Abstract
Objective:
The incidence of Alcohol Use Disorder (AUD) in human immunodeficiency virus (HIV) infection is twice that of the rest of the population. This study documents complex radiologically-identified, neuroanatomical effects of AUD+HIV comorbidity by identifying structural brain systems that predicted diagnosis on an individual basis.
Method:
Applying novel machine learning analysis to 549 participants (199 controls, 222 AUD, 68 HIV, 60 AUD+HIV), 298 MRI brain measurements were automatically reduced to small subsets per group. Significance of each diagnostic pattern was inferred from its accuracy in predicting diagnosis and performance on 6 cognitive measures.
Results:
While all three diagnostic patterns predicted the learning and memory score, the AUD+HIV pattern was the largest and had the highest predication accuracy (78.1%).
Conclusions:
Providing a roadmap for analyzing large, multimodal data sets, the machine learning analysis revealed imaging phenotypes that predicted diagnostic membership of MRIs of individuals with AUD, HIV, and their comorbidity.
Keywords: alcoholism, HIV infection, comorbidity, machine learning, disease patterns, brain imaging
1. Introduction
Alcohol Use Disorder (AUD) is common (1), and its comorbidity in individuals with human immunodeficiency virus (HIV) infection is high (2–4), occurring at a rate twice that of the general population (5). AUD and HIV-infection each disrupts brain structural integrity with the likely outcome of reducing health-related quality of life and cognition (3, 4). AUD targets, among other regions, frontal cortices (6–8) and cerebellum (9, 10). HIV similarly compromises frontal cortices, but also cingulate and parietal regions, e.g., (11). AUD and HIV are independently associated with volume deficits in thalamus, hippocampus, caudate and putamen (e.g., AUD: (4, 10, 12); HIV: (4, 11, 13)). Relatively few brain studies have examined the heightened burden of disease comorbidity (2, 14), which has the potential to exacerbate the untoward effects on neural systems through synergistic or additive processes (15, 16). HIV+AUD show moderate to severe abnormalities especially in frontal cortices and thalamus (17).
One possible solution to enhance understanding of the complex neurological effects of AUD+HIV comorbidity is to encode the architecture of the whole brain of an individual through large numbers of measures extracted from a fine-grained parcellation of brain regions. To date, however, morphometric brain studies on AUD and HIV have relied on univariate testing of relatively few MRI metrics that are separately related to diagnostic groups (2, 3, 11, 14–16, 18–21). Alternatively, machine learning approaches (22–25) can jointly analyze a large number of metrics by combining them into a single score. To highlight the power of such multivariate analysis (22, 26) for expanding knowledge about neuropsychiatric disorders, we derived diagnostic scores to predict diagnosis from MRIs collected in individuals with AUD, HIV, or their comorbidity.
To test the hypothesis that MRIs can be used to predict diagnosis and cognitive measures in individuals with AUD, HIV, or their comorbidity, we first improved on a technology called sparse classification (27–29). We then applied the corresponding novel machine learning analysis to a dataset of 549 MRIs of controls and individuals diagnosed with one of the three disorders, taken from our previous report on 30 regional volumes (17). Each MRI was now quantified in terms of 298 brain regional metrics of volume, surface area, thickness, and curvature. For each disorder, our data-driven approach first identified a diagnostic pattern by automatically reducing the large number of MRI metrics to a small subset affected by the disorder. The subset of metrics was then applied to each individual MRI to compute a diagnostic score to predict diagnosis and cognitive ramifications in the corresponding participant. By doing so, our machine learning analysis not only identified structural differences from controls in AUD individuals (as in (30)) but also in HIV and their comorbidity.
2. Materials and Methods
2.1. Participants
The four groups comprised 199 healthy controls (CTRL), 222 AUD individuals, 68 HIV-infected individuals (HIV), and 60 subjects with both AUD and HIV infection (AUD+HIV). Participants ranged in age between 25 – 75 years.
AUD participants were screened to meet DSM-IV criteria for Alcohol Dependence or Abuse and DSM5 criteria for AUD, to have ≥10 years of heavy drinking, and habitually consume ≥150 drinks a month for men or ≥90 for women. The study recorded their Days After Last Drink (DALD), total lifetime alcohol consumed in kilograms (Alc Kg), and alcohol consumed in the past year (Alc py).
Participants in non-AUD groups (e.g., HIV and CTRL) did not meet DSM-IV criteria for Alcohol Dependence or Abuse or DSM5 for AUD. HIV subjects were seropositive for the HIV-infection with CD4 count (average: 303.0) and had a Karnofsky score ≥70 (31). Participants in the CTRL group had never met DSM-IV or DSM5 criteria for any neuropsychiatric disorder and tested negative for HIV infection. The two HIV groups had higher mean Veterans Aging Cohort Study (VACS) Indices (32) than either the control or AUD groups (Table 1). Note that VACS defines a score based on pre-assigned points for age, HIV indicators (CD4 count and HIV-1 RNA), and general indicators of organ system injury (see Supplement for details).
Table 1:
Demographic information and the statistics of the cognitive and clinical measures (mean ± standard deviation) for each group. Each of the composite scores was the mean of available test measures for each participant (refer to the text for details). Group differences are measured for each diagnosis group with respect to the CTRL group by χ2-test (for sex, ethnicity, HAART medication, and AIDS status) and t-test (for other measures), and are considered not significant (NS) if p-value > 0.05.
| Measure | CTRL | AUD | p-value (vs. CTRL) | HIV | p-value (vs. CTRL) | AUD+HIV | p-value (vs. CTRL) | Pair-v/ise group differences* | |
|---|---|---|---|---|---|---|---|---|---|
| Total Subjects | 199 | 222 | - | 68 | - | 60 | - | - | |
| Demographic | Sex F/M (F% / M%) | 92/107 (46%/54%) | 66/156 (30%/70%) | 4.8108 × 10−4 | 2¼7 (31%/69%) | 0.0017 | 22/38 (37%/ 63%) | <0.00001 | X2= 13.4. p=0.004 |
| Age (years) | 46.7±14.2 | 48.4±9.9 | NS | 51.4±8.7 | 0.0102 | 51.7±6.9 | 0.0118 | CTRL = AUD < HIV = AUD+HIV | |
| Education (years) | 16.0±2.3 | 13.4±2.4 | <0.00001 | 13.5±2.4 | <0.00001 | 13.0±2.1 | <0.00001 | CTRL > AUD = HIV = AUD+HIV | |
| Socioeconomic status (lower is better) | 25.5±11.6 | 40.9±14.4 | <0.00001 | 40.7±14.2 | <0.00001 | 45.2±12.2 | <0.00001 | CTRL < AUD = HIV = AUD+HIV | |
| Body mass index | 25.9±4.2 | 26.8±4.8 | 0.0408 | 26.6±4.7 | NS | 26.8±4.9 | NS | CTRL = AUD = HIV = AUD+HIV | |
| Ethnicity Asian/African American/Caucasian/Other |
28/28/127/16 | 4/71/117/30 | <0.00001 | 0/31/34/3 | <0.00001 | 0/38/17/5 | <0.00001 | X2=97.80. p<0.0001 | |
| Clinical | Days After Last Drink (DALD) | - | 19o.9±507.9 | - | - | 398.9±1126.8 | - | AUD=AUD+HIV | |
| Total Alcohol Consumed (Ale Kg) | 34.0 ±57.0 | 1206.2±885.7 | 7.4523 × 10−40 | 110.5±240.7 | NS | 1081 0±916.1 | 4.9219 ×10−28 | CTRL = HIV < AUD = AUD+HIV | |
| Alcohol consumed in past year | - | 42.2±45.7 | - | - | - | 14.0±17.7 | - | AUD > AUD+HIV | |
| VACS Index | 13.8±12.3 | 15.0+12.7 | NS | 27.3±18.1 | 4.6496 × 10−7 | 32.8±22.5 | 5.1148 × 10* | CTRL = AUD < HIV = AUD+HIV | |
| CD4 cell count (100/mm3) | - | - | - | 303.0± 188.7 | - | 278.9±216.5 | - | HIV = AUD+HIV | |
| sladir CD4 (100/mm3) | - | - | - | 202.0±176.4 | - | 208.5± 174.4 | - | HIV = AUD+HIV | |
| Viral Load | - | - | - | 2.13+1.14 | - | 2.24±1.18 | - | HIV = AUD+HIV | |
| Percentage on HAART Medication | - | - | - | 88% | - | 87% | - | HIV = AUD+HIV (x2=0.0457) | |
| Percentage with AIDS Status | - | - | - | 53% | - | 60% | - | HIV = AUD+HIV (x2=0.9968) | |
| Cognitive | Verbal Language (VL) | −0.10±0.86 | −0.70±0.96 | 2.0424 × 10−5 | −0.92±1.14 | 3.7039 × 10−6 | −0.71 ±0.99 | 2.5637 × 10−4 | CTRL > AUD = HIV = AUD+HIV |
| Executive Function (EXF) | −0.04±0.90 | −0.70±1.12 | 3.7239 × 10−6 | −0.98±1.55 | 4.7593 × 10−6 | −1.12±1.43 | 9.3434×10−8 | CTRL > AUD = HIV = AUD+HIV | |
| Learning and Memory (LM) | −0.08±0.80 | −0.95±0.86 | 8.5698 × 10−11 | −0.84±0.97 | 2.8087 × 10−6 | −1.08±0.88 | 7.0479 ×10−10 | CTRL > AUD = HIV = AUD+HIV | |
| Speed of Information Processing (SIP) | 0.14±0.69 | −0.39±0.90 | 5.8493 × 10−6 | −0.51 ±0.85 | 8.5445 × 10−7 | −0.82±1.01 | 4.6449 × 10−10 | CTRL > AUD = HIV = AUD+HIV | |
| Motor Skills (MS) | −0.10±0.77 | −0.72±0.90 | 4.1689×10−7 | −0.93±1.36 | 5.6897 × 10−6 | −0.96±1.44 | 1.0281 × 10−5 | CTRL > AUD = HIV = AUD+HIV | |
| Quality of Social Functioning (QSF) | 0.15±0.75 | −2.00±1.63 | 2.2572×10−38 | −1.94±1.47 | 1.6003× 1030 | −2.80±1.96 | 4.8170×10−36 | CTRL > AUD = HIV = AUD+HIV | |
‘=‘ not significantly different
‘<‘ or ‘>‘ significantly different (p < 0.05)
Participants completed neuropsychological tests assessing six cognitive, motor, and social functional domains. Composite scores of each domain were derived from age- and education- corrected z-scores based on control performance: Verbal Language (VL), Executive Function (EXF), Learning and Memory (LM), Speed of Information Processing (SIP), Motor Skills (MS), and Quality of Social Functioning (QSF). Table 1 lists their mean±SD as well as the medication history of participants (see Supplement for detail).
The neuropsychological scores were based on the means of composite scores representing 6 functional domains (cf., 33, 34) described previously (Supplemental Material 17). Raw scores from tests included in each composite score were age-corrected based on 66 male and 85 female healthy controls, aged 20 to 67 at their first examination and expressed as standardized z-scores. All metrics (e.g., speed scores such as Trails A and B) were transformed so that higher scores were in the direction of better performance. Each of the composite scores was the mean of the z-scores of available test measures for each participant: EXF comprised Trails B (35) or Color Trails 2 (36) time, Wechsler Memory Scale-Revised (WMS-R) (37) or MicroCog (38) forward and backward digit span, and the Golden Stroop Color Word raw score (39); LM comprised the Rey-Osterrieth Complex Figure Test immediate recall raw score (40) and WMS-R Logical Memory (immediate recall total raw score) (37) or MicroCog Memory (immediate recognition score) (38); VL comprised FAS letter fluency total score (41) and National Adult Reading Test (42), Peabody (43), or Wechsler Test of Adult Reading (44) total score; SIP comprised Trails A (35) or Color Trails 1 (36) time, Digit Symbol (45) or Symbol Digit (46) raw score, and Golden Stroop Color raw score (40); MS comprised Grooved Pegboard mean of left and right hand scores (47), Fine Finger Movement mean of all conditions (48), and Ataxia mean score of standing on the left and right legs separately (49); QSF comprised Quality of Life SF-21 total raw score (50), Global Assessment of Functioning score (current) (51), and Activities of Daily Living (combined Performance and Instrumental scores) (52). Participants underwent different cognitive tests for some domains as some tests were replaced during the longitudinal study.
2.2. MRI Data Acquisition and Preprocessing
Imaging data were acquired on a 3T General Electric (GE; Waukesha, WI) SIGNA system using an 8-channel Array Spatial Sensitivity Encoding Technique (ASSET) coil for parallel and accelerated imaging. Inversion Recovery-SPoiled Gradient Recalled (IR-SPGR) echo sequence (TR=7.068ms, TI=300ms, TE = 2.208ms, flip angle=15◦, matrix=256 × 256, slice dimensions=1.25 × 0.9375 × 0.9375mm, 124 slices) were collected in the sagittal plane.
Processing of a T1-weighted (T1w) MR image (see Supplement for detail) resulted in the supratentorial volume (svol) according to the SRI24 atlas (53) and the z-scores of 298 morphometric measurements extracted by Freesurfer (54–56). Note that baseline volumetric MRI data of the 549 participants were previously published (17) but were derived solely from 30 ROIs of the SRI24 atlas (57) rather than the FreeSurfer atlas used herein.
The morphometric measurements of the CTRL group varied significantly with age, sex, and svol (Pearson correlation p-value < 0.005). These confounding factors were regressed out from the morphometric measurements by parameterizing a general linear model (58) on the controls of the training data. Details can be found in Supplement.
2.3. Machine Learning for Statistical Analysis
The data were divided into three diagnosis-specific sets: AUD(N=222) vs. CTRL(N=199), HIV(N=68) vs. CTRL(N=199), and AUD+HIV(N=60) vs. CTRL(N=199). With respect to each data set, our analysis followed the three steps outlined in Figure 1. Specifically, we first identified a diagnostic pattern by applying a multivariate machine learning method (see (27) and Supplement for detail) to the entire data set (Step 1), which also automatically matched the sample size of the two cohorts. The resulting pattern was applied to individual MRIs producing a diagnostic score, which was the prediction of an individual having the diagnosis based solely on brain MRI measurements. The perfect diagnostic score for CTRLs was 0.0; for any individual from the diagnostic group, the perfect diagnostic score was 1.0. The diagnostic pattern was also correlated with the 6 cognitive scores recorded for members of that diagnostic group (Step 2). Correlations that were positive and had a p-value < 0.05 were reported. Step 3 measured the accuracy of the machine learning method via 10-fold cross-validation (59). The balanced accuracy (BAcc) (27), specificity, and sensitivity in predicting the diagnosis of each test subject were recorded. Furthermore, the significance of the accuracy (p-value < 0.001) was inferred using the Fisher exact test (60). A detailed description of the three steps appears in the Supplement.
Figure 1.
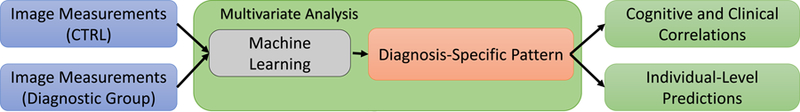
The analysis approach used for identifying diagnostic pattern and score specific to AUD, HIV, and AUD+HIV. It includes three major steps: multivariate analysis for identification of diagnostic pattern and score (Step 1), cognitive correlations (Step 2), and individual-level predictions (Step 3).
3. Results
3.1. Multivariate Analysis: Diagnostic Patterns
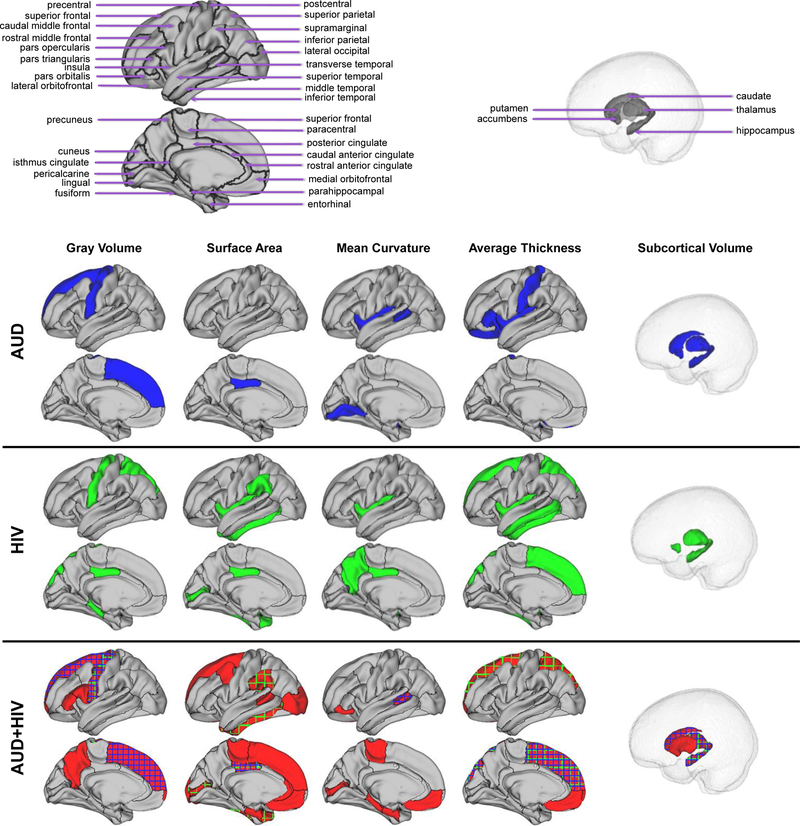
The diagnostic patterns are listed in Table 2 and visualized in Figure 2 (omitting white matter hypointensities). The AUD-specific pattern consisted of measures from 13 unique brain ROIs, the HIV-specific pattern of 15 brain ROIs, and the AUD+HIV-specific pattern of 25 brain ROIs. All patterns included the surface area of the posterior cingulate and the volumes of the WM hypointensities, precental gyurs, thalamus, and hippocampus. 7 additional measures from the AUD+HIV pattern overlapped with either the AUD- or the HIV-pattern and included the mean curvature of the banks of the superior temporal sulcus and the average thickness of the superior frontal and superior parietal gyri.
Table 2:
Measures associated with each diagnostic pattern. Bold-Italic entries denote measures in the AUD or HIV pattern that were also in the AUD+HIV pattern.
| Volume | Surface Area | Mean Curvature | Average Thickness | |
|---|---|---|---|---|
| AUD |
superior frontal
precentral gyrus thalamus caudate hippocampus accumbens WM hypointensities |
posterior cingulate |
bankssts*
lingual insula |
lateral orbitofrontal pars triangularis postcentral insula |
| HIV | Parahippocampal posterior cingulate precentral gyrus superior parietal thalamus hippocampus accumbens WM hypointensities |
supramarginal
inferior temporal temporal pole pericalcarine posterior cingulate insula |
precuneus posterior cingulate insula |
superior frontal
superior parietal inferior temporal middle temporal insula |
| AUD+HIV |
superior frontal
frontal pole pars opercularis pars triangularis precentral gyrus precuneus thalamus caudate putamen hippocampus WM hypointensities |
superior frontal caudal middle frontal paracentral medial orbitofrontal supramarginal bankssts* entorhinal inferior temporal temporal pole lateral occipital pericalcarine posterior cingulate rostral anterior cingulate |
medial orbitofrontal paracentral pars orbitalis bankssts* entorhinal parahippocampal pericalcarine |
superior frontal
frontal pole medial orbitofrontal superior parietal |
bankssts = banks of the superior temporal sulcus
Figure 2.
The diagnostic patterns (see also Table 2) for alcohol use dependency (AUD), HIV-infection (HIV), and the comorbidity (AUD+HIV). Measures in the HIV (purple) or AUD pattern (green) also appearing in the comorbidity pattern are shown in plaid in AUD+HIV.
3.2. Cognitive Correlations
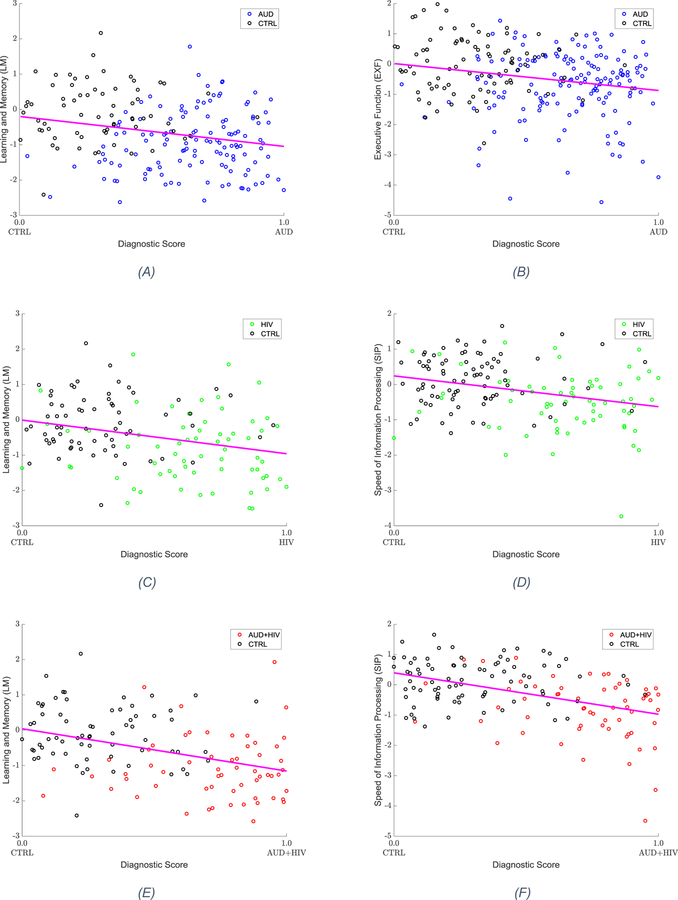
Table 3 summarizes the correlations between the diagnostic patterns and the 6 cognitive measures. All three diagnostic patterns significantly predicted lower Learning/Memory (LM) scores. Furthermore, executive function (EXF) was significantly correlated with the AUD-pattern, whereas speed of information processing (SIP) was significantly correlated with the HIV and AUD+HIV patterns. As shown in Figure 3, these performance scores had lower values with higher diagnostic scores, i.e., greater certainty of an individual to be diagnosed with the condition. Testing the diagnostic pattern of AUD with lifetime alcohol consumption and that of HIV with VACS and CD4 measures did not reveal significant correlations.
Table 3:
Correlation between the pattern identified for each diagnosis group and the six cognitive scores. Cognitive scores were measured through tests outlined in the Supplement.
| Verbal Language | Executive Function | Learning and Memory | Speed of Information Processing | Motor Skills | Quality of Social Functioning | |
|---|---|---|---|---|---|---|
| AUD | ✓ | ✓ | ||||
| HIV | ✓ | ✓ | ||||
| AUD+HIV | ✓ | ✓ |
significant correlation (p-value < 0.05)
Figure 3.
Cognitive measures significantly correlating with the diagnostic scores. The cognitive scores decline with increasing diagnostic scores. (a) Learning and Memory (LM) measure as a function of diagnostic score, for AUD vs. CTRL (b) Executive Function (EXF) measure as a function of diagnostic score, for AUD vs. CTRL (c) Learning and Memory (LM) measure as a function of diagnostic score, for HIV vs. CTRL (d) Speed of Information Processing (SIP) measure as a function of diagnostic score, for HIV vs. CTRL (e) Learning and Memory (LM) measure as a function of diagnostic score, for AUD+HIV vs. CTRL (f) Speed of Information Processing (SIP) measure as a function of diagnostic score, for AUD+HIV vs. CTRL
3.3. Individual-Level Prediction
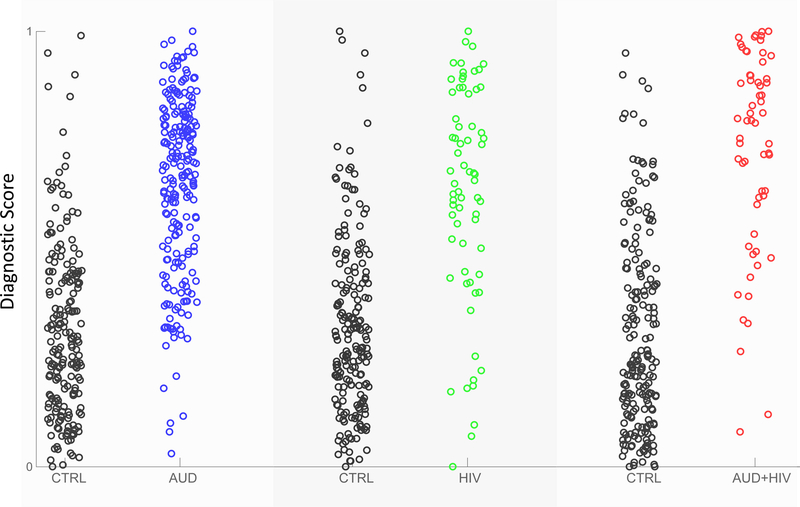
All three diagnostic scores predicted the diagnosis of individuals with significantly high accuracies. The balanced accuracy of the diagnostic score for AUD vs. CTRL was 70.1%, for HIV vs. CTRL was 76.2%, and for AUD+HIV vs. CTRL was 78.1%. The ranking of the three accuracy scores agreed with the size of the diagnostic pattern, i.e., the AUD pattern was the smallest (with 15 regional measurements), followed by HIV (22 measurements), and AUD+HIV (35 measurements) (Table 2). As each pattern inferred its own diagnostic score, each subject of the control group had a diagnostic score specific to each diagnosis. As expected, diagnostic scores of the CTRLs were generally lower than those of disease-affected participants (Figure 4).
Figure 4.
Diagnostic scores for each sample with respect to the three diagnosis-specific group comparisons (CTRL vs. AUD, CTRL vs. HIV, CTRL vs. AUD+HIV).
4. Discussion
The outcome of this machine learning analysis supported the hypothesis that MRIs alone can predict diagnosis and cognitive scores of AUD, HIV, or AUD+HIV. Our approach reduced the 298 brain measures to those most informing diagnosis, i.e., the diagnostic pattern and then applied the pattern to the MRIs of individuals to compute each person’s diagnostic score. Doing so preserved the statistical power of the data because testing for significance did not require multiple comparison correction as it is the case for conventional univariate analysis. Conventional studies minimize the number of comparisons by pre-selecting measurements (e.g., gray matter volume (11) or average thickness (61)) or creating summary scores (17) deemed informative according to expert domain-knowledge. Scores then rely on univariate testing that entails a group-level analysis to correlate each score with each diagnosis. A powerful alternative to this conventional analysis is the proposed machine learning technology, which, as noted in Supplement, was also more accurate in predicting diagnosis than other multivariate approaches. Not only did the novel machine learning approach predict AUD or HIV diagnosis of individuals based solely on their MRIs, but the predictive power of the identified patterns was measured on unseen data (i.e., data not used for optimizing the approach) so that the patterns could serve as imaging phenotypes in other MRI studies of AUD or HIV.
We recognize that this paper presents a secondary analysis of data previously published in (17). Unlike the original study we omitted Hepatitis C coinfection from the analysis but our analysis was still confined by the number of HIV-infected patients (HIV and AUD+HIV) being much smaller than the control cohort and the AUD group. For our analysis to be impartial to this issue, the machine learning method automatically selected an equal number of samples from each group and trained its model on this balanced data set. Furthermore, we measured the prediction power using the balanced accuracy (BAcc) metric, which accounted for unequal sample sizes. The scores indicated that our approach produced accurate findings even in case of imbalanced data.
Human MRI studies of HIV or AUD have used machine learning analysis to predict the age of participants (62, 63) or to select MRI metrics related to a diagnosis (64–68). A hallmark of the proposed analysis was the diagnostic score of individuals (see Figure 4), which was a continuous score directly linking variation in MRI metrics to diagnosis. This link enabled a refined interpretation of significant correlations between diagnostic patterns and functional ramifications of the condition. For example, all three diagnostic patterns predicted the Learning/Memory score (see Table 3) and consisted of regions closely linked to this brain function, namely, the hippocampus (69–71), thalamus (72, 73), and posterior cingulate cortex (74, 75). The hippocampus and thalamus have been identified as targets of AUD (76), HIV (13, 77, 78), and AUD+HIV comorbidity has been shown to principally affect the thalamus (4, 79). Cingulate volume is more frequently reported as compromised in the HIV relative to the AUD literature, e.g., (79–83). While the imaging literature has typically reported on gray matter volume effects, studies that assess cortical thickness rather than cortical volume can show different results. For example, HIV has been shown to compromise cortical thickness of areas such as the insula and temporal cortices (84–86).
Also reported for all three conditions was the link between lower learning and memory scores and higher diagnostic scores (Figure 3). According to our machine learning model, a higher diagnostic score reflected a greater impact of the disorder on the regional measures defining the diagnostic pattern. Thus, the diagnostic scores and patterns accurately summarized the magnitude of the impact that each disorder had on an affected metric.
An interesting observation was the inclusion of the hippocampus in the patterns of all groups. As it is reported in the literature (13, 87), this region supports learning and memory. Based on the statistics reported in Table 1, this cognitive measure was also significantly impaired across all three groups compared to the CTRL cohort.
Critically, our findings support a compounding effect of AUD and HIV on the neural systems of individuals diagnosed with both conditions. Among the three conditions, the diagnostic pattern of the comorbidity was the largest consisting of 35 regional measures. Several of these measures featured as part of the AUD and HIV pattern and were selected by the AUD+HIV pattern with the exception of the mean curvature and average thickness of the insula (see Table 2); however, neither mean curvature nor average thickness measure was selected by all three patterns. Rather, the patterns of the three diagnoses converged on volumes of four regions (precentral gyrus, hippocampus, thalamus, WM hypointensities) and one surface area (posterior cingulate cortex). Consistent with the size of the diagnostic patterns was the prediction accuracy of the diagnostic scores, which was most accurate for AUD+HIV comorbidity (78.1% BAcc). While the accuracy scores might be further improved based on the discussion in Supplement, all these findings indicate that the combined impact of AUD and HIV on the brain system was more extensive than either condition alone.
In addition to overlap among the three diagnostic patterns, the comorbidity pattern contained elements specific each single diagnosis. The HIV and AUD+HIV patterns were highly accurate in predicting speed of information processing performance, which is known to decline faster in patients with HIV (88, 89) and alcohol (90) than the healthy individuals. Speed of information processing has been also linked to regions that were part of both patterns, notably, the superior frontal cortex (91, 92), precentral gyrus (93), superior parietal lobe (94, 95), inferior temporal lobe (91, 96, 97), pericalcarine gyrus (91), supramarginal gyrus (98, 99), and the temporal pole (100). Featured in both patterns was the thalamus, whose volume has been reported to be significantly smaller in the HIV population, with (4, 81) or without (4, 11, 13) AUD comorbidity. Appearing in both the AUD and the comorbidity pattern was the volume of the superior frontal cortex (17, 101), which has been observed to be smaller in AUD than controls (18, 102). The significant correlation between executive function and the diagnostic pattern was only reported with respect to the AUD group but not for the comorbidity cohort. This inconsistency might be explained by the executive functioning being negatively affected by alcohol consumption, which was more recent and prevalent in the AUD cohort. Their “days after last drink” was significantly shorter (p=0.0012; two-sided t-test) and the “alcohol consumed in past year” was significantly greater (p < 0.001; two-sided t-test) than for the AUD+HIV cohort. Interestingly, these findings further supported the compounding effect of AUD and HIV as their combined effects lead to a higher prediction accuracy than the AUD-specific pattern extracted on a cohort with higher alcohol consumption.
Note that we only reported on the compounding effect as the machine learning analysis could not quantitatively assess the additive or interactive characteristic of an effect. Furthermore, the subject-level inference from this type of analysis often does not accord to the results of group-level analysis (103). For instance, “quality of social functioning” was the cognitive score most strongly differentiating between controls and the three cohorts. However, the score did not significantly correlate with any of the diagnostic patterns or in distinguishing individuals. In this data set, a better predictor for identifying significant correlations was the variance of a score within a diagnostic group. For each diagnostic group, the corresponding diagnostic patterns significantly correlated with the cognitive scores having the smallest variation, which was Speed of Information Processing for the HIV cohort and Learning/Memory for the other two diagnoses. This observation is in line with the analysis performed in Step 2 of Figure 1 as the corresponding correlation was sensitive towards the within-class covariance.
Another limitation of this study was the assumption that samples were healthy or diagnosed with HIV, AUD, or their comorbidity. One could thus increase the significance of the data-driven predictions by measuring the prediction accuracies of the diagnostic scores on the MRIs of participants with other diagnoses. However, the prediction accuracy was determined on unseen data so that the findings of this study should apply to other MRI studies adhering to this assumption. Specifically, we measured the accuracy of our machine learning method using 10- fold cross-validation (Step 3 in Figure 1). To avoid reporting overly optimistic findings, cross-validation parameterized the z-scores (with respect to the controls) and the proceeding method (including sample selection) on a subset of the data (training) and then the accuracy of the method was measured on the remaining data, which avoided reporting overly optimistic findings. One drawback of this process is that our method identified a unique diagnostic pattern for each training run. Discussing the common denominator of the 10 different patterns is complex and requires statistics over the entire data set. To simplify, we focused the discussion for each diagnosis on an example of a diagnostic pattern, which was created by applying the machine learning approach on the entire diagnostic data set (Step 1 in Figure 1).
Finally, we caution against drawing conclusions about measurements omitted from diagnostic patterns presented here as these measurements can also be informative with respect to a diagnosis. However, they were not picked by the machine learning approach, which identified a constellation of measurements that achieved a higher accuracy in labeling the individuals of the training data. Changing the training data (as done in cross-validation) can lead to selecting a different pattern. Thus, the diagnostic patterns presented in this article should be viewed as an example of a family of patterns that lead to similar prediction accuracy.
5. Conclusion
We report on the diagnostic patterns and scores based on MRI data that predicted diagnostic classification of individuals with AUD, HIV, or their comorbidity relative to control patterns. Novel machine learning technology automatically reduced 298 MRI brain measures to small subsets implicated by each diagnostic group, eliminating the need for expert-driven input. The impact of a disorder on the diagnostic pattern was summarized by a diagnostic score, which revealed an exacerbated effect of AUD+HIV comorbidity. The diagnostic patterns and scores also predicted cognitive performance of individuals and their accuracy was measured on unseen data. Thus, they could serve as imaging phenotypes for studies investigating AUD, HIV, and their comorbidity. The entire analysis was data-driven so that the novel machine learning approach is readily applicable to MRI studies of other neuropsychiatric conditions also enabling repurposing of multi-metric data.
Supplementary Material
Acknowledgments
Funding for this study was received from the U.S. National Institute on Alcohol Abuse and Alcoholism (AA017347, AA005965, AA010723, AA017168, AA026762), and the Moldow Women’s Hope and Healing Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Organization WH, Unit WHOMoSA (2014): Global status report on alcohol and health, 2014 World Health Organization. [Google Scholar]
- 2.Gongvatana A, Morgan EE, Iudicello JE, Letendre SL, Grant I, Woods SP, et al. (2014): A history of alcohol dependence augments HIV-associated neurocognitive deficits in persons aged 60 and older. J Neurovirol 20:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fama R, Sullivan EV, Sassoon SA, Pfefferbaum A, Zahr NM (2016): Impairments in Component Processes of Executive Function and Episodic Memory in Alcoholism, HIV Infection, and HIV Infection with Alcoholism Comorbidity. Alcohol Clin Exp Res 40:2656–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfefferbaum A, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, et al. (2012): Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry 72:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Justice A, Sullivan L, Fiellin D, Veterans Aging Cohort Study Project T (2010): HIV/AIDS, comorbidity, and alcohol: can we make a difference? Alcohol Res Health 33:258–266. [PMC free article] [PubMed] [Google Scholar]
- 6.Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW (2005): Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res 138:115–130. [DOI] [PubMed] [Google Scholar]
- 7.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO (1997): Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alc Clin Exp Research 21:521–529. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ (2007): Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage 34:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan EV, Rose J, Pfefferbaum A (2006): Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cerebral cortex 16:1077–1086. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan EV, Pfefferbaum A (2009): Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol 44:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, et al. (2014): Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging 35:1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Berre AP, Pitel AL, Chanraud S, Beaunieux H, Eustache F, Martinot JL, et al. (2014): Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Hum Brain Mapp 35:4635–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wade BS, Valcour V, Busovaca E, Esmaeili-Firidouni P, Joshi SH, Wang Y, et al. (2015): Subcortical shape and volume abnormalities in an elderly HIV+ cohort. Proc SPIE Int Soc Opt Eng 9417. [DOI] [PMC free article] [PubMed]
- 14.Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, Sullivan EV (2009): Working and episodic memory in HIV infection, alcoholism, and their comorbidity: baseline and 1-year follow-up examinations. Alcohol Clin Exp Res 33:1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardin F, Maheut-Bosser A, Paille F (2014): Cognitive impairments in alcohol-dependent subjects. Front Psychiatry 5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. (2011): HIV- associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Zahr NM, Sassoon SA, Kwon D, Pohl KM, Sullivan EV (2018): Accelerated and Premature Aging Characterizing Regional Cortical Volume Loss in Human Immunodeficiency Virus Infection: Contributions From Alcohol, Substance Use, and Hepatitis C Coinfection. Biol Psychiatry Cogn Neurosci Neuroimaging [DOI] [PMC free article] [PubMed]
- 18.Manzo G, De Gennaro A, Cozzolino A, Serino A, Fenza G, Manto A (2014): MR imaging findings in alcoholic and nonalcoholic acute Wernicke’s encephalopathy: a review. Biomed Res Int 2014:503596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avants BB, Epstein CL, Grossman M, Gee JC (2008): Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crovitz HF, Zener K (1962): A group-test for assessing hand- and eye-dominance. Am J Psychol 75:271–276. [PubMed] [Google Scholar]
- 21.Rosenbloom MJ, Sullivan EV, Sassoon SA, O’Reilly A, Fama R, Kemper CA, et al. (2007): Alcoholism, HIV infection, and their comorbidity: factors affecting self-rated health-related quality of life. J Stud Alcohol Drugs 68:115–125. [DOI] [PubMed] [Google Scholar]
- 22.Bzdok D (2017): Classical Statistics and Statistical Learning in Imaging Neuroscience. Front Neurosci 11:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SM, Nichols TE (2018): Statistical Challenges in “Big Data” Human Neuroimaging. Neuron 97:263–268. [DOI] [PubMed] [Google Scholar]
- 24.Varoquaux G, Thirion B (2014): How machine learning is shaping cognitive neuroimaging. Gigascience 3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castiglioni I, Salvatore C, Ramirez J, Gorriz JM (2018): Machine-learning neuroimaging challenge for automated diagnosis of mild cognitive impairment: Lessons learnt. J Neurosci Methods 302:10–13. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Kwon D, Esmaeili-Firidouni P, Pfefferbaum A, Sullivan EV, Javitz H, et al. (2016): Extracting patterns of morphometry distinguishing HIV associated neurodegeneration from mild cognitive impairment via group cardinality constrained classification. Hum Brain Mapp 37:4523–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adeli E, Li X, Kwon D, Zhang Y, Pohl KM (2018): Logisitc Regression Confined by Cardinality- Constrained Sample and Feature Selection. Accepted in IEEE Transactions to Pattern Analysis and Machine Intelligence [DOI] [PMC free article] [PubMed]
- 28.Adeli E, Shi F, An L, Wee CY, Wu G, Wang T, et al. (2016): Joint feature-sample selection and robust diagnosis of Parkinson’s disease from MRI data. Neuroimage 141:206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hastie T, Tibshirani R, Wainwright M (2015): Statistical learning with sparsity: the lasso and generalizations CRC Press. [Google Scholar]
- 30.Guggenmos M, Scheel M, Sekutowicz M, Garbusow M, Sebold M, Sommer C, et al. (2018): Decoding diagnosis and lifetime consumption in alcohol dependence from grey-matter pattern information. Acta Psychiatr Scand 137:252–262. [DOI] [PubMed] [Google Scholar]
- 31.Ohya T, Kikuchi S (1982): [Clinical evaluation of chemotherapeutic agents in the treatment of primary liver cancer]. Gan To Kagaku Ryoho 9:1623–1627. [PubMed] [Google Scholar]
- 32.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. (2006): Veterans Aging Cohort Study (VACS): Overview and description. Med Care 44:S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. (2007): Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. (2016): Prevalence of HIV- associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tombaugh TN (2004): Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists 19:203–214. [DOI] [PubMed] [Google Scholar]
- 36.D’Elia L, Satz P (1989): Color Trails 1 and 2 Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- 37.Bowden SC, Bell RC (1992): Relative usefulness of the WMS and WMS-R: a comment on D’Elia et al. (1989). Journal of clinical and experimental neuropsychology 14:340–346. [DOI] [PubMed] [Google Scholar]
- 38.Elwood RW (2001): MicroCog: assessment of cognitive functioning. Neuropsychology review 11:89–100. [DOI] [PubMed] [Google Scholar]
- 39.Golden C (1978): Stroop Color and Word Test: A Manual for Clinical and Experimental Uses Chicago: Stoelling Co. [Google Scholar]
- 40.Bennett-Levy J (1984): Determinants of performance on the Rey-Osterrieth complex figure test: An analysis, and a new technique for single-case assessment. British Journal of Clinical Psychology 23:109–119. [DOI] [PubMed] [Google Scholar]
- 41.Borkowski JG, Benton AL, Spreen O (1967): Word fluency and brain damage. Neuropsychologia 5:135–140. [Google Scholar]
- 42.Nelson HE (1982): The National Adult Reading Test (NART) Windsor, Canada: Nelson Publishing Company. [Google Scholar]
- 43.Dunn LM, Dunn ES (1997): Peabody Picture Vocabulary Test - Third Edition Circle Pines, MN: American Guidance Service. [Google Scholar]
- 44.Wechsler D (2001): Wechsler Test of Adult Reading: WTAR San Antonio, TX: Pearson Education, Inc. [Google Scholar]
- 45.Hart R, Kwentus J, Wade J, Hamer R (1987): Digit symbol performance in mild dementia and depression. Journal of Consulting and Clinical Psychology 55:236–238. [DOI] [PubMed] [Google Scholar]
- 46.Smith A (1973): The Symbol Digit Modalities Test Manual Los Angeles: Western Psychological Services. [Google Scholar]
- 47.Trites RL (1977): The Grooved Pegboard Test. Neuropsychological Test Manual Ontario, Canada: Royal Ottawa Hospital. [Google Scholar]
- 48.Fama R, Eisen JC, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, et al. (2007): Upper and lower limb motor impairments in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res 31:1038–1044. [DOI] [PubMed] [Google Scholar]
- 49.Fregly AR (1968): An ataxia battery not requiring rails. Aerospace Medicine 39:277–282. [PubMed] [Google Scholar]
- 50.Bozzette SA, Hays RD, Berry SH, Kanouse DE, Wu AW (1995): Derivation and properties of a brief health status assessment instrument for use in HIV disease. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association 8:253–265. [DOI] [PubMed] [Google Scholar]
- 51.Moos RH, McCoy L, Moos BS (2000): Global assessment of functioning (GAF) ratings: determinants and role as predictors of one-year treatment outcomes. Journal of Clinical Psychology 56:449–461. [DOI] [PubMed] [Google Scholar]
- 52.Katz S (1983): Assessing self-maintenance activities of daily living, mobility and instrumental activities of daily living. Journal of the American Geriatric Society 31:721–727. [DOI] [PubMed] [Google Scholar]
- 53.Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A (2010): The SRI24 multichannel atlas of normal adult human brain structure. Hum Brain Mapp 31:798–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dale AM, Fischl B, Sereno MI (1999): Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- 55.Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012): Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischl B (2012): FreeSurfer. Neuroimage 62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A (2010): The SRI24 multi-channel atlas of normal adult human brain structure. Human Brain Mapping 31:798–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madsen H, Thyregod P (2010): Introduction to general and generalized linear models CRC Press. [Google Scholar]
- 59.Arlot S, Celisse A (2010): A survey of cross-validation procedures for model selection. Statistics surveys 4:39. [Google Scholar]
- 60.Fisher RA (1935): The logic of inductive inference. Journal of the Royal Statistical Society 98:43. [Google Scholar]
- 61.Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, et al. (2005): Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A 102:15647–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhn T, Kaufmann T, Doan NT, Westlye LT, Jones J, Nunez RA, et al. (2018): An augmented aging process in brain white matter in HIV. Hum Brain Mapp 39:2532–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cole JH, Underwood J, Caan MW, De Francesco D, van Zoest RA, Leech R, et al. (2017): Increased brain-predicted aging in treated HIV disease. Neurology 88:1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Squeglia LM, Ball TM, Jacobus J, Brumback T, McKenna BS, Nguyen-Louie TT, et al. (2017): Neural Predictors of Initiating Alcohol Use During Adolescence. Am J Psychiatry 174:172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao B, Kong X, Kettering C, Yu P, Ragin A (2015): Determinants of HIV-induced brain changes in three different periods of the early clinical course: A data mining analysis. Neuroimage Clin 9:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wade BS, Valcour VG, Wendelken-Riegelhaupt L, Esmaeili-Firidouni P, Joshi SH, Gutman BA, et al. (2015): Mapping abnormal subcortical brain morphometry in an elderly HIV+ cohort. Neuroimage Clin 9:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Underwood J, Cole JH, Caan M, De Francesco D, Leech R, van Zoest RA, et al. (2017): Gray and White Matter Abnormalities in Treated Human Immunodeficiency Virus Disease and Their Relationship to Cognitive Function. Clin Infect Dis 65:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu X, Du X, Kerich M, Lohoff FW, Momenan R (2018): Random forest based classification of alcohol dependence patients and healthy controls using resting state MRI. Neurosci Lett 676:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deng W, Aimone JB, Gage FH (2010): New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petersen RC, Jack CR Jr., Xu YC, Waring SC, O’Brien PC, Smith GE, et al. (2000): Memory and MRI-based hippocampal volumes in aging and AD. Neurology 54:581–587. [DOI] [PubMed] [Google Scholar]
- 71.Bird CM, Burgess N (2008): The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci 9:182–194. [DOI] [PubMed] [Google Scholar]
- 72.Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT (2010): Hippocampal- anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci 31:2292–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Bourbon-Teles J, Bentley P, Koshino S, Shah K, Dutta A, Malhotra P, et al. (2014): Thalamic control of human attention driven by memory and learning. Curr Biol 24:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML (2011): Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci 15:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leech R, Sharp DJ (2014): The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan EV, Marsh L (2003): Hippocampal volume deficits in alcoholic Korsakoff’s syndrome. Neurology 61:1716–1719. [DOI] [PubMed] [Google Scholar]
- 77.Scott-Sheldon LA, Walstrom P, Carey KB, Johnson BT, Carey MP, Team MR (2013): Alcohol use and sexual risk behaviors among individuals infected with HIV: a systematic review and meta-analysis 2012 to early 2013. Curr HIV/AIDS Rep 10:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanford R, Fernandez Cruz AL, Scott SC, Mayo NE, Fellows LK, Ances BM, et al. (2017): Regionally Specific Brain Volumetric and Cortical Thickness Changes in HIV-Infected Patients in the HAART Era. Journal of acquired immune deficiency syndromes 74:563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janssen MA, Meulenbroek O, Steens SC, Goraj B, Bosch M, Koopmans PP, et al. (2015): Cognitive functioning, wellbeing and brain correlates in HIV-1 infected patients on long-term combination antiretroviral therapy. AIDS 29:2139–2148. [DOI] [PubMed] [Google Scholar]
- 80.Clark US, Walker KA, Cohen RA, Devlin KN, Folkers AM, Pina MJ, et al. (2015): Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia 70:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fama R, Rosenbloom MJ, Sassoon SA, Rohlfing T, Pfefferbaum A, Sullivan EV (2014): Thalamic volume deficit contributes to procedural and explicit memory impairment in HIV infection with primary alcoholism comorbidity. Brain Imaging Behav 8:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agudelo M, Khatavkar P, Yndart A, Yoo C, Rosenberg R, Devieux JG, et al. (2014): Alcohol abuse and HIV infection: role of DRD2. Curr HIV Res 12:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ances BM, Ortega M, Vaida F, Heaps J, Paul R (2012): Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of acquired immune deficiency syndromes 59:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK, et al. (2012): Regional cortical thinning associated with detectable levels of HIV DNA. Cerebral cortex 22:2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanford R, Fellows LK, Ances BM, Collins DL (2018): Association of Brain Structure Changes and Cognitive Function With Combination Antiretroviral Therapy in HIV-Positive Individuals. JAMA Neurol 75:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adeli E, Kwon D, Zhao Q, Pfefferbaum A, Zahr NM, Sullivan EV, et al. (2018): Chained regularization for identifying brain patterns specific to HIV infection. Neuroimage 183:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson EB, Grossrubatscher I, Frank L (2014): Dynamic Hippocampal Circuits Support Learning- and Memory-Guided Behaviors. Cold Spring Harb Symp Quant Biol 79:51–58. [DOI] [PubMed] [Google Scholar]
- 88.Fellows RP, Byrd DA, Morgello S (2014): Effects of information processing speed on learning, memory, and executive functioning in people living with HIV/AIDS. Journal of clinical and experimental neuropsychology 36:806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anand P, Springer SA, Copenhaver MM, Altice FL (2010): Neurocognitive impairment and HIV risk factors: a reciprocal relationship. AIDS Behav 14:1213–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tzambazis K, Stough C (2000): Alcohol impairs speed of information processing and simple and choice reaction time and differentially impairs higher-order cognitive abilities. Alcohol Alcohol 35:197–201. [DOI] [PubMed] [Google Scholar]
- 91.Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R (2009): Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron 63:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, et al. (2008): A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiol Aging 29:1547–1555. [DOI] [PubMed] [Google Scholar]
- 93.Carter CS, Mintun M, Cohen JD (1995): Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage 2:264–272. [DOI] [PubMed] [Google Scholar]
- 94.Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA (2007): Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia 45:2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nishiike S, Nakagawa S, Tonoike M, Takeda N, Kubo T (2001): Information processing of visually-induced apparent self motion in the cortex of humans: analysis with magnetoencephalography. Acta Otolaryngol Suppl 545:113–115. [DOI] [PubMed] [Google Scholar]
- 96.Ptak R, Valenza N (2005): The inferior temporal lobe mediates distracter-resistant visual search of patients with spatial neglect. J Cogn Neurosci 17:788–799. [DOI] [PubMed] [Google Scholar]
- 97.Mruczek RE, Sheinberg DL (2007): Activity of inferior temporal cortical neurons predicts recognition choice behavior and recognition time during visual search. J Neurosci 27:2825–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perianez JA, Maestu F, Barcelo F, Fernandez A, Amo C, Ortiz Alonso T (2004): Spatiotemporal brain dynamics during preparatory set shifting: MEG evidence. Neuroimage 21:687–695. [DOI] [PubMed] [Google Scholar]
- 99.Lazeron RH, Rombouts SA, Machielsen WC, Scheltens P, Witter MP, Uylings HB, et al. (2000): Visualizing brain activation during planning: the tower of London test adapted for functional MR imaging. AJNR Am J Neuroradiol 21:1407–1414. [PMC free article] [PubMed] [Google Scholar]
- 100.Decety J, Cacioppo S (2012): The speed of morality: a high-density electrical neuroimaging study. J Neurophysiol 108:3068–3072. [DOI] [PubMed] [Google Scholar]
- 101.Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, et al. (2018): The Role of Aging, Drug Dependence, and Hepatitis C Comorbidity in Alcoholism Cortical Compromise. JAMA Psychiatry 75:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zahr NM (2014): Structural and microstructral imaging of the brain in alcohol use disorders. Handb Clin Neurol 125:275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y, Kwon D, Pohl KM (2017): Computing group cardinality constraint solutions for logistic regression problems. Med Image Anal 35:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.