ABSTRACT
Malignant ovarian neoplasm is one of the most lethal malignancies among cancers of the female reproductive system. Occasionally, these tumors originate from non-ovarian organs as metastatic lesions since the ovary is a frequent metastatic target of many cancers. However, there limited clinical information on metastatic ovarian carcinoma (MOC) and its hallmarks are unknown. During the period of 1986–2015, 4,284 patients with malignant ovarian neoplasm were identified using the Tokai Ovarian Tumor Study Group (TOTSG) database. Of these, excluding borderline malignant tumor, 3,478 patients with malignant ovarian cancer were extracted. The pathological slides were evaluated under central pathological review. Among them, a total of 143 (4.1%) patients with MOC were identified. The median age of patients with MOC was 54 (29–82) years. The most and second most frequent original tumors were colorectal (43%, N=62) and gastric (29%, N=42) carcinoma, respectively. The rates of carcinoma of the appendix, breast, and pancreas were 8, 6, and 4%, respectively. This is the one of the largest studies clarifying the rates of MOC among malignant ovarian neoplasms. Although the rate is low, we should keep in mind that MOC, particularly from colorectal and gastric cancer should be considered when encountering clinical practice of ovarian cancer.
Key Words: malignant ovarian neoplasm, metastatic ovarian cancer, original organ, epidemiology
INTRODUCTION
Ovarian carcinoma is one of the most lethal malignancies among cancers of the female reproductive system. The recent Cancer Statistics in United States estimated that 22,440 women were newly diagnosed, and 14, 080 died of this tumor.1 According to Japanese Cancer Registry and Statistics, the number of cases and mortality ratio were 9,804 and 4,758, respectively in 2016.2 In contrast to other gynecologic cancers such as cervical, endometrial, and vulvar cancer, this tumor frequently causes no apparent symptoms in the early stages.3 However, an abdominal mass and/or fullness is a major symptom in women with disease as the tumor enlarges. Clinically, reflecting the fact that the ovary exists in the abdominal cavity, ovarian carcinoma can easily spread throughout the peritoneal cavity. Occasionally, these tumors originate from non-ovarian organs as metastatic lesions since ovary is a favorable site being metastasized for many cancers. However, there limited clinical information on metastatic ovarian carcinoma (MOC) and its hallmarks are unknown.
To evaluate the epidemiological hallmarks of patients with MOC, we conducted a retrospective study analyzing 4,284 patients with malignant ovarian neoplasm who were accumulated by the Tokai Ovarian Tumor Study Group (TOTSG), consisting of Nagoya University and 13 affiliated hospitals. To date, we have accumulated 143 patients with MOC in this investigation. In the current study, we overviewed and proposed the rates of MOC based on the central pathological system to promote appropriate therapeutic decisions for patients with ovarian carcinoma.
MATERIALS AND METHODS
Patient cohort
During the period of 1986–2015, 4,284 patients with malignant ovarian neoplasm were registered and accumulated by the Tokai Ovarian Tumor Study Group (TOTSG), consisting of 14 collaborating institutions; Nagoya University Hospital, Aichi Cancer Center Hospital, Anjyo Kosei Hospital, Toyohashi Municipal Hospital, Toyota Memorial Hospital, Ogaki Municipal Hospital, Nagoya First Red-cross Hospital, Nagoya Second Red-cross Hospital, Nagoya Ekisaikai Hospital, Nagoya Memorial Hospital, Okazaki Municipal Hospital, Handa City Hospital, Komaki City Hospital, and Gifu Prefectural Tajimi Hospital. All histological slides were reviewed by two expert pathologists with no knowledge of the patients’ clinical data under a central pathological review system. Data were collected from medical records and clinical follow-up visits. A total of 126 patients were excluded from this study due to having an unclassified histological type, a metastatic ovarian tumor that had originated from the uterus, or insufficient data. After excluding another 680 patients with borderline malignant tumors, 3,478 patients with malignant ovarian cancer were finally extracted (Fig.1). Furthermore, seven patients without sufficient information on the residual tumor were excluded. This study was approved by the ethics committee of Nagoya University. The histological cell types were assigned according to the criteria of the World Health Organization (WHO). Histological slides were reviewed by gynecologic pathologists under a central pathological review system with no knowledge of the patients’ clinical data.
Fig. 1.
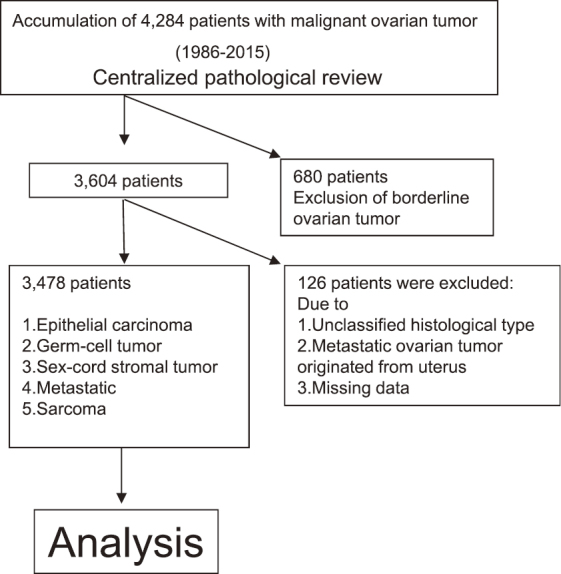
Flowchart of patient recruitment
RESULTS
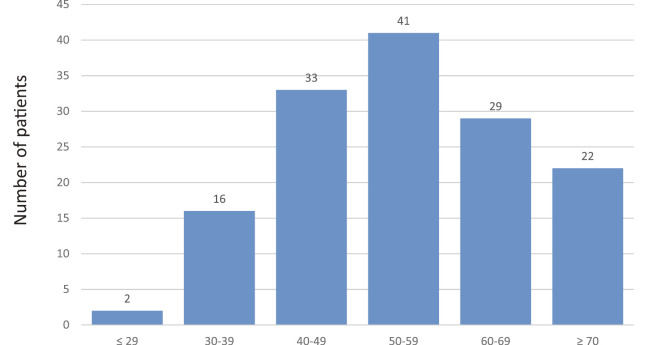
Of all 3,478 patients enrolled, the distribution of pathological types is shown in Fig.2. In total, 84.3% (N=2,932) of all patients had an epithelial ovarian carcinoma. Rates of those with a germ-cell tumor and sex cord stromal tumor were 6.8% (N=238) and 3.3% (N=115), respectively. The rates of patients with MOC excluding a uterine origin was 4.1% (N=143). Confining analysis to patients with MOC, the median age of patients was 54 (29–82) years. The age distribution is shown in Fig.3.
Fig. 2.
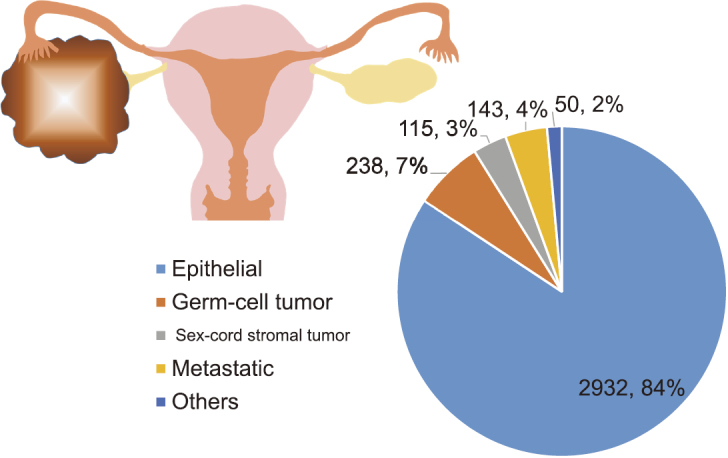
The distribution of pathological types of all 3,478 patients with malignant ovarian carcinoma
Fig. 3.
The age distribution of 143 patients with MOC
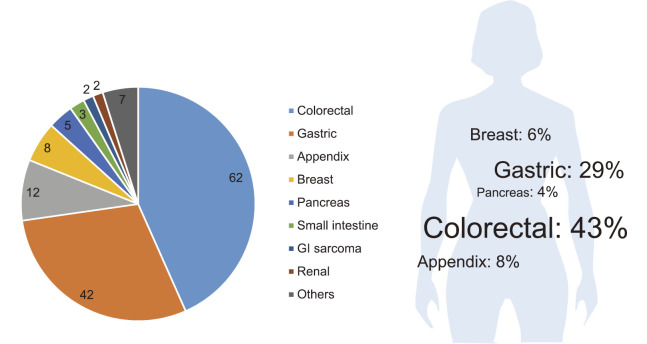
As shown in Fig.4, the most and second frequent organs of original tumor were colorectal (43%, N=62) and gastric carcinoma (29%, N=42), respectively. The rates of carcinoma of the appendix, breast, and pancreas were 8, 6, and 4%, respectively.
Fig. 4.
The original organs of 143 patients with MOC. The most and second frequent organs of original tumor were colorectal (43%, N=62) and gastric carcinoma (29%, N=42), respectively.
DISCUSSION
The ovary is a relatively common metastatic target of other primary extra-ovarian malignancies, and physicians frequently encounter non-gynecologic ovarian carcinoma.
In the present study, we enrolled 3,478 patients with malignant ovarian carcinoma extracted from the TSTOG database, and demonstrated that the rates of MOC excluding cases with a uterine origin was 4.1% (143/3,478). To date, the accurate rate of MOC has remained unknown in Japan. To our knowledge, this is the one of the largest studies to specifically investigate the rate of MOC, in spite of the fact that the retrospective nature of the study could be a possible bias. According to prior studies, the rate of such malignancies ranged from 17.8 to 30%.4-6 On the other hand, recent autopsy data showed that 10.3% (24/233) of patients who died from ovarian carcinoma were found to have ovarian metastases that were not primary but rather metastases from other primary tumors, including the colon (N=10), pancreas (N=5), stomach (N=2), extrahepatic bile ducts (N=1), cervix uterine cervix (N=3), uterine corpus (N=1), breast (N=1), and kidney (N=1).7 The inconsistency in the rate was thought to be attributable to the relatively limited patient number and retrospective nature of the study. Taken together, in this investigation, we could present the accurate rate of MOC in Japan. Ultimately, an accurate diagnosis is crucial, facilitating appropriate therapy.
According to earlier studies, the colorectum and stomach were frequent sites of the original tumors.5,8 In the current study, the primary site of the MOC was colorectal in 62 patients (43%), with 42 found in the stomach (29%), 12 in the appendix (8%), 8 in the breast (N=89), and 5 in the pancreas (4%). Our study is consistent with previous studies reporting that the most common MOC origin was the gastrointestinal tract. Consequently, the proportion of primary sites in our results may serve as a reference for Japanese patients.
In general, malignant ovarian neoplasms are heterogeneous tumors, consisting of various histologic types. Therefore, due to this heterogeneity, the hallmarks of the disease are complex and difficult to understand. Regarding the histologic features of epithelial ovarian cancer, there are four major pathological types: serous, mucinous, endometrioid, and clear-cell carcinoma.3 As aforementioned, in the current study, the majority of our cases involved mucinous carcinoma from the gastrointestinal tract. Particularly, to discriminate primary and metastatic mucinous carcinomas is of marked clinical importance. Typical characteristics of primary mucinous carcinomas are as follows: 1) relatively large, unilateral tumors, 2) with multiple smooth capsules, 3) less frequently associated with extra-ovarian metastasis, 4) more commonly associated with precursor borderline tumors, and 5) more frequently displaying confluent glandular or expansile patterns of invasion. On the other hand, representative features of metastatic mucinous carcinomas of the ovary that distinguish them from primary tumors are as follows: 1) relatively smaller-sized tumor, 2) more frequently associated with bilateral ovarian involvement, and 3) an infiltrative pattern of stromal invasion.9 As well as microscopic features, immunohistochemical staining patterns of cytokeratins 7 and 20 (CK7/20) may be effective to discriminate primary ovarian cancer, derived from MOC from gastrointestinal tract tumors.10 In the current work, permanent sections were reviewed by immunohistochemistry as much as possible. We consider it desirable to apply immunohistochemistry techniques to sections from all patients with mucinous carcinoma, because subsequent therapy should be appropriately conducted by an expert physician.
Our current work still includes several limitations due to its retrospective nature. In addition, previous studies showed that immunohistochemistry of CDX-2 (caudal related homeobox gene type2) and TTF-1 (thyroid transcription factor-1), as well as CK7/20 may be helpful diagnostic markers in making a distinction between the origin of primary and metastatic ovarian carcinoma.10-12 However, the practice of immunohistochemical examination was optional in the present study. In contrast, the strengths of our current work were firstly based on it being a regional population-based study, secondly, the performance of central pathological review by expert pathologists in gynecologic malignancy, and thirdly, the relatively large patient population.
In conclusion, the ovary is a common metastatic target for many cancers, although the precise mechanism involved has yet to be elucidated. In the present study, we overviewed 3,478 patients with malignant ovarian carcinoma from a Japanese regional population-based database under a central pathological review system, and demonstrated that the rate of MOC excluding that of a uterine origin was 4.1%. This is the one of the largest studies clarifying the rate of MOC among malignant ovarian neoplasms. Although the rate of MOC is low, we should keep in mind that the origin of MOC, particularly from colorectal and gastric cancer, should be considered when encountering ovarian cancer to ensure the appropriate clinical management of patients by an expert physician.
DISCLOSURE
The authors have nothing to disclose.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. [DOI] [PubMed]
- 2.Mathew GK, Singh SS, Swaminathan RG, Tenali SG. Laparotomy for post chemotherapy residue in ovarian germ cell tumors. J Postgrad Med. 2006;52(4):262–265. [PubMed]
- 3.Kikkawa F, Nawa A, Ino K, Shibata K, Kajiyama H, Nomura S. Advances in treatment of epithelial ovarian cancer. Nagoya J Med Sci. 2006;68(1–2):19–26. [PubMed]
- 4.Khunamornpong S, Suprasert P, Chiangmai WN, Siriaunkgul S. Metastatic tumors to the ovaries: a study of 170 cases in northern Thailand. Int J Gynecol Cancer. 2006;16 Suppl 1:132–138. [DOI] [PubMed]
- 5.Kondi-Pafiti A, Kairi-Vasilatou E, Iavazzo C, et al. Metastatic neoplasms of the ovaries: a clinicopathological study of 97 cases. Arch Gynecol Obstet. 2011;284(5):1283–1288. [DOI] [PubMed]
- 6.Yakushiji M, Tazaki T, Nishimura H, Kato T. Krukenberg tumors of the ovary: a clinicopathologic analysis of 112 cases. Nihon Sanka Fujinka Gakkai Zasshi. 1987;39(3):479–485. [PubMed]
- 7.Güth U, Arndt V, Stadlmann S, Huang DJ, Singer G. Epidemiology in ovarian carcinoma: lessons from autopsy. Gynecol Oncol. 2015;138(2):417–420. [DOI] [PubMed]
- 8.Webb MJ, Decker DG, Mussey E. Cancer metastatic to the ovary: factors influencing survival. Obstet Gynecol. 1975;45(4):391–396. [PubMed]
- 9.Yemelyanova AV, Vang R, Judson K, Wu LS, Ronnett BM. Distinction of primary and metastatic mucinous tumors involving the ovary: analysis of size and laterality data by primary site with reevaluation of an algorithm for tumor classification. Am J Surg Pathol. 2008;32(1):128–138. [DOI] [PubMed]
- 10.Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002;21(4):391–400. [DOI] [PubMed]
- 11.Hart, WR. Diagnostic challenge of secondary (metastatic) ovarian tumors simulating primary endometrioid and mucinous neoplasms. Pathol Int. 2005;55(5):231–243. [DOI] [PubMed]
- 12.Lee, KR, Young, RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol. 2003;27(3):281–292. [DOI] [PubMed]