Abstract
Current synthetic grafts for bone defect filling in the sinus can support new bone formation but lack the ability to stimulate or enhance osteogenic healing. To promote such healing, osteoblast progenitors such as human periosteum cells must undergo osteogenic differentiation. In this study, we tested the hypothesis that degradation of porous amorphous silica fibrous (PASF) scaffolds can enhance human periosteum cell osteogenic differentiation. Two types of PASF were prepared and evaluated according to their densities (PASF99, PASF98) with 99% and 98% porosity, respectively. Silicon (Si) ions were observed to rapidly release from both scaffolds within 24 hours in vitro. PASF99 Si ion release rate was estimated to be nearly double that of PASF98 scaffolds. Mechanical tests revealed a lower compressive strength in PASF99 as compared to PASF98. Osteogenic expression analysis showed that PASF99 scaffolds enhanced the expression of activating transcription factor 4, alkaline phosphatase, and collagen (Col(I)α1, Col(I)α2). Scanning electron microscopy showed cellular and extracellular matrix ingress into both scaffolds within 16 days and the formation of Ca-P precipitates within 85 days. In conclusion, this study demonstrated that PASF scaffolds enhance human periosteum cell osteogenic differentiation by releasing ionic Si, and structurally supporting cellular and extracellular matrix ingress.
Keywords: Si, scaffold, periosteum cell, Runx2, osteocalcin
1. Introduction
Bone loss due to traumatic fracture contributes to around 8 billion dollars in morbidity/mortality costs and 800 thousand procedures annually [1–3]. A significant portion of these procedures are performed within the alveolar bone within the maxilla or sinus region. At the reconstruction site of alveolar bone defects or sinus floor augmentation, autogenic bone grafting remains the primary treatment for bone defects due to their osteogenic properties [4]. In applications of bone defect filling, the procedure involves the insertion of the graft into the defect site and forced attachment of periosteum [5]. The interaction of the periosteum-derived cells, that act as progenitors of the osteoblast phenotype, are critical for the mineralization and attachment of bone and periosteum to the graft, such graft-tissue integration can occur within 3 to 6 months [6]. Yet, this grafting strategy has significant limitations, including donor site morbidity, limited quantities, inappropriate forms for the graft site and sometimes the need for increased general anesthesia during operations for their harvest from the donor site [7]. In recent years, synthetic bone substitutes have become attractive because they have the potential to provide osteoconductive and osteogenic properties necessary for healing bone without the afore-mentioned limitations [8, 9].
Calcium phosphate based materials are osteoconductive and have similar chemistry as biologic hydroxyapatite (HA) found in bone [9]. Synthetic Ca-P based materials, such as sintered HA and β-tricalcium phosphate (β-TCP), mimic their osteoconductive properties by facilitating cellular movement and extracellular matrix attachment to their matrices. Additionally, porous scaffolds made from these materials facilitate mineralized tissue ingress [10–12]. However, these materials degrade too slowly and it is difficult for the bone to completely resorb them [13, 14]. Bioactive glasses have also been used in bone defect filling applications. They are FDA approved and degrade more rapidly than Ca-P based scaffolds. They facilitate tissue attachment to their surface by forming a “bone-like” HA surface layer [15][16, 17]. Yet, these materials only partially degrade and also do not completely resorb, which leads to their microencapsulation within bone [18]. Thus, synthetic grafts that can degrade, stimulate cellular ingress, stimulate osteogenesis, and have sufficient strength to support the defect during healing and bony tissue replacement are desired.
With these criteria in mind, a silica-based three-dimensional scaffold structure with high porosity (97+% void space) was developed from high-purity amorphous silica fibers. Early experiments reported that these porous amorphous silica fibrous (PASF) scaffolds were biocompatible in vitro and promote bone healing in vivo [19]. The results from these studies suggested that the construct may alleviate many of the problems associated with the use of ceramics as bone grafts. Even with this interconnected void space (> 95%), the PASF scaffold still exhibited significant mechanical strength with a compressive strength of > 0.3 MPa and a tensile strength of > 1.4 MPa in its dry state [19]. The pore diameters within the construct can range from 150 to 300 μm. The large diameter pores and interconnected void spaces are designed to allow for the integration of vasculature simultaneous with integrating bone tissue. Biocompatibility, adequate mechanical strength, and optimized architecture suggested that the construct may offer significant benefits over the afore-mentioned synthetic grafting strategies. Yet, these early experiments did not reveal any enhancement of osteogenic marker expression and did not determine the percentage of void space necessary to enhance osteogenesis while still maintaining adequate mechanical properties. Increased pore size (>98%) will be explored here to determine if this pore size is adequate to maintain the mechanical properties for the 95% porous scaffold while enhancing osteogenic differentiation.
Human periosteum-derived cells were used in this study to determine the effect these scaffolds have on their osteogenic differentiation. Osteoprogenitor cells have been reported to be present in bone marrow [20], periosteum [21], and cortical and cancellous bone, which indicate their osteogenic potential for bone tissue engineering. Moreover, periosteum-derived cells have several advantages such as minimal invasion to obtain cells, easy cell harvest and rapid growth [22]. Osteogenic development is represented by several steps of proliferation and differentiation of cells, extracellular matrix synthesis, maturation, and mineralization, with each step accompanied by changes in the expression of various osteogenic genes. [23] In this study, the gene expression of transcription factors core binding factor a1 (Cbfa1/Runx2) and activating transcription factor 4 (ATF4) were selected for their involvement of osteogenic differentiation. Collagen type 1 (Col(Ⅰ)-α1, Col(Ⅰ)-α2) expression was selected for the biological support to which mineralized tissue nucleates and grows. Osteocalcin (OCN) is a major non-collagenous calcium-binding protein secreted by osteoblasts, which is a late marker of osteogenic differentiation. Alkaline phosphatase (ALP) is involved in dephosphorylation of collagen and an essential enzyme for calcification of osteoblasts, which is considered as an early marker of mineralized ECM [24]. These markers are typically expressed within the early stage of differentiation (< 16 days). The effect of PASF scaffolds on human periosteum cell gene expression will be explored here.
Thus, this study tests the hypothesis that PASF scaffolds release degradation products that enhance human periosteum cell osteogenic differentiation. The aim of this study was (1) to determine the mechanical and degradation properties of the porous amorphous silica scaffolds and (2) to evaluate the expression of osteoblast-specific markers during early osteogenic differentiation. The goal of this work is to demonstrate that PASF scaffolds enhance human periosteum cell osteogenic marker expression through products of scaffold degradation.
2. Materials and Methods
2.1. Study design
Two kinds of porous amorphous silica fibrous (PASF) scaffolds were prepared with 98% and 99% porosity (denoted herein as PASF98 and PASF99, respectively). The first part of this study was aimed at determining the properties of the PASF98 and PASF99 scaffolds during 90 days under cell-free in vitro environment (α-MEM alone). Scaffold morphology and structure were visualized using scanning electron microscopy (SEM). Scaffold compressive strength and elastic modulus were measured using an INSTRON® universal testing machine. Changes in ion concentration during scaffold in vitro testing were measured using inductively coupled plasma optical emission spectrometry (ICP-OES).
The second part of this study involved cell culture testing of the scaffolds. Human periosteum cells were cultured on the PASF98 and PASF99 scaffolds and amorphous silica cover slip controls for a period of 16 days in cell culture medium. Amorphous silica glass cover slips were chosen as a control surface because they are used routinely as a well-known non-degradable control for standard cell culture.
Cells were lysed for total RNA and mRNA and were used for relative gene expression analysis via quantitative polymerase chain reaction (qPCR). Gene expressions on PASF98 and 99 scaffolds were compared to those on cover slip controls at each time points. Scanning electron microscopy was also used to visualize the cell and extra cellular matrix.
2.2. PASF scaffold preparation
PASF scaffolds were prepared as described previously [19] and briefly described here. 75 wt. % of amorphous silica fiber and 25 wt. % of alumina fibers were added in distilled water. The mixture was stirred with a rotating blade in a stainless steel container (Vitamix Maxi 4000, Vitamix Corp., Cleveland, OH, USA) to break the fibers and create homogeneous slurry. To form a green body with uniform density, the slurry was poured into a compressive mold and stored under vacuum until 50% of the water was removed. The wet block was removed from the mold and dried in a standard furnace to obtain the green- body. The furnace was heated to 104ºC from room temperature in 5 minutes and remained for approximately 5 hours. Then, the temperature was raised to 204ºC in 10 minutes and remained for 1 hour. For fiber matrix fusion, the green-body was sintered at 1200ºC.
2.3. ICP-OES measurements of ion extracts from PASF scaffolds
PASF scaffolds (6 mm diameter and 12 mm length) were immersed into 1 mL alpha-minimum essential medium (α-MEM, Life Technologies Corp., Grand Island, NY, USA) in 2 mL centrifuge tubes. The test was performed for a period of 16 days and 6 samples per each time point. The ion extracts and control samples were diluted 1:50 in 2% nitric acid and ion concentrations were analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES, ICPE-9000, SHIMAZU Co., Kyoto, Japan) as described previously [16, 17, 25]. Briefly, silicon, calcium, phosphorous, and aluminum standard (Ultra Scientific, Kingston, RI) were used to create calibration curves and introduced to the Argon mini-torch via the conical nebulizer. The emission spectra generated were dispersed through an Echelle grating and collected on a 1 in 2 CCD detector. Interference from the glass tubing in the quantification of the Si emissions was corrected using three dilutions α-MEM (1:25, 1:50, 1:100) by fixing the concentration of Si to the manufacturer’s specified concentration of 0ppm. Since the media contains Ca and phosphate ions and the PASF scaffolds and control samples contain amorphous silica, total Ca, P, and Si were assayed. Al detection was used as a negative control since the PASF scaffold, control samples, and media contain no Al.
2.4. Morphological and mechanical properties of PASF scaffolds
PASF scaffolds were subjected to compressive test at a displacement rate of 0.5 mm/min by INSTRON 5567 (software version: 1.5.276, INSTRON Corp., Norwood, MA, USA) equipped with a 1kN load cell. Five samples per each time point were prepared in cylinder shape of approximately 6 mm in diameter and 12 mm in height in accordance with ASTM F 451–95 [26], all the calculations were done assuming that the section area of specimens remain unchanged during the test. Each Specimen was immersed into 1mL of α-MEM before the test. The test was performed for a period of 90 days. Specimens were wet during the compressive test and at open laboratory ambient condition. Samples were imaged at each time point using a scanning electron microscope (SEM, JEOL USA Inc., Peabody, MA, USA). Before gold-sputter coating, samples were washed with distilled water and dehydrated with 100% ethanol for 10 minutes to remove excess water. SEM observations were performed with secondary electron mode at 1.5 kV.
2.5. Cell culture
PASF scaffolds were sectioned into 10 mm × 10 mm × 5 mm rectangular shape (PASF98, PASF99). The fabricated specimens were sterilized in an autoclave at 121ºC and 2 bar for 20 minutes. Amorphous silica glass cover slip controls (Sail Boat Lab Co., Ltd, Zhejiang, China) were similarly prepared for cell culture studies
Human periosteum cells (passage 4) were obtained as previously described [27]. The guidelines were followed according to IRB protocol (Study ID STU 012011–181) [27]. These cells were cultured in 150 cm2 flasks with α-MEM containing 10% fetal bovine serum (FBS, VWR, Radnor, PA) and antibiotics (1% streptomycin/penicillin) in a humidified atmosphere of 95% air and 5% CO2 at 37ºC. The cells were seeded on each PASF scaffold and cover slip control samples in 6-well plate (BD, Franklin Lakes, NJ, USA), Replicate samples were seeded with cells at a density of 1M cells per mL media. After the cell line doubling time (48 hours), these cells were synchronized (α-MEM, 1% FBS, 1% pen-strep) for an additional 48 hours. Media was then exchanged for growth media (α-MEM, 10% FBS, 1% pen-strep) with ascorbic acid-2-phosphate (AA2P, Sigma-Aldrich Corp., St. Louis, MO, USA) and glycerol-2-phosphate (β-GP, Sigma-Aldrich Corp.) to induce differentiation. Cells on PASF scaffolds and control cover slips were removed for lysis over a 16 day time period.
2.6. Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Cells were lysed to collect mRNA (using RNeasy Mini Kit, Qiagen, Valencia, CA, USA). At the lysing cell collection, ultrasonic vibration was applied for 10 seconds with a lysis buffer, and then scaffolds were smashed manually. The recovered mRNA was converted to cDNA using reverse transcriptase (Reverse Transcription System, Promega, Madison, WI, USA) according to the manufacture’s protocol. Absorbance measurements of mRNA and cDNA samples were performed using a microvolume UV-Vis spectrophotometer (Nano Drop 2000c, Thermo Fisher Scientific Inc., Waltham, MA, USA).
Details regarding qRT-PCR were given in previous work and described below in brief [28–30]. Using glyceraldehyde phosphate dehydrogenase (GAPDH) as an internal reference gene, relative quantification of gene expression was evaluated by the comparative cycle threshold (CT) method and fold change calculated using 2−ΔΔCT. Data were normalized to GAPDH within each independent experiment and expressed as relative induction of control at corresponding time-point. The gene expression of GAPDH, osteocalcin (OCN) and core-bending factor (Cbfa1/Runx2), activating transcription factor 4 (ATF4), collagen type 1 (Col(Ⅰ)-α1, Col(Ⅰ)-α2) and alkaline phosphatase (ALP) was detected by a real-time PCR machine (CFX96, Bio-RAD Inc., Emeryville, CA) and using TaqMan assay kits (Life Technologies; Carlsbad, CA).
2.7. Scanning electron microscopy
At each time point, cell cultured samples were washed with PBS twice and transferred to a fresh well plate, and fixed with 2.5% formalin for 1 hour, excess fixative rinsed off with purified water. Samples were then sequentially dehydrated with ethanol (25, 50, 75, 90 and two changes of 100% ethanol) for 10 minutes to remove excess water. Cell migration into the scaffold structure was observed by sectioning samples after fixation. The cross-sectioned samples were then gold coated and mounted onto SEM stubs. The samples were then imaged by SEM at the conditions described above.
2.8. Data analysis and statistics
Data were presented as the mean ± standard error. One-way analysis of variance (ANOVA) and t-test comparisons were used for parametric analyses. p<0.05 was used for statistical significant. All in vitro assays, ion measurement, and mechanical testing was repeated to ensure sufficient replicate sampling for statistical power.
3. Results
3.1. Effect of PASF scaffolds on in vitro ion concentration
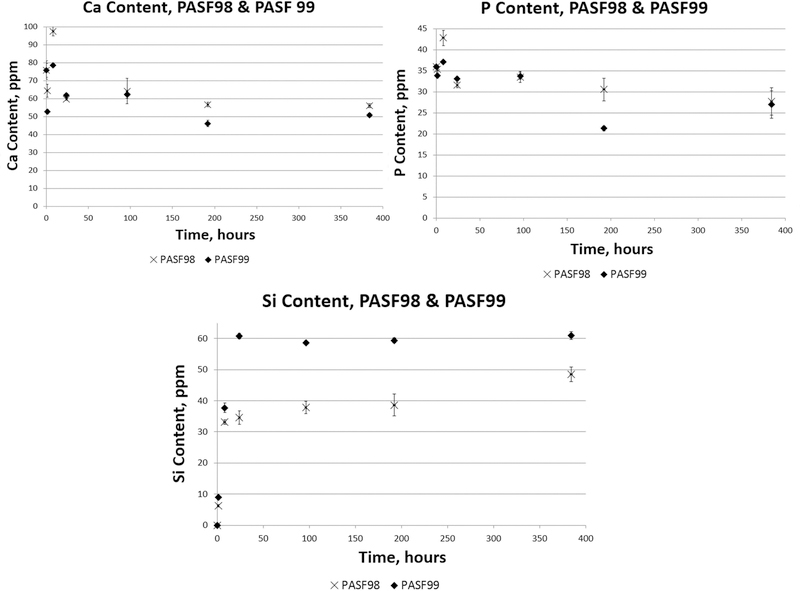
Fig. 1 shows the total Si, P, and Ca ion concentration in α-MEM during PASF in vitro immersion studies. PASF98 scaffolds showed a relatively steady release of ionic Si over the 16 day immersion period (384 hours), after which no further change in ion concentration was observed. The PASF99 scaffold had a relatively more rapid ionic Si release over a 24 hour time period, after which there was no further release of ionic Si. In the first 24 hours, each scaffold released ionic Si at a rate proportional with the square root of time, which indicated Si ion release was diffusion limited. After this initial 24 hours period, ionic Si release was found to be proportional with time, which indicated Si ion release was surface-reaction limited. Overall ionic Ca and P concentration decreased proportionally with increasing immersion time, which suggested possible precipitation of these elements onto the scaffold.
Figure 1.
ICP-OES measurement of ion concentrations of Si, P, and Ca from PASF98 and PASF99 scaffolds dissolution. For first 24 hours, Mass Transfer Coefficients of PASF 98 and 99 were calculated 7.69 ± 0.38 mg L −1 hr −1 (R2 = 0.995) and 12.81 ± 0.76 mg L −1 hr −1 (R2 = 0.993) respectively.
3.2. In Vitro Morphological Analysis
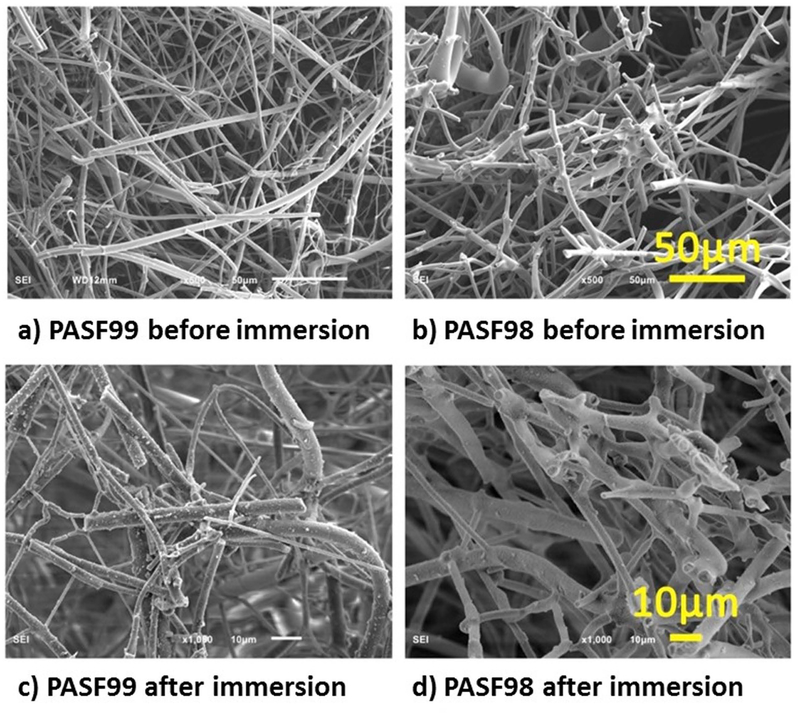
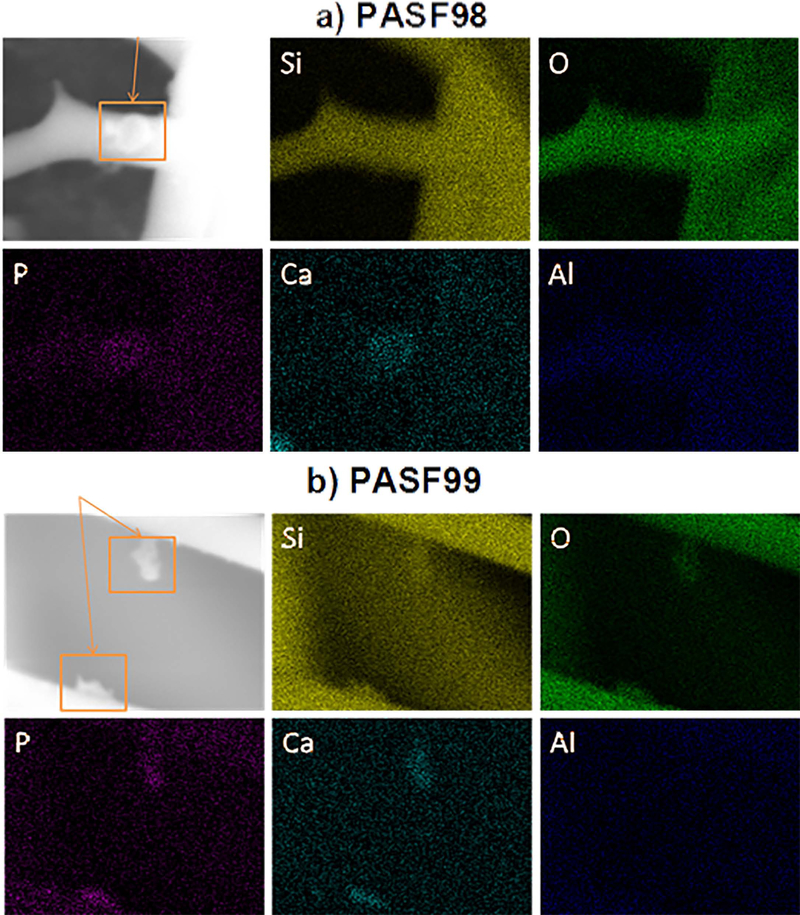
SEM images showed Morphological differences between PASF scaffolds (Fig. 2). PASF99 samples showed relatively thin and long fibrous filaments whereas PASF98 exhibited relatively short and thick fibrous filaments. Moreover, it was observed that PASF98 have a higher density of sintered cross-links between fibrous filaments. Electron dispersive x-ray analysis (EDS) of the scaffold after 85 days of immersion from the surface with some precipitates showed the presence of Ca, P, and O within these precipitates (Fig. 3).
Figure 2.
SEM images of PASF scaffolds. a) PASF99 and b) PASF98 before in vitro immersion and c) PASF99 and d) PASF98 after in vitro immersion (85 days) in α-MEM).
Figure 3.
EDS mapping images of precipitations on specimens after immersion into α-MEM for 85 days a) PASF98 b) PASF99. Si – Silicon, O – Oxygen, P – Phosphorus, Ca – Calcium, and Al – Aluminum.
3.3. Effect of in vitro environment on mechanical properties of PASF scaffolds
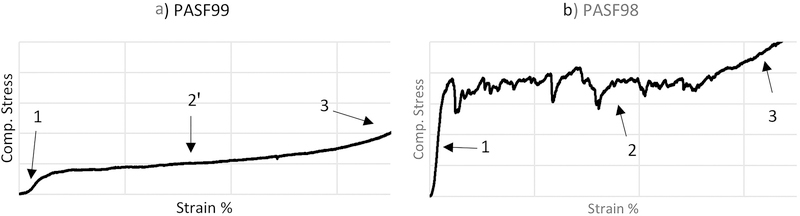
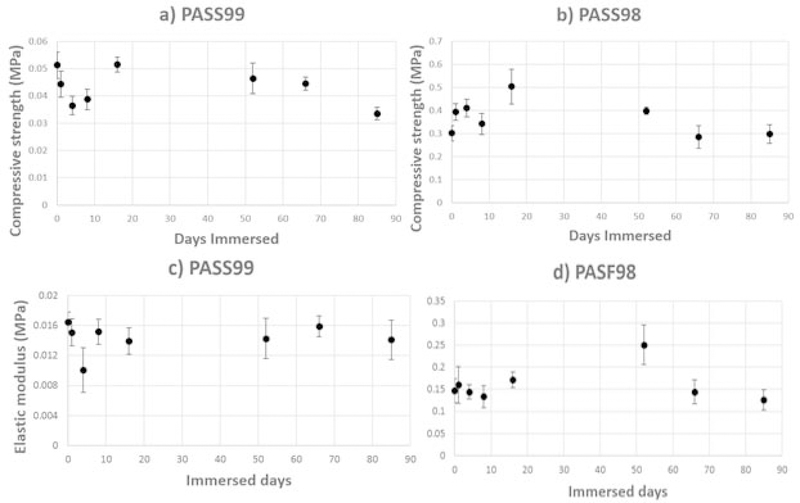
Fig. 4 shows the compressive behavior of PASF98 and PASF99 scaffolds. Three distinct regions were observed: linear elastic behavior (Region 1), structure collapse-densification plateau (Region 2), and densification (Region 3). The periodic sudden increase and decrease behavior (Region 2) for PASF98 is similar to that observed for cellular ceramics. The compressive behavior observed in Region 2 for PASF99 resembled that of a compressible material. The elastic modulus was calculated from the slope of linear elastic region and the compressive/yield strength was estimated from the point at the apex of the linear elastic region. After 85 days of immersion, no significant change in the elastic modulus and only a slight decrease in the compressive strength was observed for PASF scaffolds (Fig. 5).
Figure 4.
The typical stress strain curve of PASF99 and PASF98 scaffolds.
Figure 5.
Yield or compressive strength (a, b) and elastic modulus (c, d) of PASF99 and PASF98 scaffolds as a function of immersion time, respectively.
3.4. Effect of PASF scaffolds on Human Periosteum Cell Osteogenic Differentiation
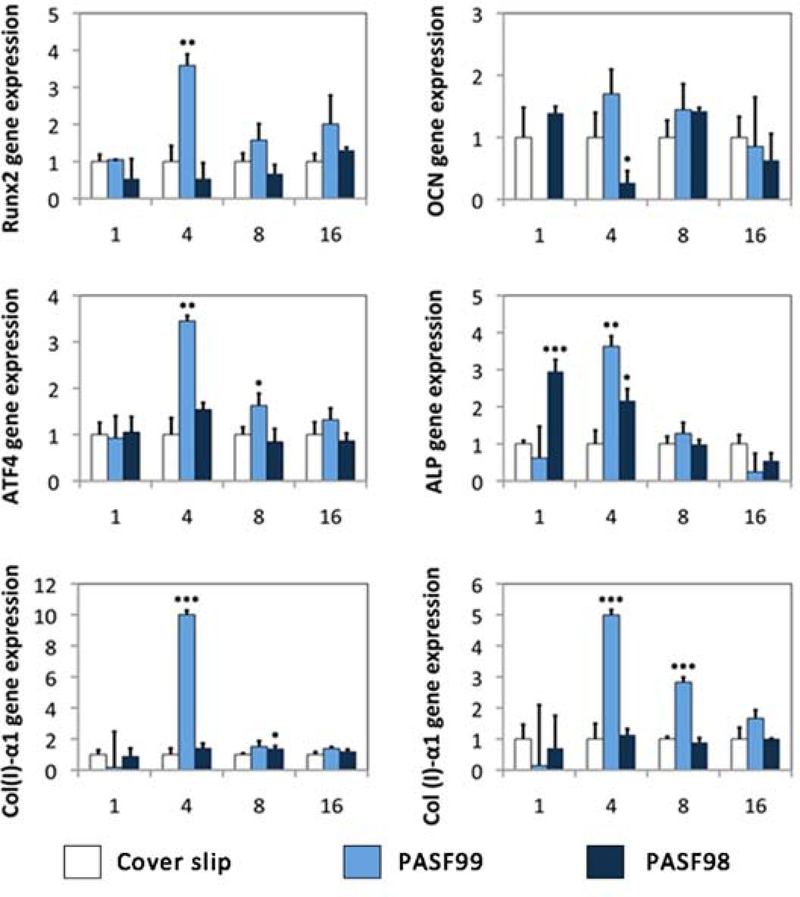
Gene expression analysis was also performed. PASF99 enhanced the osteogenic marker expression of human periosteum cells as compared to control samples (amorphous glass cover slips). PASF99 induced over-expression of Col(I)-α1 at day 4, Col(I)-α2 on day 4 and day 16 as compared to control (ANOVA, p<0.05). Moreover, PASF99 enhanced the expression of osteogenic markers that indicate the osteoblast phenotype including Runx2 (day 4) and ATF4 (day 4) (ANOVA, p<0.05), and slightly elevated the expression of OCN. PASF98 enhanced the expression of Col(I)-α1 at day8, and ALP at day1 and day4 than on control surfaces (ANOVA, p<0.05).
3.5. Effect of PASF scaffolds on human periosteum cell attachment and migration
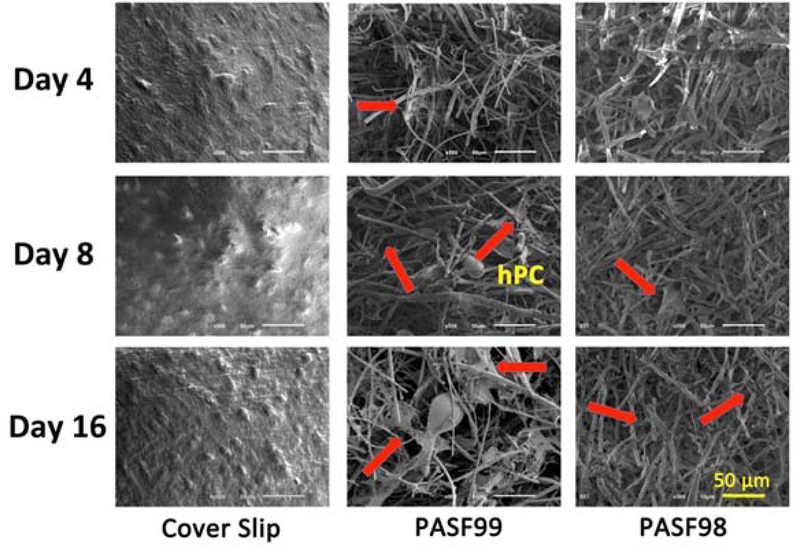
Fig. 7 shows the migration of human periosteum cells on control cover slip surfaces and PASF scaffolds at various time points during experiments. Each scaffold supported extracellular matrix (ECM) binding. No difference in cell morphology was observed between scaffolds and time points. Cells were observed to migrate into the scaffold structure with increasing time. These cells were observed to extend their filopodia onto the inner scaffold surface, which indicated their affinity for the scaffold structure.
Figure 7.
SEM images of human periosteum cells cultured on PASF99 and PASF98 scaffolds from 4 to 16 days as compared to cover slip controls.
4. Discussion
Results from the first part of this study showed that PASF98 and PASF99 were found to have notable differences in morphology, mechanical and degradation properties. Both PASF98 and PASF99 had a random fibrous structure. However, relatively shorter and thicker fibrous filaments along with higher density of sintered cross-links allowed PASF98 to endure higher stress and increased its resistance to compressive loads [31]. Furthermore, these morphological differences likely manifested as differences in compressive behavior between these scaffolds. Region (2) of the stress-strain curves were given in Fig. 4 illustrated these differences. Deformation pattern of PASF98 is similar to what has been observed by Kim et al. 2004, they suggested that the first failure initiates from the weakest point of specimen, then it propagates laterally to reach side walls and forms a compressed layer. From this point the compressed layer becomes thicker as specimen compressed more and divides the specimen to a middle dense layer and nearly untouched top and bottom layers [32]. In PASF99, a flat plateau with small positive slope on region (2̓) is likely related to dynamic evolution of microstructure which includes fibers bending and rearrangement, increasing in density of friction points and network collapsing [31, 33]. After immersion, Slight reduction rate in compressive/yield strength of both categories could be attributed to dissolution of ionic Si4+, which could have slightly weakened the network structure of specimens.
Noted differences in ionic Si dissolution were also observed. PASF99 scaffolds were observed to have a relatively rapid ionic Si release over 24 hours while PASF98 had a relatively moderate release over the same period. This was attributed to the differences in porosity between these materials. The loss of Ca and P ions in vitro could have been owed to precipitation of these ions on the PASF surface. Reflecting on earlier results from ICP-MS analysis and electron dispersive x-ray analysis (EDS), the loss of Ca and P over the dissolution time likely precipitated onto the scaffold surface as Ca-P-O aggregates (Fig. 3). The dissolution process likely followed a similar process on bioactive glass surfaces in that these aggregates formed via initial ionic silicon release, spontaneous polymerization of the released silanols, re-precipitation and eventual formation of a silica gel layer, and reprecipitation of Ca and P onto the amorphous silica surfaces [34].
Results from the second part of this study showed that PASF99 rapid ionic Si release had likely enhanced human periosteum cell osteogenic differentiation. According to our ICP results (Fig. 1), ionic Si release from PASF99 increased up to 95% of total dissolution within 24 hours of immersion. Increased expression of Runx2, ATF4, collagen type 1 and osteocalcin was associated with this ion release. On the other hand, PASF98 did not show as enhanced effect on cell differentiation. Ionic Si release from PASF98 scaffold increased up to 66% of total dissolution within 24 hours. Thus, the difference in enhanced gene expression between the scaffolds (Fig. 6) is likely attributed to the differences in the rate and total amount of dissolved ionic Si released.
Figure 6.
qRT-PCR analysis of relative gene expression of human periosteum cells exposed to PASF scaffolds. Genes (Col(Ⅰ)-α1/Col (Ⅰ)-α2), Runx2, ATF4, ALP and OCN were assayed over a 16 day period after human periosteum cells were cultured on the respective scaffolds. (ANOVA with * indicating statistical significance for p<0.05, ** indicating statistical significance for p < 0.01, and *** indicating statistical significance for p < 0.001)
Results on human periosteum cell exposure to ionic Si release were similar to those observed in our previous work with various osteoprogenitor cells exposed to bioactive glass ionic dissolution products. Bioactive glass, release of Ca2+ (0.2–1.25 mM) and Si4+ (0.14–0.4 mM), increased collagen type 1 and osteocalcin expression [17, 35]. Ca2+ or Si4+ ions enhance multiple osteogenic gene expression such as collagen type1 (Col(Ⅰ)-α1/Col (Ⅰ)-α2), core-bending factor (cbfa/Runx2) and osteocalcin (OCN), and Si4+ ion concentrations enhance the osteoblast gene expression and extracellular matrix (ECM) formation at higher levels than Ca2+ ion concentrations [29]. Thus, the release of Si ions from an amorphous silica-based scaffold that also allows cellular and extracellular matrix ingress could hasten osteogenesis and mineralized tissue ingress.
The enhanced osteogenic effect of ionic Silicon has not yet been clearly understood. In osteoblasts, it has been observed that ionic Silicon, either released as an ionic product from bioactive glass or introduced as a result of sodium metasilicate dissolution, promote the enhanced expression and cross-linking of collagen [17, 28]. This has been shown to occur via transcription factors (Runx2, OSX) and signaling proteins (SMAD 3) that regulated expression of the osteogenic phenotype, [17, 25]. In this work, a similar effect was observed Runx2 and another osteogenic transcription factor, ATF4. The connection between these transcription factors, intracellular signaling proteins, and collagen and non-collagenous marker expression as well as increased degree of cross-linking still needs to be determined. Cell signaling molecules (SMAD 1 and 5), integrins, and mitogen activated protein kinases that regulate collagenous and non-collagenous synthesis during mineralized matrix formation could be involved and may explain the results found here. These markers will be studied to isolate the mechanism of action of ionic Si on osteogenesis.
Despite the enhanced osteogenic differentiation of periosteum cells on the PASF scaffold surface, it was noted that their growth into the scaffold network was relatively low. These scaffolds were found to induce a similar growth response by MG-63 osteosarcoma cells in vitro [40]. As noted above, periosteum cells act as osteoprogenitors during defect healing and their capacity to grow into the defect depends on the amount of available Ca-P-based biomineral. In fact, Ca-P based scaffolds and nanoparticles have been noted to enhance periosteum cell growth [41, 42]. Thus, the low growth rate observed here may have been owed to the lack of an initial Ca-P-based scaffold surface chemistry. We hypothesize that incorporation of Ca-P based compounds (e.g., β-tricalcium phosphate, hydroxyapatite) can enhance periosteum cell growth on the scaffold surface. For example, nano-particles of HA could be added to the scaffolds during the sintering process. The concentration of particles in the PASF scaffold would need to be optimized such that scaffold degradation is not appreciably slowed and cell growth, differentiation, and biomineral production are maximally enhanced. Such studies could reveal the needed surface chemistry to enhance cell migration, proliferation, and biomineral production.
PASF scaffolds have sufficient strength and osteoinductive properties during degradation to potentially support bone defect healing [36]. These have important implication in clinical situation, especially in sinus floor augmentation. The key for success in sinus floor augmentation is that maintain the sinus membrane in an elevated position with blood clot [37], platelet-rich fibrin [38], autogenous bone [4], or other types of bone substitute materials [8, 9] during new bone formation and maturation [39]. The PASF scaffolds could be used as a substitute material because its osteoinductive property leads to early differentiation for osteoblast and the porous structure allows for invasion of the scaffold by osteoblast progenitor cells. Future investigation will determine the efficacy of these scaffolds to be used in vivo in the application of sinus augmentation.
5. Conclusion
In conclusion, the porous amorphous silica fibrous (PASF) scaffolds were observed to enhance human periosteum cell osteogenic differentiation. The relatively higher porosity scaffold had lower compressive strength and higher Si4+ dissolution than the less porous scaffold. While Si was released from the scaffolds, Ca and P ions were found to decrease in concentration with dissolution time. Evidence of Ca-P precipitates were observed as the dissolution period continued to increase. The relatively higher porous scaffold also enhanced human periosteum osteogenic marker expression at higher levels than was observed for the lower porosity scaffold. Despite these gene expression differences, each scaffold facilitated the ingress and attachment of human periosteum cells within their fibrous networks. Thus, this study supports the hypothesis that PASF scaffolds enhance the expression of human periosteum cell osteogenic differentiation via release of ionic Si from their surface.
6. Acknowledgements
The authors would like to acknowledge the following contributors to this work; Andrea Shiakolas, Ikuya WATANABE, and Takashi SAWASE. The authors would also like to thank the Texas A&M University for their kind support through the Research Development and Enhancement Award Program (RDEAP Grant # 304–244441, PI: Varanasi) and the National Institutes of Health/National Institute for Dental and Craniofacial Research (Grant # 1R03DE023872–01).
7. References
- [1].National Ambulatory Medical Care Survey 1998–2006. National Center for Health Statistics; Centers for Disease Control and Prevention U.S; Department of Health and Human Services; 2006. [Google Scholar]
- [2].Dhanwal DK, Dennison EM, Harvey NC, Cooper C. Epidemiology of hip fracture: Worldwide geographic variation. Indian journal of orthopaedics 2011;45:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Montjovent MO, Mathieu L, Hinz B, Applegate LL, Bourban PE, Zambelli PY, et al. Biocompatibility of bioresorbable poly(L-lactic acid) composite scaffolds obtained by supercritical gas foaming with human fetal bone cells. Tissue engineering 2005;11:1640–9. [DOI] [PubMed] [Google Scholar]
- [4].Pape HC, Evans A, Kobbe P. Autologous bone graft: properties and techniques. J Orthop Trauma 2010;24 Suppl 1:S36–40. [DOI] [PubMed] [Google Scholar]
- [5].Hoffman MD, Xie C, Zhang X, Benoit DS. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials 2013;34:8887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Canullo L, Dellavia C. Sinus lift using a nanocrystalline hydroxyapatite silica gel in severely resorbed maxillae: histological preliminary study. Clinical implant dentistry and related research 2009;11 Suppl 1:e7–13. [DOI] [PubMed] [Google Scholar]
- [7].Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976) 1995;20:1055–60. [DOI] [PubMed] [Google Scholar]
- [8].Burg KJ, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials 2000;21:2347–59. [DOI] [PubMed] [Google Scholar]
- [9].Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials 2003;24:2161–75. [DOI] [PubMed] [Google Scholar]
- [10].Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res 2002:44–52. [DOI] [PubMed] [Google Scholar]
- [11].Guda T, Walker JA, Pollot BE, Appleford MR, Oh S, Ong JL, et al. In vivo performance of bilayer hydroxyapatite scaffolds for bone tissue regeneration in the rabbit radius. J Mater Sci Mater Med 2011;22:647–56. [DOI] [PubMed] [Google Scholar]
- [12].Ono D, Jimbo R, Kawachi G, Ioku K, Ikeda T, Sawase T. Lateral bone augmentation with newly developed beta-tricalcium phosphate block: an experimental study in the rabbit mandible. Clin Oral Implants Res 2011;22:1366–71. [DOI] [PubMed] [Google Scholar]
- [13].Shih TC, Teng NC, Wang PD, Lin CT, Yang JC, Fong SW, et al. In vivo evaluation of resorbable bone graft substitutes in beagles: histological properties. Journal of biomedical materials research Part A 2013;101:2405–11. [DOI] [PubMed] [Google Scholar]
- [14].Farina NM, Guzon FM, Pena ML, Cantalapiedra AG. In vivo behaviour of two different biphasic ceramic implanted in mandibular bone of dogs. J Mater Sci Mater Med 2008;19:1565–73. [DOI] [PubMed] [Google Scholar]
- [15].Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF, et al. Bioactive glass in tissue engineering. Acta Biomater 2011;7:2355–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun J, Wei L, Liu X, Li J, Li B, Wang G, et al. Influences of ionic dissolution products of dicalcium silicate coating on osteoblastic proliferation, differentiation and gene expression. Acta Biomater 2009;5:1284–93. [DOI] [PubMed] [Google Scholar]
- [17].Varanasi VG, Saiz E, Loomer PM, Ancheta B, Uritani N, Ho SP, et al. Enhanced osteocalcin expression by osteoblast-like cells (MC3T3-E1) exposed to bioactive coating glass (SiO2-CaO-P2O5-MgO-K2O-Na2O system) ions. Acta Biomater 2009;5:3536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kobayashi H, Turner AS, Seim HB 3rd, Kawamoto T, Bauer TW. Evaluation of a silica-containing bone graft substitute in a vertebral defect model. Journal of biomedical materials research Part A 2010;92:596–603. [DOI] [PubMed] [Google Scholar]
- [19].Lyles MB; Ritsco RG, Ceramic fused fiber enhanced dental materials, U.S. Patent 5951295 A, September 14, 1999.
- [20].Bianco P, Riminucci M, Gronthos S, Robey P. Bone marrow stromal stem cells- nature, biology, and potential applications. Stem Cells 2001;19:180–92. [DOI] [PubMed] [Google Scholar]
- [21].Hutmacher D, Sittinger M. Periosteal cells in bone tissue engineering. Tissue engineering 2003;9:S45–64. [DOI] [PubMed] [Google Scholar]
- [22].Ng AM, Saim AB, Tan KK, Tan GH, Mokhtar SA, Rose IM, et al. Comparison of bioengineered human bone construct from four sources of osteogenic cells. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association 2005;10:192–9. [DOI] [PubMed] [Google Scholar]
- [23].Karner E, Backesjo CM, Cedervall J, Sugars RV, Ahrlund-Richter L, Wendel M. Dynamics of gene expression during bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Biochimica et biophysica acta 2009;1790:110–8. [DOI] [PubMed] [Google Scholar]
- [24].Bae SE, Bhang SH, Kim BS, Park K. Self-assembled extracellular macromolecular matrices and their different osteogenic potential with preosteoblasts and rat bone marrow mesenchymal stromal cells. Biomacromolecules 2012;13:2811–20. [DOI] [PubMed] [Google Scholar]
- [25].Saffarian Tousi N, Velten MF, Bishop TJ, Leong KK, Barkhordar NS, Marshall GW, et al. Combinatorial effect of Si4+, Ca2+, and Mg2+ released from bioactive glasses on osteoblast osteocalcin expression and biomineralization. Materials science & engineering C, Materials for biological applications 2013;33:2757–65. [DOI] [PubMed] [Google Scholar]
- [26].Khan MN, Islam JM, Khan MA. Fabrication and characterization of gelatin-based biocompatible porous composite scaffold for bone tissue engineering. Journal of biomedical materials research Part A 2012;100:3020–8. [DOI] [PubMed] [Google Scholar]
- [27].Kim H, Oxendine I, Kamiya N. High-Concentration of BMP2 Reduces Cell Proliferation and Increases Apoptosis via DKK1 and SOST in Human Primary Periosteal Cells. Bone 2013. 54:141–50. [DOI] [PubMed] [Google Scholar]
- [28].Varanasi VG, Leong KK, Jue SM, Dominia LM, Loomer PM, Marshall GW. Si and Ca Combinatorially Target and Enhance Early MC3T3-E1 Osteoblast Expression of Osteocalcin. Journal of Oral Implantology 2012;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Varanasi VG, Leong KK, Dominia LM, Jue SM, Loomer PM, Marshall GW. Si and Ca individually and combinatorially target enhanced MC3T3-E1 subclone 4 early osteogenic marker expression. The Journal of oral implantology 2012;38:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Qiu H, Durand K, Rabinovitch-Chable H, Rigaud M, Gazaille V, Clavère P, et al. Gene expression of HIF-1α and XRCC4 measured in human samples by real-time RT-PCR using the sigmoidal curve-fitting method. BioTechniques 2007;42:355–62. [DOI] [PubMed] [Google Scholar]
- [31].Masse JP, Poquillon D. Mechanical behavior of entangled materials with or without cross-linked fibers. Scripta Materialia 2013;68:39–43. [Google Scholar]
- [32].Kim HS, Plubrai P. Manufacturing and failure mechanisms of syntactic foam under compression. Composites Part A: Applied Science and Manufacturing 2004;35:1009–15. [Google Scholar]
- [33].Kucheyev SO, Stadermann M, Shin SJ Jr. JHS, Gammon SA, Letts SA, et al. Super-Compressibility of Ultralow-Density Nanoporous Silica. Advanced Materials 2012;24:5. [DOI] [PubMed] [Google Scholar]
- [34].Hench LL. Bioceramics. J Am Ceram Soc 1998;81:1705–28. [Google Scholar]
- [35].Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass (R) 45S5 dissolution. J Biomed Mater Res 2001;55:151–7. [DOI] [PubMed] [Google Scholar]
- [36].Choi Y, Yun JH, Kim CS, Choi SH, Chai JK, Jung UW. Sinus augmentation using absorbable collagen sponge loaded with Escherichia coli-expressed recombinant human bone morphogenetic protein 2 in a standardized rabbit sinus model: a radiographic and histologic analysis. Clin Oral Implants Res 2012;23:682–9. [DOI] [PubMed] [Google Scholar]
- [37].Lundgren S, Andersson S, Gualini F, Sennerby L. Bone reformation with sinus membrane elevation A new surgical technique for maxillary sinus floor augmentation. Clinical implant dentistry and related research 2004;6:165–73. [PubMed] [Google Scholar]
- [38].Tajima N, Ohba S, Sawase T, Asahina I. Evaluation of sinus floor augmentation with simultaneous implant placement using platelet-rich fibrin as sole grafting material. The International journal of oral & maxillofacial implants 2013;28:77–83. [DOI] [PubMed] [Google Scholar]
- [39].Kim HR, Choi BH, Xuan F, Jeong SM. The use of autologous venous blood for maxillary sinus floor augmentation in conjunction with sinus membrane elevation: an experimental study. Clin Oral Implants Res 2010;21:346–9. [DOI] [PubMed] [Google Scholar]
- [40].Lyles M; McLaughlin C; Halff G; Mallow W, Implantable system for cell growth control, U.S. Patent 20020120251 A1, August 29, 2002.
- [41].Chai Yoke Chin, Kerckhofs Greet, Roberts Scott J., Van Bael Simon, Schepers Evert, Vleugels Jozef, Luyten Frank P., Schrooten Jan, Ectopic bone formation by 3D porous calcium phosphate-Ti6Al4V hybrids produced by perfusion electrodeposition, Biomaterials, Volume 33, Issue 16, June 2012, Pages 4044–4058. [DOI] [PubMed] [Google Scholar]
- [42].Chai YC, Roberts SJ, Schrooten J, Luyten FP. Probing the osteoinductive effect of calcium phosphate by using an in vitro biomimetic model. Tissue Eng Part A. 2011. April;17(7–8):1083–97. [DOI] [PubMed] [Google Scholar]