Abstract
Background:
Accurate and expeditious diagnosis and treatment of pulmonary embolism in cancer patients improves patient outcomes. D-dimer is often used to rule out pulmonary embolism. However, this test is less accurate in cancer patients, and it is unclear whether cancer patients with normal D-dimer levels can present with pulmonary embolism.
Methods:
All consecutive patients who presented to The University of Texas MD Anderson Cancer Center in Houston, Texas, USA, between May 2009 and November 2015 who underwent computed tomography pulmonary angiography and plasma D-dimer level measurement were retrospectively reviewed. Patients with suspected pulmonary embolism and normal D-dimer levels were identified.
Results:
Among the 8023 cancer patients identified, 1156 (14%) had pulmonary embolism. Only 35 patients with pulmonary embolism (3%) had normal plasma D-dimer levels. Twenty-six of these patients had acute pulmonary embolism and the other nine had subacute or chronic pulmonary embolism. Thirteen of the 26 acute cases were in patients with hematological cancer. Most patients (23/35, 66%) had subsegmental or segmental pulmonary embolism. Only one patient had pulmonary embolism in the main pulmonary arteries.
Conclusions and Relevance:
Although it is uncommon (3%), cancer patients with radiologic evidence of pulmonary embolism can present with normal D-dimer levels. Recognizing the possibility of this uncommon occurrence is critical in the decision process for ordering diagnostic tests for evaluation of suspected pulmonary embolism.
BACKGROUND
Pulmonary embolism (PE) is more common in cancer patients than in the general population.[1] Upon presentation and clinical suspicion of PE, clinical assessment followed by diagnostic laboratory and imaging studies helps physicians make a definitive diagnosis.[2] Validated clinical decision rules stratify patients into different risk groups for PE.[3,4] Normal plasma D-dimer levels in patients with low or intermediate risk for PE can help exclude PE given the relatively high sensitivity of the test. However, baseline D-dimer levels are often increased in cancer patients, and the diagnostic accuracy of this crucial clotting biomarker is lower in cancer patients than in the general population.[5,6] Recently we showed that D-dimer perform poorly in excluding venous thromboembolism (VTE) among leukemia and lymphoma patients.[7] Here, we sought to determine whether and how often cancer patients with PE can present with normal plasma D-dimer levels.
METHODS
All consecutive patients who visited The University of Texas MD Anderson Cancer Center in Houston, Texas, USA, between May 1, 2009, and November 1, 2015, who underwent computed tomography pulmonary angiography (CTPA) and had D-dimer laboratory results were identified by querying the institution’s radiology, billing, and laboratory databases. Non-cancer patients and patients with D-dimer levels measured more than 24 hours prior to the CTPA study were excluded. The institution’s electronic medical record system was used to collect patient demographic, clinical, and radiologic data. An incidence of PE during the hospital visit, as the outcome of interest, was determined by reviewing the CTPA reports. Acute PE was defined as newly found PE with presentation and radiological evidence suggestive of acute PE such as central location of filling defect, expansion of the involved vessel, or complete occlusion of the vessel lumen, while subacute/chronic PE was defined as filling defects in similar locations as on prior CT studies and/or by linear/web like configuration or eccentric location of filling defect on CTPA.[8] A board-certified thoracic radiologist with 10 years of experience further reviewed questionable radiologic results and classified them as either positive or negative for PE. Plasma D-dimer levels for all patients were measured using the Liatest D-Di immuno-turbidimetric assay (Diagnostica Stago), with ≥0.5 μg/mL as the cutoff value for elevated levels. Patients with confirmed radiologic evidence of PE were first identified. Among these patients, those who had normal D-dimer levels (<0.5 μg/mL) were further reviewed.
RESULTS
Eligibility and final cases
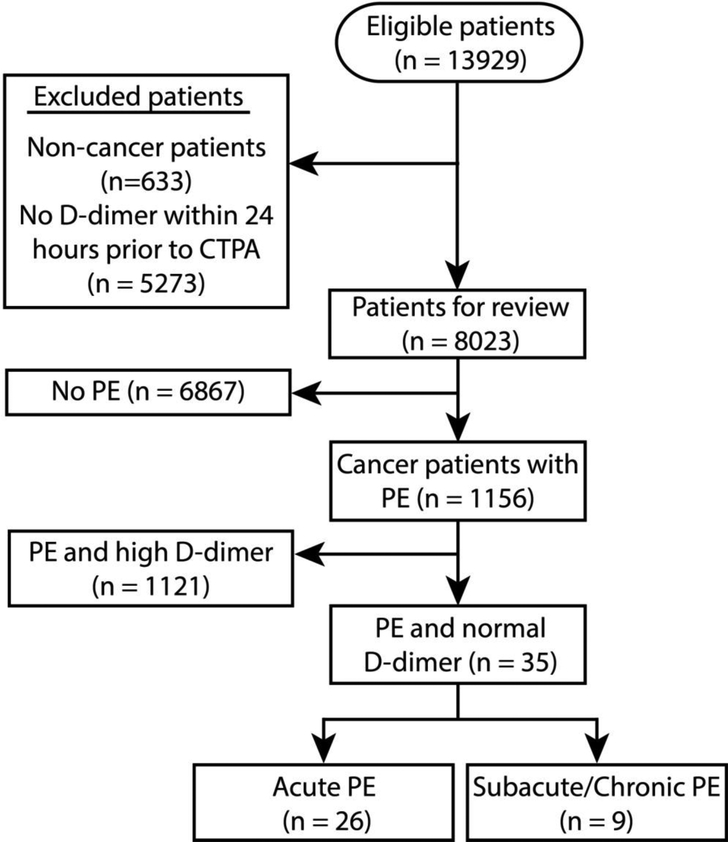
Of the 8023 cancer patients who underwent CTPA and plasma D-dimer measurement, 1156 (14%) had PE (Figure 1), while D-dimer was normal in only 926 (12%) patients. Of the patients with radiologic evidence of PE, only 35 (3.0%) had normal D-dimer. Table 1 shows the clinical characteristics of these patients. Twenty-six patients had acute PE and the remaining nine had subacute or chronic PE. Thirteen of the acute cases (50%) were in patients with liquid cancer.
Figure 1.
Flow chart of exclusion criteria to determine eligible cases (CTPA, computed tomography pulmonary angiography; PE, pulmonary embolism).
Table 1.
Patient demographic and clinical characteristics (n = 35)
| No. of patients (%) | ||
|---|---|---|
| Characteristic | Acute PE | Subacute/chronic PE |
| Total patients | 26 (74) | 9 (26) |
| Median age (range) | 58 years (23–85 years) | 54 years (27–70 years) |
| Age group | ||
| ≥50 years | 20 (77) | 5 (56) |
| <50 years | 6 (23) | 4 (44) |
| Sex | ||
| Female | 11 (42) | 5 (56) |
| Male | 15 (58) | 4 (44) |
| Race | ||
| White | 17 (65) | 4 (44) |
| Non-white | 9 (35) | 5 (56) |
| Cancer type | ||
| Lymphoma | 8 (31) | 2 (22) |
| Lung | 5 (19) | 1 (11) |
| Leukemia | 3 (12) | 2 (22) |
| Multiple myeloma | 2 (8) | 0 (0) |
| Sarcoma | 2 (8) | 1 (11) |
| Brain and spinal cord | 2 (8) | 0 (0) |
| Breast | 1 (4) | 1 (11) |
| Testicular | 1 (4) | 1 (11) |
| Esophageal | 1 (4) | 0 (0) |
| Colon | 1 (4) | 0 (0) |
| Endometrial | 0 (0) | 1 (11) |
| Cancer stage | ||
| Liquid/hematologic | 13 (50) | 4 (44) |
| IV | 5 (19) | 5 (56) |
| III | 3 (12) | 0 (0) |
| II | 2 (8) | 0 (0) |
| I | 3 (12) | 0 (0) |
| Median D-dimer level (range) | 0.38 μg/mL (0.17–0.49 μg/mL) | 0.33 μg/mL (0.17–0.42 μg/mL) |
| Wells score clinical probability | ||
| Low | 0 (0) | 0 (0) |
| Intermediate | 19 (73) | 8 (89) |
| High | 7 (27) | 1 (11) |
| Simplified revised Geneva score clinical probability | ||
| Low | 2 (8) | 0 (0) |
| Intermediate | 23 (89) | 8 (89) |
| High | 1 (4) | 1 (11) |
| Pulmonary Embolism Rule-out Criteria (PERC) | ||
| Meet all | 0 (0) | 0 (0) |
| Does not meet all | 26 (100) | 9 (100) |
| Highest PE location | ||
| Subsegmental | 9 (35) | 2 (22) |
| Segmental | 10 (38) | 2 (22) |
| Lobar | 5 (19) | 2 (22) |
| Interlobar | 1 (4) | 3 (33) |
| Main | 1 (4) | 0 (0) |
| Laterality | ||
| Right | 17 (65) | 5 (56) |
| Left | 5 (19) | 2 (22) |
| Bilateral | 4 (15) | 2 (22) |
| Count | ||
| Single | 17 (65) | 5 (56) |
| Multiple | 9 (35) | 4 (44) |
| History of VTE | ||
| No | 15 (58) | 0 (0) |
| Yes | 11 (42) | 9 (100) |
| Active treatment with an anticoagulant | ||
| No | 15 (58) | 3 (33) |
| Yes | 11 (42) | 6 (67) |
PE; pulmonary embolism, VTE; venous thromboembolism.
Eleven (42%) of the patients with acute PE and six (67%) of the patients with subacute or chronic PE were receiving active treatment with an anticoagulant. Most patients (23/35, 66%) had subsegmental or segmental PE. Twenty-two patients (63%) had a single PE. Only one patient had PE in the main pulmonary arteries. The majority of the patients had intermediate pretest probability in the clinical prediction scores. Below we report three cases representative of three different scenarios: 1) Acute PE and normal D-dimer, 2) acute PE and normal D-dimer while receiving active treatment with an anticoagulant, and 3) subacute or chronic PE and normal D-dimer.
Case scenario 1: acute PE
A 76-year-old woman presented to the emergency department at night complaining of abdominal pain, nausea, vomiting, and shortness of breath. All symptoms had started 3 days prior to presentation, but the shortness of breath had worsened since the morning of that day. The patient had multiple myeloma and was receiving treatment with pomalidomide. Her medical history included hypertension, hypothyroidism, and right bundle branch block. Upon arrival, her blood pressure was 122/65 mm Hg, heart rate 80 beats/minute, respiratory rate 18 breaths/minute, and O2 saturation 99% on room air. She was alert, oriented, and in no acute distress. She had no chest pain, cough, or hemoptysis. Her D-dimer level was 0.28 μg/mL. Electrocardiography showed a stable right bundle branch block. CTPA showed central filling defects within the right interlobar and segmental branches of the right lower lobe pulmonary arteries, consistent with acute PE. There was no evidence of right ventricular strain. The patient started treatment with enoxaparin (70 mg, subcutaneously, twice daily) and was admitted to the inpatient service for further care.
Case scenario 2: acute PE while receiving active treatment with an anticoagulant
A 24-year-old white women with stage II low-grade osteosarcoma of the sphenoid sinusdiagnosed 4 years prior to presentation and under ongoing close observation, visited outpatient clinic for follow-up. The patient complained of 5 hours of chest pain. She described the pain as dull continuous pain in her left chest, measuring 8 out of 10 on the numeric rating scale for pain. The patient had also experienced shortness of breath for a few days prior to evaluation but denied any hemoptysis, loss of consciousness, or any constitutional symptoms.
The patient was alert and oriented. Peripheral pulses were present and capillary refill normal. The chest was clear bilaterally according to auscultation. All other physical examination yielded unremarkable results. Upon arrival, her heart rate was 112 beats/minute, respiratory rate 22 breaths/minute, blood pressure 138/90 mm Hg, and O2 saturation 100% on nasal cannula oxygen (2 L/minute). Her medical history included deep venous thrombosis, PE, irritable bowel syndrome, seizures, and peripheral neuropathies. Both deep venous thrombosis and PE events occurred 2 months prior to presentation and resulted in a 12-day hospital admission, including an intensive care unit stay. Since that time, she had been receiving treatment with warfarin. Her laboratory results were as follows: D-dimer 0.19 μg/mL, prothrombin time 26.9 seconds, international normalized ratio 2.41, and partial thromboplastin time 52.4 seconds. CTPA showed acute central PE within the right and left main pulmonary arteries with no radiologic evidence of right heart strain. The patient was sent to the emergency department and subsequently discharged home to continue the warfarin regimen (7.5 mg, orally, once daily).
Case scenario 3: Subacute or chronic PE
A 37-year-old man presented to the emergency department for evaluation of chest pain that had been going on for 5 days. The pain was localized in the left mid-costal sternal junction, not radiating elsewhere, and not associated with shortness of breath, hemoptysis, cough, or fever. He described his pain as a burning sensation, 6 out of 10. His cancer history consisted of stage IV non-small cell lung cancer with brain metastasis, not receiving any active treatment. Other comorbidities included a history of PE, pericardial effusion, and hypercholesterolemia. The PE occurred approximately 10 months prior to presentation, for which he had been treated with dalteparin. He had stopped dalteparin therapy 2 weeks prior to his visit. Upon physical examination, the patient was in no acute distress and was alert and oriented. His vital signs were as follows: blood pressure 122/84 mm Hg, heart rate 90 beats/minute, respiratory rate 18 breaths/minute, and O2 saturation 100% on room air. His D-dimer level was 0.28 μg/mL. CTPA showed a defect in the left segmental pulmonary artery consistent with subacute or chronic PE, but there was no evidence of acute PE. The patient was discharged home to resume dalteparin therapy.
DISCUSSION
The records of patients who presented to a large comprehensive cancer center during the period studied with suspicion of PE were reviewed to determine whether PE can present in cancer patients with normal D-dimer levels. A total of 1156 patients had PE, and only 35 of those (3.0%) had normal D-dimer levels. Of these, 26 had acute PE, 9 had subacute or chronic PE. D-dimer was normal in only 926 patients, having 4% (35) of patients with normal D-dimer to have PE.
After the activation of the coagulation cascade, both the thrombus and D-dimer are formed in parallel.[9] This allows D-dimer, the main biomarker of blood clots, to be an effective biomarker in excluding VTE because of its high sensitivity. This useful biomarker, in combination with validated clinical prediction rules, is effective in estimating pretest probability in patients with suspected PE[2] and preventing unnecessary diagnostic imaging studies.[10] According the American College of Physicians’ guideline for the evaluation of patients with suspected pulmonary embolism,[2] plasma D-dimer measurement should be obtained in all patients with intermediate pretest probability of PE and patients with low pretest probability of PE who do not meet all Pulmonary Embolism Rule-Out Criteria. Accurate and fast diagnosis of PE, especially in high-risk groups such as cancer patients, is critical to avoid significant morbidities and mortality.[11,12] Cancer patients have a higher risk of VTE, higher baseline D-dimer levels, and lower predictive values of D-dimer in detecting VTE when compared with the general population.[1,6,5] It has been known that some hematological malignancies secrete proteolytic factors,[13] and we speculate that some patients with hematological malignancies and pulmonary embolism have normal D-dimer levels because of accelerated degradation of D-dimer. Other factors that may also influence the sensitivity of D-dimer, includes age, thrombus burden and fibrinolytic activity, duration of symptoms, previous VTE and inflammatory state.[14] In addition, anticoagulants may affect the results of common coagulation assays.[15,16] The complexity of factors that exist in cancer patients, from comorbidities to medications received to the cancer itself, may affect the sensitivity of D-dimer as a biomarker, and identifying cancer patients with PE and normal D-dimer is crucial. The results from this case series and our previous analysis[7] suggest that D-dimer measurement is less useful in exclusion of PE in patients with hematological malignancies, and when the suspicion of PE is intermediate based on the pre-test probability, the decision to order CTPA should be based more heavily on clinical presentation and assessment than the D-dimer level. Subsequent multicenter study with larger number of patients can further help identify predictors of PE in patients with normal D-dimer, including cancer related factors as half of the patients with acute PE and normal D-dimer levels in our study had liquid tumors.
In conclusion, we observed cases of PE in cancer patients with normal D-dimer levels. Although the incidence of PE in cancer patients with normal D-dimer levels was only 3%, this possibility in cancer patients should be recognized. Failure to do so may negatively influence outcomes in a population that already faces high comorbidities, including the cancer itself.
KEY POINTS.
D-dimer is less accurate in cancer patients, and it is unclear whether cancer patients with normal D-dimer levels can present with pulmonary embolism.
Three percent (3.0%) of cancer patients with radiologic evidence of pulmonary embolism found on computed tomography pulmonary angiography during the period studied had normal D-dimer levels.
Despite the high sensitivity of D-dimer in detecting pulmonary embolism, a percentage of cancer patients may have normal plasma D-dimer levels in the presence of pulmonary embolism.
Failure to recognize this group of patients may negatively influence outcomes in a population that already faces high comorbidities, including the cancer itself.
Acknowledgments
The authors acknowledge Erica Goodoff, ELS, for editorial support.
Funding: None
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: Dr. Yeung is the principal investigator of an investigator-initiated clinical trial supported by DepoMed and a retrospective clinical study supported by Bristol-Myers Squibb through ARISTA-USA (BMS/Pfizer American Thrombosis Investigator Initiated Research Program). All other authors declare no competing financial or non-financial interests. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: The study was approved by the institutional review boards of MD Anderson; which granted waivers of informed consent.
REFERENCES
- 1.Lee AY, Levine MN (2003) Venous thromboembolism and cancer: risks and outcomes. Circulation 107 (23 Suppl 1):I17–21. doi: 10.1161/01.CIR.0000078466.72504.AC [DOI] [PubMed] [Google Scholar]
- 2.Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD, Clinical Guidelines Committee of the American College of P (2015) Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 163 (9):701–711. doi: 10.7326/M14-1772 [DOI] [PubMed] [Google Scholar]
- 3.Le Gal G, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H, Perrier A (2006) Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 144 (3):165–171 [DOI] [PubMed] [Google Scholar]
- 4.Wells PS, Ginsberg JS, Anderson DR, Kearon C, Gent M, Turpie AG, Bormanis J, Weitz J, Chamberlain M, Bowie D, Barnes D, Hirsh J (1998) Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med 129 (12):997–1005 [DOI] [PubMed] [Google Scholar]
- 5.Lee AY, Julian JA, Levine MN, Weitz JI, Kearon C, Wells PS, Ginsberg JS (1999) Clinical utility of a rapid whole-blood D-dimer assay in patients with cancer who present with suspected acute deep venous thrombosis. Ann Intern Med 131 (6):417–423 [DOI] [PubMed] [Google Scholar]
- 6.Righini M, Le Gal G, De Lucia S, Roy PM, Meyer G, Aujesky D, Bounameaux H, Perrier A (2006) Clinical usefulness of D-dimer testing in cancer patients with suspected pulmonary embolism. Thromb Haemost 95 (4):715–719 [PubMed] [Google Scholar]
- 7.Qdaisat A, Al Soud R, Wu CC, Rojas Hernandez CM, Li J, Meng QH, Abdel-Razeq H, Yeung SJ (2019) Poor performance of D-Dimer in excluding venous thromboembolism among patients with lymphoma and leukemia [published online ahead of print, Jaunary 10, 2019]. Haematologica. doi: 10.3324/haematol.2018.211466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter BW, Lichtenberger JP 3rd, Wu CC(2014) Acquired abnormalities of the pulmonary arteries. AJR Am J Roentgenol 202 (5):W415–421. doi: 10.2214/AJR.13.11760 [DOI] [PubMed] [Google Scholar]
- 9.Adam SS, Key NS, Greenberg CS (2009) D-dimer antigen: current concepts and future prospects. Blood 113 (13):2878–2887. doi: 10.1182/blood-2008-06-165845 [DOI] [PubMed] [Google Scholar]
- 10.Righini M, Perrier A, De Moerloose P, Bounameaux H (2008) D-Dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost 6 (7):1059–1071. doi: 10.1111/j.1538-7836.2008.02981.x [DOI] [PubMed] [Google Scholar]
- 11.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH (2007) Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 5 (3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x [DOI] [PubMed] [Google Scholar]
- 12.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH (2007) Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 110 (10):2339–2346. doi: 10.1002/cncr.23062 [DOI] [PubMed] [Google Scholar]
- 13.Colombo R, Gallipoli P, Castelli R (2014) Thrombosis and hemostatic abnormalities in hematological malignancies. Clin Lymphoma Myeloma Leuk 14 (6):441–450. doi: 10.1016/j.clml.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 14.Siragusa S, Terulla V, Pirrelli S, Porta C, Falaschi F, Anastasio R, Guarnone R, Scarabelli M, Odero A, Bressan MA (2001) A rapid D-dimer assay in patients presenting at the emergency room with suspected acute venous thrombosis: accuracy and relation to clinical variables. Haematologica 86 (8):856–861 [PubMed] [Google Scholar]
- 15.Couturaud F, Kearon C, Bates SM, Ginsberg JS (2002) Decrease in sensitivity of D-dimer for acute venous thromboembolism after starting anticoagulant therapy. Blood Coagul Fibrinolysis 13 (3):241–246 [DOI] [PubMed] [Google Scholar]
- 16.Schutgens RE, Esseboom EU, Haas FJ, Nieuwenhuis HK, Biesma DH (2002) Usefulness of a semiquantitative D-dimer test for the exclusion of deep venous thrombosis in outpatients. Am J Med 112 [DOI] [PubMed] [Google Scholar]