Abstract
Feasibility of endoscopic retrograde cholangiopancreatography (ERCP) for biliary drainage is not always applicable due to anatomical alterations or to inability to access the papilla. Percutaneous transhepatic biliary drainage has always been considered the only alternative for this indication. However, endoscopic ultrasonography-guided biliary drainage represents a valid option to replace percutaneous transhepatic biliary drainage when ERCP fails. According to the access site to the biliary tree, two kinds of approaches may be described: the intrahepatic and the extrahepatic. Endoscopic ultrasonography-guided rendez-vous transpapillary drainage is performed where the second portion of the duodenum is easily reached but conventional ERCP fails. The recent introduction of self-expandable metal stents and lumen-apposing metal stents has improved this field. However, the role of the latter is still controversial. Echoendoscopic transmural biliary drainage can be challenging with potential severe adverse events. Therefore, trained endoscopists, in both ERCP and endoscopic ultrasonography are needed with surgical and radiological backup.
Keywords: Endoscopic ultrasonography-guided biliary drainage, EUS, Percutaneous transhepatic biliary drainage, Endoscopic ultrasonography-guided hepatogastric anastomosis, Endoscopic ultrasonography-guided antegrade stent placement, Endoscopic ultrasonography-guided choledochoduodenostomy, Endoscopic ultrasonography-guided transgallbladder, Endoscopic ultrasonography-guided rendezvous
Core tip: Feasibility of endoscopic retrograde cholangiopancreatography for biliary drainage is not always applicable due to anatomical alterations or to inability to access the papilla. Percutaneous transhepatic biliary drainage has always been considered the only alternative for this indication. Endoscopic ultrasonography-guided biliary drainage represents a valid option to replace the other two methods.
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) with stent placement represents standard treatment for the management of benign and malignant biliary obstructions. Approximately 500000 ERCPs are performed annually in the United States alone with a failure rate that varies between 5% and 7%[1]. ERCP-guided biliary drainage is performed by direct cannulation of the papilla under endoscopic vision via the duodenoscope with the assistance of radiological cholangiography. Once the biliary tract has been reached, the biliary drainage can be obtained with different devices and technique (with stent positioning) depending on the underlying disease. In light of this, the papilla must necessarily be endoscopically reachable. Therefore, reasons for failure depend mainly on whether the papilla is endoscopically accessible or not. In the first case, ampullary pathology, periampullary diverticulum, and ampullary neoplastic infiltration can cause failure. In the second case, benign (peptic stenosis) and malignant duodenal stenosis or postsurgical anatomy such as a gastrointestinal bariatric bypass, a Roux-en Y gastric bypass or a Billroth II gastroenterostomy may prevent the access to the papilla causing unsuccessful procedures (Table 1).
Table 1.
Current indications for endoscopic ultrasonography-guided biliary drainage after failure of endoscopic retrograde cholangiopancreatography in referral centers
| Accessible papilla |
| Ampullary pathology |
| Periampullary diverticulum |
| Ampullary neoplastic infiltration |
| Non-accessible papilla |
| Peptic GI stenosis |
| Malignant GI strictures |
| Gastrointestinal bariatric bypass |
| Roux-en Y gastric by-pass |
| Billroth II gastroenterostomy |
GI: Gastrointestinal.
Until a few years ago, percutaneous transhepatic biliary drainage (PTBD) has been the only possible procedure in case of ERCP failure. PTBD involves the direct transhepatic puncture of the biliary system with consequent cholangiography and positioning of a drainage catheter. According to the literature, this procedure is associated with a morbidity rate up to 33%, including catheter dislocation, infection, bleeding, biliary leakages, acute cholangitis, and pneumothorax[2,3]. An alternative to PTBD is endoscopic ultrasonography-guided biliary drainage (EUS-BD). EUS-BD has several advantages such as internal drainage and a single procedure performed by the same operator without the discomfort of an external catheter. The feasibility of cholangiogram under endoscopic ultrasonography guidance was first reported in 1996 by Wiersema et al[4].
EUS-guided bilio-digestive anastomosis, first published by Giovanni et al[5] in 2001, is performed worldwide with reported cumulative technical success and post-procedure adverse events of 90% and 17%, respectively[6]. A recent systematic review and meta-analysis[7-15] by Sharaiha et al[12] included nine studies comparing the efficacy and safety of EUS-BD and PTBD[16]: three RCTs[7,11,15] and six retrospective studies[8-10,12-14]. All studies included patients undergoing EUS-BD in tertiary centers. One study[11] included both benign and malignant etiologies of biliary obstruction, whereas the remaining studies only included patients with malignant etiologies.
EUS-BD and PTBD showed equivalent technical success (OR: 1.78; 95%CI: 69-4.59; I2 = 22%). However EUS-BD was associated with a better clinical success (OR: 0.45; 95%CI: 0.23-0.89; I2 = 0%), less post-procedure adverse events (OR: 0.23; 95%CI: 0.12-047; I2 = 57%), and lower reintervention rates (OR: 0.13; 95%CI: 0.07-0.24; I2 = 0%). No significant differences were observed for the duration of hospital stay between EUS-BD and PTBD, but EUS-BD was more cost-effective.
TECHNIQUES
EUS-BD should be performed by experienced endoscopists who have performed at least 20 procedures under tutor supervision[17], and who are trained in both EUS and ERCP. Skilled staff is needed for guidewire manipulation, and carbon dioxide insufflation is compulsory to reduce the risk of pneumoperitoneum.
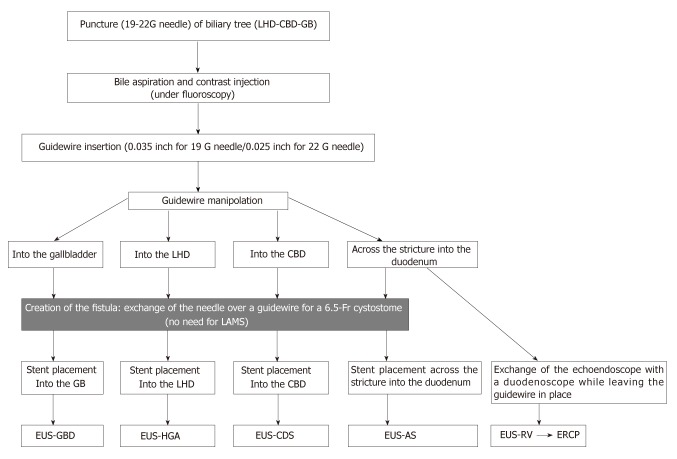
According to the access to the biliary tree, two approaches can be applied: the intrahepatic approach [hepatogastric anastomosis (EUS-HGA) or antegrade stent placement] or the extrahepatic approach [choledochoduodenostomy (EUS-CDS) or transgallbladder (EUS-GBD)] (Figure 1).
Figure 1.
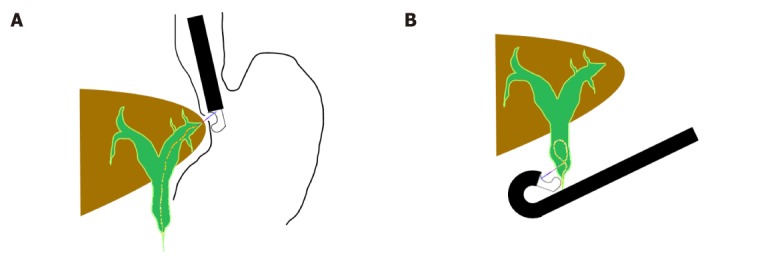
The access points of different endoscopic ultrasonography-guided biliary drainage procedures. A: The intrahepatic approach; B: The extrahepatic approach.
EUS-guided rendezvous (EUS-RV) transpapillary drainage is performed where the second portion of the duodenum is easily accessible but conventional ERCP failed. In EUS-RV, the biliary duct is punctured by using a fine needle aspiration needle from the upper gastrointestinal tract under EUS guidance followed by guidewire placement into the duodenum through the needle. After exchanging the endoscope with the ERCP duodenoscope, biliary cannulation is then reattempted by using the EUS-placed guidewire.
The intrahepatic approach
Such approach is typically preferred in cases where the papilla is not endoscopically accessible due to gastric outlet obstruction, to an obstructing proximal duodenal tumor, or in patients with surgically altered anatomy. Dilatation of intrahepatic ducts is compulsory to perform this approach. Cancer infiltration of the gastric wall within the planned path of approach to the biliary ducts or massive ascites and coagulopathy are contraindications to this type of approach.
With the tip of the echoendoscope positioned along the lesser curvature of the stomach, the dilated left hepatic duct (segment III) can be correctly visualized. Transgastric needle (19-22 G) insertion into the left hepatic duct and contrast injection clearly show the biliary tree under fluoroscopy. The next step is to exchange the needle over a guidewire for a 6.5-Fr cystotome used to create the fistula between the stomach and the left hepatic duct with a cutting current. Either plastic stents or self-expandable metal stents (SEMS) are then positioned over the guidewire (hepatic-gastric stent) or advancing the guidewire across the stricture and the papilla to complete an antegrade stent placement.
This kind of technique is not actually standardized, and no pooled data is available comparing the efficacy of different devices. The choice of the needle is still in debate because some operators suggest the 19 G needle because the large diameter reduced the risk of shearing the guidewire coating during manipulation although the 19 G needle can be stiffer and more difficult to handle compared to a 22 G needle. Usually a hydrophilic guidewire is preferred because strictures can be crossed more easily. The 0.025-inch guidewire, which fits a 22 G needle, can help during manipulation maneuvers due to its flexibility. However, it can make the stent insertion challenging due to the lack of stiffness and the less stable scope position.
The optimal biliary access points and learning curves for technically successful EUS-HGA have been evaluated by Oh et al[18] in 129 consecutive patients who underwent EUS-HGA. For each EUS-HGA session the following measurements were taken: intrahepatic bile duct diameter at the point of puncture, the hepatic portion length and bile duct segment for each needle puncture attempt, and procedure times (from initial bile duct puncture to final transmural stenting).
In the logistic regression model, low technical success rates were related with intrahepatic bile duct diameter of puncture site ≤ 5 mm (OR: 3.7; 95%CI: 1.71–8.1; P < 0.01) and hepatic portion length > 3 cm (OR: 5.7; 95%CI: 2.7–12; P < 0.01). The learning curve for technical success was evaluated by measuring procedure time and adverse events by using the moving average method and cumulative sum analysis, respectively. Procedure times and adverse events were shorter after 24 cases had been performed by the same operator and became stable at 33 cases of EUS-HGA.
These data suggest that a bile duct diameter > 5 mm and hepatic portion length 1 cm to ≤ 3 cm on EUS may guide the choice for the optimal site of puncture for successful EUS-HGA and that 33 cases of EUS-HGA are needed to achieve technical proficiency.
A crucial step for technical success is the creation of a fistula, which can potentially have an impact on complications such as biliary leakage, bilioperitoneum, or perforation. In order to insert the stent, the dilatation of the fistula is compulsory and can be performed by using balloon dilatators, stiff gradual catheters, needle knives, and cystotomes with cutting current. Advancing of stiff catheter may create tissue resistance forming a gap between the stomach and the liver with post-procedural biliary leak. Balloon dilatation also generates radial force, which is why some endoscopists prefer 6.5-Fr cystotomes. In a recent meta-analysis of EUS-BD technique, Wang et al[19] reported adverse event rates of 19.68% (49/249) with needle knife, 20.37% (44/216) with balloon catheter, and 38.46% (10/26) with cystotome.
The choice of the stent depends on the indication (benign vs malignant), the degree of ductal dilatation, whether the wire could cross the anastomosis, the length of fistula tract, and surgical indication for the patient[20]. In the first reported cases of HGA, plastic stents involved significant post-procedural biliary leakage. The use of fully covered self-expandable metal stents may cause side biliary duct obstruction, cholangitis, and significant stent migration. To prevent these complications, Giovannini et al[11] used the “stent-in-stent technique” with insertion of two metal stents: firstly, an uncovered metal 8-10 cm stent is placed to prevent migration and to occlude side biliary branch; secondly, a fully covered 6 cm stent is placed in the uncovered stent to prevent the biliary leakage. Recently, Song et al[21] reported no proximal or distal stent migration in any of the 27 patients who had undergone EUS-BD using a hybrid metal stent (Standard Sci Tech Inc, Seoul, South Korea) partially covered by SEMS (uncovered in the intrahepatic portion and covered in the transmural distal). SEMS are considered an interesting option compared to plastic stents due to a bigger caliber and longer patency especially when reintervention for stent substitution is not required.
The extrahepatic approach
The extrahepatic approach, including EUS-CDS and, when feasible, choledochoantrostomy, is usually performed in case of failure of selective cannulation of the common biliary duct because of ampullary neoplasm, neoplastic infiltration from pancreatic cancer, or when the access to the papilla is prevented by benign (peptic stenosis) or malignant duodenal stenosis. In all these cases, there is no consensus about the choice between the intrahepatic or the extrahepatic approach depending on the endoscopist’s discretion and expertise. More recently some authors have described gallbladder drainage for biliary drainage in patients with distal biliary obstruction and patent cystic duct[22,23] meaning that this technique can be literally considered an extrahepatic approach.
The tip of the echoendoscope is advanced to the duodenal bulb or, when feasible, to the antrum wall where the dilated common biliary duct is closer to the wall. Likewise, in the extrahepatic approach technique, the access to the bile duct is achieved with a 19-gauge EUS needle with subsequent bile aspiration, 0.035-inch guidewire manipulation into the intrahepatic tree, dilatation of the fistula, and stent insertion. Because stent migration is the main post procedural complication, similarly to HGA, some endoscopists prefer 4 cm or more, fully covered biliary metal stents. However, the use of these stents can make reintervention more difficult, and duodenal trauma and even perforation can be caused by the distal portion of the stent.
Clinical efficacy and safety of EUS-CDS versus endoscopic transpapillary stenting (ETS) as first-line treatment were tested by Kawakubo et al[24] in 82 patients with distal malignant biliary obstruction. The found equivalent clinical success rates (EUS-CDS 96.2%, ETS 98.2%; P = 0.54) and overall adverse event rates (EUS-CDS 26.9%, ETS 35.7%; P = 0.46). However, a shorter mean procedural time was found with EUS-CDS rather than with ETS (19.7 min vs 30.2 min; P < 0.01)[24]. These data were confirmed by Nakai et al[25] in a prospective multicenter study.
Lumen-apposing metal stents (LAMS) were first introduced to drain peripancreatic fluid collections, but recently they have been used for EUS-BD. The stent includes a full silicone covered, wider lumen and bigger flanges to prevent tissue ingrowth, provide fast drainage, reduce the risk of migration with biliary leakage, and allow removability. New cautery-enhanced delivery systems (Hot AXIOS device, Boston Scientific) are available allowing EUS-BD in one step with no need for prior needle puncture, guidewire insertion, or fluoroscopy. Biliary duct dilatation and a distance of no more than 10 mm are required to avoid stent migration, leakage, and pressure necrosis.
EUS-CDS using a LAMS was proposed as an alternative approach for patients with malignant obstructive jaundice and ERCP failure. Tsuchiya et al[26] evaluated prospectively the long-term outcome (median: 184 d; range: 12-819) in 19 patients undergoing EUS-CDS using a fully covered LAMS with a cautery-enhanced delivery system. Technical success was achieved in all patients and jaundice improvement in 95% of patients (18/19).
No intraprocedural adverse events were recorded, but the post procedure related adverse events ratio was 15.8% [3/19; acute cholangitis (n = 2) and fever (n = 1)]. Five patients had secondary stent obstruction because of food residue (n = 2), kinking (n =1), suspected tumor ingrowth (n = 1), and spontaneous dislodgement (n = 1) with reintervention in four of these five patients. The authors suggested that food impaction and bile duct kinking were consequences of the small diameter of the LAMS (6-8 mm diameter could have shorter patency compared to 10 mm diameter) and of the absence of the spontaneous outflow of the bile after decompression. The efficacy of EUS-CDS using the LAMS was recently confirmed by Anderloni et al[27] in a retrospective analysis in 46 patients. They reported technical and clinical success rates of 93.5% and 97.7%, respectively. However, adverse events were found in five patients (11.6%) with one fatal bleeding 17 d after stent placement, three episodes of stent occlusion (food impaction), and one of spontaneous migration (all four required reintervention). Despite these encouraging results, the authors suggested a careful evaluation before using the stent in this clinical setting due to serious adverse events.
Recently, EUS-GBD was reported to be useful for acute cholecystitis in patients unfit for surgery. Jang et al[28] found that EUS-GBD was comparable to percutaneous transhepatic gallbladder drainage in terms of technical feasibility, efficacy, and safety of the procedures. In a pooled analysis on the efficacy and safety of EUS-GBD with LAMS in nonoperative candidates with acute cholecystitis, Kalva et al[29] showed that technical success represented 93.86% (95%CI: 90.56-96.49) while clinical success was obtained in 92.48% (95%CI: 88.9-95.42). The overall complication rate was 18.31% (95%CI: 13.49-23.68), and the stent related complication rate in the pooled percentage of patients was 8.16% (95%CI: 4.03-14.96).
Some authors reported encouraging results with EUS-GBD in case of failure to treat malignant distal biliary obstruction and cystic duct patent. Imai et al[22] reported technical success rates and functional success rate of 100% and 91.7%, respectively with 16.7% of adverse events in a series of 12 patients with obstructive jaundice due to unresectable malignant distal biliary stricture who underwent EUS-GBD after ERCP failure.
The rendez-vous technique
EUS-RV is considered a second-line approach in case of ERCP failure due to juxtapapillary diverticulum or ampullary cancer. Once the dilated intrahepatic or extrahepatic duct is identified and punctured with the 19-gauge EUS aspiration needle, a long (450 cm) 0.035-inch or 0.025-inch guidewire is inserted downstream through the stenosis and into the duodenum.
The echoendoscope is withdrawn leaving the wire in place, and a duodenoscope is inserted to grasp the wire into the scope channel with forceps or a snare. The traditional cannulation over the wire is then performed to access the biliary duct. Crossing the stenosis and the papilla with the guidewire can be difficult and the need to exchange endoscopes may prolong procedural time. This kind of approach is generally preferred for benign indications because there is no anatomical alteration of the biliary duct as when the fistula is created with subsequent stent placement in EUS-HGA or EUS-CDS. The site of the puncture (duodenal bulb, second portion, and stomach) has been examined by Iwashita et al[30] in 20 patients after failed cannulation. The guidewire was successfully manipulated in 100% (10/10) with the second portion (D2) approach, and 66.7% (6/9) with other approaches, thus suggesting that the extrahepatic approach from D2 may improve the success rate of EUS-RV.
EUS-RV seems to be the safest of all three approaches[31] and has been supported by several studies. Safety and efficacy of EUS–RV have been evaluated by Iwashita et al[32] in 40 patients who underwent salvage EUS–RV immediately after failed biliary cannulation. Successful manipulation of the guidewire into the small intestine was achieved in 29 of 40 patients. Five patients (13%) had complications including pancreatitis, abdominal pain, pneumoperitoneum, and sepsis/death, which were believed to be unrelated to the procedure.
The algorithm for EUS-BD guidance
The choice of approach is still under debate and is mainly based on anatomical factors, indication of the procedure, and the endoscopist's experience. Ascites or non-dilated intrahepatic left biliary ducts are conditions for an extrahepatic approach, while for benign indications (e.g., biliary duct stone removal) a mini-invasive approach like the rendez-vous technique is recommended.
Artifon et al[33] compared the outcomes of EUS-HGA and EUS-CDS in a prospective randomized trial of 49 patients with distal malignant biliary obstruction. The technical success rate was 96% versus 91% with a clinical success rate of 91% versus 77% and similar procedural time. The overall adverse event rates were 16.3% (20% for the HGA group and 12.5% for the CDS group). These data show no significant differences between the two techniques.
These data have been confirmed by Khashab et al[34] in an international multicenter comparative trial with 121 patients who underwent EUS-BD (CDS: 60, HGA: 61). However, CDS was found to be associated with shorter hospital stay, improved stent patency, and fewer procedural and stent-related complications[34].
The anatomical site of transmural biliary drainage was also evaluated in a review by Wang et al[19], which included 42 studies with 1192 patients. The cumulative technical success rate, the functional success rate, the adverse event rate of EUS-BD, and the pooled odds ratio of technical success rate, functional success rate, and adverse event rates of the transduodenal approach versus transgastric approach were calculated. No significant difference was found.
Some authors have suggested different algorithms to guide the choice of approach (Table 2). Park et al[35] evaluated an algorithm based on enhanced guidewire manipulation for EUS-BD after ERCP failure in 45 patients achieving overall technical and functional success rates of 91% (intention to treat, 41/45) and 95% (per protocol, 39/41), respectively. More recently other authors have suggested an algorithm for biliary drainage based on patient anatomy[20].
Table 2.
Algorithms for guidance endoscopic ultrasonography-guided biliary drainage
| Ref. | Design | Proposed algorithm | No. of patients | Technical success rate | Complication rate |
| Park et al[35] | PS | “Enhanced guidewire manipulation protocol” EUS-RV/EUS-AS with guidewire manipulation protocol as a first-line In case of failure or duodenal invasion, transmural EUS-BD | 45 | 91% | 11% |
| Tyberg et al[20] | PS | “Patient anatomy” Dilated IHBT on cross-sectional imaging, received IHa Nondilated IHBT on cross-sectional imaging, received EHa In case of failure of IHa, conversion to an EHa | 52 | 96% | 10% |
PS: Prospective study; EUS-RV: Endoscopic ultrasonography-guided rendez; EUS-AS: Endoscopic ultrasonography-guided antegrade stent placement; EUS-BD: Endoscopic ultrasonography-guided biliary drainage; IHBT: Intrahepatic biliary tree; IHa: Intrahepatic approach; EHa: Extrahepatic approach.
Patients with a dilated intrahepatic biliary tree on cross-sectional imaging received an intrahepatic approach, while patients with a nondilated intrahepatic biliary tree on cross-sectional imaging underwent an extrahepatic approach. In case of failure of intrahepatic drainage, conversion to an extrahepatic approach was proposed. Following this algorithm, technical success in 50/52 patients (96%) was reported with adverse events in five patients (10%).
A recent worldwide multi-institutional survey[36] consisting of ten questions related to the practice of EUS-BD among regional experts revealed the general feeling that EUS-BD could replace PTBD after ERCP failure and that the rendez-vous stenting technique should be first choice. Most endoscopists recommended the use of SEMS for EUS-BD while there was no agreement about the superiority of partially-covered SEMS over fully covered SEMS for EUS-HGA. Regarding the length of the stent, 8-10 cm SEMS were recommended for EUS-HGA while 6 cm SEMS for EUS-CDS. There was general agreement about the use of 6-Fr cystotomes for fistula creation.
There are no prospective studies evaluating the role of EUS-BD as a primary drainage technique in comparison to ERCP. The ERCP related complications like pancreatitis in difficult cannulation might suggest the role of EUS-BD as a good primary alternative in these setting or in patients with altered anatomy or malignant obstruction. However, the use of advanced ERCP techniques in a tertiary-care center usually provides high technical success rate so that EUS-BD is required in a very limited number of cases (only 0.6% of native papilla ERCPs according to the authors)[37].
CONCLUSION
PTBD represents a rescue procedure for ERCP failure. The technical success rate of PTBD is over 95% with a 33% or higher overall adverse event rate including bleeding, infection, dislodgement, biliary leak, and tract seeding[2]. Moreover, this technique can be uncomfortable for the patient due to an external drainage catheter and is contraindicated with ascites or multiple liver metastasis. EUS-BD has become an evolving alternative to PTBD with a better clinical success rate (OR: 0.45), fewer adverse events (OR: 0.23), and fewer reinterventions (OR: 0.13)[16] (Tables 3 and 4). EUS biliary drainage can be achieved by puncturing the intrahepatic duct in the III segment (intrahepatic approach) and inserting an HGA stent, advancing a guidewire across the stricture and the papilla to complete an antegrade stent placement, or by puncturing the common bile duct, or the gallbladder (extrahepatic approach) with CDS or GBD (Figure 2). When the papilla is accessible, puncturing the biliary tree (intrahepatic or extrahepatic) and inserting the guidewire into the small intestine to cannulate with the rendez-vous technique (EUS-RV) represents the most appropriate and safe route.
Table 3.
Comparative studies among different techniques of biliary drainage
| Ref. | Design | Technique | No. of patients | Technical success rate | Complication rate |
| Artifon et al[33] | PS | EUS-HGA vs EUS-CDS | 49 | 96% vs 91% | 20% vs 12.5% |
| Khashab et al[34] | PS | EUS-HGA vs EUS-CDS | 121 | 91.8 vs 93.3% | 19.6% vs 13.3% |
| Sharaiha et al[16] | RS rev | PTBD vs EUS-BD | 60 | 84.6% vs 91.4% | 25% vs 13% |
| Artifon et al[7] | PS | PTBD vs EUS-CDS | 25 | 100% vs 100% | 25% vs 15.3% |
| Bapaye et al[8] | RS | PTBD vs EUS-BD | 50 | 100% vs 92% | 46% vs 20% |
| Bill et al[10] | RS | PTBD vs EUS-RV | 50 | 100% vs 76% | 17% vs 28% |
| Jang et al[28] | PS | PTGD vs EUS-GBD | 29 | 97% vs 97% | 3% vs 7% |
| Khashab et al[9] | PS | PTBD vs EUS-BD | 73 | 100% vs 86.4% | 39.2% vs 18.2% |
PS: Prospective study; RS: Retrospective study; Rev: Review; EUS-BD: Endoscopic ultrasonography-guided biliary drainage; EUS-HGA: Endoscopic ultrasonography-guided hepatogastric anastomosis; EUS-CDS: Endoscopic ultrasonography-guided choledochoduodenostomy; EUS-RV: Endoscopic ultrasonography-guided rendezvous; EUS-GBD: Echoendoscopic transgallbladder drainage.
Table 4.
Advantages and disadvantages of the different techniques
| Advantages | Disadvantages | |
| ERCP | Widely available Relative low complication rate (compared to PTBD and EUS-BD) | Not feasible in case of inaccessible papilla |
| PTBD | Available rescue therapy for ERCP failure | High complication rate (bleeding-infection) External catheter Contraindicated if ascites |
| EUS | Different possible approaches (HGA, CDS, GBD, RV) Internal drainage Same session of failed ERCP Fewer re-interventions | Not widely available High endoscopic ERCP/EUS expertise required Not yet standardized algorithm |
ERCP: Endoscopic retrograde cholangiopancreatography; PTBD: Percutaneous transhepatic biliary drainage; EUS: Endoscopic ultrasonography; EUS-BD: Endoscopic ultrasonography-guided biliary drainage; HGA: Hepatogastric anastomosis; CDS: Choledochoduodenostomy; RV: Rendezvous; GBD: Transgallbladder drainage.
Figure 2.
Steps for endoscopic ultrasonography-guided biliary drainage: The crucial step for complication is enhanced in red. LHD: Left hepatic duct; CBD: Common bile duct; GB: Gallbladder; LAMS: Lumen apposing metal stent; EUS-BD: Endoscopic ultrasonography-guided biliary drainage; EUS-HGA: Endoscopic ultrasonography-guided hepatogastric anastomosis; EUS-CDS: Endoscopic ultrasonography-guided choledochoduodenostomy; EUS-RV: Endoscopic ultrasonography-guided rendez-vous.
There is general agreement that EUS-BD may replace PTBD as a drainage method after failure of ERCP[36]. There is no formal consensus on how to choose between the intrahepatic or the extrahepatic approach or rendezvous technique. Algorithms for biliary drainage based on patient anatomy[20] or guidewire manipulation[35] have been developed with encouraging results, but probably the appropriate approach should be decided on a case-to-case basis according to the patient’s anatomy and condition. The most crucial step for both approaches is represented by the dilatation of the fistula that potentially can impact the technical success or failure of the drainage procedure. For this reason, most operators prefer transpapillary (rendezvous) EUS-BD or the antegrade technique because the post-procedure biliary leak risk is inferior. The recent introduction of LAMS has improved this field by reducing leakage and the mean procedural time, however potential severe adverse events can occur and need to be carefully evaluated[27].
In 2011, a consortium involving 40 international experts decided upon a standardized terminology, nomenclature, and indications for EUS-BD concluding that due to the potential serious adverse events associated with the procedure, EUS-BD should only be performed by endoscopists trained in both EUS and ERCP, performing pancreatic-biliary EUS and fine needle aspiration with large ERCP and EUS experience of at least 4-5 years (at least 200-300 EUS and ERCP each year) with a 95% to 98% success rate for standard ERCP, with a surgical and interventional radiology backup[38]. Therefore, the endoscopist must have mastery of multiple techniques to be able to fully perform EUS-BD.
Footnotes
Conflict-of-interest statement: All authors have no conflicts of interest to report.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: March 15, 2019
First decision: April 11, 2019
Article in press: May 1, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kamimura K, Yeh HZ S-Editor: Ji FF L-Editor: Filipodia E-Editor: Xing YX
Contributor Information
Raffaele Salerno, Gastroenterology and Digestive Endoscopy Unit, ASST Fatebenefratelli Sacco - Department of Biochemical and Clinical Sciences “L. Sacco”, University of Milan, Milano 20100, Italy.
Sophia Elizabeth Campbell Davies, Hospital Pharmacy, ASST Fatebenefratelli Sacco, Piazza Principessa Clotilde, Milan 20121, Italy.
Nicolò Mezzina, Gastroenterology and Digestive Endoscopy Unit, ASST Fatebenefratelli Sacco - Department of Biochemical and Clinical Sciences “L. Sacco”, University of Milan, Milano 20100, Italy.
Sandro Ardizzone, Gastroenterology and Digestive Endoscopy Unit, ASST Fatebenefratelli Sacco - Department of Biochemical and Clinical Sciences “L. Sacco”, University of Milan, Milano 20100, Italy.
References
- 1.Coté GA, Singh S, Bucksot LG, Lazzell-Pannell L, Schmidt SE, Fogel E, McHenry L, Watkins J, Lehman G, Sherman S. Association between volume of endoscopic retrograde cholangiopancreatography at an academic medical center and use of pancreatobiliary therapy. Clin Gastroenterol Hepatol. 2012;10:920–924. doi: 10.1016/j.cgh.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Yarmohammadi H, Covey AM. Percutaneous biliary interventions and complications in malignant bile duct obstruction. Chin Clin Oncol. 2016;5:68. doi: 10.21037/cco.2016.10.07. [DOI] [PubMed] [Google Scholar]
- 3.Nennstiel S, Weber A, Frick G, Haller B, Meining A, Schmid RM, Neu B. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis Over 10 Years. J Clin Gastroenterol. 2015;49:764–770. doi: 10.1097/MCG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 4.Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK. Endosonography-guided cholangiopancreatography. Gastrointest Endosc. 1996;43:102–106. doi: 10.1016/s0016-5107(06)80108-2. [DOI] [PubMed] [Google Scholar]
- 5.Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 6.Khan MA, Akbar A, Baron TH, Khan S, Kocak M, Alastal Y, Hammad T, Lee WM, Sofi A, Artifon EL, Nawras A, Ismail MK. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:684–703. doi: 10.1007/s10620-015-3933-0. [DOI] [PubMed] [Google Scholar]
- 7.Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–774. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 8.Bapaye A, Dubale N, Aher A. Comparison of endosonography-guided vs. percutaneous biliary stenting when papilla is inaccessible for ERCP. United European Gastroenterol J. 2013;1:285–293. doi: 10.1177/2050640613490928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khashab MA, Valeshabad AK, Afghani E, Singh VK, Kumbhari V, Messallam A, Saxena P, El Zein M, Lennon AM, Canto MI, Kalloo AN. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557–565. doi: 10.1007/s10620-014-3300-6. [DOI] [PubMed] [Google Scholar]
- 10.Bill J, Darcy M, Fujii-Lau LL, Mullady D, Gaddam S, Murad F, Early DS, Edmundowicz SA, Kushnir VM. Tu1617 A Comparison Between Endoscopic Ultrasound Guided Rendezvous and Percutaneous Biliary Drainage After Failed ERCP for Malignant Biliary Obstruction. Gastrointest Endosc. 2015;81:AB531. doi: 10.1055/s-0042-112584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giovannini M, Bories E, Napoleon B, Barthet M, Caillol F, Pesenti C. 855 Multicenter Randomized Phase II Study: Percutaneous Biliary Drainage vs EUS Guided Biliary Drainage : Results of the Intermediate Analysis. Gastrointest Endosc. 2015;81:AB174. [Google Scholar]
- 12.Sharaiha RZ, Kumta NA, Desai AP, DeFilippis EM, Gabr M, Sarkisian AM, Salgado S, Millman J, Benvenuto A, Cohen M, Tyberg A, Gaidhane M, Kahaleh M. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg Endosc. 2016;30:5500–5505. doi: 10.1007/s00464-016-4913-y. [DOI] [PubMed] [Google Scholar]
- 13.Torres-Ruiz M-F, De La Mora-Levy J-G, Alonso-Larraga JO, Del Monte JS, Hernandez-Guerrero A. Su1337 Biliary Drainage in Malignant Obstruction: A Comparative Study Between EUS-Guided vs Percutaneous Drainage in Patients With Failed ERCP. Gastrointest Endosc. 2016;83:AB356. [Google Scholar]
- 14.Sportes A, Camus M, Grabar S, Leblanc S, Coriat R, Hochberger JH, Chaussade S, Prat F. Mo2084 Comparative Trial of EUS-Guided Hepatico-Gastrostomy and Percutaneous Transhepatic Biliary Drainage for Malignant Obstructive Jaundice After Failed ERCP. Gastrointest Endosc. 2016;83:AB522–AB523. [Google Scholar]
- 15.Lee TH, Choi JH, Park do H, Song TJ, Kim DU, Paik WH, Hwangbo Y, Lee SS, Seo DW, Lee SK, Kim MH. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin Gastroenterol Hepatol. 2016;14:1011–1019.e3. doi: 10.1016/j.cgh.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Sharaiha RZ, Khan MA, Kamal F, Tyberg A, Tombazzi CR, Ali B, Tombazzi C, Kahaleh M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–914. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Hara K, Yamao K, Mizuno N, Hijioka S, Imaoka H, Tajika M, Tanaka T, Ishihara M, Okuno N, Hieda N, Yoshida T, Niwa Y. Endoscopic ultrasonography-guided biliary drainage: Who, when, which, and how? World J Gastroenterol. 2016;22:1297–1303. doi: 10.3748/wjg.v22.i3.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh D, Park DH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017;10:42–53. doi: 10.1177/1756283X16671671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Zhu J, Xing L, Wang Y, Jin Z, Li Z. Assessment of efficacy and safety of EUS-guided biliary drainage: a systematic review. Gastrointest Endosc. 2016;83:1218–1227. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Tyberg A, Desai AP, Kumta NA, Brown E, Gaidhane M, Sharaiha RZ, Kahaleh M. EUS-guided biliary drainage after failed ERCP: a novel algorithm individualized based on patient anatomy. Gastrointest Endosc. 2016;84:941–946. doi: 10.1016/j.gie.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Song TJ, Lee SS, Park DH, Seo DW, Lee SK, Kim MH. Preliminary report on a new hybrid metal stent for EUS-guided biliary drainage (with videos) Gastrointest Endosc. 2014;80:707–711. doi: 10.1016/j.gie.2014.05.327. [DOI] [PubMed] [Google Scholar]
- 22.Imai H, Kitano M, Omoto S, Kadosaka K, Kamata K, Miyata T, Yamao K, Sakamoto H, Harwani Y, Kudo M. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016;84:147–151. doi: 10.1016/j.gie.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Itoi T, Binmoeller K, Itokawa F, Umeda J, Tanaka R. Endoscopic ultrasonography-guided cholecystogastrostomy using a lumen-apposing metal stent as an alternative to extrahepatic bile duct drainage in pancreatic cancer with duodenal invasion. Dig Endosc. 2013;25 Suppl 2:137–141. doi: 10.1111/den.12084. [DOI] [PubMed] [Google Scholar]
- 24.Kawakubo K, Kawakami H, Kuwatani M, Kubota Y, Kawahata S, Kubo K, Sakamoto N. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy. 2016;48:164–169. doi: 10.1055/s-0034-1393179. [DOI] [PubMed] [Google Scholar]
- 25.Nakai Y, Isayama H, Kawakami H, Ishiwatari H, Kitano M, Ito Y, Yasuda I, Kato H, Matsubara S, Irisawa A, Itoi T. Prospective multicenter study of primary EUS-guided choledochoduodenostomy using a covered metal stent. Endosc Ultrasound. 2019;8:111–117. doi: 10.4103/eus.eus_17_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuchiya T, Teoh AYB, Itoi T, Yamao K, Hara K, Nakai Y, Isayama H, Kitano M. Long-term outcomes of EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction: a prospective multicenter study. Gastrointest Endosc. 2018;87:1138–1146. doi: 10.1016/j.gie.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Anderloni A, Fugazza A, Troncone E, Auriemma F, Carrara S, Semeraro R, Maselli R, Di Leo M, D'Amico F, Sethi A, Repici A. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest Endosc. 2019;89:69–76. doi: 10.1016/j.gie.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 28.Jang JW, Lee SS, Song TJ, Hyun YS, Park DY, Seo DW, Lee SK, Kim MH, Yun SC. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012;142:805–811. doi: 10.1053/j.gastro.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 29.Kalva NR, Vanar V, Forcione D, Bechtold ML, Puli SR. Efficacy and Safety of Lumen Apposing Self-Expandable Metal Stents for EUS Guided Cholecystostomy: A Meta-Analysis and Systematic Review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwashita T, Yasuda I, Mukai T, Iwata K, Ando N, Doi S, Nakashima M, Uemura S, Mabuchi M, Shimizu M. EUS-guided rendezvous for difficult biliary cannulation using a standardized algorithm: a multicenter prospective pilot study (with videos) Gastrointest Endosc. 2016;83:394–400. doi: 10.1016/j.gie.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Savides TJ, Varadarajulu S, Palazzo L EUS 2008 Working Group. EUS 2008 Working Group document: evaluation of EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2009;69:S3–S7. doi: 10.1016/j.gie.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 32.Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, Chang KJ. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60–65. doi: 10.1055/s-0030-1256871. [DOI] [PubMed] [Google Scholar]
- 33.Artifon EL, Marson FP, Gaidhane M, Kahaleh M, Otoch JP. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: is there any difference? Gastrointest Endosc. 2015;81:950–959. doi: 10.1016/j.gie.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 34.Khashab MA, Messallam AA, Penas I, Nakai Y, Modayil RJ, De la Serna C, Hara K, El Zein M, Stavropoulos SN, Perez-Miranda M, Kumbhari V, Ngamruengphong S, Dhir VK, Park DH. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc Int Open. 2016;4:E175–E181. doi: 10.1055/s-0041-109083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park DH, Jeong SU, Lee BU, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video) Gastrointest Endosc. 2013;78:91–101. doi: 10.1016/j.gie.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 36.Guo J, Giovannini M, Sahai AV, Saftoiu A, Dietrich CF, Santo E, Fusaroli P, Siddiqui AA, Bhutani MS, Bun Teoh AY, Irisawa A, Arturo Arias BL, Achanta CR, Jenssen C, Seo DW, Adler DG, Kalaitzakis E, Artifon E, Itokawa F, Poley JW, Mishra G, Ho KY, Wang HP, Okasha HH, Lachter J, Vila JJ, Iglesias-Garcia J, Yamao K, Yasuda K, Kubota K, Palazzo L, Sabbagh LC, Sharma M, Kida M, El-Nady M, Nguyen NQ, Vilmann P, Garg PK, Rai P, Mukai S, Carrara S, Parupudi S, Sridhar S, Lakhtakia S, Rana SS, Ogura T, Baron TH, Dhir V, Sun S. A multi-institution consensus on how to perform EUS-guided biliary drainage for malignant biliary obstruction. Endosc Ultrasound. 2018;7:356–365. doi: 10.4103/eus.eus_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holt BA, Hawes R, Hasan M, Canipe A, Tharian B, Navaneethan U, Varadarajulu S. Biliary drainage: role of EUS guidance. Gastrointest Endosc. 2016;83:160–165. doi: 10.1016/j.gie.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, Binmoeller KF, Giovannini M. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372–1379. doi: 10.3748/wjg.v19.i9.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]