Abstract
Sensory hair cells of the inner ear are exposed to continuous mechanical stress, causing damage over time. The maintenance of hair cells is further challenged by damage from a variety of other ototoxic factors, including loud noise, aging, genetic defects, and ototoxic drugs. This damage can manifest in many forms, from dysfunction of the hair cell mechanotransduction complex to loss of specialized ribbon synapses, and may even result in hair cell death. Because mammalian hair cells do not regenerate, the repair of hair cell damage is important for continued auditory function throughout life. Here we discuss how several key hair cell structures can be damaged, and what is known about how they are repaired.
Keywords: Hair cell, tip link, F-actin core, ribbon synapse, stereocilia, mechanotransduction
Hair Cell Maintenance and Repair
Hearing loss is the third most common health defect in adults, after heart disease and arthritis, affecting almost half of individuals over the age of 75 [1]. Damage from a variety of intrinsic and extrinsic sources can lead to a buildup of damage over time, leading to decreased hearing ability in the elderly (Figure 1, Key Figure). This review will discuss various types of hair cell injury, including tip link breakage, stereocilia core damage, and synapse loss, and what is known about their repair.
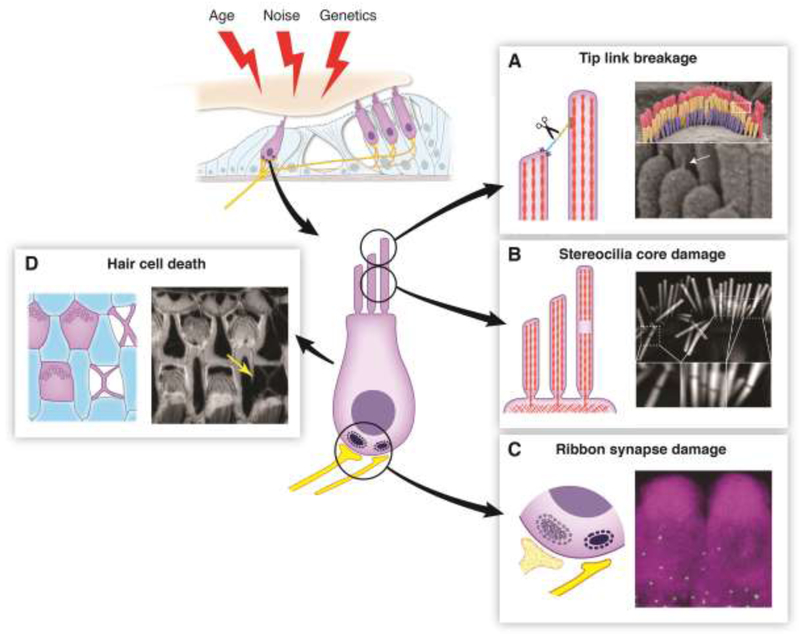
Figure 1, Key Figure. An Overview of Hair Cell Damage.
(Top Left) Hair cells can accumulate damage stemming from a variety of factors including age, noise, and genetics. (Center) Diagram of a hair cell indicating sites vulnerable to damage. (A) Tip link breakage. Left: Tip links can be broken by overstimulation or by in vitro calcium chelation, leading to a loss of tension on the MET channel complex and subsequent loss of the MET current. Right: Scanning electron micrograph showing an outer hair cell bundle. Tip links (white arrow) are visible at higher magnification below. (B) Stereocilia core damage. Left: Overstimulation of hair bundles causes the appearance of gaps in staining of the stereocilia F-actin core. These gaps likely represent sites of F-actin depolymerization, which would decrease bundle rigidity. Right: Phalloidin staining of a noise-damaged inner hair cell bundle. Gaps in the staining are visible at higher magnification below. (C) Ribbon synapse damage. Left: Ribbon synapses can be lost due to exposure to loud noise or prolonged exposure to milder noise, even in absence of permanent hearing threshold shift. Their loss can reduce hearing ability in noisy environments, known as “hidden hearing loss”. Right: Immunolabeling of CTBP2 (a component of ribbon synapses, green puncta) at the base of hair cells labeled by MYO7A immunostaining (magenta). (D) Hair cell death. Left: Auditory sensory epithelium with F-actin scars at the sites of missing hair cells. When hair cells die, they are extruded from the epithelium. Nearby supporting cells fill in the hole, leaving a cross-shaped F-actin scar. Right: Phalloidin labeling of F-actin in the organ of Corti from an aged mouse. An F-actin scar is seen at the site of a missing hair cell (yellow arrow). All images were taken in the Shin lab.
Mechanical sound stimuli are transduced into an electrochemical signal by hair cells in the cochlea. The signal is further transmitted to afferent neurons, which carry the signal to the brain, allowing for its perception [2,3]. A specialized mechanosensitive organelle at the apical surface of hair cells, known as the hair bundle (Glossary), is deflected in response to these auditory and vestibular stimuli. The hair bundle is composed of actin-based protrusions called stereocilia arranged into a staircase-like array [4,5]. The F-actin cores of the stereocilia taper at their base, where they insert into an actin mesh structure known as the cuticular plate [6]. The stereocilia are connected by a series of extracellular links along their length. The tip link is mechanically coupled to the mechanoelectrical transduction (MET) channel at the tip of a shorter row stereocilium and extends to the side of the stereocilium in the next tallest row [7–9]. Deflection of the hair bundle increases tension on tip links, opening MET channels and depolarizing the hair cell, leading to rapid neurotransmitter release from the ribbon synapses at the hair cell base. Hair cells are sensitive to a variety of genetic and environmental insults, e.g. mutations in genes encoding hair bundle proteins and noise trauma, respectively. In humans and other mammals, any damage that results in hair cell death can lead to irreparable hearing loss due to the inability of mature mammalian hair cells to regenerate [10]. In contrast, the hair cells of many non-mammalian vertebrates, such as birds and amphibians, have a significant regenerative capacity [11]. In these species, within days of severe acoustic trauma, the majority of hair cells can be mitotically replaced by supporting cells. It has been speculated that mammals lost the ability to regenerate hair cells as a consequence of adaptations of the organ of Corti to sense higher frequency stimuli, including the rise of distinct subtypes of supporting cells. Additionally, there was likely minimal negative selection against age-related hair cell loss, as most of this loss occurs after mammals have reached reproductive age [12]. The inability of mammals to replace dead and dying hair cells necessitates mechanisms to preserve the function of hair cells throughout the life of the organism and repair minor damages that build up over time.
To date, our understanding of the repair and maintenance mechanisms in hair cells is limited. When considering these mechanisms, it might be helpful to draw an analogy to facilities management. To ensure smooth operations of facilities and buildings, the ideal maintenance plan must be built on four pillars: Robustness of build, system redundancies, reactive maintenance, and preventive maintenance. With regard to hair cells, robust subcellular structures would obviously contribute to longevity of the cells, but hair cell MET, in its essence, depends on gracile mechanical parts to sense displacements that approach the scale of Brownian motion. The need for system redundancies might have given rise to the multitude of stereocilia in a hair bundle, and on the molecular and genetic level, to paralogous genes with overlapping functions. In a previous study, we showed for example that 31 of 56 actin and actin-binding proteins have paralogs present in the hair bundle [13]. Reactive maintenance, operating on a run-to-failure strategy, enjoys the benefit of low energetic cost, but has the potential of increased downtime, even catastrophic failure, unless redundancies are provided. Finally, a preventive mode of maintenance will most likely involve a continuous turnover of the functionally important proteins and structures, especially those sensitive to wear-and-tear. The turnover would need to be executed in a manner that does not disrupt MET function, possibly achieved by redundancy. With this analogy in mind, this review addresses the molecules and mechanisms involved in hair cell repair, and discusses implications for understanding mechanisms of hearing loss and for developing therapies.
Breakage and Repair of Tip Links
Tip link breakage
One of the best-characterized nonlethal forms of hair cell damage is the breakage of tip links. The tip link, composed of a heterotetramer of protocadherin 15 (PCDH15) and cadherin 23 (CDH23) [14–16], is vital for coupling mechanical stimuli to the opening of the MET channel [17]. PCDH15, at the lower end of the tip link, associates with the MET channel complex and interacts in a calcium-dependent manner with CDH23 at the upper end of the tip link, which is connected with the actin stereocilia core. A myosin motor at the upper tip link insertion, suggested to be either Myosin Ic [18] or Myosin VIIa [19], provides resting tension on the MET channel, making hair cells exquisitely sensitive to small deflections of the bundle [18,19]. Intense hair bundle stimulation, such as from exposure to prolonged or intense noise, can increase the force on tip links and cause their disruption [20]. Tip links can also be broken in vitro by overstimulation with a fluid jet or stiff probe or by the disruption of cadherin associations with a calcium chelator [17]. Tip link ablation uncouples the MET channel complex from mechanical stimuli, leading to the loss of mechanotransduction [17].
Tip link regeneration
In both avian [21] and mammalian species [22,23], tip links can be regenerated within 24 hours after in vitro breakage, accompanied by a restoration of mechanotransduction. Additionally, tip link numbers were shown to increase in vivo in chick hair cells over several time points after noise exposure [24,25]. In chickens, in vitro tip link recovery was unaffected by the presence of inhibitors of transcription and translation, suggesting that repair occurs without the production of new tip link components. The regeneration of tip links is suggested to be stimulated by decreased Ca++ influx through the MET channel, but direct evidence for this is lacking [21].
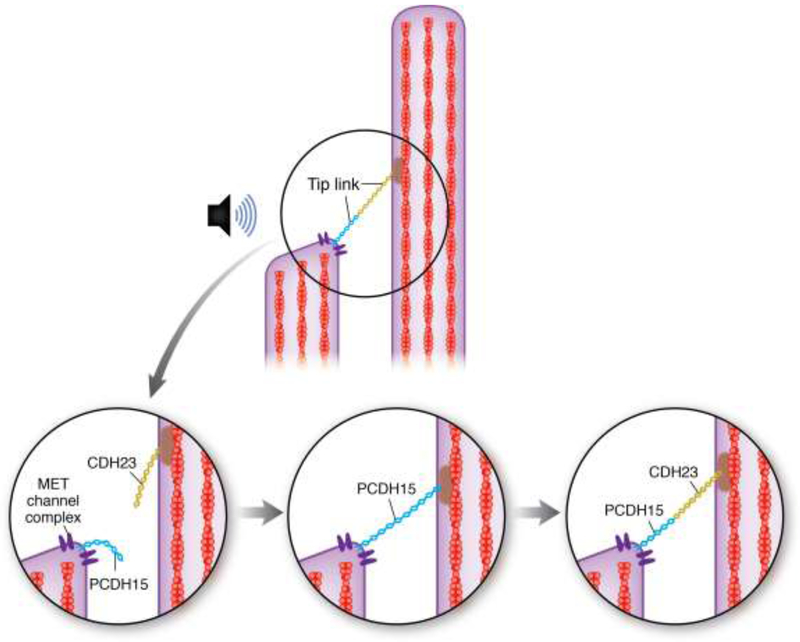
A more recent study in mammalian hair cells found that tip link regeneration occurs in a two-step process in which a temporary tip link, consisting of only PCDH15, is formed, followed by subsequent replacement of the upper half of the tip link by CDH23 (Figure 2). The MET current amplitude is fully restored in 12 hours by the temporary PCDH15-PCDH15 tip link, but adaptation of the MET current was still impaired for up to 36 hours, suggesting that the temporary tip link is not fully functional [23]. While considerable work remains to be done to characterize tip link regeneration, especially in in vivo models, it has been suggested that the breakage of tip links and their repair could contribute to the temporary threshold shift (TTS) observed after noise trauma [21].
Figure 2. Schematic model of Tip Link Repair.
(Top) A tip link, consisting of Protocadherin 15 (blue) and Cadherin 23 (gold) connects the MET complex at the top of one stereocilium to the actin core of the stereocilium in the next tallest row. Overstimulation, such as from loud noise, can cause tip link breakage (bottom left). According to [23], a temporary tip link, consisting of only Protocadherin 15, is formed within about 12 hours of damage, partially reestablishing MET channel function (bottom middle). Within about 36 hours after damage, the Protocadherin 15 at the top half of the tip link is replaced by Cadherin 23 to allow full restoration of MET function (bottom right).
One remaining question is whether tip link replacement is purely a mode of reactive maintenance or if tip links are turned over on a continual basis for preventative maintenance. For the latter option to work without downtime, each stereocilium (or sets of mechanically-connected stereocilia) must be able to execute the recovery by itself. One can speculate that this requirement for stereocilia-autonomous recovery is the reason that stereociliar tip link recovery evolved to be independent from a cellular transcriptional or translational response, as shown for chick basilar papilla hair cells [21]. The tip link components must therefore be recycled or derived from a pool of replacement proteins nearby, but direct evidence for such a mechanism is still lacking.
Stereocilia core damage and remodeling
Reversible MET current-dependent stereocilia F-actin core remodeling
The length of the F-actin cores of shorter row stereocilia has been shown to be regulated by the MET current [26,27]. Regression of shorter row stereocilia is seen in mutant mice in which tip links are lost postnatally. The specific localization of MET channel complexes at the tips of shorter row stereocilia implicated the MET current, which is absent when tip links are broken [17], in the regulation of their length. Two possible explanations were given to explain the phenomenon. The first explanation suggested that decreases in the local Ca++ concentration due to MET channel closure could alter the activity of actin regulatory proteins and lead to changes in stereocilia length. For example, the activity of myosin IIIa, which transports espin to the stereocilia tips, is likely dependent on Ca++ [28,29]. Reduced Ca++ concentrations, therefore, would prevent the elongation promoted by espin tip localization. The second explanation suggests that the tenting of the membrane caused by the pulling of the tip link reduces the resisting membrane tension at the stereocilia tip, allowing for elongation through a Brownian ratchet mechanism [30]. When tip links are broken and the membrane becomes rounded, this effect would be reversed.
The suggested role of the MET current in regulating the length of mechanotransducing shorter row stereocilia was tested experimentally by Velez-Ortega and colleagues [27]. Chemically blocking MET channels led to a specific and reversible shortening of the transducing stereocilia, without significantly changing the length of the tallest row. MET channel blockage also caused thinning of transducing row stereocilia tips, which they claimed was due to Ca++-dependent actin remodeling, rather than changes in membrane tension. This dynamic regulation of stereocilia actin polymerization by the MET current could be important for the regulation of stereocilia length and recovery of hair bundles after damage. For example, it is possible that this localized F-actin remodeling at stereocilia tips is necessary for the reestablishment of the MET channel complex after tip link breakage.
Mechanical damage to stereocilia F-actin core
Mechanical overstimulation in vitro has been shown to decrease the stiffness of the hair bundle [31,32] The decrease was shown to be reversible and independent of tip link breakage and it was suggested to be caused by damage to the stereocilia F-actin core or rootlet [32]. However, the relevance of in vitro stimulation with a fluid jet for studying noise-induced bundle damage is unclear. Many other changes in stereocilia F-actin cores have been observed using transmission electron microscopy (TEM) and scanning electron microscopy (SEM) following traumatic noise exposure including actin depolymerization, loss of F-actin crosslinkers, rootlet breakage, and stereocilia fusion [33–36]. A reduction in stereocilia number, variability in stereocilia height and width, and gaps in fluorescent phalloidin signal in the F-actin stereocilia core have also been shown following noise exposure [37]. The physiological relevance of these gaps is not known, but they likely represent areas of F-actin depolymerization or disorganization, which would decrease bundle stiffness and sensitivity. One caveat in the interpretation of this data is that the noise exposure protocols varied widely in severity between studies, and multiple animal models were used, including both mammalian and non-mammalian species. A pair of studies partially addressed this by comparing stereocilia damage in feline hair cells following either a mild, TTS-inducing [36] or a severe, permanent threshold shift (PTS)-inducing noise exposure. Following the TTS-inducing noise exposure, only minor changes were observed in the stereocilia core, including a shortening of stereocilia rootlets [36]. After PTS-inducing noise trauma, more severe damage was observed in the rootlets, along with stereocilia fusion, and holes in the stereocilia actin matrix [35].
Repair of stereocilia F-actin damage: Preventative turnover or reactive repair?
Until recently, stereocilia F-actin cores were thought to turn over through treadmilling within 48–72 hours, similar to, although somewhat slower than, filopodia and microvilli [38–40]. Fast turnover would presumably make the active repair of stereocilia cores unnecessary, as newly polymerized actin could quickly replace damaged structures. However, more recent studies have concluded that, in contrast, stereocilia core actin is extremely stable [41–43].
The original studies reporting fast turnover relied on imaging of fixed hair cells after transfection of β-actin-GFP, observing hair bundles labeled along their length with GFP within 48 hours of the transfection. Additionally they observed stereocilia with GFP labeling extending from stereocilia tips to partially down the core, as would be expected if the stereocilia actin were treadmilling [38,39].
In 2012, the treadmilling hypothesis was challenged by Zhang et al. [41], who presented several lines of evidence demonstrating that actin turnover in the stereocilia is restricted to the tips. First, they observed that stereocilia incorporated newly synthesized protein much more slowly than in the rest of cell, except at their very tips, which is inconsistent with an actin turnover rate of 2–3 days. They also showed that a bleached fiducial line in β-actin-GFP expressing hair bundles did not move closer to the rootlet over several days in culture, as would be expected if treadmilling were occurring. Finally, they showed that β- and γ- actin remained in stereocilia, except for a small region at tips, for at least 18 weeks after inducible knockout of either isoform [41]. The slow stereocilia actin turnover model was further supported by two additional reports in 2015 [42,43]. The first report demonstrated that newly synthesized GFP-β-actin localized to stereocilia tips, with minimal incorporation in the stereocilia core over 40 weeks [44]. The second report used live imaging of hair cells transfected with GFP-β-actin to demonstrate tip localization of new actin incorporation. Using this technique, the authors were able to explain the seemingly contradictory conclusion of fast turnover, determining that the hair cells with GFP-β-actin extending partially down the stereocilia core were immature. The labeled upper portion of the stereocilia represented growth of the F-actin core that occurred after transfection [42].
The stability of stereocilia F-actin over months implies that any structural damage must be repaired to preserve the integrity and rigidity of hair bundles. Little is known about such repair mechanisms, but Belyantseva et al. [45] suggested that γ-actin might be involved. Phalloidin-negative gaps were observed in stereocilia after noise trauma, and the authors saw an enrichment of γ-actin immunostaining in these gaps. Similar gaps were observed in γ-actin knockout mice without exposure to damaging noise, further supporting a role for γ-actin in their repair. This study also reported an enrichment of DNaseI staining, as well as espin, cofilin, and β-actin immunostaining in the gaps. The enrichment of DNaseI staining, which labels monomeric actin [46], along with β- and γ-actin suggests that globular actin is present at higher levels at these sites, whether due to depolymerization of the previously existing F-actin core or due to recruitment to the site for repolymerization. The presence of cofilin, which has both F-actin nucleating and severing roles [47], and espin, which has actin bundling activity [48], suggests that localized F-actin remodeling is occurring at the phalloidin-negative gaps to repair noise-induced damage to the paracrystalline F-actin stereocilia core [45] (Figure 3). Recent data suggest that despite the stability of actin filaments in cores, stereocilia F-actin crosslinkers are continuously turned over. The transience of crosslinkers might allow for the localized F-actin depolymerization and repolymerization necessary for repair [49]. Repair of these sites could contribute to recovery from temporary hearing threshold shifts (TTS), similar to tip link regeneration. However, it is still unclear whether these sites are being repaired structurally and/or functionally.
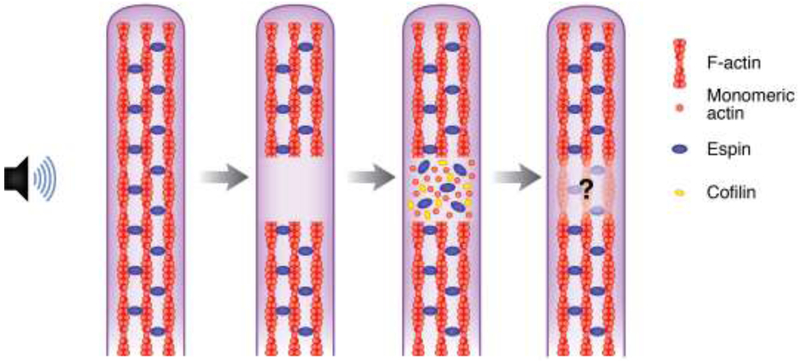
Figure 3. A Potential Mechanism for the Repair of Stereocilia F-actin Core Damage.
Overstimulation can cause the appearance of gaps in phalloidin staining of the F-actin cores of cochlear hair cell stereocilia. Gaps in F-actin staining in damaged stereocilia likely represent areas of disorganized or depolymerized actin. Immunostaining for β- and γ-actin is enriched at these sites, along with cofilin and espin. DNaseI staining is also observed, indicating that the actin is monomeric. The presence of monomeric actin, along with cofilin, which can nucleate actin at high concentrations, and espin, an actin crosslinker, suggests that localized F-actin remodeling is occurring to repair the damage [45]. However, there is no definite evidence that the damage is actually repaired.
Repair of F-actin structures in other cell types has been described. Strain sites in stress fibers, indicated by decreased signal of fluorescently labeled actin, are repaired through the recruitment of zyxin and paxillin. Zyxin and paxillin subsequently recruit the F-actin nucleator VASP and the F-actin crosslinker α-actinin to repair the strained site with localized F-actin polymerization to prevent a catastrophic breakage of the fiber [50,51]. It remains to be investigated whether analogous mechanism may be occurring in damaged stereocilia F-actin cores.
Can entire hair bundles be replaced?
Hair cells in regenerative species, such as frogs and chickens, can replace the entire hair bundle after nonlethal insults. For example, low doses of ototoxic aminoglycosides caused hair bundle loss in the bullfrog saccule. The bundleless hair cells were able to survive for at least a week and produce new hair bundles [52]. Evidence for such radical makeover in mammals is limited. After hair bundle ablation, hair cells in mammalian organs of Corti survived in culture for up to two weeks, but the bundles were not regenerated, despite evidence of functional maturation of the surviving cells [22]. A follow-up study suggested that virus-mediated expression of Atoh1, a transcription factor that regulates hair cell differentiation, can improve hearing preservation in noise-exposed guinea pigs, but since Atoh1 expression was limited to supporting cells, the underlying mechanism is unclear, and further characterization is needed [53].
Noise-Induced Synapse Damage and Repair
Ribbon synapse reduction and hidden hearing loss
Inner hair cell synapses with type I auditory spiral ganglion neurons (SGNs) are characterized by presynaptic structures called ribbons [54]. Neurotransmitter-filled vesicles are clustered around scaffolding proteins [55,56], which are linked to the presynaptic membrane [57]. The clustering of vesicles promotes rapid neurotransmitter release and allows for a graded response to stimuli [58]. Intense hair cell stimulation can cause damage to these presynaptic ribbons, as well as to postsynaptic terminals [59]. Glutamate-mediated excitotoxicity contributes to the postsynaptic damage [60,61], but the mechanism of presynaptic ribbon damage is not clearly understood. Potential causes include the loss of contact between pre- and post-synaptic densities and disassembly of ribbon subunits in response to intense stimulation [62]. Incomplete recovery of SGNs and ribbon synapses after damage is associated with a phenomenon known as hidden hearing loss [59,63,64]. While there is no PTS in these patients detected by auditory brainstem response (ABR), a decrease in amplitude of the signal from the auditory nerve is observed. This is likely due to a selective loss of low spontaneous rate auditory nerve fibers, which would cause difficulty in hearing in noisy environments [59,65,66].
Repair of synapses
Controversy exists on whether SGNs are able to repair damaged synapses. Kujawa and Liberman [59] observed no significant recovery of lost synapses between inner hair cell ribbons and SGNs within 8 weeks of noise exposure in mice. They also describe late onset SGN loss, corresponding to the degree of synapse loss. However, in guinea pigs, other groups have observed partial or complete recovery of synapse numbers after noise exposure [67,68], in agreement with older ultrastructural data, which used both a glutamate excitotoxicity model and noise exposure model to damage synapses [61,69]. These discrepancies could be explained, in part, by differences in synapse recovery between species. Efforts to resolve this issue have not yet delivered a clear answer [63,70]. Despite the controversy regarding the ability of IHC synapses to be repaired, there is agreement that there is a permanent decrease in the amplitude of auditory nerve response [59,70,71]. This might be due to a lack of synapse recovery and subsequent neuron loss [59,63], or alternatively, by incomplete function of recovered synapses [70,71]. Whichever the case, the unrepaired or improperly repaired synapses likely contribute to hidden hearing loss.
To prevent potentially irreversible loss of synapses, it would be beneficial for hair cells to have an upstream mechanism to prevent further stimulation and limit the insult to the hair cell. One might speculate that tip link breakage may act in this manner. Tip links could be considered a “circuit breaker”, to prevent further damage to other cellular structures vulnerable to overstimulation, like the synapses. While the fragility of tip links may seem like an inherent flaw in hair cell design, this quality may actually serve to protect hair cells from extensive irreparable damage, as the tip links themselves can be repaired. However, the MET current would have to be quickly restored to preserve synapse function, as it’s been shown that cells in which the MET current is lost revert to a pre-hearing pattern of innervation [72]. A similar feedback mechanism involving efferent innervation of outer hair cells has also been reported to protect synapses. Maison and colleagues [73]demonstrated that ablation of efferent innervation dramatically increased synapse loss after prolonged noise exposure, suggesting that the increased firing rate of these neurons to suppress outer hair cell amplification during loud noise exposure is protective against noise-induced cochlear neuropathy.
Hair cell death and wound healing
ROS-induced hair cell damage and endogenous antioxidant systems
Many factors causing hair cell death and damage have been described, including, but not limited to genetic abnormalities, loud or prolonged noise exposure [74,75], and ototoxic drugs [76] [77]. Hair cells can undergo cell death through apoptosis or necrosis, depending in part on the cause of cellular stress (reviewed in [78]). A common element in most causes of hair cell death is the production of reactive oxygen species (ROS). For example, noise trauma has been shown to elevate calcium levels in hair cells, which can subsequently lead to the stimulation of ROS production by the mitochondria [79,80]. Additionally it has also been suggested that higher metabolic demand in hair cells during exposure to intense stimulation could also lead to increased ROS levels, which can cause various forms of damage in the cell, including the activation of cell death pathways [81]. The endogenous antioxidant systems of the cochlea, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GR) enzymes, are capable of neutralizing ROS generated during normal hair cell activity. However, during noise trauma, these enzymes may become overwhelmed, leading to a buildup of ROS in hair cells [82]. Thus, antioxidants have been proposed as therapeutic agents to prevent ROS accumulation in hair cells after noise [83]. N-acetyl cysteine [84], as well as several other antioxidants, has been shown to attenuate noise-induced hearing loss when given either before or after noise exposure [85].
Preservation of the sensory epithelium cytoarchitecture after hair cell death
When a hair cell dies, the whole dying cell or its apical surface is extruded from the sensory epithelium. Remaining cell fragments are phagocytosed by supporting cells, which also seal the hole in the epithelium, leaving a scar and preserving the epithelial cytoarchitecture and organ of Corti barrier integrity [86–88]. In the avian inner ear, an actin cable assembled by supporting cells constricts around the hair bundle of dying hair cells, causing its excision. The remaining portion of the cell is then subsequently phagocytosed by surrounding supporting cells [89]. While repairing the epithelium is necessary for continued function, it may be possible to intervene before cell death occurs, preventing the extrusion of cells and allowing more time for repair of the damaged cells.
Concluding Remarks and Future Perspectives
Unlike many lower vertebrate species [11,90], mammals are unable to regenerate hair cells (reviewed in [10]) and appear to have a reduced capacity for intracellular repair of hair bundle damage [22]. Some mechanisms by which mammalian hair cells are maintained and repair minor damage have been described [22,23,27] and were discussed in this review. These mechanisms are likely essential for preserving hearing function in mammals, but much remains unknown (see Outstanding Questions). Recovery of tip links and the actin core provides a clear example of a possible contributing factor to the recovery from temporary threshold shift, while inefficient repair of hair bundle damage, such as F-actin core depolymerization, or the death of hair cells likely cause progressive and permanent hearing loss. Characterizing these maintenance and repair mechanisms will be important for the development of therapies for various forms of hearing loss.
Highlights.
Hair cells acquire damage from a variety of environmental and genetic factors. To fully maintain auditory function, damaged hair cells must be repaired.
Broken tip links are repaired in both regenerating and non-regenerating hair cells.
Intense noise exposure damages the stereocilia F-actin core, which may be repaired by localized F-actin remodeling.
Ribbon synapse loss, which can reduce hearing ability in noisy environments, may or may not be reversible.
Holes in the sensory epithelium caused by the extrusion of dying hair cells are sealed by projections from nearby supporting cells to preserve barrier integrity.
Acknowledgements
We thank Ting-Ting Du and Sihan Li (University of Virginia) for providing images used in this manuscript and for providing helpful commentary. The figure illustrations were created by Anita Impagliazzo. Funding was provided by NIH/NIDCD grants F31DC017370 (ELW) and R01DC014254 (JBS).
Glossary
- Hair bundle:
staircase-shaped structure at the apex of hair cells that is deflected in response to stimulation, leading to the opening of mechanosensitive ion channels
- Hair cell mechanoelectrical transduction (MET):
conversion of sound or gravity-induced deflection of the hair bundle into an electrical signal in the form of an influx of cations through the MET channel
- Permanent Threshold Shift (PTS):
a permanent change in hearing threshold following exposure to loud or prolonged noise
- Ribbon synapse:
a specialized synapse in hair cells and other cell types in which neurotransmitter-filled vesicles clustered to facilitate their rapid release
- Spiral ganglion neurons:
neurons innervating auditory hair cells; their axons form the auditory branch of the eighth cranial nerve
- Stereocilia:
actin-based protrusion that arrange into a staircase-like array to build the hair bundle
- Temporary Threshold Shift (TTS):
a temporary change in hearing threshold following exposure to loud or prolonged noise
- Tip link:
extracellular link connecting the MET channel at the tips of shorter row stereocilia to the F-actin core of the stereocilium in the next tallest row
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no competing interests.
References
- 1.Blackwell DL, Lucas JW,CT (2014) Summary health statistics for US adults: National Health Interview Survey. Vital Heal. Stat 10 260, 1–161 [PubMed] [Google Scholar]
- 2.Hudspeth AJ (2005) How the ear ‘s works work: mechanoelectrical transduction and amplification by hair cells. C.R. Biol 328, 155–162 [DOI] [PubMed] [Google Scholar]
- 3.Fettiplace R (2017) Hair cell transduction, tuning, and synaptic transmission in the mammalian cochlea. Compr. Physiol 7, 1197–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flock A (1964) Structure of the macula utriculi with special reference to directional interplay of sensory resoneses as revealed by morphological polarization. J. Cell Biol. 22, 413–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilney LG et al. (1980) The organization of actin filaments in the stereocilia of cochlear hair cells. J. Cell Biol. 86, 244–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeRosier DJ and Tilney LG (1989) The structure of the cuticular plate, an in vivo actin gel. J. Cell Biol. DOI: 10.1083/jcb.109.6.2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickles JO et al. (1984) Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res 15, 103–112 [DOI] [PubMed] [Google Scholar]
- 8.Beurg M et al. (2009) Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat. Neurosci DOI: 10.1038/nn.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurima K et al. (2015) TMC1 and TMC2 Localize at the Site of Mechanotransduction in Mammalian Inner Ear Hair Cell Stereocilia. Cell Rep. 12, 1606–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns JC and Corwin JT (2013) A historical to present-day account of efforts to answer the question: “What puts the brakes on mammalian hair cell regeneration?.” Hear. Res 297, 52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corwin JT and Cotanche D. a (1988) Regeneration of sensory hair cells after acoustic trauma. Science DOI: 10.1126/science.3381100 [DOI] [PubMed] [Google Scholar]
- 12.Groves AK (2010) The challenge of hair cell regeneration. Exp. Biol. Med. (Maywood). 235, 434–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin J-B et al. (2013) Molecular architecture of the chick vestibular hair bundle. Nat. Neurosci 16, 365–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siemens J et al. (2004) Cadherin 23 Is a component of the tip link in hair-cell stereocilla. Nature 428, 950–955 [DOI] [PubMed] [Google Scholar]
- 15.Ahmed ZM et al. (2006) The Tip-Link Antigen, a Protein Associated with the Transduction Complex of Sensory Hair Cells, Is Protocadherin-15. J. Neurosci DOI: 10.1523/JNEUROSCI.1163-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazmierczak P et al. (2007) Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature DOI: 10.1038/nature06091 [DOI] [PubMed] [Google Scholar]
- 17.Assad JA et al. (1991) Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron DOI: 10.1016/0896-6273(91)90343-X [DOI] [PubMed] [Google Scholar]
- 18.Holt JR et al. (2002) A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell DOI: 10.1016/S0092-8674(02)00629-3 [DOI] [PubMed] [Google Scholar]
- 19.Grati M and Kachar B (2011) Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc. Natl. Acad. Sci. U. S. A 108, 11476–11481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickles JO et al. (1987) Vulnerability of tip links between stereocilia in the guinea pig to acoustic trauma. Hear. Res 25, 173–183 [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y-D et al. (1996) Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc. Natl. Acad. Sci 94, 15469–15474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia S et al. (2009) Fate of mammalian cochlear hair cells and stereocilia after loss of the stereocilia. J. Neurosci 29, 15277–15285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Indzhykulian AA et al. (2013) Molecular Remodeling of Tip Links Underlies Mechanosensory Regeneration in Auditory Hair Cells. PLoS Biol. 11, e1001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husbands JM et al. (1999) Tip-link integrity on chick tall hair cell stereocilia following intense sound exposure. Hear. Res 135, 135–145 [DOI] [PubMed] [Google Scholar]
- 25.Kurian R et al. (2003) Tip link loss and recovery on chick short hair cells following intense exposure to sound. Hear. Res DOI: 10.1016/S0378-5955(03)00165-5 [DOI] [PubMed] [Google Scholar]
- 26.Caberlotto E et al. (2011) Coupling of the mechanotransduction machinery and F-actin polymerization in the cochlear hair bundles. Bioarchitecture 1, 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vélez-Ortega AC et al. (2017) Mechanotransduction current is essential for stability of the transducing stereocilia in mammalian auditory hair cells. Elife 6, pii: e24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salles FT et al. (2009) Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat. Cell Biol. DOI: 10.1038/ncb1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merritt RC et al. (2012) Myosin IIIB uses an actin-binding motif in its espin-1 cargo to reach the tips of actin protrusions. Curr. Biol 22, 320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peskin CS et al. (1993) Cellular Motions and Thermal Fluctuations: The Brownian Ratchet. Biophys. J 65, 316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders JC et al. (1986) Growth of threshold shift in hair-cell stereocilia following overstimulation. Hear. Res 23, 245–255 [DOI] [PubMed] [Google Scholar]
- 32.Duncan RK and Saunders JC (2000) Stereocilium injury mediates hair bundle stiffness loss and recovery following intense water-jet stimulation. J. Comp. Physiol.- A Sensory, Neural, Behav. Physiol 186, 1095–1106 [DOI] [PubMed] [Google Scholar]
- 33.Tilney LG et al. (1982) Changes in the organization of actin filaments in the stereocilia of noise-damaged lizard cochleae. Hear. Res DOI: 10.1016/0378-5955(82)90013-2 [DOI] [PubMed] [Google Scholar]
- 34.Engström B et al. (1983) Ultrastructural studies of stereocilia in noise-exposed rabbits. Hear. Res DOI: 10.1016/0378-5955(83)90110-7 [DOI] [PubMed] [Google Scholar]
- 35.Liberman MC (1987) Chronic ultrastructural reconstruction changes in acoustic trauma: Serial-section of stereocilia and cuticular plates. Hear. Res 26, 65–88 [DOI] [PubMed] [Google Scholar]
- 36.Liberman MC and Dodds LW (1987) Acute ultrastructural changes in acoustic trauma: Serial-section reconstruction of stereocilia and cuticular plates. Hear. Res 26, 45–64 [DOI] [PubMed] [Google Scholar]
- 37.Avinash GB et al. (1993) 3-D analysis of F-actin in stereocilia of cochlear hair cells after loud noise exposure *. Hear. Res 67, 139–146 [DOI] [PubMed] [Google Scholar]
- 38.Schneider ME et al. (2002) Rapid renewal of auditory hair bundles. Nature DOI: 10.1038/418837a [DOI] [PubMed] [Google Scholar]
- 39.Rzadzinska AK et al. (2004) An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J. Cell Biol. 164, 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manor U and Kachar B Dynamic length regulation of sensory stereocilia. , Seminars in Cell and Developmental Biology, 19 (2008), 502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang D et al. (2012) Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature 481, 520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drummond MC et al. (2015) Live-cell imaging of actin dynamics reveals mechanisms of stereocilia length regulation in the inner ear. Nat. Commun 6, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narayanan P et al. (2015) Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament severing proteins. Nat Commun. 6, 554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narayanan P et al. (2015) Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nat. Commun 6, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belyantseva IA et al. (2009) Gamma-actin is required for cytoskeletal maintenance but not development. Proc. Natl. Acad. Sci. U. S. A 106, 9703–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mannherz HG et al. (1975) A Specific 1:1 G-Actin:DNAase I Complex Formed by the Action of DNAase I on F-Actin. FEBS Lett. 60, 34–38 [DOI] [PubMed] [Google Scholar]
- 47.Andrianantoandro E and Pollard TD (2006) Mechanism of Actin Filament Turnover by Severing and Nucleation at Different Concentrations of ADF / Cofilin. DOI: 10.1016/j.molcel.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 48.Loomis PA et al. (2003) Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. 163, 1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy P and Perrin BJ (2018) The stable actin core of mechanosensory stereocilia features continuous turnover of actin cross-linkers. Mol. Biol. Cell 29, 1856–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith MA et al. (2010) A Zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev. Cell 19, 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith MA et al. (2013) LIM Domains Target Actin Regulators Paxillin and Zyxin to Sites of Stress Fiber Strain. PLoS One 8, e69378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gale JE et al. (2002) Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog’s saccule. J. Neurobiol 50, 81–92 [DOI] [PubMed] [Google Scholar]
- 53.Yang SM et al. (2012) Regeneration of Stereocilia of Hair Cells by Forced Atoh1 Expression in the Adult Mammalian Cochlea. PLoS One 7, e46355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith C and Sjostrand F (1961) Structure of the nerve endings on the external hair cells of the guinea pig cochlea as studied by serial sections. J. Ultrastruct. Res 5, 523–56 [DOI] [PubMed] [Google Scholar]
- 55.Schmitz F et al. (2000) RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron DOI: 10.1016/S0896-6273(00)00159-8 [DOI] [PubMed] [Google Scholar]
- 56.Dick O et al. (2001) Localization of the presynaptic cytomatrix protein Piccolo at ribbon and conventional synapses in the rat retina: Comparison with Bassoon. J. Comp. Neurol DOI: 10.1002/cne.1344 [DOI] [PubMed] [Google Scholar]
- 57.Khimich D et al. (2005) Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature DOI: 10.1038/nature03418 [DOI] [PubMed] [Google Scholar]
- 58.Safieddine S et al. (2012) The Auditory Hair Cell Ribbon Synapse: From Assembly to Function. Annu. Rev. Neurosci 35, 509–28 [DOI] [PubMed] [Google Scholar]
- 59.Kujawa SG and Liberman MC (2009) Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci DOI: 10.1523/JNEUROSCI.2845-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puel JL et al. (1996), Excitotoxicity and plasticity of IHC-auditory nerve contributes to both temporary and permanent threshold shift , in Scientific Basis of Noise-induced Hearing Loss, pp. 36–42 [Google Scholar]
- 61.Puel JL et al. (1998) Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport DOI: 10.1097/00001756-199806220-00037 [DOI] [PubMed] [Google Scholar]
- 62.Shi L et al. (2016) Cochlear Synaptopathy and Noise-Induced Hidden Hearing Loss. Neural Plast. DOI: 10.1155/2016/6143164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin HW et al. (2011) Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. JARO - J. Assoc. Res. Otolaryngol DOI: 10.1007/s10162-011-0277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaette R and McAlpine D (2011) Tinnitus with a Normal Audiogram: Physiological Evidence for Hidden Hearing Loss and Computational Model. J. Neurosci DOI: 10.1523/JNEUROSCI.2156-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furman AC et al. (2013) Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol DOI: 10.1152/jn.00164.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young ED and Barta PE (1986) Rate responses of auditory nerve fibers to tones in noise near masked threshold. J. Acoust. Soc. Am DOI: 10.1121/1.393530 [DOI] [PubMed] [Google Scholar]
- 67.Liu L et al. (2012) Silent Damage of Noise on Cochlear Afferent Innervation in Guinea Pigs and the Impact on Temporal Processing. PLoS One DOI: 10.1371/journal.pone.0049550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi L et al. (2013) Ribbon synapse plasticity in the cochleae of guinea pigs after noise-induced silent damage. PLoS One DOI: 10.1371/journal.pone.0081566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pujol R and Puel JL (1999) Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: A review of recent findings. Ann. N. Y. Acad. Sci DOI: 10.1111/j.1749-6632.1999.tb08646.x [DOI] [PubMed] [Google Scholar]
- 70.Shi L et al. (2015) Noise induced reversible changes of cochlear ribbon synapses contribute to temporary hearing loss in mice. Acta Otolaryngol. DOI: 10.3109/00016489.2015.1061699 [DOI] [PubMed] [Google Scholar]
- 71.Shi L et al. (2016) Coding deficits in noise-induced hidden hearing loss may stem from incomplete repair of ribbon synapses in the cochlea. Front. Neurosci DOI: 10.3389/fnins.2016.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corns LF et al. (2018) Mechanotransduction is required for establishing and maintaining mature inner hair cells and regulating efferent innervation. Nat. Commun 9, 4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maison S et al. (2013) Efferent Feedback Minimizes Cochlear Neuropathy from Moderate Noise Exposure. J. Neurosci 33, 5542–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liberman MC (2017) Noise-induced and age-related hearing loss: new perspectives and potential therapies. F1000Research 6, 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohlemiller KK Recent findings and emerging questions in cochlear noise injury. , Hearing Research, 245 (2008), 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schacht J et al. (2012) Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat. Rec.(Hoboken) 295, 1837–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng AG et al. (2005) Mechanisms of hair cell death and protection. Curr. Opin. Otolaryngol. Head Neck Surg. 13, 343–8 [DOI] [PubMed] [Google Scholar]
- 78.Dinh CT et al. (2015) Molecular regulation of auditory hair cell death and approaches to protect sensory receptor cells and/or stimulate repair following acoustic trauma. Front. Cell. Neurosci DOI: 10.3389/fncel.2015.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng T-I and Jou M-J (2010) Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci 1201, 183–188 [DOI] [PubMed] [Google Scholar]
- 80.Bottger EC and Schacht J (2013) The mitochondrion: A perpetrator of acquired hearing loss. Hear. Res 303, 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Henderson D et al. (2006) The Role of Oxidative Stress in Noise-Induced Hearing Loss. Ear Hear. 27, 1–19 [DOI] [PubMed] [Google Scholar]
- 82.Wong ACY and Ryan AF (2015) Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci. 7, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oishi N and Schacht J (2011) Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs 16, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bielefeld EC et al. (2007) Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Otolaryngol. 127, 914–919 [DOI] [PubMed] [Google Scholar]
- 85.Le TN et al. (2017) Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol. Neck Surg. 46, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raphael Y and Altschuler RA (1991) Scar formation after drug-induced cochlear insult. Hear. Res DOI: 10.1016/0378-5955(91)90034-7 [DOI] [PubMed] [Google Scholar]
- 87.Forge A (1985) Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear. Res DOI: 10.1016/0378-5955(85)90121-2 [DOI] [PubMed] [Google Scholar]
- 88.Anttonen T et al. (2014) How to Bury the Dead: Elimination of Apoptotic Hair Cells from the Hearing Organ of the Mouse. JARO - J. Assoc. Res. Otolaryngol DOI: 10.1007/s10162-014-0480-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bird JE et al. (2010) Supporting Cells Eliminate Dying Sensory Hair Cells to Maintain Epithelial Integrity in the Avian Inner Ear. J. Neurosci 30, 12545–12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cotanche DA (1987) Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear. Res DOI: 10.1016/0378-5955(87)90135-3 [DOI] [PubMed] [Google Scholar]