multiple processes. The authors hypothesised that these mutations increase KCC2 affinity for Cl−. KCC2 Thr906A/ Thr1007A variant-carrying neurons reached Cl− equilibrium at a more negative EGABA than the wild type control. When Cl− admittance is low, the increased Cl− affinity displayed by these variants aids extrusion at levels beyond the wild-type threshold [67]. This increase in KCC2 function was sufficient to reduce chemoconvulsant-induced seizure activity and severity [67], suggesting that the cotransporter has therapeutic potential as a seizure limiting drug target.
Recent data provided by Friedel et al. (2015) showed the protein, With-no-lysine kinase 1 (WNK1) stimulated phosphorylation of both Thr906 and Thr1007 by means of the Kinase, Ste20-related proline alanine-rich kinase (SPAK) [69]. SPAK was phosphorylated and subsequently activated by WNK1 inhibiting KCC2 activity [69]. SPAK function and phosphorylation may also fluctuate throughout development depending on WNK1 activity [73]. Should phosphorylation of KCC2 residues Thr906 and Thr1007 occur in immature brains but fall during development, it may explain why KCC2-dependent Cl− extrusion dominates in the adult CNS [69]. WNK1 is, therefore, a key regulator of KCC2 activity and a potential therapeutic target for the treatment of excitatory/inhibitory disorders.
Interestingly, Friedel et al. (2015) also found that inhibition of WNK1 dephosphorylated KCC2 at Thr906 and Thr1007 [69]. This relationship was noted in other studies suggesting a regulatory role of WNK1 in KCC2 activity. KCC2 activity assays showed the amino acid taurine significantly inhibited KCC2 via serine/threonine phosphorylation compared to control and also activated WNK1 [74]. This corroborates Friedel et al. (2015) who showed that inhibition of WNK1 increased [Cl−]i extrusion in a KCC2-dependent manner in cultured rat hippocampal and cortical neurons [69]. Genetic studies examining changes in WNK1 activity may elucidate the aetiology of many neurological diseases.
Using the organic compound N-ethylmaleimide (NEM), Conway et al. (2017) increased KCC2 activity through increased S940 phosphorylation and decreased Thr1007 phosphorylation [72]. Interestingly, NEM was found to potentiate KCC2 activity in neurons, particularly in cells with higher pThr1007 levels or lower pS940 [72]. Furthermore, KCC2 mutation S932D could abolish further stimulation by NEM, whereas T1008A by another KCC2 activator, staurosporine [70]. Such findings provide valuable insight into therapeutic limitations as drugs that act to modulate KCC2 surface levels or intrinsic conformational change through phosphorylation [33, 34] would only be effective in cases of high pThr1007 or high pThr1008 and low pSer940 or low pSer932. These attributes are more common in cases of spinal cord injury. Nevertheless such drugs may be of some use in the treatment of neurological disorders. Despite this limitation, their work suggests that manipulation of Thr1007 phosphorylation may prove relevant to the advancement of neurological therapeutics.
An independent study identified the regulatory role of five phosphosites Ser31, Thr34, Ser932, Thr999, and Thr1008 using alanine and aspartine mutants [70]. Substitution of Ser31, Thr34, and Thr999 did not affect KCC2 activity. Ser932D (mimicking phosphorylation) and Thr1008A (mimicking dephosphorylation), however, increased transporter activity [70]. In addition, treatment with the known KCC2 activators NEM or staurosporine was ineffective in activating Ser31D, Thr34A, Ser932A/D, Thr999A, Thr1008A/D or Ser31A, Thr31D, Ser932D KCC2 variants, respectively [70]. These results demonstrated the existence of phosphosensitive sites that regulate KCC2 activities via the integration of various signalling pathways.
4.2. Trophic Factors
KCC2 activity is modulated by a number of trophic (growth) factors including TGF-β2 [75], neurotrophic factor [76], and brain-derived neurotrophic factor (BDNF) [57]. Of these, BDNF is the most well-studied modulator of KCC2 activation.
BDNF is a 27-kDa polypeptide involved in neuronal survival, differentiation, and growth [77]. Its role in KCC2 regulation was first discovered by Aguado et al. (2003) who noted that KCC2 mRNA levels increased with overexpression of the BDNF gene in developing neurons. This process was later found to utilise the Tropomyosin-related kinase (Trk) pathway, as deletion of the TrkB isoform decreased KCC2 mRNA [78]. These data suggest a proregulatory role of BDNF in immature neurons. In mature neurons, however, BDNF downregulates KCC2 at both the protein and RNA levels [79, 80].
Recently, Huang and colleagues noted BDNF-KCC2 regulation was injury dependent. In intact animals, BDNF downregulated membrane-bound KCC2. In animals with spinal cord injury, however, BDNF upregulated the cotransporter [81]. Reasons for these differences are not yet understood, although the authors suggested one hypothesis based on BDNF-TrKB receptor binding. This causes activation of signal pathway components such as PLCγ; BDNF downregulates KCC2 in the presence of PLCγ but upregulates it when PLCγ is lacking. Given that spinal cord injury has previously been found to decrease PLCγ expression [80], it may play a logical role in injury-dependent KCC2 regulation. Interestingly, a separate study has shown that BDNF plays a crucial role in KCC2 upregulation after seizure-induced neuronal insult [57]. Together, these studies suggest that targeting BDNF may be of therapeutic value in the treatment of diseases involving KCC2 downregulation.
4.3. Transcriptional and Translational Regulation
KCC2 expression is exclusive to neuronal cells, as dictated by the activity of a neuron-restrictive silencing element (NRSE) acting at the first intron of SLC12A5 [82, 83]. A 1.4 kb promoter fragment is also implicated in KCC2 neuron expression. This was identified in a transgenic model lacking NRSE. Cells lacking NRSE showed increased levels of KCC2 expression and also expressed the active 1.4 kb promoter fragment [39]. The transcription factor Erg4 has since been found to bind to this promotor fragment and regulate KCC2 expression [40]. SLC12A5 also displays a second binding site within its promoter region known as the E-box region, which binds upstream stimulating factors (USF) 1 and 2. USF1 is negatively regulated by amyloid precursor protein (APP) which simultaneously downregulates KCC2 [84]. USF1 is, therefore, a potentially key component in the expression pattern of KCC2. Regulatory proteins such as APP and USF1 may act as biomarkers for the early identification of neurological and epileptic disease.
For successful Cl− extrusion, KCC2 must be expressed at the cell surface. A further role in which APP is implicated is the stabilisation of KCC2 at the cell membrane. Direct binding of APP to KCC2 blocks phosphorylation of the tyrosine residues (903, 1087) which normally promote transporter internalisation and degradation [85]. In this way, APP acts as both a pre- and posttranslational regulator of KCC2 activity and displays strong therapeutic potential for the treatment and/or diagnosis of diseases associated with KCC2 dysfunction.
Surface expression of the KCC2 cotransporter is regulated by kainate receptors, through formation of molecular complexes between the kainate receptor subunit GluK2 and KCC2 [86, 87]. Phosphorylation of Gluk2 by PKC increases KCC2 activity, but PKC can also act directly on the cotransporter due to activation of group 1 metabotropic glutamate receptors (mGluRs). Through induction of Ca2+ release from internal stores, these receptors increase intracellular levels of the cation [88]. PKC is a Ca2+-sensitive kinase meaning its subsequent activation by group 1 mGluRs is an important component of KCC2 recorded activity. In this way, glutamatergic signalling can indirectly enhance inhibitory GABAergic signalling through increased KCC2 activity [88]. This process is implicated in maintaining equilibrium between excitatory and inhibitory signals [88]. Many neurological diseases are attributed to imbalance of these signals. This indirect mechanism of KCC2 regulation, therefore, presents a potential therapeutic pathway for drug targeting.
5. The Role of KCC2 in the Development of Epilepsy
The role of KCC2 mutants in epilepsy development was discovered in two separate studies conducted on patients displaying different epileptic symptoms. The first studied an Australian family suffering from febrile seizures and identified an arginine-to-histidine substitution at position 952. This missense mutation, formally named R952H, caused a substantial decrease in KCC2 membrane expression compared to the wild-type control [89]. The second, conducted by Kahle and colleagues, investigated idiopathic generalised epilepsy in a cohort of Canadian patients displaying the same mutation, c.2855G>A (R952H) [90]. Companion studies noted a significant decrease in Cl− extrusion compared to control indicative of KCC2 impairment [90].
Kahle et al. (2014) also found a second KCC2 variant, R1049C, with a cysteine substitution at the 1049 position. According to in silico bioinformatics programmes, this mutation is predicted to possess pathogenic properties that correlate with KCC2 dysfunction [90]. In accordance with the findings of Puskarjov et al. (2014), Kahle and colleagues showed that R952H mutants had a significantly lower level of KCC2 expressed at the cell surface. In R1049C mutants, however, KCC2 levels were not noticeably different to control [89, 90]. R1049C reduced KCC2 efficacy for Cl− extrusion, resulting in higher basal [Cl−]i levels and membrane depolarisation at the previously inhibitory synapse [90]. Both variants also displayed a significant (>50%) decrease in S940 phosphorylation. Thus, both R952H and R1049C C-terminal mutations reduce KCC2 activity. This, in part, may be due to a decrease in stimulatory S940 phosphorylation [90]. Alternatively, interaction of these variants with the ISO domain (a unique 15 amino acid region on the KCC2 C-terminal domain) which has previously been identified as a vital component to KCC2 isotonic activity may cause the observed reduction in KCC2 function [91].
More recently, Stödberg and colleagues identified an autosomal recessive heterozygous loss-of-function mutation in the SLC12A5 gene in children from two separate families [92]. In both families, two children developed clinical features of epilepsy of infancy with migrating focal seizures (EIMFS). All mutated residues were of KCC2b lineage: L288H, L403P, and G528D. Of the four children examined, two had compound heterozygous mutations, c.1208T>C (p.L403P) and c.1583G>A (p.G528D), whilst the others had homozygous missense mutations, c.863T>A (p.L288H) [92]. L403P and G528D mutants displayed complete loss of KCC2-mediated Cl− extrusion, whilst the homozygous L403P mutant had reduced surface expression and glycosylation leading to partial loss of function [92]. Their data further contributes to the growing evidence that disruption of KCC2 activity is implicated in epilepsy. Research into additional mutations affecting SLC12A5 may provide novel insight into the individual application of antiepileptic strategies.
There are, however, limitations to the data collected here that cannot be overlooked. All variants described in these studies were only identified through examination of the SLC12A5 gene sequence. The need for whole genome sequencing intervention to identify other variants or alleles not encoded by SLC12A5 but that augment KCC2 activity was raised by these studies [89, 90, 92].
Another study conducted by Saitsu et al. (2016) also identified four previously undiscovered KCC2 variants that resulted in EIMFS [93]. In a sample of ten sporadic and one familial case of EIMFS, whole exome sequencing identified compound heterozygous SLC12A5 variants in two families: c.279 + 1G > C causing skipping of exon 3 in the transcript (p.E50_Q93del), c.572 C >T (p.A191V) in two siblings, and c.967T > C (p.S323P) and c.1243 A > G (p.M415V) in another individual. Another patient with migrating multifocal seizures carrying compound heterozygous mutations, c.953G>C (p.W318S) and c.2242_2244delTCC (p.S748del), was also identified from whole exome sequencing data of 526 patients and targeting of the SLC12A5 sequence from a cohort of 141 patients with infantile epilepsy [93]. Gramicidin-perforated patch-clamp analysis identified a reduction in Cl− extrusion of E50_Q93del and M415V mutants, with mildly impaired function of A191V and S323P mutants. Membrane expression of these KCC2 variants did not differ from control. Heterologous expression of two KCC2 variants, however, mimicking the patients' status, showed significantly higher [Cl−]i levels than wild-type KCC2 but lower levels compared to the group lacking KCC2 [93]. These findings indicate that even partial disruption to neuronal Cl− extrusion, mediated by two impaired variants of SLC12A5, causes EIMFS.
Since these discoveries, gene panel sequencing of an EIMFS patient from an unrelated family found a compound heterozygous constellation of variants in SLC12A5 consisting of a maternally inherited p.Ser399Leu and a de novo p.Arg880Leu mutation in human KCC2b [93]. Such mutations may be pathogenic.
6. KCC2 in Neurodevelopmental Disorders
KCC2's C-terminal domain is encoded at the 3' end of the SLC12A5 gene [90]. Recently, Merner et al. (2015) investigated KCC2 regulatory variation using Sanger sequencing to investigate the coding nucleotides 21-25 of the SLC12A5 gene [94]. The authors screened a total of 427 autism spectrum disorder (ASD), 143 schizophrenic, and 190 intellectual disability cases [94]. R952H and R1049C were among the mutations found in ASD cases. Interestingly, R952H was also implicated in schizophrenia, suggesting overlap between these disorders. Different phenotypic outcomes from R952H mutation (i.e., which disease the patient has) are likely dependent on other allele interactions.
Thorough understanding of how risk alleles contribute to disease is not yet established. In polygenic disease models, causality is never attributed to just one variant [95]. Merner showed that patients with ASD carried rare KCC2 variants that affected CpG sites [94]. CpG sites are prone to methylation, a process that can alter the expression pattern of the gene [96]. Variation in SLC12A5 expression in patients with ASD may, therefore, be the consequence of epigenetic interactions, which represent a potentially valuable focus for future research.
7. KCC2 in Neuropathic Pain
Neuropathic pain (NP) is characterised by spontaneous pain sensations and tactile allodynia. The system of pain detection requires a balance of excitatory and inhibitory signals. When this balance is disrupted either through injury or psychogenic insult, it can lead to NP. In both the spinal cord and dorsal horn, synaptic transmission patterns vary between NP models [97, 98]. This pain has been attributed to dysfunctional inhibitory mechanisms in the spinal cord. In fact, pharmacological disruption of synaptic inhibition within the dorsal horn induces symptoms commonly attributed to NP [99]. Reduction of the Cl− gradient across the neuronal membrane has since been identified as the cause of NP initiated by peripheral nerve injury [100]. This is the result of downregulation of KCC2. During NP pathogenesis, an array of cellular mechanisms converge causing a reduction in KCC2 expression and function and increase in neuronal [Cl−]i [100]. The need to identify cellular mechanisms that increase KCC2 activity during neuropathic episodes is, therefore, crucial to the advancement of therapeutics in this field.
Increasing KCC2 activity presents a very prudent area of research [101–103]; the ability to restore normal inhibitory function in neurological conditions associated with impaired Cl− transport may prove to be an effective therapeutic strategy. High-throughput screening assays have now identified KCC2 activators that reduce [Cl−]i. Gagnon et al. (2013) optimised a first-in-class arylmethylidine family of compounds (CLP257) to lower [Cl−]i [104]. CLP257 rescued KCC2 plasma expression, renormalised stimulated recall responses in spinal nociceptive pathways sensitized after nerve injury, and reduced hypersensitivity of NP rat models [104]. The results of Cardarelli et al. (2017), displaying CLP257 as a direct KCC2 activator, were not replicable [105] but do reveal the compounds' ability to potentiate GABAAR activity [105]. Furthermore, GABAAR-dependent synaptic inhibition by KCC2 antagonist, gabazine could actually tune KCC2 activity via the Cl−-sensitive WNK1 kinase [106]. Oral treatment of the CLP257 prodrug equivalent, CLP290, showed similar efficacy to their control of pregabalin, a drug commonly used in the treatment of epilepsy and anxiety [104, 107]. Side effects of pregabalin include dizziness and sedation causing motor function disturbance [107]. Such side effects were not present during treatment with CLP290 [104]. These results highlight KCC2 as a plausible target for NP drug therapy and may provide further insight into the treatment of other neurological disorders.
8. Therapeutic Potential of KCC2
KCC2's interaction with Cl− importer GABAAR makes it a potential target for the treatment of several neurological diseases. Currently, phenobarbital (PB), a barbiturate that delays the closing of GABAAR, is the most common first-line drug used for the treatment of seizures [108]. Hypoxic-ischaemic encephalopathy is a major contributor to the onset of neonatal seizures, with over 50% of patients displaying electrographic seizures even after treatment with PB [109]. Interruption to the expression and/or function of either KCC2 or NKCC1 affects the antiseizure efficacy of GABAAR agonists [110]. The higher [Cl−]i within immature neurons potentially contributes to resistance to pharmacological first-line antiseizure GABAAR agonists in the immature brain [111].
Recently, a translational model for age-dependent PB-resistant seizures was developed by Kang et al. (2015) [112]. Using a permanent unilateral carotid-ligation model of neonatal ischaemic-seizures in CD-1 pups, the authors investigated the ability of the NKCC1 antagonist bumetanide to rescue PB-resistance. Bumetanide failed to rescue PB as an antiseizure therapeutic [112]. A number of preclinical models show that the severity of seizure and mechanism of damage can influence the efficacy of antiseizure drugs and alter cotransporter expression [113–116]. Kharod et al. (2018) noted model-specific insults modulated both expression and function of the NKCC1 and KCC2 cotransporters. Using a pentylenetetrazol-induced seizure model, they identified a significant upregulation of KCC2. In contrast, ischaemia-induced seizures significantly downregulated KCC2 [117]. These data combined reveal KCC2 expression to be insult specific and may explain why some anticonvulsant therapies display variable efficacy during first-line treatment.
Activation of the Trk isoform TrkB has been shown to induce phosphorylation of phospholipase C-γ1 which is linked to the downregulation of KCC2 and development of epilepsy [118, 119]. Carter et al. (2018) showed that TrkB antagonist, ANA12, increases the efficacy of PB in CD1 mice at doses as low as 2.5 mg/kg. ANA12 also rescued KCC2 expression after postnatal ischaemia [120]. Unlike current clinical antagonists (e.g. bumetanide, furosemide), ANA12 is capable of passing through the blood-brain-barrier [121], allowing it to have greater therapeutic impact on KCC2 activity as this has previously been a limiting factor for treatments [122]. ANA12 may, therefore, have therapeutic benefit by preventing downregulation of KCC2, thus maintaining low [Cl−]i.
9. Conclusion
KCC2 is a key player in the maintenance of neuronal Cl− homeostasis. A plethora of studies identify KCC2 dysfunction and misregulation as a key component in the development and onset of many neurological diseases. KCC2 is a strong candidate for therapeutic targeting and should be further considered by pharmaceutical investors. It should be noted that the majority of these findings are not made in human neuronal cell lines and are, therefore, limited in their ability to determine the immediate effects of targeting KCC2. Despite this, the data collected from human participants indicates that there is a place for KCC2 pharmaceuticals in the treatment of epilepsy. Continued research in human neuronal cell types may reveal more opportunities for drug development.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Succol F., Fiumelli H., Benfenati F., Cancedda L., Barberis A. Intracellular chloride concentration influences the GABAA receptor subunit composition. Nature Communications. 2012;3:p. 738. doi: 10.1038/ncomms1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valdivieso Á. G., Clauzure M., Massip-Copiz M., Santa-Coloma T. A. The chloride anion acts as a second messenger in mammalian cells - modifying the expression of specific genes. Cellular Physiology and Biochemistry. 2016;38(1):49–64. doi: 10.1159/000438608. [DOI] [PubMed] [Google Scholar]
- 3.Ruffin V. A., Salameh A. I., Boron W. F., Parker M. D. Intracellular pH regulation by acid-base transporters in mammalian neurons. Frontiers in Physiology. 2014;5:p. 43. doi: 10.3389/fphys.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sardini A., Amey J. S., Weylandt K.-H., Nobles M., Valverde M. A., Higgins C. F. Cell volume regulation and swelling-activated chloride channels. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2003;1618(2):153–162. doi: 10.1016/j.bbamem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Marín O. Interneuron dysfunction in psychiatric disorders. Nature Reviews Neuroscience. 2012;13(2):107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 6.Nelson S. B., Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87(4):684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sgado P., Dunleavy M., Genovesi S., Provenzano G., Bozzi Y. The role of GABAergic system in neurodevelopmental disorders: a focus on autism and epilepsy. International Journal of Physiology, Pathophysiology and Pharmacology. 2011;3(3):223–235. [PMC free article] [PubMed] [Google Scholar]
- 8.Costa E. From GABAA receptor diversity emerges a unified vision of GABAergic inhibition. Annual Review of Pharmacology and Toxicology. 1998;38:321–350. doi: 10.1146/annurev.pharmtox.38.1.321. [DOI] [PubMed] [Google Scholar]
- 9.Sivilotti L., Nistri A. GABA receptor mechanisms in the central nervous system. Progress in Neurobiology. 1991;36(1):35–92. doi: 10.1016/0301-0082(91)90036-Z. [DOI] [PubMed] [Google Scholar]
- 10.Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. The Journal of Physiology. 1987;385(1):243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzhala V. I., Talos D. M., Sdrulla D. A., et al. NKCC1 transporter facilitates seizures in the developing brain. Nature Medicine. 2005;11(11):1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 12.Rivera C., Voipio J., Payne J. A., et al. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 13.Hausselt S. E., Euler T., Detwiler P. B., Denk W. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biology. 2007;5(7):p. e185. doi: 10.1371/journal.pbio.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khirug S., Yamada J., Afzalov R., Voipio J., Khiroug L., Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. The Journal of Neuroscience. 2008;28(18):4635–4639. doi: 10.1523/jneurosci.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glickfeld L. L., Roberts J. D., Somogyi P., Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nature Neuroscience. 2009;12(1):21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowery N. G., Brown D. A., Collins G. G. S., Galvan M., Marsh S., Yamini G. Indirect effects of amino‐acids on sympathetic ganglion cells mediated through the release of γ‐aminobutyric acid from glial cells. British Journal of Pharmacology. 1976;57(1):73–91. doi: 10.1111/j.1476-5381.1976.tb07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeb T. Z., Lee H. H., Walker J. A., Davies P. A., Moss S. J. Hyperpolarizing GABAergic transmission depends on KCC2 function and membrane potential. Channels (Austin) 2014;5(6):475–481. doi: 10.4161/chan.5.6.17952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright R., Newey S. E., Ilie A., et al. Neuronal chloride regulation via KCC2 is modulated through a GABAB receptor protein complex. The Journal of Neuroscience. 2017;37(22):5447–5462. doi: 10.1523/JNEUROSCI.2164-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plotkin M. D., Snyder E. Y., Hebert S. C., Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. Journal of Neurobiology. 1997;33(6):781–795. doi: 10.1002/(SICI)1097-4695(19971120)33:6<781::AID-NEU6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Yamada J., Okabe A., Toyoda H., Kilb W., Luhmann H. J., Fukuda A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. The Journal of Physiology. 2004;557, part 3:829–841. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanhatalo S., Matias Palva J., Andersson S., Rivera C., Voipio J., Kaila K. Slow endogenous activity transients and developmental expression of K+–Cl− cotransporter 2 in the immature human cortex. European Journal of Neuroscience. 2005;22(11):2799–2804. doi: 10.1111/j.1460-9568.2005.04459.x. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Zhou J., Chen Z., Chen S., Zhu F., Zhou L. Long-term expressional changes of Na+-K+-Cl- co-transporter 1 (NKCC1) and K+-Cl- co-transporter 2 (KCC2) in CA1 region of hippocampus following lithium-pilocarpine induced status epilepticus (PISE) Brain Research. 2008;1221:141–146. doi: 10.1016/j.brainres.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Wake H., Watanabe M., Moorhouse A. J., et al. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. The Journal of Neuroscience. 2007;27(7):1642–1650. doi: 10.1523/jneurosci.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coe B. P., Girirajan S., Eichler E. E. The genetic variability and commonality of neurodevelopmental disease. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2012;160C(2):118–129. doi: 10.1002/ajmg.c.31327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen I., Navarro V., Clémenceau S., Baulac M., Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298(5597):1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 26.D'Antuono M., Louvel J., Köhling R., et al. GABAA receptor-dependent synchronization leads to ictogenesis in the human dysplastic cortex. Brain. 2004;127(7):1626–1640. doi: 10.1093/brain/awh181. [DOI] [PubMed] [Google Scholar]
- 27.Huberfeld G., Wittner L., Clemenceau S., et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. The Journal of Neuroscience. 2007;27(37):9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dargaei Z., Bang J. Y., Mahadevan V., et al. Restoring GABAergic inhibition rescues memory deficits in a Huntington’s disease mouse model. Proceedings of the National Acadamy of Sciences of the United States of America. 2018;115(7):E1618–E1626. doi: 10.1073/pnas.1716871115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uvarov P., Ludwig A., Markkanen M., et al. A novel N-terminal isoform of the neuron-specific K-Cl cotransporter KCC2. The Journal of Biological Chemistry. 2007;282(42):30570–30576. doi: 10.1074/jbc.M705095200. [DOI] [PubMed] [Google Scholar]
- 30.Uvarov P., Ludwig A., Markkanen M., et al. Coexpression and heteromerization of two neuronal K-Cl cotransporter isoforms in neonatal brain. The Journal of Biological Chemistry. 2009;284(20):13696–13704. doi: 10.1074/jbc.M807366200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois C. J., Cardoit L., Schwarz V., et al. Role of the K(+)-Cl(-) cotransporter KCC2a isoform in mammalian respiration at birth. ENeuro. 2018;5(5) doi: 10.1523/ENEURO.0264-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne J. A., Stevenson T. J., Donaldson L. F. Molecular characterization of a putative K-Cl cotransporter in rat brain. a neuronal-specific isoform. The Journal of Biological Chemistry. 1996;271(27):16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 33.Kahle K. T., Deeb T. Z., Puskarjov M., et al. Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends in Neurosciences. 2013;36(12):726–737. doi: 10.1016/j.tins.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina I., Friedel P., Rivera C., et al. Current view on the functional regulation of the neuronal K(+)-Cl(-) cotransporter KCC2. Frontiers in Cellular Neuroscience. 2014;8:p. 27. doi: 10.3389/fncel.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber M., Hartmann A.-M., Beyer T., Ripperger A., Nothwang H. G. A novel regulatory locus of phosphorylation in the C terminus of the potassium chloride cotransporter KCC2 that interferes with n-ethylmaleimide or staurosporine-mediated activation. The Journal of Biological Chemistry. 2014;289(27):18668–18679. doi: 10.1074/jbc.M114.567834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silayeva L., Deeb T. Z., Hines R. M., et al. KCC2 activity is critical in limiting the onset and severity of status epilepticus. Proceedings of the National Acadamy of Sciences of the United States of America. 2015;112(11):3523–3528. doi: 10.1073/pnas.1415126112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaesse P., Guillemin I., Schindler J., et al. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. The Journal of Neuroscience. 2006;26(41):10407–10419. doi: 10.1523/JNEUROSCI.3257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agez M., Schultz P., Medina I., et al. Molecular architecture of potassium chloride co-transporter KCC2. Scientific Reports. 2017;7(1):p. 16452. doi: 10.1038/s41598-017-15739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uvarov P., Pruunsild P., Timmusk T., Airaksinen M. S. Neuronal K+/Cl- co-transporter (KCC2) transgenes lacking neurone restrictive silencer element recapitulate CNS neurone-specific expression and developmental up-regulation of endogenous KCC2 gene. Journal of Neurochemistry. 2005;95(4):1144–1155. doi: 10.1111/j.1471-4159.2005.03434.x. [DOI] [PubMed] [Google Scholar]
- 40.Uvarov P., Ludwig A., Markkanen M., Rivera C., Airaksinen M. S. Upregulation of the neuron-specific K+/Cl- cotransporter expression by transcription factor early growth response 4. The Journal of Neuroscience. 2006;26(52):13463–13473. doi: 10.1523/JNEUROSCI.4731-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonislawski D. P., Schwarzbach E. P., Cohen A. S. Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiology of Disease. 2007;25(1):163–169. doi: 10.1016/j.nbd.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaenisch N., Witte O. W., Frahm C. Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke. 2010;41(3):e151–e159. doi: 10.1161/STROKEAHA.109.570424. [DOI] [PubMed] [Google Scholar]
- 43.Wu H., Shao A., Zhao M., et al. Melatonin attenuates neuronal apoptosis through up-regulation of K(+) -Cl(-) cotransporter KCC2 expression following traumatic brain injury in rats. Journal of Pineal Research. 2016;61(2):241–250. doi: 10.1111/jpi.12344. [DOI] [PubMed] [Google Scholar]
- 44.Lin K.-C., Lin H.-J., Chang C.-P., Lin M.-T. Decreasing or increasing heat shock protein 72 exacerbates or attenuates heat-induced cell death, respectively, in rat hypothalamic cells. FEBS Open Bio. 2015;5:724–730. doi: 10.1016/j.fob.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papp E., Rivera C., Kaila K., Freund T. F. Relationship between neuronal vulnerability and potassium-chloride cotransporter 2 immunoreactivity in hippocampus following transient forebrain ischemia. Neuroscience. 2008;154(2):677–689. doi: 10.1016/j.neuroscience.2008.03.072. [DOI] [PubMed] [Google Scholar]
- 46.Gagnon K. B., Di Fulvio M. A molecular analysis of the Na(+)-independent cation chloride cotransporters. Cellular Physiology and Biochemistry. 2013;32(7):14–31. doi: 10.1159/000356621. [DOI] [PubMed] [Google Scholar]
- 47.Kaila K., Price T. J., Payne J. A., Puskarjov M., Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nature Reviews Neuroscience. 2014;15(10):637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uvarov P., Llano O., Ludwig A., Airaksinen M. S., Rivera C. Multiple Roles of KCC2 in the Developing Brain. San Diego, Calif, USA: Academic Press; 2013. [Google Scholar]
- 49.Stein V., Hermans-Borgmeyer I., Jentsch T. J., Hübner C. A. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. Journal of Comparative Neurology. 2004;468(1):57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- 50.Kanaka C., Ohno K., Okabe A., et al. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104(4):933–946. doi: 10.1016/S0306-4522(01)00149-X. [DOI] [PubMed] [Google Scholar]
- 51.Hübner C. A., Stein V., Hermans-Borgmeyer I., Meyer T., Ballanyi K., Jentsch T. J. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30(2):515–524. doi: 10.1016/S0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 52.Balakrishnan V., Becker M., Löhrke S., Nothwang H. G., Güresir E., Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. The Journal of Neuroscience. 2003;23(10):4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sedmak G., Jovanov-Milošević N., Ulamec M. P. M., Krušlin B., Kaila K., Judaš M. Developmental expression patterns of KCC2 and functionally associated molecules in the human brain. Cerebral Cortex. 2016;26(12):4574–4589. doi: 10.1093/cercor/bhv218. [DOI] [PubMed] [Google Scholar]
- 54.Clancy B., Darlington R. B., Finlay B. L. Translating developmental time across mammalian species. Neuroscience. 2001;105(1):7–17. doi: 10.1016/S0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 55.Erecinska M., Cherian S., Silver I. A. Energy metabolism in mammalian brain during development. Progress in Neurobiology. 2004;73(6):397–445. doi: 10.1016/j.pneurobio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Puskarjov M., Ahmad F., Kaila K., Blaesse P. Activity-dependent cleavage of the K-Cl cotransporter KCC2 mediated by calcium-activated protease calpain. The Journal of Neuroscience. 2012;32(33):11356–11364. doi: 10.1523/jneurosci.6265-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puskarjov M., Ahmad F., Khirug S., Sivakumaran S., Kaila K., Blaesse P. BDNF is required for seizure-induced but not developmental up-regulation of KCC2 in the neonatal hippocampus. Neuropharmacology. 2015;88:103–109. doi: 10.1016/j.neuropharm.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Jantzie L. L., Getsy P. M., Firl D. J., Wilson C. G., Miller R. H., Robinson S. Erythropoietin attenuates loss of potassium chloride co-transporters following prenatal brain injury. Molecular and Cellular Neuroscience. 2014;61:152–162. doi: 10.1016/j.mcn.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakajima E., Hammond K. B., Rosales J. L., Shearer T. R., Azuma M. Calpain, not caspase, is the causative protease for hypoxic damage in cultured monkey retinal cells. Investigative Ophthalmology & Visual Science. 2011;52(10):7059–7067. doi: 10.1167/iovs.11-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blomgren K., Hallin U., Andersson A.-L., et al. Calpastatin is up-regulated in response to hypoxia and is a suicide substrate to calpain after neonatal cerebral hypoxia-ischemia. The Journal of Biological Chemistry. 1999;274(20):14046–14052. doi: 10.1074/jbc.274.20.14046. [DOI] [PubMed] [Google Scholar]
- 61.Rosenkranz K., May C., Meier C., Marcus K. Proteomic analysis of alterations induced by perinatal hypoxic-ischemic brain injury. Journal of Proteome Research. 2012;11(12):5794–5803. doi: 10.1021/pr3005869. [DOI] [PubMed] [Google Scholar]
- 62.Kaila K., Ruusuvuori E., Seja P., Voipio J., Puskarjov M. GABA actions and ionic plasticity in epilepsy. Current Opinion in Neurobiology. 2014;26:34–41. doi: 10.1016/j.conb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Lee H. H. C., Walker J. A., Williams J. R., Goodier R. J., Payne J. A., Moss S. J. Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. The Journal of Biological Chemistry. 2007;282(41):29777–29784. doi: 10.1074/jbc.M705053200. [DOI] [PubMed] [Google Scholar]
- 64.Lee H. H. C., Jurd R., Moss S. J. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Molecular and Cellular Neuroscience. 2010;45(2):173–179. doi: 10.1016/j.mcn.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leonzino M., Busnelli M., Antonucci F., Verderio C., Mazzanti M., Chini B. The timing of the excitatory-to-inhibitory GABA switch is regulated by the oxytocin receptor via KCC2. Cell Reports. 2016;15(1):96–103. doi: 10.1016/j.celrep.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bos R., Sadlaoud K., Boulenguez P., et al. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proceedings of the National Acadamy of Sciences of the United States of America. 2013;110(1):348–353. doi: 10.1073/pnas.1213680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore Y. E., Deeb T. Z., Chadchankar H., Brandon N. J., Moss S. J. Potentiating KCC2 activity is sufficient to limit the onset and severity of seizures. Proceedings of the National Acadamy of Sciences of the United States of America. 2018;115(40):10166–10171. doi: 10.1073/pnas.1810134115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Los Heros P., Alessi D. R., Gourlay R., et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl- co-transporters. Biochemical Journal. 2014;458(3):559–573. doi: 10.1042/BJ20131478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Friedel P., Kahle K. T., Zhang J., et al. WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons. Science Signaling. 2015;8(383):p. ra65. doi: 10.1126/scisignal.aaa0354. [DOI] [PubMed] [Google Scholar]
- 70.Cordshagen A., Busch W., Winklhofer M., Nothwang H. G., Hartmann A. Phosphoregulation of the intracellular termini of K(+)-Cl(-) cotransporter 2 (KCC2) enables flexible control of its activity. The Journal of Biological Chemistry. 2018;293(44):16984–16993. doi: 10.1074/jbc.RA118.004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Titz S., Sammler E. M., Hormuzdi S. G. Could tuning of the inhibitory tone involve graded changes in neuronal chloride transport? Neuropharmacology. 2015;95:321–331. doi: 10.1016/j.neuropharm.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 72.Conway L. C., Cardarelli R. A., Moore Y. E., et al. N-Ethylmaleimide increases KCC2 cotransporter activity by modulating transporter phosphorylation. The Journal of Biological Chemistry. 2017;292(52):21253–21263. doi: 10.1074/jbc.M117.817841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vitari A. C., Deak M., Morrice N. A., Alessi D. R. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochemical Journal. 2005;391(1):17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inoue K., Furukawa T., Kumada T., et al. Taurine inhibits K+-Cl- cotransporter KCC2 to regulate embryonic Cl- homeostasis via with-no-lysine (WNK) protein kinase signaling pathway. The Journal of Biological Chemistry. 2012;287(25):20839–20850. doi: 10.1074/jbc.M111.319418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roussa E., Speer J. M., Chudotvorova I., et al. The membrane trafficking and functionality of the K+-Cl- co-transporter KCC2 is regulated by TGF-beta2. Journal of Cell Science. 2016;129(18):3485–3498. doi: 10.1242/jcs.189860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z., Wang X., Wang W., Lu Y.-G., Pan Z. Z. Brain-derived neurotrophic factor-mediated downregulation of brainstem K+-Cl− cotransporter and cell-type-specific GABA impairment for activation of descending pain facilitation. Molecular Pharmacology. 2013;84(4):511–520. doi: 10.1124/mol.113.086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barde Y.-A., Davies A. M., Johnson J. E., Lindsay R. M., Thoenen H. Brain derived neurotrophic factor. Progress in Brain Research. 1987;71(C):185–189. doi: 10.1016/S0079-6123(08)61823-3. [DOI] [PubMed] [Google Scholar]
- 78.Carmona M. A., Pozas E., Martínez A., Espinosa-Parrilla J. F., Soriano E., Aguado F. Age-dependent spontaneous hyperexcitability and impairment of GABAergic function in the hippocampus of mice lacking trkB. Cerebral Cortex. 2006;16(1):47–63. doi: 10.1093/cercor/bhi083. [DOI] [PubMed] [Google Scholar]
- 79.Shulga A., Thomas-Crusells J., Sigl T., et al. Posttraumatic GABA(A)-mediated [Ca2+]i increase is essential for the induction of brain-derived neurotrophic factor-dependent survival of mature central neurons. The Journal of Neuroscience. 2008;28(27):6996–7005. doi: 10.1523/JNEUROSCI.5268-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rivera C., Voipio J., Thomas-Crusells J., et al. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. The Journal of Neuroscience. 2004;24(19):4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang Y.-J., Lee K. H., Grau J. W. Complete spinal cord injury (SCI) transforms how brain derived neurotrophic factor (BDNF) affects nociceptive sensitization. Experimental Neurology. 2017;288:38–50. doi: 10.1016/j.expneurol.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Karadsheh M. F., Delpire E. Neuronal restrictive silencing element is found in the KCC2 gene: Molecular basis for KCC2-specific expression in neurons. Journal of Neurophysiology. 2001;85(2):995–997. doi: 10.1152/jn.2001.85.2.995. [DOI] [PubMed] [Google Scholar]
- 83.Schoenherr C. J., Anderson D. J. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267(5202):1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- 84.Doshina A., Gourgue F., Onizuka M., et al. Cortical cells reveal APP as a new player in the regulation of GABAergic neurotransmission. Scientific Reports. 2017;7(1):p. 370. doi: 10.1038/s41598-017-00325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen M., Wang J., Jiang J., et al. APP modulates KCC2 expression and function in hippocampal GABAergic inhibition. eLife. 2017;6 doi: 10.7554/eLife.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahadevan V., Pressey J. C., Acton B. A., et al. Kainate receptors coexist in a functional complex with KCC2 and regulate chloride homeostasis in hippocampal neurons. Cell Reports. 2014;7(6):1762–1770. doi: 10.1016/j.celrep.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pressey J. C., Mahadevan V., Khademullah C. S., et al. A kainate receptor subunit promotes the recycling of the neuron-specific K(+)-Cl(-) co-transporter KCC2 in hippocampal neurons. The Journal of Biological Chemistry. 2017;292(15):6190–6201. doi: 10.1074/jbc.M116.767236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banke T. G., Gegelashvili G. Tonic activation of group I mGluRs modulates inhibitory synaptic strength by regulating KCC2 activity. The Journal of Physiology. 2008;586(20):4925–4934. doi: 10.1113/jphysiol.2008.157024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Puskarjov M., Seja P., Heron S. E., et al. A variant of KCC2 from patients with febrile seizures impairs neuronal Cl- extrusion and dendritic spine formation. EMBO Reports. 2014;15(6):723–729. doi: 10.1002/embr.201438749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kahle K. T., Merner N. D., Friedel P., et al. Genetically encoded impairment of neuronal KCC2 cotransporter function in human idiopathic generalized epilepsy. EMBO Reports. 2014;15(7):766–774. doi: 10.15252/embr.201438840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Acton B. A., Mahadevan V., Mercado A., et al. Hyperpolarizing GABAergic transmission requires the KCC2 C-terminal ISO domain. The Journal of Neuroscience. 2012;32(25):8746–8751. doi: 10.1523/JNEUROSCI.6089-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stödberg T., McTague A., Ruiz A. J., et al. Mutations in SLC12A5 in epilepsy of infancy with migrating focal seizures. Nature Communications. 2015;6:p. 8038. doi: 10.1038/ncomms9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saitsu H., Watanabe M., Akita T., et al. Impaired neuronal KCC2 function by biallelic SLC12A5 mutations in migrating focal seizures and severe developmental delay. Scientific Reports. 2016;6:p. 30072. doi: 10.1038/srep30072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Merner N. D., Chandler M. R., Bourassa C., et al. Regulatory domain or CPG site variation in SLC12A5, encoding the chloride transporter KCC2, in human autism and schizophrenia. Frontiers in Cellular Neuroscience. 2015;9:p. 386. doi: 10.3389/fncel.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klassen T., Davis C., Goldman A., et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145(7):1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jabbari K., Bernardi G. Cytosine methylation and CpG, TpG (CpA) and TpA frequencies. Gene. 2004;333:143–149. doi: 10.1016/j.gene.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 97.Woolf C. J., Mannion R. J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. The Lancet. 1999;353(9168):1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 98.Zeilhofer H. U. The glycinergic control of spinal pain processing. Cellular and Molecular Life Sciences. 2005;62(18):2027–2035. doi: 10.1007/s00018-005-5107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loomis C. W., Khandwala H., Osmond G., Hefferan M. P. Coadministration of intrathecal strychnine and bicuculline effects synergistic allodynia in the rat: An isobolographic analysis. The Journal of Pharmacology and Experimental Therapeutics. 2001;296(3):756–761. [PubMed] [Google Scholar]
- 100.Coull J. A. M., Boudreau D., Bachand K., et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424(6951):938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 101.Doyon N., Prescott S. A., Castonguay A., et al. Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis. PLoS Computational Biology. 2011;7(9):p. e1002149. doi: 10.1371/journal.pcbi.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Koninck Y. Altered chloride homeostasis in neurological disorders: a new target. Current Opinion in Pharmacology. 2007;7(1):93–99. doi: 10.1016/j.coph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 103.Kahle K. T., Staley K. J., Nahed B. V., et al. Roles of the cation-chloride cotransporters in neurological disease. Nature Clinical Practice Neurology. 2008;4(9):490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- 104.Gagnon M., Bergeron M. J., Lavertu G., et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nature Medicine. 2013;19(11):1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cardarelli R. A., Jones K., Pisella L. I., et al. The small molecule CLP257 does not modify activity of the K+-Cl- co-transporter KCC2 but does potentiate GABAA receptor activity. Nature Medicine. 2017;23(12):1394–1396. doi: 10.1038/nm.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heubl M., Zhang J., Pressey J. C., et al. GABAA receptor dependent synaptic inhibition rapidly tunes KCC2 activity via the Cl−-sensitive WNK1 kinase. Nature Communications. 2017;8(1):p. 1776. doi: 10.1038/s41467-017-01749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: Current evidence and future directions. Current Opinion in Anaesthesiology. 2007;20(5):456–472. doi: 10.1097/ACO.0b013e3282effaa7. [DOI] [PubMed] [Google Scholar]
- 108.Gilman J. T., Gal P., Duchowny M. S., Weaver R. L., Ransom J. L. Rapid sequential phenobarbital treatment of neonatal seizures. Pediatrics. 1989;83(5):674–678. [PubMed] [Google Scholar]
- 109.Boylan G. B., Stevenson N. J., Vanhatalo S. Monitoring neonatal seizures. Seminars in Fetal and Neonatal Medicine. 2013;18(4):202–208. doi: 10.1016/j.siny.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Khirug S., Ahmad F., Puskarjov M., Afzalov R., Kaila K., Blaesse P. A single seizure episode leads to rapid functional activation of KCC2 in the neonatal rat hippocampus. The Journal of Neuroscience. 2010;30(36):12028–12035. doi: 10.1523/JNEUROSCI.3154-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kirmse K., Witte O. W., Holthoff K. GABAergic depolarization during early cortical development and implications for anticonvulsive therapy in neonates. Epilepsia. 2011;52(9):1532–1543. doi: 10.1111/j.1528-1167.2011.03128.x. [DOI] [PubMed] [Google Scholar]
- 112.Kang S. K., Markowitz G. J., Kim S. T., Johnston M. V., Kadam S. D. Age- and sex-dependent susceptibility to phenobarbital-resistant neonatal seizures: role of chloride co-transporters. Frontiers in Cellular Neuroscience. 2015;9:p. 173. doi: 10.3389/fncel.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dzhala V. I., Brumback A. C., Staley K. J. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Annals of Neurology. 2008;63(2):222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 114.Cleary R. T., Sun H., Huynh T., et al. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PLoS ONE. 2013;8(3):p. e57148. doi: 10.1371/journal.pone.0057148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Löscher W., Puskarjov M., Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology. 2013;69:62–74. doi: 10.1016/j.neuropharm.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 116.Kang S., Kadam S. Pre-clinical models of acquired neonatal seizures: differential effects of injury on function of chloride co-transporters. Austin Journal of Cerebrovascular Disease & Stroke. 2014;1(6) [PMC free article] [PubMed] [Google Scholar]
- 117.Kharod S. C., Carter B. M., Kadam S. D. Pharmaco-resistant neonatal seizures: critical mechanistic insights from a chemoconvulsant model. Developmental Neurobiology. 2018;78(11):1117–1130. doi: 10.1002/dneu.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He X. P., Pan E., Sciarretta C., Minichiello L., McNamara J. O. Disruption of TrkB-mediated phospholipase Cgamma signaling inhibits limbic epileptogenesis. The Journal of Neuroscience. 2010;30(18):6188–6196. doi: 10.1523/JNEUROSCI.5821-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heinrich C., Lähteinen S., Suzuki F., et al. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiology of Disease. 2011;42(1):35–47. doi: 10.1016/j.nbd.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Carter B. M., Sullivan B. J., Landers J. R., Kadam S. D. Dose-dependent reversal of KCC2 hypofunction and phenobarbital-resistant neonatal seizures by ANA12. Scientific Reports. 2018;8(1):p. 11987. doi: 10.1038/s41598-018-30486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cazorla M., Prémont J., Mann A., Girard N., Kellendonk C., Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. The Journal of Clinical Investigation. 2011;121(5):1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aird R. B. A study of intrathecal, cerebrospinal fluid-to-brain exchange. Experimental Neurology. 1984;86(2):342–358. doi: 10.1016/0014-4886(84)90192-4. [DOI] [PubMed] [Google Scholar]