Abstract
The hexanucleotide repeat expansion in the C9orf72 gene is the most common genetic variant found in individuals with sporadic amyotrophic lateral sclerosis (ALS), occurring at a frequency of between 7-11% in cohorts of European ancestry. While limited data suggest that C9-expansions (>30 repeats) are less frequent in African-Americans with ALS, there is no data on the frequency of C9-expansions among ALS subjects residing in Africa. We therefore investigated the frequency of this expansion mutation (using repeat-primed PCR) in a cohort of 143 South Africans (SA) with ALS. The cohort included different genetic ancestry subgroups who self-identified as black African (n=24), Cape mixed-African (M/A) (n=65), white European ancestry (n=51), and Indian ancestry (n=3). Three M/A individuals had a family history of ALS (2%) and all had normal C9orf72 alleles. Of the 140 individuals with sporadic ALS who were successfully genotyped, 10 (7%) carried pathogenic C9-expansions; four white and six M/A ancestry individuals, respectively. Our results highlight the importance of including Africans in genetic studies aimed at unravelling the genomic architecture in ALS and suggest pathogenetic mechanisms other than the C9orf72 expansion in black Africans with ALS.
Keywords: Africa, motor neuron disease, C9orf72, mutation, gene
1. Introduction
The hexanucleotide repeat expansion in the C9orf72 gene is the most common genetic variant identified in individuals with familial amyotrophic lateral sclerosis (ALS) and fronto-temporal dementia (FTD)[1, 2]. The normal (wild-type) repeat region comprises less than 10 repeats and more than 30 repeats are considered pathogenic [3]. Although the function of the C9orf72 protein remains largely unknown, and it is not clear whether the pathogenic consequence of the C9orf72 expansion is related to loss or gain of function, it does appear to be relevant to the underlying pathogenesis of ALS in a substantial proportion of familial and sporadic cases [3]. A prominent pathogenic mechanism in C9orf72 associated ALS is proposed to be related to nuclear inclusions formed by the expanded repeats encoding dipeptide repeat proteins [4].
The frequency of pathogenic C9orf72 repeat expansions, hereafter referred to as C9-expansions, among sporadic ALS cases is much lower compared to those with a family history of ALS. In addition, the frequencies of C9-expansions differ substantially among different European populations ranging between 4% in Italy and 21% in Finland, although most frequently ranges between 7-10% among sporadic ALS cases with European genetic ancestry (in [3, 5, 6]). There is little data regarding C9-expansions among African-Americans with ALS and no published data from Africa [3]. As the C9orf72 expansion mutation is the most common variant found in ALS cohorts we proceeded to assess the frequency of this expansion mutation in a cohort of South Africans with ALS.
2. Methods
2.1. Study subjects
One hundred and forty-three South African (SA) patients attending ALS clinics at two academic centres in the Cape Town region, Groote Schuur Hospital and Tygerberg Hospital, underwent genotyping for the C9orf72 repeat expansion. Subjects were diagnosed by a neurologist and categorized as either classic ALS or one of the phenotypic subtypes: primary lateral sclerosis (PLS), progressive muscular atrophy (PMA) or flail arm (FA) ALS [7]. Participants categorized themselves according to the following SA racial census categories (http://www.statssa.gov.za): indigenous black African (17%), Cape mixed-African ancestry (M/A)(44%), white European genetic ancestry (36%), or SA Indian ancestry (2%). Cape M/A is a term used in South Africa for persons of predominantly Khoisan (>60% genetic contribution) and black African genetic ancestry as well as smaller genetic contributions from Europeans more than Southeast Asians [8]. White refers to individuals of European genetic ancestry. The black Africans are largely Xhosa-speaking migrants from the Eastern Cape region and ‘Indian’ refers to those with recent ancestors immigrating from the subcontinent. Family history was obtained after a structured interview according to a standardized data capture form. Non-ALS South African controls (SA-controls) of black African and Cape mixed-African ancestry included 25 individuals with myasthenia gravis (MG) and 20 healthy individuals [9, 10].
Ethics approval for this study was obtained from the University of Cape Town’s Health Sciences Faculty research ethics committee (HREC 351/2016).
2.2. DNA extraction and determination of C9orf72 repeat length
Genomic DNA from ALS and SA-control subjects was extracted from buffy coats of nucleated cells obtained from anticoagulated whole blood. Three DNA samples with pathogenic C9orf72 expansions (ND11252*A1, ND11081*A1 and ND10284*A1) were donated by the Coriell Institute of Medical Research and included as positive controls.
2.2.1. Repeat primed PCR (RP-PCR)
For ALS subjects, a two-step protocol was designed for characterising the C9orf72 G4C2 hexanucleotide repeat in the first intron. Step one involved amplification of the expanded alleles by RP-PCR using primer sequences and reaction conditions described by Renton et al. [1] which allows for the detection of up to about 30 repeats. The second step allowed for the amplification of unexpanded alleles only (up to 12 repeats) and the determination of zygosity by combining the fluorescently labelled sequence specific primer from the RP-PCR and a sequence specific primer designed with Primer3Plus [11] (5’-CACAGTACTCGCTGAGGGTG-3’). This second reaction was optimised to include 50 ng genomic DNA, 1X Failsafe Premix J buffer (Lucigen), 1.25 U GoTaq G2 flexi DNA polymerase (Promega Corporation) and 10 pmol of each primer in a 15 μL reaction volume. Cycling conditions involved an initial denaturation at 95°C for 2 minutes followed by 35 cycles consisting of 95°C for 30 seconds, 60 °C for 30 seconds and 72°C for 1 minute. Amplification was concluded with a final extension at 72°C for 10 minutes.
Amplified products were analysed on a 3130xl Genetic Analyzer with a GeneScan™500 internal size standard using standard electrophoresis conditions for fragment analysis on a 36cm capillary array with POP7 polymer (Thermo Fisher Scientific). Electropherograms were visualised with GeneMapper v4 software and the fragment sizes recorded. The smaller allele sizes were confirmed for the sequence specific amplified products, using the BigDye™ terminator v3.1 cycle sequencing kit according the manufacturer’s instructions and capillary electrophoresis on a 3130xl Genetic Analyzer (Thermo Fisher Scientific).
The repeat sizes were calculated by creating a bin set for the sequence specific PCR with the smallest peak corresponding to two repeats. Additional bins were created in 6bp increments. The repeat primed primer binds exactly three repeats in addition to a partial repeat (C4G), however, it will also bind and amplify two repeats and it is therefore not possible to distinguish two and three repeats with this primer set [12]. Sizes were verified by applying a formula to the fragment sizes obtained from the chromatograms which takes primer and flanking sequences into account and incorporates a correction factor to allow for the mobility shifts observed with capillary electrophoresis.
In samples with low DNA concentrations and indeterminate results with the above mentioned RP-PCR protocol, we used the AmplideX™ PCR/CE C9orf72 kit (Asuragen, Austin, USA) according to the manufacturers’ instructions to determine the expanded repeat sizes (analytic range 2-145 repeats).
2.2.2. Whole genome sequencing (WGS)
We were able to examine the WGS data of a sample of South African ALS (n=25) and control subjects (n=45) [9, 10], for C9orf72 repeat expansions and the surrogate ‘risk’ marker, rs3849942 T allele, which is associated with the C9-expansion haplotype [12, 13]. The C9orf72-allele length was determined from PCR-free 30X coverage WGS data using ExpansionHunter [14] which validated the RP-PCR results from 25 ALS subjects.
2.3. Statistical analyses
Allele frequencies were calculated in Microsoft® Excel for Mac version 16.18. Group comparisons of continuous variables were compared with Student’s t test (2 groups) or one-way ANOVA (>2 groups). A Wilcoxon matched-pairs signed rank test was performed to compare allele frequencies between the two control groups (MG and healthy controls) and a Fischer exact test was used to compare C9-risk allele frequencies between black vs M/A groups. A 2-sided p value <0.05 was considered significant. Graphs were generated in Prism 7 for Mac OS X version 7.0c
3. Results
C9orf72 repeat length was successfully determined in 143 samples. The cohort contained three subjects with familial ALS (2%) (2 families with either PLS ± progressive aphasia (one family), and PMA at presentation (two individuals from the same family).
The clinical characteristics of sporadic ALS cases are summarized according to C9orf72 repeat length in table 1. Men were more frequent in the sample (64%). The median age at onset was 55 years (interquartile range (IQR) 47–66) and was younger in men compared to women (54 vs 59 years, p=0.023) and in those with African genetic ancestry; 50 years in black African-, 54 years in Cape M/A-, and 61 years in white patients (p<1×10−4). Among those with African genetic ancestry (black + M/A; n=89) the disease phenotype at presentation was largely ALS (n=74; 83%) and the most frequent clinical anatomical region involved first by history was lumbosacral (n=42; 47%) followed by cervical involvement (n=37; 42%) and bulbar onset occurred in 12% (n=10).
Table 1.
Clinical characteristics of South African ALS patients according to C9orf72 allele repeat size.
| Clinical characteristics | <20 repeats n (%) |
>30 repeats n |
|---|---|---|
| Disease phenotype | ||
| ALS | 123 (86) | 8 |
| PMA | 9 (6) | 1 |
| PLS | 7 (5) | 1 |
| FA | 4 (3) | 0 |
| Clinical presentation | ||
| lumbosacral | 65 (45) | 7 |
| cervical | 56 (39) | 2 |
| bulbar | 18 (13) | 1 |
| respiratory | 3 (2) | 0 |
| behavioral | 1 (1) | 0 |
| Genetic ancestry | ||
| black African | 24 (17) | 0 |
| Cape M/A | 65 (45) | 6 |
| white | 51 (36) | 4 |
| Indian | 3 (2) | 0 |
Legend: n refers to the number of individuals with one allele in a particular size range.
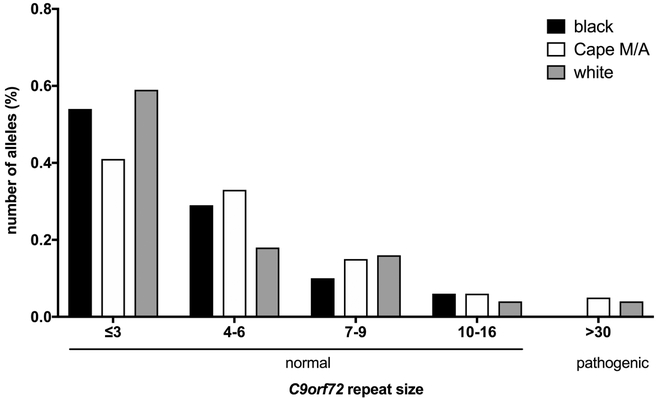
In the overall cohort who were genotyped (n=143), 93% of the cases had C9orf72 alleles within the normal range (<30 repeats) and the distribution of the normal allele sizes was not significantly different between the ancestry groups (figure 1, one-way ANOVA test p=0.51). Of the sporadic ALS cases (n=140), 10 (four white/European genetic ancestry and six Cape M/A patients), carried pathogenic C9-expansions of >30 repeats (7%) and none of these were black ALS cases. There was no difference in the ages at symptoms onset between those with normal repeat allele sizes (<20) and those with C9-expansions (>30)(p=0.87). All three ALS cases with familiy members with ALS (Cape M/A genetic ancestry) had normal C9orf72 alleles (≤7 repeats). Therefore, amongst those who self identified as Cape mixed-African ancestry, the frequency with C9-expansions of >30 repeats was 9%. Three of the Cape M/A subjects with pathogenic C9-expansions underwent WGS, and we confirmed the rs3849942 T/T genotype which associated with the C9orf72 risk haplotype.
Figure 1.
Distribution of C9orf72 alleles in each South African subpopulation according to repeat size. Normal refers to ≤30 repeats and pathogenic refers to >30 repeats. Three individuals of SA ‘Indian’ ancestry are not shown (all had normal alleles).
All 45 SA-control subjects had unexpanded C9orf72 alleles. In this group, the distribution of normal allele sizes was not significantly different between MG and healthy controls (supplementary Fig 1; p=0.98). Furthermore, amongst 142 chromosomes without C9-expansions the rs3849942 T allele frequencies were similar amongst the M/A and black African genomes (T allele frequency 16% M/A group vs 18% black group; p>0.99).
4. Discussion
This clinic-based series of South Africans (SA) with ALS showed a similar frequency of C9orf72 expanded alleles overall compared to most European cohorts. However, although those with Cape mixed-African genetics had a similar frequency to some European cohorts (6/65; 9%) none of the 24 black Africans carried an expanded C9-allele. This is interesting as the Cape mixed-African subpopulation derives most of their genetic ancestry from Khoisan and black Africans. Few data are available in black populations with ALS. Two cohorts from North America (US) included 71 and 49 black people, respectively, of whom four (3%) had an expanded C9-allele [6, 15]. Although not directly comparable, but to provide some context, previous studies [in 8] and a pharmacogenetic study by our group [in 16] suggested similar European admixture proportions (≈25%) amongst the Cape mixed-African genetic ancestry group and African-Americans. Combining the US data with ours show that 10/206 (5%) individuals with sporadic ALS and African genetic ancestry have been found to have the expanded C9-alelle which may be attributed to European genetic admixture.
We found a similar frequency of C9-expansions among the white cases with European genetic ancestry (8%) compared to a recent large European cohort (10%) [5], but this frequency varies considerably amongst Europeans (4% to 21 %)[3]. C9-expansion frequencies of ≈5% have been reported in ALS cases from India and East-Asia [3] and rarely in ALS cases from mainland China (0.3%)[17]. Although these frequencies reflect the prevalence of the expanded C9-allele among individuals without a family history of ALS (sALS), European populations also appear to have a higher frequency of familial ALS in their samples (10-13%)[6, 18, 19] compared to Africans (this report; 2%), Indians and Chinese (1-3%)[17, 20]. This observation may reflect the lower frequency of expanded C9orf72 expansion mutations, but it may also be the result of non-biological or socio-economic effects resulting in lower life expectancy and poorer access to specialist diagnostic health care. However, it is noteworthy that both this African cohort (Cape M/A and black), and a report from China [17] showed lower frequencies of bulbar onset ALS compared to cohorts with European genetic ancestors (13-14% vs 25-30%) [6, 15]. Although some European ancestry cohorts showed associations with C9-expansions and bulbar onset ALS and earlier age at onset [21], others [12] and this study did not replicate this finding.
There are rare cases in which C9-expansion alleles have been found in European controls (16/10,992, 0.1 %)[6, 12, 18, 21]. In addition, low frequencies of C9-expansion alleles were found in Europeans with other neurodegenerative diseases including Huntington’s disease-like syndromes (7/421; 1.6%) [3]. A study confined to black South Africans (n=97), in which C9orf72 alleles were sized in a lab-based sample of possible “Huntington disease” (HD) phenocopies (both HD1 and HD2 mutations were excluded), found all the C9orf72 repeat alleles in that cohort within the 2-11 repeat range [22]. Here we add an 45 additional SA-controls with African genetic ancestry, who underwent WGS, and found no C9orf72 expansion mutations.
Majounie et al. studied the haplotype of the region adjoining the expanded C9orf72 locus and found evidence to suggest a common ancestral founder effect in which Europeans with the C9-expanded allele shared at least part of the haplotype [6]. However, using more polymorphic microsatellite markers within a 300kb region flanking the C9orf72 locus, Beck et al. reported that the C9-expanded alleles were found on several unrelated haplotypes suggesting mutiple mutational events and arguing against a shared common ancestor [12]. In this report the three individuals with mixed African genetic ancestry and the C9 mutation were also homozygous for the C9-associated rs 3849942 T-allele despite a similar frequency of the T allele among the South African sub-populations.
A potential limitation of our study is that we have not confirmed our RP-PCR results with Southern blotting. However, positive controls with known expanded C9-alleles were included in each experiment and the accuracy of the assay was confirmed by independent sequencing of 3 samples. It is worth highlighting that we found false ‘intermediate alleles’ when RP-PCR was performed on samples with low concentrations of DNA (results not shown), but these were resolved using a commercial kit which was optimized for low quantities of DNA.
5. Conclusion
This ALS cohort comprising 89 individuals with African genetic ancestry showed no C9 expansion mutations among black South Africans with ALS, but the Cape M/A group, who have European admixture, showed similar frequencies to European populations. We highlight the importance of including populations from Africa and Asia in studies aimed at unravelling the genomic architecture in ALS.
Supplementary Material
Supplementary Figure 1. Distribution of C9orf72 alleles in non-ALS South African controls according to repeat size.
Highlights:
143 South Africans with ALS underwent C9orf72 repeat expansion genotyping
Familial ALS was uncommon (2%) and these cases had normal C9orf72 alleles
C9-expansions (>30 repeats) were found in 7% of cases with sporadic ALS
C9-expansions were not found in 24 black Africans with ALS
Acknowledgements
We thank the JCN Foundation for financial support and Celtic Molecular Diagnostics and Asuragen for donating the AmplideX® PCR/CE C9orf72 Kit. We thank the Coriell Institute of Medical Research for sharing DNA samples from three patients with ALS. We thanks Ansie Wichers for her assistance. We acknowledge the support of Prof N Mulder who funded the whole genome sequencing of ALS cases (Human Genome Research Insitute: U24HG006941). We also thank the Southern African Human Genome programme (SAHGP) participants. The South African WGS dataset was generated by the national SAHGP initiative funded by the Department of Science and Technology of South Africa. JMH receives funding from the National Research Foundation of South Africa (no. 113416). MN receives financial support from the University of Cape Town’s Health Sciences Faculty and the UCT Neuroscience Institute.
List of abbreviations
- ALS
amyotrophic lateral sclerosis
- FTD
fronto-temporal dementia
- SA
South Africa(n)
- PLS
primary lateral sclerosis
- PMA
progressive muscular atrophy
- FA
flail arm
- M/A
mixed-African
- RP-PCR
repeat primed polymerase chain reaction
- ANOVA
analysis of variance
- US
United States
- HD
Huntington’s disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Declarations of interest: None
References
- 1.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C90RF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011;72(2):257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C90RF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011;72:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woollacott IO, Mead S. The C90RF72 expansion mutation: gene structure, phenotypic and diagnostic issues. Acta Neuropathol 2014;127:319–32. [DOI] [PubMed] [Google Scholar]
- 4.Gendron TF, Belzil VV, Zhang YJ, Petrucelli L. Mechanisms of toxicity in C9FTLD/ALS. Acta Neuropathol 2014;127:359–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan S, Shatunov A, Sproviero W, Jones AR, Shoai M, Hughes D, et al. A comprehensive analysis of rare genetic variation in amyotrophic lateral sclerosis in the UK. Brain 2017;140:1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 2012;11:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravits J, Appel S, Baloh RH, Barohn R, Brooks BR, Elman L, et al. Deciphering amyotrophic sclerosis: what phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotroph Lateral Scler Frontotemporal Degener 2013;14 Suppl 1:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintana-Murci L, Harmant C, Quach H, Balanovsky O, Zaporozhchenko V, Bormans C, et al. Strong maternal Khoisan contribution to the South African coloured population: a case of gender-biased admixture. Am J Hum Genet. 2010;86:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhury A, Ramsay M, Hazelhurst S, Aron S, Bardien S, Botha G, et al. Whole-genome sequencing for an enhanced understanding of genetic variation among South Africans. Nat Commun 2017;8(1):2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nel M, Mulder N, Europa TA, Heckmann JM. Using Whole Genome Sequencing in an African Subphenotype of Myasthenia Gravis to Generate a Pathogenetic Hypothesis. Frontiers in genetics 2019;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 2007;35(Web Server issue):W71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck J, Poulter M, Hensman D, Rohrer JD, Mahoney CJ, Adamson G, et al. Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am J Hum Genet 2013;92:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok K, Traynor BJ, Schymick J, Tienari PJ, Laaksovirta H, Peuralinna T, et al. Chromosome 9 ALS and FTD locus is probably derived from a single founder. Neurobiology of aging 2012;33:209 e3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolzhenko E, van Vugt J, Shaw RJ, Bekritsky MA, van Blitterswijk M, Narzisi G, et al. Detection of long repeat expansions from PCR-free whole-genome sequence data. Genome Res 2017;27:1895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umoh ME, Fournier C, Li Y, Polak M, Shaw L, Landers JE, et al. Comparative analysis of C9orf72 and sporadic disease in an ALS clinic population. Neurology. 2016;87(10):1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heckmann JM, Lambson EM, Little F, Owen EP. Thiopurine methyltransferase (TPMT) heterozygosity and enzyme activity as predictive tests for the development of azathioprine-related adverse events. J Neurol Sci 2005;231:71–80. [DOI] [PubMed] [Google Scholar]
- 17.Zhang K, Liu Q, Liu K, Shen D, Tai H, Shu S, et al. ANXA11 mutations prevail in Chinese ALS patients with and without cognitive dementia. Neurol Genet 2018;4:e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Redondo A, Dols-Icardo O, Rojas-Garcia R, Esteban-Perez J, Cordero-Vazquez P, Munoz-Blanco JL, et al. Analysis of the C9orf72 gene in patients with amyotrophic lateral sclerosis in Spain and different populations worldwide. Hum Mutat 2013;34:79–82. [DOI] [PubMed] [Google Scholar]
- 19.Gijselinck I, Van Langenhove T, van der Zee J, Sleegers K, Philtjens S, Kleinberger G, et al. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol 2012;11:54–65. [DOI] [PubMed] [Google Scholar]
- 20.Narain P, Gomes J, Bhatia R, Singh I, Vivekanandan P. C9orf72 hexanucleotide repeat expansions and Ataxin 2 intermediate length repeat expansions in Indian patients with amyotrophic lateral sclerosis. Neurobiology of aging 2017;56:211 e9–e14. [DOI] [PubMed] [Google Scholar]
- 21.Millecamps S, Boillee S, Le Ber I, Seilhean D, Teyssou E, Giraudeau M, et al. Phenotype difference between ALS patients with expanded repeats in C9ORF72 and patients with mutations in other ALS-related genes. J Med Genet 2012;49(4):258–63. [DOI] [PubMed] [Google Scholar]
- 22.Baine FK, Peerbhai N, Krause A. A study of Huntington disease-like syndromes in black South African patients reveals a single SCA2 mutation and a unique distribution of normal alleles across five repeat loci. J Neurol Sci 2018;390:200–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Distribution of C9orf72 alleles in non-ALS South African controls according to repeat size.