Abstract
Background: Oral squamous cell carcinoma (OSCC) is one of the most common cancers, with high metastasis and mortality. Licochalcone A (LCA) is a chalconoid from the root of Glycyrrhiza inflata, which has anti-tumor, anti-inflammatory, anti-angiogenesis effects in many cancers. However, the mechanism that underlies LCA regulating cell proliferation, migration, and invasion in OSCC remains poorly understood.
Methods: LY294002 or insulin-like growth factor 1 (IGF-1) were used to block or stimulate the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) pathway in OSCC cells. Cell proliferation was investigated by MTT assay and proliferating cell nuclear antigen (PCNA) protein level using Western blot. The expression of metastasis-related protein was detected via Western blot. Cell migration and invasion abilities were evaluated by trans-well assay. A murine xenograft model of OSCC was established to investigate the anti-tumor effect of LCA in vivo.
Results: Treatment of LCA inhibited cell proliferation in SCC4 and CAL-27 cells. Moreover, PI3K/AKT signaling was blocked by LY294002, and activated by IGF-1. LCA could suppress proliferation, migration, and invasion of OSCC cells, which was similar to the treatment of LY294002. In addition, LCA decreased IGF-1-induced OSCC progression. In a murine xenograft model, LCA treatment protected against tumor growth and metastasis in vivo.
Conclusions: LCA might inhibit cell proliferation, migration, and invasion through regulating the PI3K/AKT pathway in OSCC, developing a potential chemotherapeutic agent for OSCC.
Keywords: oral squamous cell carcinoma, Licochalcone A, PI3K/AKT, PCNA, migration, invasion
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common cancers, and accounts for 90% of oral cancer.1 Its invasive ability exacerbates tumor malignancy and its factors may serve as potential diagnostic and therapeutic targets of OSCC.2 With the advances in cancer diagnosis and treatment, OSCC has gained more attention, while the 5-year survival rate remains unsatisfactory.3 Hallmarks of proliferation, growth, inflammation, invasion, migration, as well as cell death play essential roles in the prognosis of OSCC.4 The surgery, radiotherapy, and chemotherapy have gained more attention for OSCC treatment in recent years, whereas the role of the therapy remains controversial.5,6 Hence, development of therapeutic agents is required for greater efficacy in OSCC treatment.
Licochalcones (LCs) are a class of natural bioactive compounds, which have most important anti-inflammatory, anti-oxidant, anti-cancer, anti-microbial, and anti-viral roles.7 LCD might induce apoptosis and suppress cell migration and invasion in human melanoma cells.8 LCA have been reported to inhibit cell migration and invasion by down-regulating mitogen-activated protein kinase kinase-4 (MKK4) and its substrate c-Jun N-terminal kinase (JNK) and urokinase plasminogen activator (uPA) expression in human hepatocellular carcinoma.9 Moreover, LCA suppresses cell viability, enhanced autophagy and apoptosis by regulating the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) pathway/mTOR pathway in breast cancer cells.10 Notably, LCA plays an important role in cell viability and apoptosis by regulating extracellular signal-regulated kinase1/2 (ERK1/2) and p38-mediated tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression in head and neck squamous carcinoma FaDu cells.11 More importantly, LCA is suggested to induce apoptosis through regulating Sp1 and Sp1 regulatory proteins expression in OSCC.12 However, the mechanism allows LCA regulating migration, and invasion of OSCC remains largely unclear.
The PI3K/AKT pathway has been regarded as one of the key mechanisms involved in cell migration, invasion, and epithelial-mesenchymal transition in lung cancer.13 Moreover, the PI3K/AKT signaling pathway is regarded to associate with proliferation and metastasis in renal cell carcinoma.14 In addition, the PI3K/AKT pathway is suggested to be involved in cell apoptosis in human pharyngeal squamous carcinoma FaDu cells.15 The previous effort suggests the PI3K/AKT pathway is required for cell growth in oral cancer.16 Hence, we assumed that the PI3K/AKT pathway might be associated with LCA-mediated progression of OSCC. In the present study, we investigated the effect of LCA on proliferation, migration, and invasion in OSCC cells. Moreover, we explored whether it was associated with the PI3K/AKT pathway. In addition, the potential anti-tumor effect of LCA was evaluated in vivo by murine xenograft model of OSCC.
Materials and methods
Cell culture and treatment
Human OSCC cell lines SCC4 and CAL-27 cells were purchased from American Tissue Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI-1640 cell medium (Invitrogen, Carlsbad, CA, USA) containing 10% FBS (Gibco, Carlsbad, CA, USA), 1% penicillin, and streptomycin (Thermo Fisher, Wilmington, DE, USA) in a humidified incubator at 37°C with 5% CO2.
To evaluate the effect of LCA on OSCC progression, different concentrations (0, 25, 50, 100 μM) of LCA (Sigma, St. Louis, MO, USA) were introduced into cells for 24 hours or 48 hours. To block the PI3K/AKT pathway, 50 μM LY294002 (Sigma) was added to cells 2 hours before treatment of LCA. However, cells were incubated with 100 ng/mLof insulin-like growth factor 1 (IGF-1) (Sigma) for 20 minutes to activate the PI3K/AKT pathway.
Cell viability
Cells were seeded at a density of 1×104 cells/well in 96-well plates overnight and then treated with the indicated concentration of LCA, LY294002, or IGF-1. After the incubation, cells were incubated with 5 mg/mLMTT (Sigma) for 4 hours, followed by crystals dissolved in 100 μL/well dimethyl sulfoxide (DMSO, Sigma). The absorbance was measured at 570 nm with a microplate reader (Bio-Rad, Hercules, CA, USA). All experiments were performed three times.
Western blots
Total protein was prepared from OSCC cells or tumor tissues in cell lysis buffer (Thermo Fisher) and then quantified by BCA assay kit (Sigma). Following denatured and separated by SDS-PAGE gel, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA) and then blocked with blocking reagent (Thermo Fisher). Subsequently, the membranes were incubated with primary antibodies against proliferating cell nuclear antigen (PCNA) (#13110), p-AKT (#4060), AKT (#4691), PI3K (#4249), matrix metalloproteinases-2 (MMP-2) (#87809), MMP-9 (#13667), or β-actin (#4970) (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C and secondary antibodies (#5127) (Cell Signaling Technology) for 2 hours. The protein blots were visualized using enhanced chemiluminescence (Thermo Fisher) and normalized by β-actin as a standard for band intensities.
Trans-well assay
To investigate cell migration and invasion ability, trans-well assay was conducted in SCC4 and CAL-27 cells. In migration assay, cells were seeded in the upper chambers (Costar, Corning, NY, USA) containing serum-free medium and cultured at 37°C in 5% CO2 for 8 hours. Migrated cells were fixed with 100% methanol for 10 minutes, then stained with 0.1% crystal violet (Sigma) and counted using a microscope (Olympus, Tokyo, Japan). In invasion assay, the trans-well chambers were coated with Matrigel (BD, San Jose, CA, USA) and then used for subsequent assay following a similar approach.
Murine xenograft model of OSCC
Animal experiments were conducted in accordance with the guidelines for Care and Use of Laboratory Animals approved by the Animal Research committee of Yuhuangding Hospital. BALB/c nude mice (male, 6-week-old) were purchased from Vital River Laboratory Animal Technology (Beijing, China). SCC4 cells (6×106 cells) were subcutaneously injected into nude mice (n=8 per group). One week after cell implantation, 10 mg/kg LCA dissolved in 5% ethanol or 5% ethanol were introduced into the experiment or control group by intraperitoneal injection, respectively, every 2 days for 4 weeks. Tumors were examined every week, and tumor volume was calculated with slide calipers as V=0.5×length×width×height. Mice were killed at 35 days after cell implantation, and tumor specimens were weighted and then collected for molecular analyses.
Statistical analysis
Data were expressed as the mean±standard error of the mean (SEM) from three independent experiments. Difference was evaluated by paired Student’s t-test or one-way ANOVA using SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA). Statistically significant was considered as P-values less than 0.05.
Results
LCA inhibits cell proliferation in OSCC cells
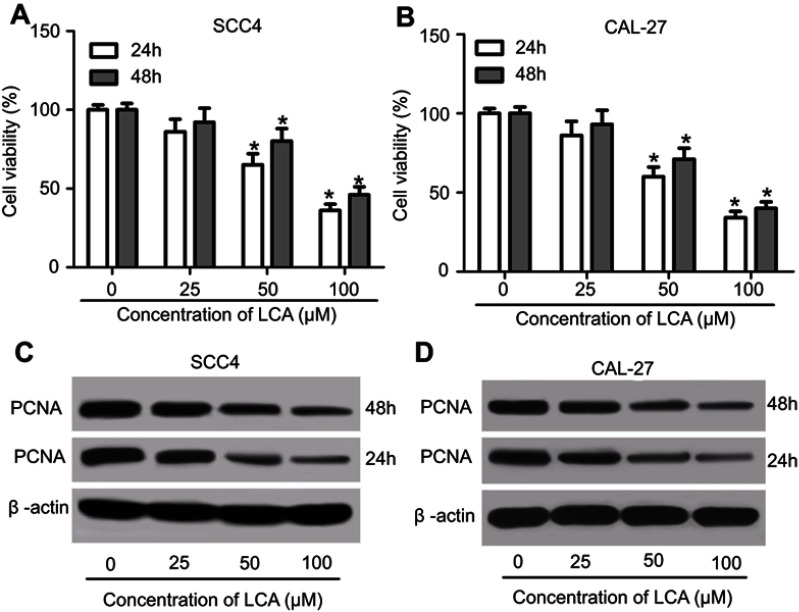
To investigate the cytotoxic effect of LCA on OSCC cells, SCC4 and CAL-27 cells were exposed to different concentrations (0, 25, 50, and 100 μM) of LCA for 24 hours or 48 hours. After the treatment, cell viability was obviously decreased in SCC4 and CAL-27 cells after 50 or 100 μM LCA treatment for 24 or 48 hours in a concentration dependent manner (Figures 1A and B). Moreover, the abundances of PCNA protein were measured in the two cells, and results showed that the PCNA level was progressively reduced in SCC4 and CAL-27 cells at 24 or 48 hours in response to different concentrations of LCA stimulation (Figures 1C and D). Besides, a 24 hour-incubation with LCA led to severer loss of cell viability and PCNA protein abundance than exposure for 48 hours in SCC4 and CAL-27 cells (Figures 1A–D). Hence, Cells with exposure of 100 μM LCA for 24 hours were used for further study. These data demonstrate that LCA suppressed cell proliferation in OSCC cells.
Figure 1.
LCA inhibited cell proliferation of OSCC cells. (A and B) The cell viability of SCC4 and CAL-27 were detected at 24 and 48 hours after exposure of 0, 25, 50, 100 μM LCA. (C and D) The expression of PCNA protein was examined in SCC4 and CAL-27 at 24 and 48 hours after different concentrations of LCA treatment. *P<0.05.
Abbreviations: LCA, Licochalcone A; OSCC, Oral squamous cell carcinoma; PCNA, proliferating cell nuclear antigen.
LCA inhibits OSCC progression by blocking the PI3K/AKT pathway
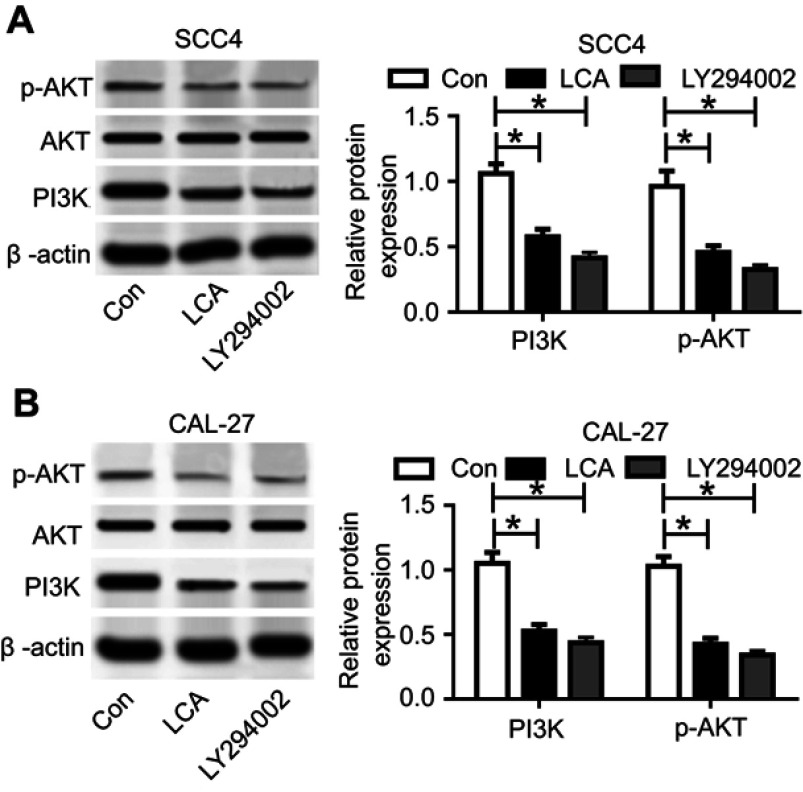
To probe the molecular mechanism of LCA regulating OSCC progression, we investigated the effect of LCA on the PI3K/AKT pathway in OSCC cells. The inhibitor of PI3K/AKT signaling LY294002 was used as a positive control. After the exposure of 100 μM LCA for 24 hours or 50 μM LY294002 for 2 hours, the expression levels of p-AKT and PI3K protein were significantly reduced in SCC4 and CAL-27 cells, while the total AKT expression level showed few alterations, suggesting that LCA treatment inhibited the PI3K/AKT pathway (Figures 2A and B).
Figure 2.
LCA blocked PI3K/AKT pathway in OSCC cells. (A and B) The expression of PI3K/AKT-related proteins was detected in SCC4 and CAL-27 cells after exposure of 100 μM LCA for 24 hours or 50 μM LY294002 for 2 hours. *P<0.05.
Abbreviations: Con, control; LCA, Licochalcone A; OSCC, Oral squamous cell carcinoma.
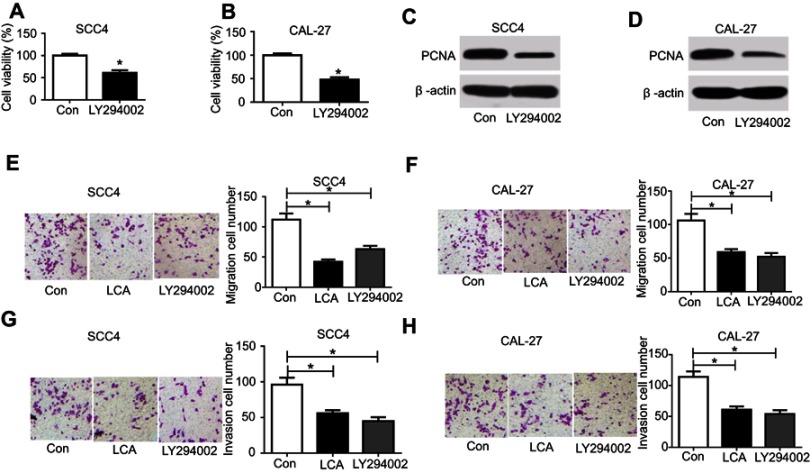
To investigate whether the PI3K/AKT pathway was associated with LCA-mediated OSCC progression, SCC4 and CAL-27 cells were treated with 100 μM LCA for 24 hours or 50 μM LY294002 for 2 hours. As shown in Figures 3A–D, inhibition of PI3K/AKT by LY294002 significantly decreased cell proliferation. Furthermore, the analysis of trans-well revealed that LCA markedly repressed cell migration and invasion in SCC4 and CAL-27 cells, which is similar to the effect of LY294002 (Figures 3E–H). These results indicated that LCA suppressed cell proliferation, migration, and invasion by blocking the PI3K/AKT pathway.
Figure 3.
LCA suppressed cell proliferation, migration, and invasion in OSCC cells. Cells were treated with 100 μM LCA for 24 hours or 50 μM LY294002 for 2 hours. The effect of LY294002 on cell viability (A and B) and PCNA protein level (C and D) was investigated in SCC4 and CAL-27 cells. (E–H) The effect of LCA and LY294002 on cell migration and invasion was analyzed in SCC4 and CAL-27 cells. *P<0.05.
Abbreviations: Con, control; LCA, Licochalcone A; OSCC, Oral squamous cell carcinoma; PCNA, proliferating cell nuclear antigen.
To further investigate this mechanism, SCC4 and CAL-27 cells were exposed to 100 ng/mL IGF-1 for 20 minutes, leading to the activation of the PI3K/AKT pathway in OSCC cells, which was confirmed in Figures 4A and B. However, introduction of LCA attenuated IGF-1-induced PI3K/AKT pathway activation (Figures 4A and B). With respect to cell proliferation, the promotion of the PI3K/AKT pathway using IGF-1 significantly enhanced the expression of PCNA protein, whereas LCA treatment weakened PCNA level in SCC4 and CAL-27 cells (Figures 4C and D). Moreover, the presence of IGF-1 effectively promoted cell migration and invasion in SCC4 and CAL-27 cells, while exposure of LCA mitigated this effect (Figures 4E–H). Together, these results further revealed that LCA hindered OSCC progression through mediating the PI3K/AKT pathway.
Figure 4.
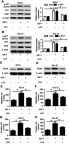
LCA inhibited IGF-1-induced cell viability, migration, and invasion by regulating the PI3K/AKT pathway. Cells were stimulated with 100 ng/mL of IGF-1 for 20 minutes and then treated with 100 μM LCA for 24 hours. (A and B) The effect of LCA on IGF-1-mediated PI3K/AKT pathway was investigated in SCC4 and CAL-27 cells. (C and D) The expression of PCNA protein was detected in SCC4 and CAL-27 cells after IGF-1 and LCA treatment. (E and F) The number of migration cells was analyzed in SCC4 and CAL-27 cells. (G and H) The ability of invasion was investigated in cells with IGF-1 and LCA exposure. *P<0.05.
Abbreviations: LCA, Licochalcone A; PCNA, proliferating cell nuclear antigen.
LCA delays tumor growth and metastasis in vivo
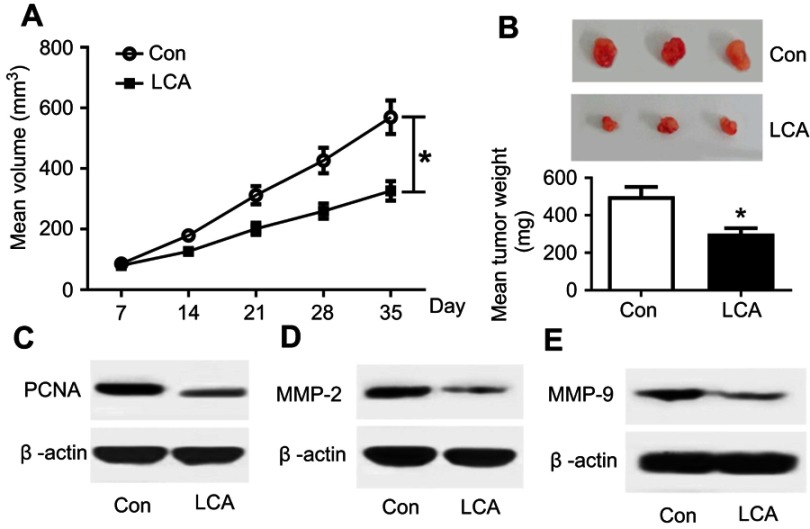
To further evaluate the anti-tumor effect of LCA on OSCC in vivo, 16 nude mice were injected with SCC4 cells and then treated with or without LCA. As a result, LCA treatment significantly lessened the tumor volume compared with the control group (n=8 per group) (Figure 5A). Moreover, tumor weight was also obviously decreased by LCA treatment (Figure 5B). Besides, the protein factors of proliferation and metastasis were investigated in tumor samples. As demonstrated in Figure 5C, LCA treatment resulted in a strong loss of PCNA protein abundance. Furthermore, the expression levels of metastasis markers (MMP-2 and MMP-9) were evidently down-regulated in tumor tissues after LCA treatment (Figures 5D and E). These findings uncovered that LCA treatment had a limited capacity for tumor growth and metastasis of OSCC in vivo.
Figure 5.
LCA decreased tumor growth and metastasis in vivo. (A) Tumor volume was detected every week after LCA or control treatment. (B) Tumor weight was measured at the end point. (C–E) The expression levels of PCNA, MMP-2, and MMP-9 protein were examined in tumor tissues after LCA insult. *P<0.05.
Abbreviations: Con, control; LCA, Licochalcone A; PCNA, proliferating cell nuclear antigen.
Discussion
OSCC is one common lethal disease with increasing incidence threatening a large number of people all over the world.17 Hence, a novel effective and low-toxicity therapeutic agent is expected for OSCC treatment. Licorice is obtained from the roots of Glycyrrhiza species, which exhibits potential beneficial effects to prevent or treat oro-dental diseases.18 LCA is one of the bioactive ingredients of licorice, which was suggested as a compound with effective and low-toxicity features against Toxoplasma gondii.19 Moreover, LCA played anti-tumor and anti-metastatic effects in colorectal cancer by regulating proliferation and metastasis in preclinical studies.20 Notably, LCA might mediate cell apoptosis through regulating Sp1 expression or caspase-dependent FasL pathway in OSCC.12,21 However, the mechanism that underlies LCA involved in cell proliferation, migration, and invasion of OSCC cells remains unclear. In this study, we found that LCA suppressed cell proliferation, migration, and invasion in OSCC. Moreover, we demonstrated that it was associated with the PI3K/AKT signaling pathway.
We first investigated the effect of LCA on cell proliferation in SCC4 and CAL-27 cells. PCNA is a proliferation-related protein, which has been suggested to positively correlate with cell proliferation in OSCC.22 Hence, the cell viability and PCNA protein abundance were investigated for cell proliferation in our works. Results showed that LCA decreased cell viability and PCNA level in OSCC cells, suggesting that LCA inhibited cell proliferation of OSCC cells, which is also in agreement with the previous studies displaying the anti-proliferation role of LCA in glioma and lung cancer cells.23,24 Moreover, migration and invasion also aggravated the tumor malignancy. Trans-well assay showed that LCA blocked the migrated and invasive abilities of OSCC cells in this study, which is consistent with the effect in former work revealing that LCA repressed cell migration and invasion in human hepatocellular carcinoma cells.9 Accordingly, a promising signaling pathway is needed to better understand the mechanism in the study.
The PI3K/AKT pathway has been reported to be activated in multiple types of tumor malignancies.25 Moreover, the PI3K/AKT pathway was associated with migration and invasion in many cancers, such as prostate cancer, lung cancer, and gastric cancer.26–28 We hypothesized that the PI3K/AKT pathway might be involved in migration and invasion in our effort. In our research, we found that LCA treatment blocked the PI3K/AKT pathway through inhibiting p-AKT and PI3K protein expressions in OSCC cells, which is also in agreement with previous studies in skin cancer and gastric cancer.29,30 Notably, the regulatory effect of the PI3K/AKT pathway on cell viability, migration, and invasion was directly investigated by inhibition or activation of the pathway in OSCC cells. LY294002 was regarded as a common inhibitor of the PI3K/AKT pathway.31 In addition, IGF-1 was used as an activator of the PI3K/AKT pathway in a previous study.32 By using LY294002 or IGF-1, this study showed that the PI3K/AKT pathway was positively correlated with OSCC cell proliferation, migration, and invasion, which is similar to former works.33,34 Furthermore, the effect of LCA was similar to the role of LY294002, and it could attenuate the effect of IGF-1, suggesting that LCA treatment blocked PI3K/AKT signaling to suppress cell proliferation, migration, and invasion in OSCC cells.
Preclinical investigation was necessary for evaluating the anti-tumor effect of LCA on OSCC by murine xenograft model.11,35 Hence, SCC4 cells were introduced into nude mice and then treated by LCA. Results uncovered that LCA inhibited tumor growth. Moreover, LCA treatment suppressed PCNA, MMP-2, and MMP-9 abundances, suggesting that LCA might inhibit proliferation and metastasis in vivo. This is similar to the results in such findings that LCA decreased MMPs in rat chondrocytes.36 Functional LCA was realized by a combination with clinical drugs.19 Hence, the effect of combination of LCA and clinical drugs on OSCC is needed to be investigated by in-depth preclinical experiments in a further study. Furthermore, this study just investigated the signaling pathway involved in LCA-addressed progression by using pharmacological inhibitors of PI3K (LY294002) or activator (IGF-1). To better understand the mechanism, the experiments should be performed using siRNA ablation or overexpression in future.
In the present study, we demonstrated that LCA treatment inhibited cell proliferation, migration and invasion in OSCC, possibly associated with the PI3K/AKT pathway, providing a novel therapeutic agent for OSCC treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.D’souza S, Addepalli V. Preventive measures in oral cancer: an overview. Biomed Pharmacother. 2018;107:72–80. doi: 10.1016/j.biopha.2018.07.114 [DOI] [PubMed] [Google Scholar]
- 2.Siriwardena S, Tsunematsu T, Qi G, Ishimaru N, Kudo Y. Invasion-related factors as potential diagnostic and therapeutic targets in oral squamous cell Carcinoma-A review. Int J Mol Sci. 2018;19(5):1462. doi: 10.3390/ijms19051462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JW, Park Y, Roh JL, et al. Prognostic value of glucosylceramide synthase and P-glycoprotein expression in oral cavity cancer. Int J Clin Oncol. 2016;21(5):883–889. doi: 10.1007/s10147-016-0973-1 [DOI] [PubMed] [Google Scholar]
- 4.Sasahira T, Kirita T. Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int J Mol Sci. 2018;19(8):2413. doi: 10.3390/ijms19082413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu T, Chua B, Batstone M. Postoperative radiotherapy for oral squamous cell carcinoma with histologic risk factors: are we over-treating? J Oral Maxillofac Surg. 2018;76(7):1565–1570. doi: 10.1016/j.joms.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 6.Hartner L. Chemotherapy for oral cancer. Dent Clin North Am. 2018;62(1):87–97. doi: 10.1016/j.cden.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 7.Gatta D, Franceschelli S, Felaco M, Speranza L. Biological effects of licochalcones. Mini Rev Med Chem. 2019;19(8):647–656. doi: 10.2174/1389557518666180601095420 [DOI] [PubMed] [Google Scholar]
- 8.Si L, Yan X, Hao W, et al. Licochalcone D induces apoptosis and inhibits migration and invasion in human melanoma A375 cells. Oncol Rep. 2018;39(5):2160–2170. doi: 10.3892/or.2018.6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai J, Hsiao P, Yang S, et al. Licochalcone A suppresses migration and invasion of human hepatocellular carcinoma cells through downregulation of MKK4/JNK via NF-κB mediated urokinase plasminogen activator expression. PLoS One. 2014;9(1):e86537. doi: 10.1371/journal.pone.0086537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue L, Zhang W, Fan Q, Wang L. Licochalcone A inhibits PI3K/Akt/mTOR signaling pathway activation and promotes autophagy in breast cancer cells. Oncol Lett. 2018;15(2):1869–1873. doi: 10.3892/ol.2017.7451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park M, Kim S, Cho I, et al. Licochalcone-A induces intrinsic and extrinsic apoptosis via ERK1/2 and p38 phosphorylation-mediated TRAIL expression in head and neck squamous carcinoma FaDu cells. Food Chem Toxicol. 2015;77:34–43. doi: 10.1016/j.fct.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho J, Chae J, Yoon G, et al. Licochalcone A, a natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma. Int J Oncol. 2014;45(2):667–674. doi: 10.3892/ijo.2014.2461 [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, He J, Xing X, et al. Mn12Ac inhibits the migration, invasion and epithelial-mesenchymal transition of lung cancer cells by downregulating the Wnt/β-catenin and PI3K/AKT signaling pathways. Oncol Lett. 2018;16(3):3943–3948. doi: 10.3892/ol.2018.9136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie J, Lin W, Huang L, et al. Bufalin suppresses the proliferation and metastasis of renal cell carcinoma by inhibiting the PI3K/Akt/mTOR signaling pathway. Oncol Lett. 2018;16(3):3867–3873. doi: 10.3892/ol.2018.9111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi M, Moon S, Lee S, et al. Adenosine induces intrinsic apoptosis via the PI3K/Akt/mTOR signaling pathway in human pharyngeal squamous carcinoma FaDu cells. Oncol Lett. 2018;15(5):6489–6496. doi: 10.3892/ol.2018.8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Ren X, Zhang L, Li Y, Cheng B, Xia J. Oridonin inhibits oral cancer growth and PI3K/Akt signaling pathway. Biomed Pharmacother. 2018;100:226–232. doi: 10.1016/j.biopha.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 17.Thomson P. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J Oral Pathol Med. 2018;47(9):803–807. doi: 10.1111/jop.12733 [DOI] [PubMed] [Google Scholar]
- 18.Messier C, Epifano F, Genovese S, Grenier D. Licorice and its potential beneficial effects in common oro-dental diseases. Oral Dis. 2012;18(1):32–39. doi: 10.1111/j.1601-0825.2011.01842.x [DOI] [PubMed] [Google Scholar]
- 19.Si H, Xu C, Zhang J, et al. Licochalcone A: an effective and low-toxicity compound against Toxoplasma gondii in vitro and in vivo. Int J Parasitol Drugs Drug Resist. 2018;8(2):238–245. doi: 10.1016/j.ijpddr.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Shin E, Park J, Kim Y, Park J. Antitumor and antimetastatic effects of licochalcone A in mouse models. J Mol Med. 2010;88(8):829–838. doi: 10.1007/s00109-010-0625-2 [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Park M, Lee S, et al. Licochalcone A induces apoptosis in KB human oral cancer cells via a caspase-dependent FasL signaling pathway. Oncol Rep. 2014;31(2):755–762. doi: 10.3892/or.2013.2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gkouveris I, Nikitakis N, Aseervatham J, Ogbureke K. The tumorigenic role of DSPP and its potential regulation of the unfolded protein response and ER stress in oral cancer cells. Int J Oncol. 2018;53(4):1743–1751. doi: 10.3892/ijo.2018.4484 [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Wu G, Chen R, et al. Licochalcone A attenuates glioma cell growth in vitro and in vivo through cell cycle arrest. Food Funct. 2018;9(8):4500–4507. doi: 10.1039/c8fo00728d [DOI] [PubMed] [Google Scholar]
- 24.Qiu C, Zhang T, Zhang W, et al. Licochalcone A inhibits the proliferation of human lung cancer cell lines A549 and H460 by inducing G2/M cell cycle arrest and ER stress. Int J Mol Sci. 2017;18(8):1761. doi: 10.3390/ijms18081761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorpe L, Yuzugullu H, Zhao J. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15(1):7–24. doi: 10.1038/nrc3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamidi A, Song J, Thakur N, et al. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci Signal. 2017;10(486):eaal4186. doi: 10.1126/scisignal.aal4186 [DOI] [PubMed] [Google Scholar]
- 27.Baek S, Ko J, Lee J, et al. Ginkgolic acid inhibits invasion and migration and TGF-β-induced EMT of lung cancer cells through PI3K/Akt/mTOR inactivation. J Cell Physiol. 2017;232(2):346–354. doi: 10.1002/jcp.25426 [DOI] [PubMed] [Google Scholar]
- 28.Qin L, Jia Z, Xie D, Liu Z. Knockdown of long noncoding RNA urothelial carcinoma-associated 1 inhibits cell viability, migration, and invasion by regulating microRNA-182 in gastric carcinoma. J Cell Biochem. 2018;119(12):10075–10086. doi: 10.1002/jcb.27344 [DOI] [PubMed] [Google Scholar]
- 29.Song N, Kim J, Park J, et al. Licochalcone A, a polyphenol present in licorice, suppresses UV-induced COX-2 expression by targeting PI3K, MEK1, and B-Raf. Int J Mol Sci. 2015;16(3):4453–4470. doi: 10.3390/ijms16034453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao W, Yuan X, Yu L, et al. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci Rep. 2015;5:10336. doi: 10.1038/srep10336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu H, Jiang T, Ren K, et al. RUNX2 plays an oncogenic role in esophageal carcinoma by activating the PI3K/AKT and ERK signaling pathways. Cell Physiol Biochem. 2018;49(1):217–225. doi: 10.1159/000492872 [DOI] [PubMed] [Google Scholar]
- 32.Zhao S, Wang L, Zhang C, et al. Inhibitor of growth 3 induces cell death by regulating cell proliferation, apoptosis and cell cycle arrest by blocking the PI3K/AKT pathway. Cancer Gene Ther. 2018;25:240–247. doi: 10.1038/s41417-018-0023-4 [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Deng X, Zhang J, et al. Elevated expression of zinc finger protein 703 promotes cell proliferation and metastasis through PI3K/AKT/GSK-3β signalling in oral squamous cell carcinoma. Cell Physiol Biochem. 2017;44(3):920–934. doi: 10.1159/000485360 [DOI] [PubMed] [Google Scholar]
- 34.Zhu L, Shen Y, Sun W. Paraoxonase 3 promotes cell proliferation and metastasis by PI3K/Akt in oral squamous cell carcinoma. Biomed Pharmacother. 2017;85:712–717. doi: 10.1016/j.biopha.2016.11.084 [DOI] [PubMed] [Google Scholar]
- 35.Ishida K, Tomita H, Nakashima T, et al. Current mouse models of oral squamous cell carcinoma: genetic and chemically induced models. Oral Oncol. 2017;73:16–20. doi: 10.1016/j.oraloncology.2017.07.028 [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Hu Z, Jin L, Wu L. Licochalcone A inhibits MMPs and ADAMTSs via the NF-κB and Wnt/β-catenin signaling pathways in rat chondrocytes. Cell Physiol Biochem. 2017;43(3):937–944. doi: 10.1159/000481645 [DOI] [PubMed] [Google Scholar]