Abstract
Background and objectives: Vitexin is a natural flavonoid glycoside mainly extracted from the leaves of vitex, which has a variety of physiological activities. For example, vitexin has antitumor and anti-inflammation activities, and it can also promote blood circulation in the body. However, the function and mechanism of vitexin in nasopharyngeal carcinoma (NPC) are still unclear.
Materials and methods: Cell Counting Kit-8 assay and cell cycle analysis were performed to examine cell survival in response to vitexin. Immunoblotting was used to analyze relative proteins’ expression. NPC xenograft models were established to assess the effect of vitexin in vivo. The luciferase activity of pNFκB-Luc was analyzed by using Dual-Luciferase Reporter Assay System. Quantitative real-time polymerase chain reaction was performed to detect relative genes’ expression. Kinase activity of IKKβ was analyzed in a cell-free system.
Results: In this study, vitexin was found to display significant antitumor activity in NPC in vitro and in vivo. In NPC cells, vitexin inhibited cell cycle progression in NPC cells and induced the cleavages of PARP and inhibited antiapoptotic proteins’ expression, including Bcl-2 and Mcl1. Further studies indicated that vitexin significantly suppressed the luciferase activity of pNF-κB-Luc and inhibited the activation of NF-κB key regulators, including p65, IκBα and IKKs in NPC cells. Moreover, the kinase activity of IKKβ could be suppressed by vitexin in a cell-free system, and overexpression of CA-IKKβ could attenuate the inhibitory effect of vitexin on p65 phosphorylation.
Conclusion: These results indicated that vitexin displayed antitumor activity by suppressing NF-κB signaling in NPC, which suggested that vitexin could be as a potential drug for the treatment of NPC in the future.
Keywords: vitexin, NF-κB, nasopharyngeal carcinoma, cell apoptosis, target
Introduction
Nasopharyngeal carcinoma (NPC) is a rare type of head and neck malignancies, and it arises from the epithelium of the nasopharynx.1 Both of the environmental and genetic risk factors can promote the tumorigenesis of NPC, such as occupational exposure to formaldehyde, Epstein–Barr virus infection, cigarette smoking and other dietary factors or genetic polymorphisms.2 The occurrence of NPC is relatively common in southern China and southeast Asia with an incidence rate of 15/100,000–50/100,000, but it is not prevalent in Europe and the USA.3 Nowadays, radiotherapy (RT) is the primary treatment for NPC, and the five-year overall survival rate of the early-stage NPC is about 90% after treating by RT alone, but about 70% NPC patients were at the locally advanced stage and the five-year survival treated by RT alone is very poor, which prompts researchers to add chemotherapy or targeted therapy to improve the efficacy of RT on NPC.4 Although great progress has been done, a truly effective chemotherapy regimen for NPC has not been identified. These clinical observations prompted us to investigate a potential drug as an alternative option for NPC treatment.
A recent paper reported that ovatodiolide significantly inhibited the viability of NPC cells, attenuated NPC stem cell tumorigenicity, suppressed tumor growth and enhanced the sensitivity of NPC cells to cisplatin treatment by dysregulating JAK/STAT signaling pathway, which could be as a putative inhibitor of JAK2 and STAT3 for future NPC treatment.5 Another paper reported that Polyphyllin I (PP I) extracted from natural herb Paris polyphylla suppressed tumor growth and induced apoptosis of NPC in vitro and in vivo by downregulating lncRNA-ROR, subsequently upregulating p53 signaling.6 In this study, vitexin, a natural flavonoid glycoside, was found to display effective anti-NPC activity both in vitro and in vivo. And further studies showed that vitexin significantly inhibited p65 activation by suppressing IKK phosphorylation in NPC cells, which indicated that vitexin could be as a novel NF-κB inhibitor. These results showed that vitexin could be purposed as the chemotherapeutics for the treatment of NPC in clinic.
Materials and methods
Cell culture and reagents
NPC cells CNE1, CNE2, HK1 and HNE1 were cultured in high-glucose DMEM supplemented with 10% fetal bovine serum, and 1% penicillin/streptomycin (Beyotime, Nantong, China) in 5% CO2 at 37°C. All NPC cells were kindly provided from the Cancer Hospital of Shanghai Fudan University (Shanghai, China). The use of the NPC cell lines was approved by the institutional review board and ethics committee of Suzhou Municipal Hospital, the Affiliated Suzhou Hospital of Nanjing Medical University. Vitexin (VTX) was purchased from Sigma-Aldrich (St Louis, MO, USA).
Cell viability
NPC cells were seeded in 96-well plates at a density of 4000 cells/100 μL and cultured overnight at 37°C with 5% CO2. Then, NPC cells were incubated with different concentrations of vitexin (0, 5, 10, or 20 μM) for 24 hrs in the 96-well plates. Cell survival was examined by Cell Counting Kit-8 (CCK-8) assay according to the manufacturer’s instructions (Biotool, Houston, USA).
Cell cycle analysis
NPC cells CNE1 and HK1 were seeded in 6-well plates at a density of 500,000 cells/mL and cultured overnight at 37°C with 5% CO2. Then, NPC cells were treated with vehicle or 10 μM vitexin for 24 hrs before cell cycle analysis. Subsequently, NPC cells were fixed with 70% cold ethanol overnight and washed with cold PBS, followed by being resuspended in 100 μL PBS containing 100 μg/mL RnaseA (Beyotime, Nantong, China) for 30 mins at 37°C. Then, NPC cells were washed with cold PBS and incubated with propidium iodide for 5 mins at room temperature. Finally, the cell cycle was analyzed on a flow cytometer (Attune® NxT; Life Technologies).
Immunoblotting
Immunoblotting was performed as described previously.7 Equal amounts of total proteins (30 μg) were subjected to SDS-PAGE, transferred onto PVDF membrane, and immunoblotted with specific antibodies. The primary antibodies against PARP, Bcl-2, Mcl1, phospho-p65 (p-p65), p65, p-IκBα, IκBα, p-IKK, IKKα and IKKβ were purchased from Cell Signaling Technology (Danvers, MA). Anti-Flag tag and GAPDH antibodies were purchased from Sigma-Aldrich (St Louis, MO, USA).
Xenograft studies
The human NPC cells CNE1 (8×106 cells/site) were injected subcutaneously in the right flanks of female nude mice purchased from Shanghai Slac Laboratory Animal Co. Ltd., Shanghai, China. Mice were randomly divided into two groups when tumors became palpable. One group was orally received vitexin (30 mg/kg) in PBS containing 10% Tween 80 and 10% DMSO daily for continuous two weeks, and the other was given vehicle. Tumor volumes were monitored by using vernier caliper every other day. This animal study was approved by the Review Board of Animal Care and Use of Suzhou Municipal Hospital, the Affiliated Suzhou Hospital of Nanjing Medical University.
Luciferase assay
CNE1 cells were seeded in 6-well plates at a density of 500,000 cells/mL and cultured overnight at 37°C with 5% CO2. Subsequently, the luciferase reporters driven by NF-κB response elements (pNFκB-LUC) or empty vector along with the internal control renilla plasmids were transfected into CNE1 cells by Lipofectamine® 2000 (Invitrogen) according to the manufacturer’s instruction. Twenty-four hours later, cells were incubated with increasing concentrations of vitexin overnight, and then cells were harvested and lysed for luciferase assay by using Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA) as described previously.8
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted by using the TRIzol® Reagent (Takara, Japan) according to the manufacturer’s instructions. Then, cDNA was synthesized from equal quantities of total RNA by using the PrimeScriptTM RT reagent Kit with gDNA Eraser (Takara, Japan). To determine the mRNA levels of Cyclin D1 and Bcl-2, qRT-PCR was performed by using SYBR Green qPCR Master Mix (Roche, Basel, Switzerland) as described previously.9 The primers used were as follows: Cyclin D1, forward 5ʹ-GCGAGGAACAGAAGTGCG-3ʹ and reverse 5ʹ-TGGAGTTGTCGGTGTAGATGC-3ʹ; Bcl-2, forward 5ʹ-CTGGGAGAACAGGGTACGATAA-3ʹ and reverse 5ʹ-CGGGCTGGGAGGAGAAGAT-3ʹ; GAPDH, forward 5ʹ-GCACCGTCAAGGCTGAGAAC-3ʹ and reverse 5ʹ-TGGTGAAGACGCCAGTGGA-3ʹ.
Kinase activity in cell-free assay
Kinase activities in the presence of different concentrations of vitexin were examined by using the Kinase-Glo Luminescent Kinase Assay Kit (Promega, Madison, WI, USA) according to the manufacturer’s instruction as described previously.10
Plasmids construction and gene transfection
The human CA-IKKβ (constitutively activated IKKβ) gene was amplified by PCR and subcloned into pcDNA3.1 plasmids with a Flag tag as previously described.11 The pNFκB-LUC plasmid was purchased from Beyotime Biotechnology, Inc. (Nantong, China). Plasmids were transiently transfected into CNE1 cells by Lipofectamine® 2000 (Invitrogen) according to the manufacturer’s instruction.
Statistical analysis
Data was recorded as the mean ± SD of at least three independent experiments. All the experiments were repeated three times. The Student’s t-test was used for comparison of two groups in the experiments, and a p-value <0.05 was supposed to be statistically significant.
Results
Vitexin inhibited cell growth by inducing cell apoptosis in MPC
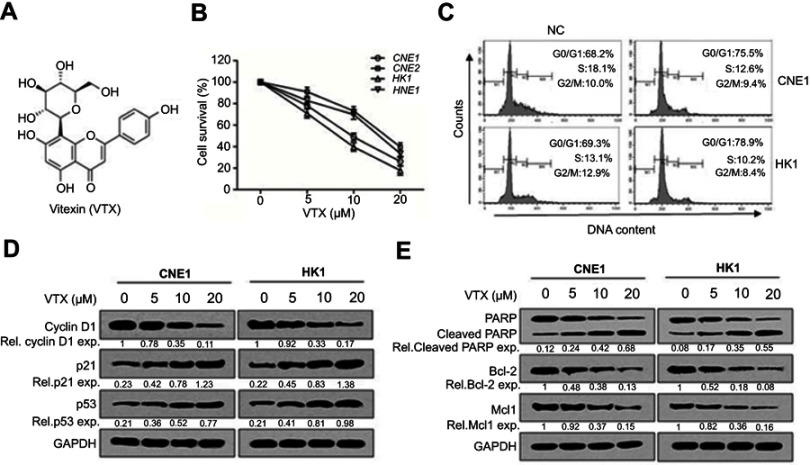
To assess the antitumor effect of vitexin on NPC cells, CCK-8 assay was performed. As shown in Figure 1A and B, four NPC cell lines were collected and incubated with increasing concentrations of vitexin for 24 hrs, and CCK-8 assay showed that vitexin significantly inhibited NPC cell survival. Subsequently, cell survival was also assessed by cell cycle analysis. As shown in Figure 1C, vitexin significantly induced cell cycle arrest at G0/G1 phase in CNE1 and HK1 cells. And vitexin also inhibited the expression levels of cell cycle-associated proteins, including Cyclin D1, and upregulated p21 and p53 expression in NPC cells (Figure 1D). Finally, the status of caspase substrate PARP was assessed by immunoblotting. As shown in Figure 1E, the cleavages of PARP could be also induced by vitexin dose-dependently in CNE1 and HK1 cells, and the antiapoptotic proteins Bcl-2 and Mcl1 were also downregulated by vitexin dose-dependently. These results indicated that vitexin inhibited NPC cell survival by inducing cell apoptosis in vitro.
Figure 1.
Vitexin inhibited cell growth by inducing cell apoptosis in nasopharyngeal carcinoma. (A) The chemical structure of vitexin (VTX). (B) Four NPC cell lines were incubated with indicated concentrations of vitexin for 24 hrs, followed by CCK-8 assay. (C) CNE1 and HK1 cells were treated with vehicle (NC) or 10 μM vitexin for 24 hrs, followed by PI staining and analysis on a flow cytometer. (D) CNE1 and HK1 cells were treated with 0, 5, 10 or 20 μM vitexin for 24 hrs, followed by immunoblotting against Cyclin D1, p21 and p53. GAPDH was used as an internal control. (E) CNE1 and HK1 cells were treated with increasing concentrations of vitexin for 24 hrs, followed by immunoblotting against PARP, Bcl-2, Mcl1 and GAPDH.
Vitexin inhibited NPC cell growth in vivo
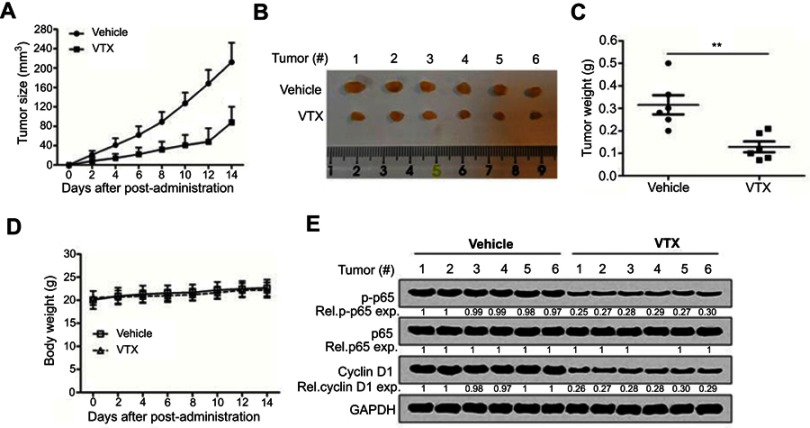
To evaluate the antitumor activity of vitexin in vivo, a NPC cell line-derived xenograft model was established. As shown in Figure 2A, vitexin significantly suppressed the tumor growth at the beginning of the 4th day. At the end of the experiment, tumors were excised and taken photos, and the tumors in vitexin-treated group were obviously smaller than the control (Figure 2B). And the tumor weight of vitexin-treated group was also lighter than the control (Figure 2C). Interestingly, the body weights of the mice were not changed during the process of drug administration (Figure 2D). Finally, the excised tumors were lysed for immunoblotting, and the results showed that the phosphorylated p65 and Cyclin D1 were significantly downregulated in vitexin-treated group, which suggested that vitexin may inhibit NF-κB signaling in NPC (Figure 2E).
Figure 2.
Vitexin inhibited nasopharyngeal carcinoma cell growth in vivo. (A) NPC cells CNE1 (8×106) were inoculated subcutaneously into nude mice. When tumors were palpable, mice were randomly divided into two groups (n=6/group). One group was orally administrated with vitexin (30 mg/kg) daily for continuous 2 weeks, and the other was given vehicle as a control group. Tumor volumes were monitored every other day. (B) At the end of the experiment, tumors were excised and taken photos. (C) Tumor weight was measured. (D) Body weight was also measured during the process. **p<0.01. (E) The excised tumors were lysed for immunoblotting against p-p65, p65 and Cyclin D1. GAPDH was used as an internal control.
Vitexin suppressed NF-κB signaling in NPCcells
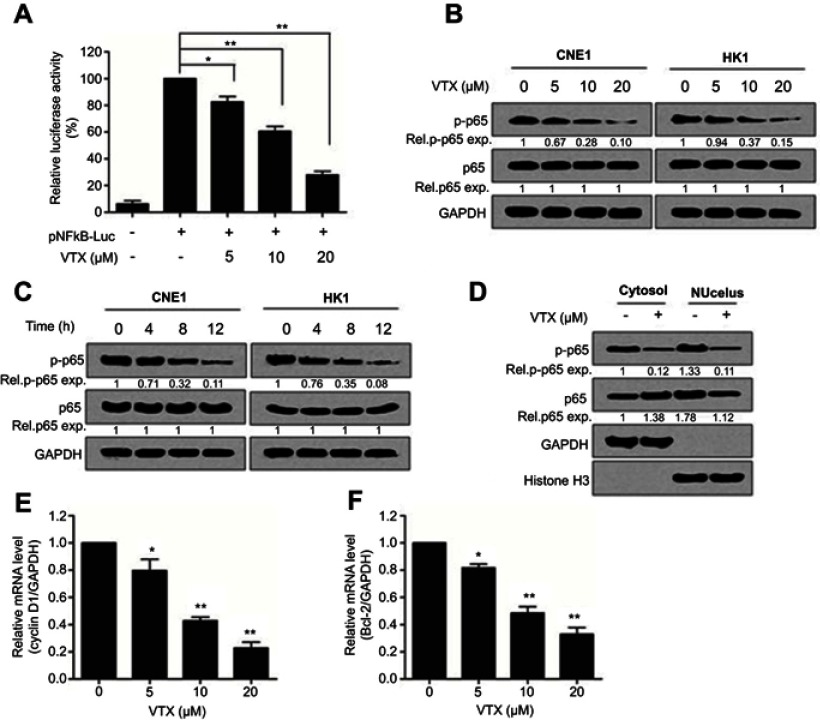
Further studies showed that vitexin significantly suppressed the luciferase activity of pNF-κB-Luc, which further suggested that vitexin may inhibit NF-κB signaling (Figure 3A). To elucidate it, two NPC cells were treated with increasing concentrations of vitexin for 24 hrs, and immunoblotting revealed that the phosphorylation of NF-κB p65 could be obviously suppressed by vitexin in a dose-dependent manner (Figure 3B). Moreover, the phosphorylation of p65 could be also inhibited by vitexin in a time-dependent manner in CNE1 and HK1 cells (Figure 3C). In addition, vitexin could affect the nuclear translocation of NF-κB p65 in NPC cells (Figure 3D). At the same time, the target genes of NF-κB signaling pathway, including Cyclin D1 and Bcl-2, were also downregulated after treated with vitexin (Figure 3E and F). Above studies demonstrated that vitexin inhibited NF-κB signaling in NPC cells.
Figure 3.
Vitexin suppressed NF-κB signaling in nasopharyngeal carcinoma cells. (A) The luciferase reporters driven by NF-κB response elements (pNFκB-LUC) were transfected into CNE1 cells for 24 hrs, and then cells were incubated with vitexin overnight. Luciferase activity was analyzed by using Dual-Luciferase Reporter Assay System. (B) CNE1 and HK1 cells were treated with increasing concentrations of vitexin for 24 hrs, followed by immunoblotting against p-p65, p65 and GAPDH. (C) CNE1 and HK1 cells were treated with 20 μM vitexin for indicated time, followed by immunoblotting against p-p65, p65 and GAPDH. (D) CNE1 cells were treated with 10 μM vitexin. Twenty-four hours later, cells were lysed for cytoplasmic and nuclear protein isolation, followed by immunoblotting against p-p65, p65, GAPDH and Histone H3. (E and F) CNE1 cells were treated with indicated concentrations of vitexin. Twenty-four hours later, cells were prepared for qRT-PCR against Cyclin D1 (E) and Bcl-2 (F). GAPDH was used as an internal control. *p<0.05; **p<0.01.
Vitexin inhibits IKK activation in NPC
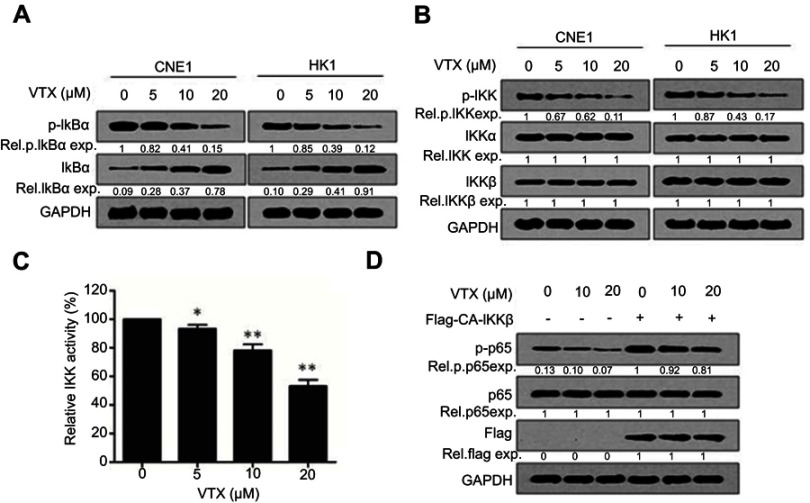
To further elucidate how vitexin downregulated p65 activation, the statuses of upstream kinases were evaluated. As shown in Figure 4A, the phosphorylation of IκBα, an inhibitor of NF-κB, was downregulated by vitexin, along with the upregulation of total IκBα in CNE1 and HK1 cells. Furthermore, the phosphorylation of IKK, the kinase of IκB, was also suppressed by vitexin, but the total proteins of IKKα and IKKβ were not changed (Figure 4B). And the in vitro kinase assay showed that the kinase activity of IKKβ was also suppressed by vitexin in a cell-free system (Figure 4C). To further assess it, the constitutively activated IKKβ (CA-IKKβ) plasmid was constructed, and we found that overexpression of CA-IKKβ significantly attenuated the inhibitory effect of vitexin on NF-κB p65 phosphorylation (Figure 4D). These results demonstrated that vitexin inhibited NF-κB signaling by suppressing IKK activation.
Figure 4.
Vitexin inhibits IKK activation in nasopharyngeal carcinoma. (A) CNE1 and HK1 cells were treated with increasing concentrations of vitexin for 24 hrs, followed by immunoblotting against p-IκBα, IκBα and GAPDH. (B) CNE1 and HK1 cells were treated with increasing concentrations of vitexin for 24 hrs, followed by immunoblotting against p-IKK, IKKα, IKKβ and GAPDH. (C) Increasing concentrations of vitexin were incubated with the recombinant IKKβ, and kinase activity of IKKβ was then analyzed in a cell-free system. *p<0.05; **p<0.01. (D) CNE1 cells were transfected with empty vector or Flag-CA-IKKβ for 24 hrs, followed by the treatment of increasing concentrations of vitexin for 24 hrs. Then, cells were prepared for immunoblotting against p-p65, p65, Flag and GAPDH.
Discussion
The secondary metabolites of plants have been revealed to possess positive effects on many malignancies, and numerous plant extracts have been assessed for possible application in cancer therapy.12 In this study, vitexin, a natural flavonoid glycoside, was shown to display significant antitumor activity in NPC both in vitro and in vivo. Interestingly, vitexin has been used as a traditional Chinese medicine for the treatment of a variety of diseases for a long year,13 and previous studies have also shown that vitexin has effective antitumor roles in some other tumors, including colorectal cancer,14 leukemia,15 glioblastoma,16 bladder cancer17 and breast cancer,18 which further supported our research that vitexin had significant antitumor activity. Additionally, vitexin was reported to participate in the main events involved in the process of neurotoxicity.19 Vitexin enhanced the production of neuroprotective factors and reduced neurodegeneration, such as abnormal protein aggregation, neuroinflammation, redox imbalance, etc.19
NF-κB signaling is overexpressed or overactivated in many cancers and promotes tumorigenesis.20 It is also involved in the drug resistance in the chemotherapy of many cancers.21 Therefore, targeting NF-κB signaling has been an effective strategy for antitumor drug discovery.22 In mamalian cells, NF-κB signaling is strictly regulated by several important steps, including extracellular stimuli, IKKs activation, IκBα phosphorylation and degradation, NF-κB nuclear translocation, DNA binding and transactivation, which can be all used as the targets for the development of NF-κB inhibitors.23 For example, bortezomib could suppress the degradation of IκBα and thus inhibited NF-κB activation.24 Another paper showed that the antimalarial drug mefloquine could significantly inhibit NF-κB signaling by suppressing IKK activation and induced cell apoptosis in colorectal cancer.25 In this study, vitexin was also found to suppress NF-κB signaling by inhibiting IKK activation in NPC cells. And many inhibitors have been also reported to focus on IKK complex because of the critical roles of IKKs as the integrator of many signals that regulate NF-κB.26
In addition to affect NF-κB signaling, vitexin was also reported to be involved in regulating several other pathways in tumors. For example, vitexin was reported to induce cell apoptosis by suppressing PI3K/AKT/mTOR signaling in human non-small cell lung cancer A549 cells.27 And another paper also showed that vitexin-induced antitumor effect was exerted by proapoptotic process, and it was mediated by a decreased Bcl-2/Bax ratio and activation of caspases in breast, prostate, liver, and cervical cancers.28 These studies provide substantial evidences to support the scientific exploration of vitexin in tumors, but the specific mechanism of vitexin in tumors is still unclear.
Therefore, this study showed that vitexin could be a novel NF-κB inhibitor by suppressing IKK activation and displayed significant antitumor activity in NPC. Vitexin could be further developed as a potential drug for the treatment of NPC in the future.
Conclusion
In conclusion, this paper demonstrated that the natural flavonoid glycoside vitexin exerted its antitumor activity by suppressing NF-κB signalling in NPC cells. This study suggests that vitexin could be further used for NPC treatment in clinic in the future.
Acknowledgment
This study was supported by the funding of the Natural Science Foundation of Jiangsu Province (BK20141177).
Animal ethics
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Akbas U, Koksal C, Kesen ND, et al. Nasopharyngeal carcinoma radiotherapy with hybrid technique. Med Dosim. 2018. pii: S0958-3947(18)30106-7. doi: 10.1016/j.meddos.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya T, Babu GKainickal CT. Current role of chemotherapy in nonmetastatic nasopharyngeal cancer. J Oncol. 2018;2018:3725837. doi: 10.1155/2018/3725837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Chen QY, Liu H, et al. Emerging treatment options for nasopharyngeal carcinoma. Drug Des Devel Ther. 2013;7:37–52. doi: 10.2147/DDDT.S30753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu SC, Huang CM, Bamodu OA, et al. Ovatodiolide suppresses nasopharyngeal cancer by targeting stem cell-like population, inducing apoptosis, inhibiting EMT and dysregulating JAK/STAT signaling pathway. Phytomedicine. 2018;56:269–278. doi: 10.1016/j.phymed.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 6.Hong F, Jiang J, Liu X, et al. Anticancer activity of Polyphyllin I in nasopharyngeal carcinoma by modulation of lncRNA ROR and P53 signaling. J Drug Target;2019. 1–22. doi: 10.1080/1061186X.2018.1561887 [DOI] [PubMed] [Google Scholar]
- 7.Han K, Xu X, Chen G, et al. Identification of a promising PI3K inhibitor for the treatment of multiple myeloma through the structural optimization. J Hematol Oncol. 2014;7:9. doi: 10.1186/1756-8722-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Han K, Zhu J, et al. An inhibitor of cholesterol absorption displays anti-myeloma activity by targeting the JAK2-STAT3 signaling pathway. Oncotarget. 2016;7(46):75539–75550. doi: 10.18632/oncotarget.12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Han K, Tang X, et al. The ring finger protein RNF6 induces leukemia cell proliferation as a direct target of pre-B-cell leukemia homeobox 1. J Biol Chem. 2016;291(18):9617–9628. doi: 10.1074/jbc.M115.701979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato A, Kudo C, Yamakoshi H, et al. Curcumin analog GO-Y030 is a novel inhibitor of IKKbeta that suppresses NF-kappaB signaling and induces apoptosis. Cancer Sci. 2011;102(5):1045–1051. doi: 10.1111/j.1349-7006.2011.01886.x [DOI] [PubMed] [Google Scholar]
- 11.Delhase M, Hayakawa M, Chen Y, et al. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284(5412):309–313. [DOI] [PubMed] [Google Scholar]
- 12.Ganesan K, Xu B. Molecular targets of vitexin and isovitexin in cancer therapy: a critical review. Ann N Y Acad Sci. 2017;1401(1):102–113. doi: 10.1111/nyas.13446 [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Zu Y, Liu W, et al. Preparative separation of vitexin and isovitexin from pigeonpea extracts with macroporous resins. J Chromatogr A. 2007;1139(2):206–213. doi: 10.1016/j.chroma.2006.11.015 [DOI] [PubMed] [Google Scholar]
- 14.Bhardwaj M, Cho HJ, Paul S, et al. Vitexin induces apoptosis by suppressing autophagy in multi-drug resistant colorectal cancer cells. Oncotarget. 2018;9(3):3278–3291. doi: 10.18632/oncotarget.22890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling T, Lang W, Feng X, et al. Novel vitexin-inspired scaffold against leukemia. Eur J Med Chem. 2018;146:501–510. doi: 10.1016/j.ejmech.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Li D, Chen H, et al. Vitexin induces G2/Mphase arrest and apoptosis via Akt/mTOR signaling pathway in human glioblastoma cells. Mol Med Rep. 2018;17(3):4599–4604. doi: 10.3892/mmr.2018.8394 [DOI] [PubMed] [Google Scholar]
- 17.Scarpa ES, Emanuelli M, Frati A, et al. Betacyanins enhance vitexin-2-O-xyloside mediated inhibition of proliferation of T24 bladder cancer cells. Food Funct. 2016;7(12):4772–4780. doi: 10.1039/c6fo01130f [DOI] [PubMed] [Google Scholar]
- 18.Epstein Shochet G, Drucker L, Pasmanik-Chor M, et al. First trimester human placental factors induce breast cancer cell autophagy. Breast Cancer Res Treat. 2015;149(3):645–654. doi: 10.1007/s10549-015-3266-x [DOI] [PubMed] [Google Scholar]
- 19.Lima LKF, Pereira SKS, Junior RDSS, et al. A brief review on the neuroprotective mechanisms of vitexin. Biomed Res Int. 2018;2018:4785089. doi: 10.1155/2018/4785089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YH, Kim JH, Kim BG, et al. Tauroursodeoxycholic acid attenuates colitis-associated colon cancer by inhibiting nuclear factor kappaB signaling. J Gastroenterol Hepatol. 2019;34(3):544–551. doi: 10.1111/jgh.14526 [DOI] [PubMed] [Google Scholar]
- 21.Yu C, Chen S, Guo Y, et al. Oncogenic TRIM31 confers gemcitabine resistance in pancreatic cancer via activating the NF-κB signaling pathway. Theranostics. 2018;8:3224–3236. doi: 10.7150/thno.23259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dantonio PM, Klein MO, Freire M, et al. Exploring major signaling cascades in melanomagenesis: a rationale route for targetted skin cancer therapy. Biosci Rep. 2018;38:5. doi: 10.1042/BSR20180511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G, Han K, Xu X, et al. An anti-leishmanial thiadiazine agent induces multiple myeloma cell apoptosis by suppressing the nuclear factor kappaB signalling pathway. Br J Cancer. 2014;110(1):63–70. doi: 10.1038/bjc.2013.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray RZ, Norbury C. Proteasome inhibitors as anti-cancer agents. Anticancer Drugs. 2000;11(6):407–417. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Wang J, Han K, et al. Antimalarial drug mefloquine inhibits nuclear factor kappa B signaling and induces apoptosis in colorectal cancer cells. Cancer Sci. 2018;109(4):1220–1229. doi: 10.1111/cas.13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DF, Hung MC. Advances in targeting IKK and IKK-related kinases for cancer therapy. Clin Cancer Res. 2008;14(18):5656–5662. doi: 10.1158/1078-0432.CCR-08-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Jiang Q, Liu H, et al. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol Res. 2019;52:7. doi: 10.1186/s40659-019-0214-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Liu YE, Cao J, et al. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin Cancer Res. 2009;15:5161–5169. doi: 10.1158/1078-0432.CCR-09-0661 [DOI] [PMC free article] [PubMed] [Google Scholar]