Abstract
T cell transdifferentiation to functionally distinct subsets can play a key role in balancing the protective and pathogenic features of the T cell response. In a new study, Karmaus et al showed that mTORC1 activity influences metabolic heterogeneity within a T cell population to modulate transdifferentiation and disease pathogenesis in a setting of chronic inflammation-driven autoimmunity.
Upon recognizing antigen—be it foreign antigen during pathogen infection or self-antigen during autoimmunity—CD4+ T cells are activated to undergo clonal expansion, which increases the pool of antigen-specific cells, followed by lineage differentiation and memory formation(Parkin and Cohen, 2001). Differentiation dictates the functional characteristics of the activated effector T cell population, while memory formation generates a pool of long-lived cells with stem cell-like properties that can be rapidly mobilized upon a second encounter with antigen. CD4 T cells can differentiate into several lineages distinguished by distinct cytokine profiles and functional characteristics. For example, CD4+ T cells can differentiate into TH1 T cells that produce IFN, which acts on other immune cells to amplify the inflammatory response, or into TH17 T cells that produce the cytokine IL-17(Zhou et al., 2009). While a limited TH17 T cell response plays an essential role in clearing infections by fungal pathogens and extracellular bacteria, an uncontrolled TH17 response often leads to pathogenic inflammation and autoimmunity (Stockinger and Omenetti, 2017). Intriguingly, recent studies indicate that TH17 cells are unexpectedly plastic. In a mouse model of myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalitis (EAE), fate mapping studies indicate that TH17 cells can shut off production of their signature cytokine IL-17 and switch to production of IFN γ, which presumably drives the inflammatory response to promote disease pathogenesis(Hirota et al., 2011; Kurschus et al., 2010). How such transdifferentiation is controlled was not known, but proper regulation of TH17 transdifferentiation and plasticity is likely to be pivotal in balancing the protective and pathogenic features of the TH17 T cell response.
Karmaus et al (Karmaus et al., 2018) now demonstrate that in MOG-induced EAE, metabolic heterogeneity within the TH17 T cell population influences functional heterogeneity to impact lineage plasticity and disease pathogenesis. First, these TH17 cells can be distinguished by expression of CD27, a costimulatory molecule of the TNF receptor superfamily implicated in T cell activation and differentiation. CD27+ TH17 T cells express less IFNγ but higher levels of factors associated with T cell memory, while CD27- TH17 T cells expressed higher levels of IFNγ and factors associated with effector T cells. Importantly, using single cell fate mapping to unequivocally track expression of IL-17, the authors found that CD27+ TH17 T cells encountering antigen can proliferate and convert to CD27- TH17 T cells in vitro and in vivo, while CD27- TH17 T cells remained CD27-. Together, these findings defined the CD27+ population as a subset of TH17 T cells with memory-like features, capable of in vivo persistence and conversion to a CD27-, more terminally differentiated, effector-like CD27- population.
Intriguingly, transcriptome analysis suggested differences in the metabolism of CD27+ and CD27- TH17 T cells, with the CD27- population bearing increased expression of genes related to mTORC1 signaling, MYC signaling, cholesterol metabolism, and glycolysis. mTORC1 is a key metabolic sensing pathway that integrates metabolic cues and immunological signals to determine T cell fate and immunological outcome(Chapman and Chi, 2015), prompting the authors to ask about the role of Raptor, a defining subunit of the mTORC1 complex, in regulating TH17 conversion. Importantly, Raptor deficiency in TH17 T cells that have expressed IL-17 protected mice from CNS inflammation, T cell infiltration, and clinical manifestations in MOG-induced EAE. Such TH17 T cells were defective in expression of T-bet a transcriptional “master regulator” of TH1 differentation, and of IFNγ (a transcriptional target of T-bet), leading to a loss of the transdifferentiated IL-17-IFN γ + population. Together these findings demonstrate that mTORC1 regulates transdifferentiation of TH17 T cells to a IFN γ +, TH1-like subset that mediates inflammation and pathogenesis in EAE.
Mechanistically, Raptor-deficient TH17 T cells downregulated expression of cholesterol biosynthesis pathways and MYC signaling. Deletion of MYC or of HMGCR (a rate-limiting enzyme for cholesterol biosynthesis) in TH17 T cells that have expressed IL-17 was sufficient to impair transdifferentiation, similar to Raptor deficiency, implicating the respective metabolic pathways in modulating transdifferentiation. Furthermore, Raptor deficiency perturbed reciprocal expression of T-bet and TCF-1, a transcription factor associated with memory, leading to increased levels of a TCF-1hiT-betlo subset. Finally, Raptor deletion impaired the CD27+ to CD27- conversion, leading to maintenance of the CD27+ subset, during in vitro MOG stimulation and experimental EAE.
Together, this provocative and important study showed that mTORC1 activity orchestrates transdifferentiation of TH17 T cells from a CD27+, memory-like population to a CD27-, terminally differentiated, IFN-γ producing population that mediates disease in EAE (Figure 1). Several questions arise from the study. First, does mTORC1 activity regulate TH17 transdifferentiation in other settings of chronic inflammation? In addition to TH1 T cells, TH17 cells can transdifferentiate into other lineages such as the immunosuppressive Treg lineage (Gagliani et al., 2015) —does mTORC1 activity or metabolism influence such transdifferentiation?
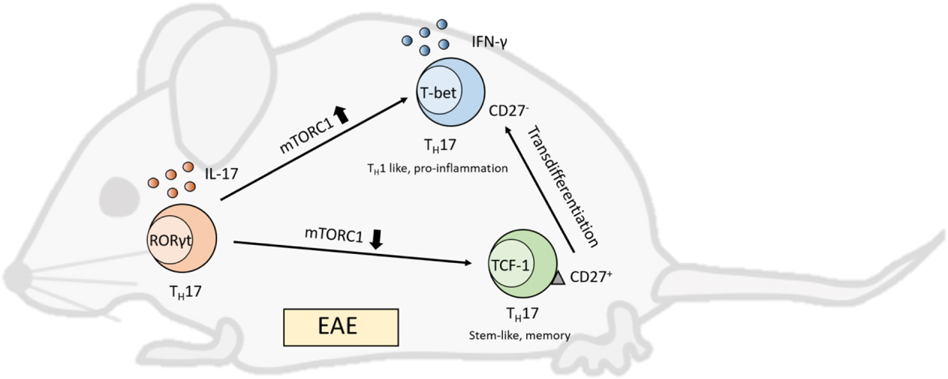
Figure 1. mTOR controls TH17 transdifferentiation.
Karmaus et.al find that TH17 cells in a mouse model of experimental autoimmune encephalitis (EAE) are heterogeneous in mTORC1 activity and function. High mTORC1 activity is associated with lack of CD27 expression and production of IFN-γ in a TH1-like, pro-inflammatory and pathogenic subset, while low mTORC1 activity maintains CD27 expression and production of IL-17 in a stem- and memory-like subset. The memory-like subset with low mTORC1 activity persists in vivo and has the capacity to transdifferentiate into the TH1-like subset with high mTORC1 activity in the chronic inflammatory environment of EAE.
Second, CD27 expression appears to distinguish TH17 cells with memory versus effector characteristics, but how CD27 heterogeneity arises within the TH17population is unclear, as well as its role (if any) in regulating transdifferentiation. The role of CD27 vis-à-vis mTORC1 activity in these processes is also outstanding. Could mTORC1 activity modulate CD27 expression, and does CD27 signaling influence mTORC1 activity? In this regard, recent studies implicating interactions between TCR signaling and mTORC1 signaling in coordinating the effector versus memory cell fate during asymmetric T cell division seem to offer an interesting parallel (Pollizzi et al., 2016; Verbist et al., 2016). These studies suggested that enhanced ligation of the T cell receptor and associated coreceptors increases mTORC1 activity in the daughter cell proximal to the antigen-bearing dendritic cell, which guides metabolic changes that instructs differentiation to an effector phenotype, while decreased mTORC1 activity in the distal daughter cell coordinates metabolic changes that influences differentiation to a memory phenotype. One can speculate that a similar reciprocal relationship between CD27 signaling and mTORC1 signaling influences TH17 transdifferentiation in EAE and other settings of chronic inflammation.
Finally, because mTORC1 is a nutrient sensing pathway, it would be interesting to determine whether mTORC1 integrates metabolic cues to modulate transdifferentiation. Do metabolic signals coordinate with TCR signaling to influence mTORC1 activity and transdifferentiation? Can amino acid levels and composition in the diet impinge on mTOR to influence TH17 transdifferentiation and thus intiation and/or progression of autoimmune diseases? We look forward to the elucidation of these and other questions as the field continues to probe the role of mTORC1 in regulating T cell biology.
Acknowledgements
T.H. was supported by R01AI102964–05 from National Institute of Health. R.W. was supported by 1UO1CA232488–01 and 1R01AI114581 from National Institute of Health, and 128436-RSG-15-180-01-LIB from the American Cancer Society.
Footnotes
Declaration of Interests
The authors declare no competing interests
References
- Chapman NM, and Chi H.J.F.i.i. (2015). mTOR links environmental signals to T cell fate decisions. 5, 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Vesely MCA, Iseppon A, Brockmann L, Xu H, Palm NW, De Zoete MR, Licona-Limón P, Paiva RS, and Ching TJN (2015). T H 17 cells transdifferentiate into regulatory T cells during resolution of inflammation. 523, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, and Menzel U.J.N.i. (2011). Fate mapping of IL-17-producing T cells in inflammatory responses. 12, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmaus PW, Chen X, Lim SA, Herrada AA, Nguyen T-LM, Xu B, Dhungana Y, Rankin S, Chen W, and Rosencrance CJN (2018). Metabolic heterogeneity underlies reciprocal fates of TH 17 cell stemness and plasticity. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurschus FC, Croxford AL, Heinen P, A., Wörtge S, Ielo D, and Waisman A.J.E.j.o.i. (2010). Genetic proof for the transient nature of the Th17 phenotype. 40, 3336–3346. [DOI] [PubMed] [Google Scholar]
- Parkin J, and Cohen BJTL (2001). An overview of the immune system. 357, 1777–1789. [DOI] [PubMed] [Google Scholar]
- Pollizzi KN, Sun I-H, Patel CH, Lo Y-C, Oh M-H, Waickman AT, Tam AJ, Blosser RL, Wen J, and Delgoffe G.M.J.N.i. (2016). Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8+ T cell differentiation. 17, 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, and Omenetti SJNRI (2017). The dichotomous nature of T helper 17 cells. 17, 535. [DOI] [PubMed] [Google Scholar]
- Verbist KC, Guy CS, Milasta S, Liedmann S, Kamiński MM, Wang R, and Green DRJN (2016). Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. 532, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chong MM, and Littman DRJI (2009). Plasticity of CD4+ T cell lineage differentiation. 30, 646–655. [DOI] [PubMed] [Google Scholar]