Abstract
A gastrointestinal (GI) transmural defect is defined as total rupture of the GI wall, and these defects can be divided into three categories: perforations, leaks, and fistulas. Surgical management of these defects is usually challenging and may be associated with high morbidity and mortality rates. Recently, several novel endoscopic techniques have been developed, and endoscopy has become a first-line approach for therapy of these conditions. The use of endoscopic vacuum therapy (EVT) is increasing with favorable results. This technique involves endoscopic placement of a sponge connected to a nasogastric tube into the defect cavity or lumen. This promotes healing via five mechanisms, including macrodeformation, microdeformation, changes in perfusion, exudate control, and bacterial clearance, which is similar to the mechanisms in which skin wounds are treated with commonly employed wound vacuums. EVT can be used in the upper GI tract, small bowel, biliopancreatic regions, and lower GI tract, with variable success rates and a satisfactory safety profile. In this article, we review and discuss the mechanism of action, materials, techniques, efficacy, and safety of EVT in the management of patients with GI transmural defects.
Keywords: Gastrointestinal, Endoscopy, Endoscopic vacuum therapy, Negative pressure therapy, Fistula, Leak, Perforation, Defect
Core tip: Gastrointestinal (GI) transmural defects, including perforations, leaks, and fistulas, are difficult to manage and are associated with high rates of morbidity and mortality. Endoscopic vacuum therapy (EVT) has developed into a valuable tool for the treatment of these conditions. EVT has proven to be an effective and safe method in the intraluminal treatment of transmural defects, as it promotes changes in perfusion, causes microdeformation and macrodeformation, and decreases bacterial contamination, secretion, and local edema to facilitate healing. In this review, we discuss the mechanism of action, materials, techniques, efficacy, and safety of EVT in the management of patients with transmural GI defects.
INTRODUCTION AND BACKGROUND
A gastrointestinal (GI) transmural defect is defined as total rupture of the GI wall and these defects can be divided into three main categories including perforation, leaks, and fistulas. Recognition of the specific classification of the defect is essential for choosing the best treatment modality. In the past, many endoscopic techniques, including clips, cap-mounted clips, covered self-expandable metal stents (CSEMS), tissue sealants, endoscopic sutures, cardiac septal defect occluders, septotomies, and internal drainage with pig-tail stents, have been shown to be effective in reducing morbidity and mortality in the treatment of transmural defects. However, the efficacy varies in most studies[1-17] and, thus, endoscopists continue to investigate novel techniques for management of these defects.
Endoscopic vacuum therapy (EVT), also known as endoscopic negative pressure therapy, Endovac therapy, and E-Vac therapy, is an innovative endoscopic option for treating transmural GI defects[18-21]. This endoscopic approach is based on the negative pressure wound therapy for treatment of non-healing wounds. The healing effect of this technique occurs through multiple mechanisms, including changes in perfusion, microdeformation, macrodeformation, exudate control, and bacterial control[22]. Although some authors use the term “negative pressure” in their description of this technique[18,19,21], we find this to be misleading, as physical pressure always has a positive value[23,24]. Thus, in this review we will use the term EVT.
The first report of EVT[25] was in the treatment of an anastomotic leak following a rectal surgery in 2003. Since then, EVT has been used in the adult population for closure of esophageal, gastric (most commonly after bariatric surgery), small bowel, pancreatic, and colorectal defects, with success rates above 70%[26-33]. Additionally, one study demonstrated the use of EVT in the pediatric population, with a high success rate in the treatment of upper GI transmural defects[34].
In this article, we review and discuss the mechanism of action, indications, materials, techniques, efficacy, and safety of EVT in the management of patients with transmural defects.
MECHANISM OF ACTION
Vacuum therapy has been commonly used for treatment of non-healing skin wounds. In management of transmural defects, EVT is thought to promote healing via similar mechanisms, including macrodeformation, microdeformation, changes in perfusion, exudate control, and bacterial clearance[35,36].
Macrodeformation
Macrodeformation occurs when suction is applied to the sponge resulting in deformational forces being exerted on the defect edges, thus drawing the edges together. Studies showed that a negative pressure of 125 mmHg can decrease the volume of a reticulated open-pore polyurethane sponge by approximately 80%, resulting in substantial shrinkage of the defect[35-39].
Microdeformation
Microdeformation describes the mechanical changes that occur on a microscopic scale when suction is applied. Mechanical strain causes a deformation of the cytoskeleton which initiates signaling cascades leading to release of growth factors which promote cell proliferation and migration, increasing the expression of extracellular matrix components and contractile elements that are necessary for healing. Factors known to affect the efficiency of this mechanism include level of suction, pore size and consistency of the sponge, type of tissue being treated, and deformability of the surrounding tissues[35,40].
Changes in perfusion
Adequate blood flow is essential for healing because it delivers oxygen and vital nutrients to the tissue in addition to removing waste products. Vacuum therapy treatment results in increased microvessel density. Vacuum therapy causes temporary hypoperfusion in the defect edges resulting in localized hypoxia-inducible factor 1α and concomitant modulation of vascular endothelial growth factor expression, leading to increase angiogenesis[22,41,42]. In healthy human skin, suction levels of up to 300 mmHg applied to a reticulated open-pore polyurethane sponge cause a fivefold increase of blood flow[43]. Additionally, other studies have demonstrated that a negative pressure of 125 mmHg considerably increased the blood vessel density, reaching a maximum of 200% in contrast to the vessel density prior to treatment[44].
Exudate control
Fluid accumulation in the extracellular space and tissue edema often occur in chronic defects, inhibiting healing by compressing local cells and tissues. It has been demonstrated that wound healing is improved following fluid removal, and although the exact mechanism for this improved healing is unclear, proposed theories include local alterations in blood flow and removal of harmful substances[22,24,45,46]. Additionally, by removing fluid, there is a reduction in the compression forces acting on the microvasculature, which allows increased blood flow and perfusion of the tissue[35].
Bacterial clearance
A high bacterial load may interfere with the process of defect healing; however, there is conflicting evidence regarding the role of vacuum therapy in decreasing bacterial contamination[22]. One randomized study reported that vacuum treatment had a positive effect on wound healing because of a significant decrease in bacterial load compared with non-vacuum–treated wounds[47]. Additionally, a second study including patients with thoracic infections showed improvement in infection control prior to definitive closure[48]. However, other studies have also shown either an increase or no change in bacterial load using this technique[49,50].
INDICATIONS
EVT represents a clinical endoscopic evolution of vacuum-assisted closure therapy, a well-established treatment for open wounds[47,49,51]. Since it is still a relatively new technique, currently no standardized indications for use have been established[51].
All patients with acute or chronic GI defects are candidates for EVT. Endoscopic evaluation is always required prior to treatment to identify the wall defect, to characterize the leak or fistula tract, and to evaluate the contaminated cavity. Larger defects, including perforations, leaks and fistulas, typically associated with fluid collections, are the most common indication for EVT, and studies have shown high efficacy rates of healing associated with this technique[26-34]. When a small defect is associated with a contaminated cavity, dilation of the defect to access the cavity is needed to place the sponge extraluminally. Additionally, small defects, less than 10 mm, without an associated cavity, can be managed with intraluminal placement of the sponge[1,10,52,53].
EVT can be used throughout the GI tract for esophageal, gastric, small bowel, biliopancreatic, and colorectal defects. The most common indications with established data are defects in the esophagus (perforations, leaks and fistulas after anastomoses), stomach (mainly after bariatric surgery), and colorectal areas (anastomotic leaks and fistulas)[26-33,51,54]. Additionally, recent data on early use of EVT in patients with anastomotic ischemic following esophagectomy has been reported with favorable results[55]. The use of EVT in GI ischemia had also been successfully reported in a case of ischemia of the blind end of the jejunal loop after Roux-en-Y gastrectomy[56].
An additional benefit is that EVT can be used in critically ill, hemodynamically unstable patients in need of infectious source control. This technique allows for control of the focus of the sepsis by removing necrotic debris, tissue, and purulent material, while promoting tissue healing and thus hopefully allowing for patient stabilization. It should be noted, however, that if the patient does not clinically respond to EVT therapy, surgical intervention may still be required[48,51,52].
Similar to alternative techniques, EVT has limited efficacy in some clinical scenarios. In defects larger than 5 cm, the sponge size may be insufficient to occlude the defect[52,57,58]. In multiloculated fluid collections, the proper placement of the sponge can be inadequate due to the septations of the collection[57]. In patients with complete dehiscence of a surgical anastomosis, EVT can be used to control sepsis; however, frequently, a second intervention, such as CSEMS or revisional surgery, is needed to restore the anastomosis and preserve continuity of the upper GI tract. Additionally, patients with anastomotic leakage after esophagectomy with necrosis of the gastric conduit usually require surgical revision[51,59]. And finally, another limitation of use of EVT occurs in patients with GI-cutaneous fistula. Mechanistically, EVT relies on the ability to create negative pressure to keep the defect and fistula tract close. Atmospheric exposure prevents this negative pressure system from occurring, and frequently results in dressing malformation and failure. While attempts to plug the fistula at the skin level with occlusive dressings or glue/tissue sealants has been used, this does not maintain an ideal negative pressure seal, which can lead to moisture buildup and eventual failure[52].
To date, contraindications to EVT remain unclear. However, it is recommended that EVT should be avoided in patients with defects in close vicinity of major vessels or those on therapeutic anticoagulants due to the risk of major bleeding[26,60-62]. Additionally, it should be avoided in patients with defects in connection to the tracheobronchial system[18].
PROCEDURE
The procedure can be performed in the operating room, endoscopy suite, or at the bed side. In those patients with upper GI defects, anesthesia with endotracheal intubation is recommended for safe airway management during the passage of the sponge. However, during exchanges, deep sedation may be preferred in certain patients. In those patients with lower GI defects, deep sedation is likely safe depending on other clinical factors. Once the patient is adequately sedated, endoscopic evaluation is required to identify and characterize the wall defect and to evaluate the contaminated cavity. Once adequately evaluated, endoscopic irrigation and debridement is recommended.
A meticulous evaluation of the cavity (with or without fluoroscopy) is performed to choose the correct sponge size; estimation of the size of the sponge can be based on the size of the endoscope or endoscopist prior experience. After these steps, the endoscope is removed, and the sponge system is prepared[18,34,52,57].
For the purposes of this review, we will explain the detailed technique for use of EVT in upper GI defects. Lower GI defects can be managed with few modifications to this technique. A silicon 16 or 18-Fr (10 to 16 Fr in children) nasogastric tube (NGT) is introduced into the patient’s nares and advanced to the posterior pharynx. Then, the NGT is retrieved though the mouth by using a finger or grasper instrument[18,34,52,57].
A custom EVT sponge is assembled using a polyurethane foam (PUF). The custom sponge is cut to size based on the defect size. Of note, the sponge size is limited to the diameter of the esophagus and overestimation of the sponge size may hinder your ability to visualize the perforation, as there is limited working space with the relatively small diameter of the normal esophagus. In general, the standard size of the sponge is 3 to 7 cm in length and 2-3 cm in diameter. After the sponge is cut to the appropriate size and positioned at the tip of the NGT, the sponge is secured using either silk ties or permanent suture (such as 2-0 or greater prolene or nylon). Finally, a stitch is placed through both the tubing and the sponge at both the proximal and distal ends. To facilitate endoscopic placement and retrieval, a permanent suture is driven to the distal part of tube and tied into a small loop[18,34,52,57].
After the customized sponge system is created, a grasper should be placed through the working channel of the endoscope before insertion into the patient mouth. Then, the short suture loop is grasped with the device. Some authors like to soak the sponge with water-soluble contrast to allow fluoroscopic-assisted placement, however, this is an optional technique. Then, the sponge and the endoscope are lubricated and inserted into the mouth. Due to the size of the system and the endoscope, introduction into the upper esophageal sphincter can be difficult and careful attention should be paid to avoid trauma during insertion[18,34,36,57].
Depending on the size of the perforation, the endoscope should either be driven to the perforation site (if smaller than 10 mm) or should be driven through the perforation into the cavity (if larger than 10 mm) (see topic below: intracavitary and intraluminal EVT). Once inside the cavity, the grasper can be advanced while the endoscope is withdrawn to the GI lumen. Then, the suture loop is released from the grasper. After placement, under endoscopic visualization, the sponge can be pushed or pulled with the grasper to ensure proper position[18,34,36,57].
Once the sponge is in proper position, the NGT is secured to the nose. The suction tubing is hooked up to the vacuum therapy unit and canister. The NGT with the sponge is then attached to the canister tubing using a custom adapter. The vacuum therapy setting frequently used in the GI tract is 125 mmHg of pressure at continuous moderate intensity, however, some authors also describe the use of a higher pressure, to 175 mmHg. If the patient is uncomfortable, or if the patient experiences pooling of secretions above the sponge on the continuous suction setting, the settings can be changed to intermittent suction (5 min on, 2 min off) at the same pressure[18,34,52,57]. It should be mentioned that in patients with a gastrostomy, the procedure described above can be performed via a retrograde fashion through the gastrostomy[34].
There is limited data regarding oral fluid intake in patients during EVT treatment. While the administration of oral fluids may be controversial, in our experience, low volume of clear fluid (for example, 50 cc of water) administered four times daily for comfort need did not impact treatment course.
Intracavitary and Intraluminal EVT
The two techniques of EVT placement, intraluminal (Figure 1A) and intracavity (Figure 1B), are based on where the sponge system is placed[53,58]. In intracavitary placement, a short sponge is typically placed into the extraluminal cavity as a long sponge would be more likely to fold on itself rendering it less effective. With continuous EVT, the cavity ultimately is drained and collapses onto the lumen, which then seals the defect, preventing further contamination. In intraluminal EVT, the sponge system is placed into the GI lumen. In this approach, frequently a long, cylindrical sponge systems is used. When the vacuum is applied, the lumen collapses over the defect zone, and the EVT system keeps the tract dry by draining GI secretions, allowing the defect to seal avoiding contamination[20,53,58]. Independent of where the sponge system is placed, the most important mechanisms of action of EVT are the simultaneous drainage and closure of the defect.
Figure 1.
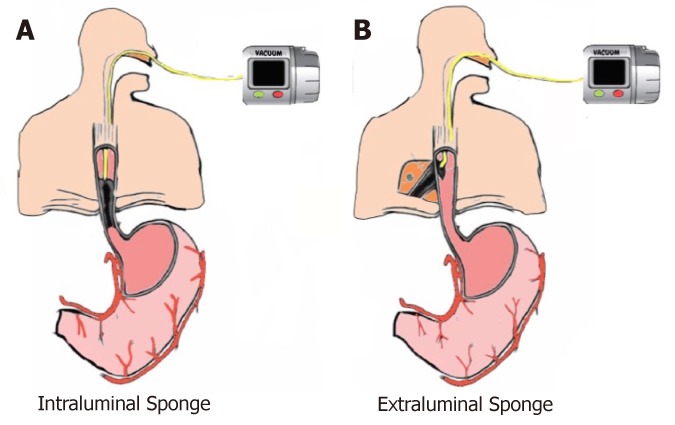
Sponge placement. A: Intraluminal endoscopic vacuum therapy (EVT); B: Intracavitary EVT.
Sponge system exchanges
The sponge system should ideally remain in place for approximately 3 to 5 d at a time. No more than 7 d is recommended. The sponge embeds into the surrounding tissue, and thus, the longer the sponge remains in place, the more difficult it will be to remove. To exchange the sponge, continuous suction should first be turned off. Then, the endoscope is used to drive between the tissue and the sponge interface to dislodge the sponge from the granulation tissue. If the sponge does not dislodge easily with gentle traction, water or saline can be infused into the NGT to disconnect the sponge from the tissue.
It is important to understand that NGT manipulation should be performed carefully because if the NGT is dislodged from the sponge, retrieving the sponge becomes very challenging. This can drastically increase procedure time and risks associated with prolonged procedures. A grasper can also be used to manipulate the sponge and to grab the loop suture in the distal part of the sponge system to remove it. Similar to insertion, the diameter of the sponge is too large to be removed through the nares with the NGT. Thus, the sponge must be removed from the mouth. Once the sponge is outside the mouth, the NGT can be cut with a blade or scissors[18,34,52,57].
Open-pore polyurethane sponge and open-pore film drains
Several open-pore polyurethane sponge drains (OPDs) (Figure 2) and open-pore film drains (OFDs) (Figure 3) have been developed with different advantages[19,20,53,63-67]. In general, short systems (< 5 cm) are used for intracavitary EVT and long systems (> 5 cm) are used for intraluminal therapy[53]. OPDs are more frequently used in EVT compared to OFDs[19,20,53].
Figure 2.
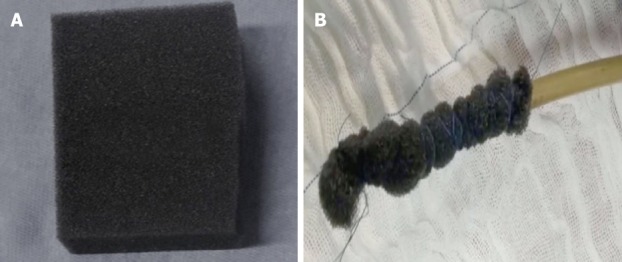
Open-pore polyurethane sponge drain. A: Open-pore polyurethane sponge; B: Open-pore polyurethane sponge drain for endoscopic vacuum therapy.
Figure 3.

Open-pore film drain. A: Open-pore film; B: Open-pore film drain for endoscopic vacuum therapy.
The only commercially available OPD for EVTis the Endosponge® (B. Braun Melsungen AG, Melsungen, Germany) which is marketed for use in the esophagus. However, no electronic pump system has been approved for GI endoscopy therapies yet[53].
OFDs are newer compared to OPDs and have been developed using a very thin open-pore, double-layer, drainage film (Suprasorb® CNP Drainage Film, Lohmann and Rauscher International GmbH and Co; Rengsdorf, Germany), which is approved for vacuum therapy in wound skin defects[19]. The film is wrapped around the openings in the NGT instead of the PUF[53]. These new drains have the advantage of a very small diameter facilitating their introduction through the nares and placement into small wall defects[65]. These drains also have the advantage to adhere well to the intended defect but adhere less tightly to the normal mucosa surrounding the defect during EVT[53]. A combination of the tools, with PUF wrapped with the open-pore film was also reported in some studies[64,66]. Nutritional support is imperative to wound healing, and thus, for EVT in upper GI defects a double lumen drain has been developed with an additional jejunal feeding tube to allow for enteral feeding access[67,68].
Notably, in our experience, we used gauze coated with perforated sterile plastic drain instead of OPDs or OFDs. This technique, described by Dr. Flaubert Sena de Medeiros, is feasible with a lower cost and non-inferior results to other drains systems[69] (Figure 4).
Figure 4.
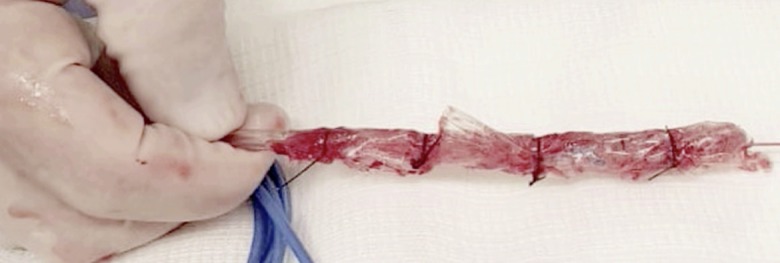
A low cost modified endoscopic vacuum therapy drain system made with a gauze coated with perforated sterile plastic.
Timing and costs
The initial endoscopic vacuum system placement takes approximately 30 to 60 min, including diagnostic endoscopy, evaluation (with or without dilation), irrigation, and placement of the sponge system. Subsequent sponge system exchanges take approximately 30 min of procedural time[57]. One study evaluated the cost of EVT use and demonstrated that for an average treatment span of 25 d, including 8 sponge exchanges per patient, the total cost per patient was approximately $10118.00[57].
EFFICACY
EVT efficacy in the treatment of transmural GI defects is well reported in case series, cohort studies and systematic reviews. To date, no randomized control trials have been published comparing EVT versus other surgical or endoscopic techniques. In this section, the efficacy of EVT will be discussed with regards to management of transmural GI defects, including those involving the esophagus, stomach (post-bariatric complications), small bowel, biliopancreatic, and lower GI tract.
Upper GI defects
The successful use of EVT in upper GI defects was first published in 2008[70]. In this report, two patients with intrathoracic anastomotic leaks after esophagectomy and gastrectomy were successfully treated with a mean of 5 sponge exchanges over a mean of 15 d, without adverse events. After this report, different centers published on the use of EVT in upper GI transmural defects. To date, the most common use of EVT in the upper GI tract has been for closure of esophageal defects[20,57-59,62,71-74]. The inspiration and expiration respiratory movements associated with EVT facilitate the extraluminal transport of even small amounts of fluids[53].
In acute perforations, EVT has shown satisfactory results in several studies. Loske et al[75] demonstrated in a series with 10 patients, including iatrogenic perforations from the cricopharyngeal to the gastroesophageal junction, that all patients were successfully treated within a median of 3 to 7 d without any associated adverse events or need for adjunctive therapy. Kuehn et al[60] demonstrated a similar clinical success rate of 100% in a separate series including 10 patients with acute perforation (8 iatrogenic and 2 Boerhaave). And finally, Heits et al[76] published their study which evaluated the efficacy of EVT in esophageal acute perforations (iatrogenic, spontaneous, and foreign body-associated), showing a primary clinical success of 90% with a mean sponge exchange of 5.4 (2 to 12) and a period of 19 ± 14.26 d.
The majority of studies on the use of EVT in upper GI endoscopy are related to the treatment of intrathoracic leaks, including the use of EVT as primary or as a rescue therapy (Figure 5). In these studies, the efficacy rate of EVT varies from 66.7% to 100%[58,59,73,77,78], with two of these studies demonstrating an efficacy of 100% without any adverse event[77,78].
Figure 5.
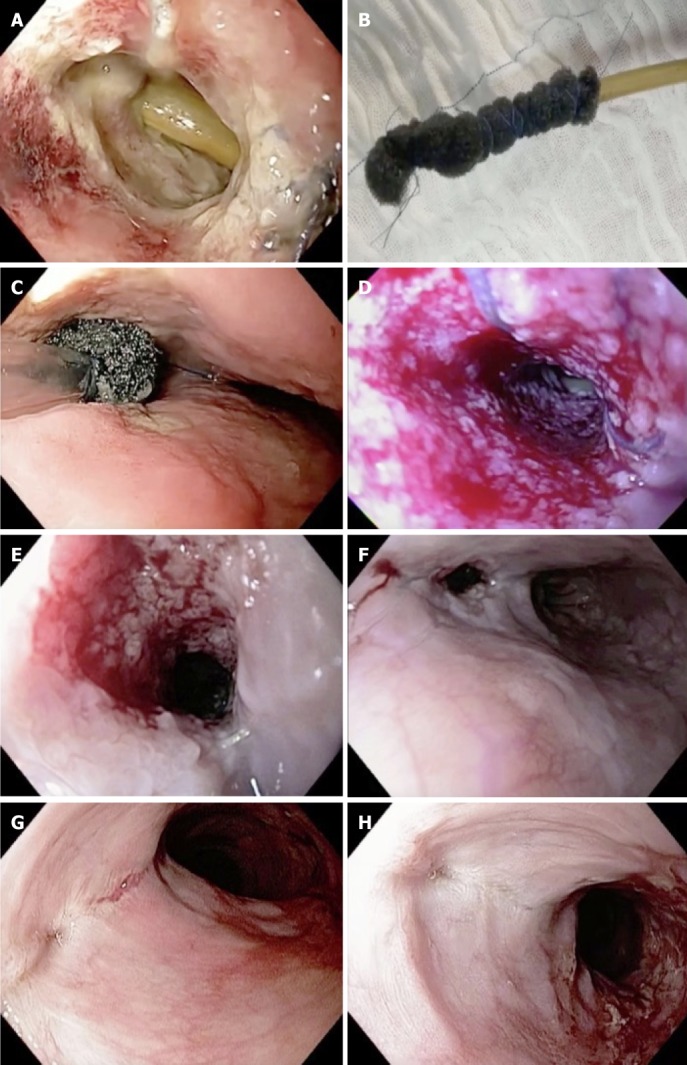
Endoscopic vacuum therapy in the management of an esophageal defect. A: Complete dehiscence of the esophageal leak and the mediastinal drainage; B: Open-pore polyurethane foam drain; C: Intracavitary sponge placement; D: Granulation tissue after second sponge exchange; E: Granulation tissue after fourth sponge exchange; F: Reduction of the defect size after seven sponge exchanges; G: Complete closure after nine sponge exchanges; H: Scar after esophageal closure with endoscopic vacuum therapy.
There are several cohort studies comparing the use of EVT with other techniques in the management of esophageal leaks[27,79-83]. In one retrospective analysis comparing EVT versus self-expandable stents (metal and plastic stents), overall closure rate was 84.4% for EVT versus 53.8% for the stent group. Additionally, a multivariate analysis showed successful defect closure was independently associated with EVT[79]. The superiority of EVT compared to SEMS was confirmed in two other comparative studies[81,82]. Additionally, Manfredi et al[34] showed the superiority of EVT compared to stents in pediatric patients (mean age 24 mo) showing successful closure in 88% of patients who underwent EVT versus 63% of patients who had stent placement. The largest series comparing EVT versus other approaches in the management of leak after esophagectomy showed that EVT is superior to surgical revision, stent placement, and conservative management[80]. These results were confirmed in a recent systematic review and meta-analysis[83], showing that the esophageal defect closure rate is significantly higher in EVT than SEMS, with a shorter treatment duration, lower major complication rate, and lower in-hospital mortality.
The indications for use of EVT in the upper GI tract are expanding to different applications. A recent series[55] demonstrated the use of EVT in the management of anastomotic ischemia, without active leak, after esophageal resections. This study showed interesting results; 75% of the patients developed complete mucosal recovery, while the other 25% of patients developed a leak during the use of EVT. However, these leaks were ultimately successfully treated with EVT. With the increase in the use of EVT, a recent study[28] evaluating patients who underwent EVT in the treatment of esophageal transmural defects concluded that EVT is well tolerated with a satisfactory long-term quality of life.
Post-bariatric surgery complications
Obesity is a pandemic and bariatric and metabolic surgery is the most effective treatment. Despite satisfactory clinical results, the number of adverse events, including leaks and fistulas, after bariatric surgery has increased[1,84-90]. Therefore, the use of EVT in the post-bariatric surgery setting is increasing. While older management algorithms published in 2015 and 2016, did not cite the EVT approach[91,92] as a management option, those from more recent years have proposed the use of EVT in both early and chronic settings[1,93].
A recent study[94], demonstrated the use of EVT in patients with early infradiaphragmatic leakage after bariatric surgery, including laparoscopic sleeve gastrectomy (LSG) and RYGB. In this series, some cases were performed with EVT alone and others with EVT with stent (stent-over-sponge). In 80% of patients, the leak was connected to abscess cavities. Clinical success, defined as no signs of persistent leakage, was achieved in all patients studied.
In a study including patients with acute, early, late, and chronic leaks after sleeve gastrectomy, the use of EVT was associated with 100% resolution of leaks confirmed by upper GI series, with an average of 10.3 sponge exchanges over an average of 50 d[95]. The satisfactory results of EVT in the management of post-LSG leaks was confirmed in other reports[30,96]. However, in contrast to those results, one report demonstrated a case in which the EVT failed to heal a staple line leak after a revisional bariatric surgery (adjustable gastric band to LSG)[97].
In terms of the RYGB subgroup, one group performed a study in a porcine model, performing 10 RYGB. The gastrojejunal anastomoses were fashioned, and a 2 cm defect was created across the staple line. Seven of the ten pigs received EVT and three were included in the control group that did not receive any therapy. All porcine treated with EVT had complete healing of the defect and all control porcines had persistent leak, demonstrating that EVT can be effective in the management of gastrojejunal anastomotic leaks[98]. In humans, while there is limited data for the use of EVT for gastrojejunal leaks, one case report demonstrated the successful use of EVT in the treatment of a post-RYGB leak which had failed prior endoscopic attempt with CSEMS[99]. Additionally, a case report[56] showed a complete reperfusion and epithelization of an ischemic blind jejunal loop after RYGB with EVT management.
Small bowel and biliopancreatic defects
There are several reports of the use of EVT in the management of duodenal wall defects[100-103], including leaks and perforation[29,64,100-103]. Depending on the location of the defect, the sponge system can be placed either via nasal/oral or via percutaneous stoma, such as gastrostomy and jejunostomy, in cases where the defect is located distal to the duodenum[92,100,104].
The use of EVT has been successfully reported in treatment of duodenal iatrogenic perforations during endoscopic procedures such as ERCP[100] and post argon plasma coagulation, after endoscopic mucosal resection of an adenocarcinoma[102], and in the management of post-surgical complications[29,101,102].
The successful use of EVT has also been reported in the management of post-surgical duodenal leaks[64,103]. Loske et al[64] reported the treatment of a duodenal leak with EVT using the pull-through technique along an intestinal-cutaneous fistula. In this case, the sponge was placed in the internal opening of the duodenal fistula. The EVT application resulted in closure of the defect next to the tube and internal drainage of the GI/pancreatobiliary secretions, immediately stopping external drainage. After 3 sponge exchanges over the course of 14 d, the EVT was removed, and at 3-mo follow-up, the defect was completed healed.
The use of EVT has also been reported in the treatment of biliopancreatic conditions including infected pancreatic fluid collections and post-pancreatic surgery[32,66,105-107]. Several case reports[66,105,106] have described the successful multi-step use of EVT in infected pancreatic collections. First, an endoscopic drainage with stent is performed. Then, after at least 1 wk, the stent is removed, followed by dilation of the tract and placement of the EVT system. However, despite the favorable results of EVT in the management of pancreatic fluid collections shown in these reports, there is a theoretical risk of massive hemorrhage when performing this technique in the region of the celiac trunk and portal venous system[66]. Due to this risk, we recommend endoscopic drainage with stents as a first approach and EVT as a rescue therapy in selected cases[108-110].
EVT has also been described in the management of complications after biliopancreatic surgery[32,106,107]. Loske et al[107] described the treatment of a dehiscence of the biliojejunal and pancreaticojejunal anastomoses with EVT in a patient with a previous gastroenterostomy. A separate report[106] showed the feasibility and efficacy of EVT using a long sponge (12 cm in length) placed in the stomach for the treatment of a pancreatic-gastric anastomosis dehiscence. Additionally, a third case report demonstrated the successful use of EVT with a two-sided sponge using the pull-through technique in the treatment of a pancreaticgastrostomy[32].
Lower GI defects
Anastomotic leak is the most significant adverse event after colorectal surgery, with a range of occurrence between 1.5% to 23%, and is considered the major cause of postoperative morbidity and mortality[111,112]. The best approach for the treatment of anastomotic leaks has not been identified yet, especially in lower anastomoses[113]. The management decision in this population must be based on the clinical condition of the patient, including operative intervention for unstable patients (i.e., those with peritonitis), and more conservative modalities for stable patients[111,112].
Endoscopic modalities, including stents, fibrin glue, clips, cap mounted clips, and double pigtail catheter drainage show variable success in the management of lower GI defects[112-117]. In 2003, Weidenhagen et al[25] described the first use of EVT in the lower GI tract for sepsis control caused by an anastomotic leak after a rectal surgery, showing a successful outcome. After this favorable report, the use of EVT in the management of lower GI defects increased and several studies were published showing a high efficacy and safety profile[113,118-120].
The first study evaluating EVT in the treatment of anastomotic leak after low anterior resection (LAR)[113] included 29 patients and showed 90.3% successful closure with a mean of 11.4 ± 6.3 sponge exchanges and a duration of 34.4 ± 19.4 d. In this study, most of patients had a protected stoma created at the primary surgery. In a retrospective study[118] including anastomotic leak after rectal resection, Hartmann’s stump insufficiency, and rectal perforation, EVT demonstrated an 83% closure rate overall. For those patients with anastomotic leak, the closure rate success was 90%, similar to several other studies[121-123]. The German multicenter study[120] using EVT in the treatment of anastomotic leakage after colorectal surgery, including patients with rectal cancer and ulcerative colitis, analyzed the use of EVT after anastomotic leakage after colorectal surgery in two groups. One group were those patients whom underwent treatment within 6 wk post-operatively and the second group after 6 wk post-operatively. Patients whom underwent the procedure within 6 wk post-surgery had a higher closure rate (75% vs 38%). In this study, closure was achieved in a median of 40 d with a mean of 13 sponge exchanges.
One concern in the use of EVT in lower GI tract is that the feces may block the vacuum system, and thus, in some centers, physicians limit the use of EVT to those patients with fecal diversions. However, several studies have included patients without fecal diversion, and have shown efficacy of the method, suggesting that the lack of fecal diversion is not an exclusion criteria for EVT[119,124-127]. A study comparing the use of EVT in patients with and without stoma is needed to confirm this hypothesis.
Recently, a systematic review[112] including 14 studies (case series and cohort studies) with a total of 197 patients with anastomotic leakage treated with EVT showed an overall successful closure rate of 88.8%, with very low rates of adverse events.
SAFETY
In general, EVT is a safe procedure with a low rate of adverse events. The most common complaint from patients during EVT treatment is related to the NGT, as this can cause significant patient discomfort, including pain, nausea, and emesis, especially in those patients with an additional nasoenteral tube. Additionally, patients have reported distress over having to undergo numerous repeat procedures for sponge exchanges[26,51,62].
The most frequent adverse events are sponge dislocation, minor bleeding after sponge exchange due to ingrowth of granulation tissue into the sponge, and anastomotic strictures. However, major bleeding events have also been reported[26,51,60,62].
One major concern regarding EVT in the upper GI tract is the risk of major bleeding, due to the risk of development of a fistula between the cavity and the aorta (or aortic branches), as well as formation and rupture of pseudoaneurysm involving vessels or heart chambers due to the ongoing inflammatory process of EVT[51,62]. Unfortunately, several studies have reported major bleeding events. A prospective study[26] including 52 patients with upper GI defects treated with EVT reported 4.1% minor adverse events, including sponge dislocations and minor bleeding after sponge removal. Minor bleeding was usually self-limited and more frequent sponge exchanges could potentially mitigate this risk.
However, more notably, in this study, two patients died due to major bleeding related to EVT. One patient died from acute hemorrhage 56 d after initial EVT placement. The other patient died 12 d after initial EVT placement due to a non-manageable hemorrhage after sponge removal during the third sponge exchange. In this case, authors believe that a rupture of the descending aorta occurred. In a case series[60] including 5 patients that were successfully treated with EVT, two anastomotic strictures were reported. In both cases dilation with bougies were performed. One of these patients had two dilations without adverse events. The other patient had severe bleeding after dilation and unfortunately died, with cause of death on autopsy being identified as an aortoesophageal fistula leading to hemorrhagic shock. In a retrospective study[62] including 21 patients, two bleeding events (10%) were reported. One bleeding event occurred from the pancreas during treatment of a posterior gastric perforation and the other bleeding event occurred from an aortic branch during treatment of an esophageal anastomotic leak. In these two cases, fresh blood was seen in the EVT output fluid and the EVT was terminated immediately. Both patients underwent surgery for aortic stenting.
Based on these major bleeding reports, if a significant bleed occurs during treatment, EVT should be stopped and a triple-phase CT performed to direct possible management. Additionally, the CT scan should be reviewed prior to starting EVT in the upper GI tract to exclude vascular issues.
CONCLUSION
EVT is a new option in the management of GI transmural defects. EVT use has been increasing and appears to be effective in the treatment of this condition as a first line therapy, as well as a salvage procedure when other options have failed. The most experience with EVT is in the treatment of esophageal transmural defects, showing better results than any other therapy. However, due to the major bleeding risks associated with this technique, patients should undergo this procedure in experienced centers and be monitored closely for adverse events.
Footnotes
Conflict-of-interest statement: Eduardo Guimaraes Hourneaux de Moura, consultant for Boston Scientific and Olympus Moura, E is a consultant to Boston Scientific; Christopher C Thompson, reports fee as a consultant for Boston Scientific and Medtronic; fees as consultant and institutional grants from USGE Medical, Olympus, and Apollo Endosurgery. All other authors declare that they have no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: April 12, 2019
First decision: April 13, 2019
Article in press: May 1, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gkekas I, Richardson WS S-Editor: Ji FF L-Editor: A E-Editor: Xing YX
Contributor Information
Diogo Turiani Hourneaux de Moura, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women’s Hospital - Harvard Medical School, Boston, MA 02115, United States; Department of Endoscopy of Clinics Hospital of São Paulo University, São Paulo 05403-000, Brazil.
Bruna Furia Buzetti Hourneaux de Moura, Esophageal and Airway Atresia Treatment Center, Boston Children's Hospital - Harvard Medical School, Boston, MA 02115, United States.
Michael A Manfredi, Esophageal and Airway Atresia Treatment Center, Boston Children's Hospital - Harvard Medical School, Boston, MA 02115, United States.
Kelly E Hathorn, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women’s Hospital - Harvard Medical School, Boston, MA 02115, United States.
Ahmad N Bazarbashi, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women’s Hospital - Harvard Medical School, Boston, MA 02115, United States.
Igor Braga Ribeiro, Department of Endoscopy of Clinics Hospital of São Paulo University, São Paulo 05403-000, Brazil.
Eduardo Guimarães Hourneaux de Moura, Department of Endoscopy of Clinics Hospital of São Paulo University, São Paulo 05403-000, Brazil.
Christopher C Thompson, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women’s Hospital - Harvard Medical School, Boston, MA 02115, United States.
References
- 1.de Moura DTH, Sachdev AH, Thompson CC. Endoscopic Full-Thickness Defects and Closure Techniques. Curr Treat Options Gastroenterol. 2018;16:386–405. doi: 10.1007/s11938-018-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goenka MK, Goenka U. Endotherapy of leaks and fistula. World J Gastrointest Endosc. 2015;7:702–713. doi: 10.4253/wjge.v7.i7.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez JM, Lorenzo D, Guilbaud T, Bège T, Barthet M. Internal endoscopic drainage as first line or second line treatment in case of postsleeve gastrectomy fistulas. Endosc Int Open. 2018;6:E745–E750. doi: 10.1055/s-0044-101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moura EG, Ferreira FC, Cheng S, Moura DT, Sakai P, Zilberstain B. Duodenal stenting for malignant gastric outlet obstruction: prospective study. World J Gastroenterol. 2012;18:938–943. doi: 10.3748/wjg.v18.i9.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Moura DT, Mestieri LH, Cheng S, Rodela GL, De Moura EG, Sakai P, Oliveira JF, Artifon EL. Natural orifice transluminal endoscopic surgery to salvage a migrated stent during EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2016;83:656–657. doi: 10.1016/j.gie.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro IB, Bernardo WM, Martins BDC, de Moura DTH, Baba ER, Josino IR, Miyahima NT, Coronel Cordero MA, Visconti TAC, Ide E, Sakai P, de Moura EGH. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E558–E567. doi: 10.1055/a-0591-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Moura DTH, Ribeiro IB, Funari MP, Baptista A, Thompson CC, de Moura EGH. Novel use of a cardiac septal occluder to treat a chronic recalcitrant bariatric fistula after Roux-en-Y gastric bypass. Endoscopy. 2019;51:E111–E112. doi: 10.1055/a-0842-6287. [DOI] [PubMed] [Google Scholar]
- 8.Rogalski P, Daniluk J, Baniukiewicz A, Wroblewski E, Dabrowski A. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol. 2015;21:10542–10552. doi: 10.3748/wjg.v21.i37.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro IB, de Moura DTH, Thompson CC, de Moura EGH. Acute abdominal obstruction: Colon stent or emergency surgery? An evidence-based review. World J Gastrointest Endosc. 2019;11:193–208. doi: 10.4253/wjge.v11.i3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bemelman WA, Baron TH. Endoscopic Management of Transmural Defects, Including Leaks, Perforations, and Fistulae. Gastroenterology. 2018;154:1938–1946.e1. doi: 10.1053/j.gastro.2018.01.067. [DOI] [PubMed] [Google Scholar]
- 11.de Moura EG, Silva GL, de Moura ET, Pu LZ, de Castro VL, de Moura DT, Sallum RA. Esophageal perforation after epicardial ablation: an endoscopic approach. Endoscopy. 2015;47 Suppl 1 UCTN:E592–E593. doi: 10.1055/s-0034-1393594. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki O, Bernardo WM, Brunaldi VO, Junior CCC, Minata MK, de Moura DTH, de Souza TF, Campos JM, Santo MA, de Moura EGH. Efficacy and Safety of Stents in the Treatment of Fistula After Bariatric Surgery: a Systematic Review and Meta-analysis. Obes Surg. 2018;28:1788–1796. doi: 10.1007/s11695-018-3236-6. [DOI] [PubMed] [Google Scholar]
- 13.Hourneaux de Moura DT, Jirapinyo P, Hathorn KE, Thompson CC. Use of a cardiac septal occluder in the treatment of a chronic GI fistula: What should we know before off-label use in the GI tract? VideoGIE. 2018;4:114–117. doi: 10.1016/j.vgie.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar N, Thompson CC. A novel method for endoscopic perforation management by using abdominal exploration and full-thickness sutured closure. Gastrointest Endosc. 2014;80:156–161. doi: 10.1016/j.gie.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baptista A, Hourneaux De Moura DT, Jirapinyo P, Hourneaux De Moura EG, Gelrud A, Kahaleh M, Salinas A, Sabagh LC, Ospina A, Rincones VZ, Doval R, Bandel JW, Thompson CC. Efficacy of the cardiac septal occluder in the treatment of post-bariatric surgery leaks and fistulas. Gastrointest Endosc. 2019;89:671–679.e1. doi: 10.1016/j.gie.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 16.Haito-Chavez Y, Kumbhari V, Ngamruengphong S, De Moura DT, El Zein M, Vieira M, Aguila G, Khashab MA. Septotomy: an adjunct endoscopic treatment for post-sleeve gastrectomy fistulas. Gastrointest Endosc. 2016;83:456–457. doi: 10.1016/j.gie.2015.08.065. [DOI] [PubMed] [Google Scholar]
- 17.de Moura EG, Galvão-Neto MP, Ramos AC, de Moura ET, Galvão TD, de Moura DT, Ferreira FC. Extreme bariatric endoscopy: stenting to reconnect the pouch to the gastrojejunostomy after a Roux-en-Y gastric bypass. Surg Endosc. 2012;26:1481–1484. doi: 10.1007/s00464-011-2060-z. [DOI] [PubMed] [Google Scholar]
- 18.Loske G, Müller CT. Tips and tricks for endoscopic negative pressure therapy. Chirurg. 2019;90:7–14. doi: 10.1007/s00104-018-0725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loske G, Schorsch T, Rucktaeschel F, Schulze W, Riefel B, van Ackeren V, Mueller CT. Open-pore film drainage (OFD): a new multipurpose tool for endoscopic negative pressure therapy (ENPT) Endosc Int Open. 2018;6:E865–E871. doi: 10.1055/a-0599-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Moura DTH, Brunaldi VO, Minata M, Riccioppo D, Santo MA, de Moura EGH. Endoscopic vacuum therapy for a large esophageal perforation after bariatric stent placement. VideoGIE. 2018;3:346–348. doi: 10.1016/j.vgie.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton NJ, Sharrock A, Rickard R, Mughal M. Systematic review of the use of endo-luminal topical negative pressure in oesophageal leaks and perforations. Dis Esophagus. 2017;30:1–5. doi: 10.1111/dote.12531. [DOI] [PubMed] [Google Scholar]
- 22.Lalezari S, Lee CJ, Borovikova AA, Banyard DA, Paydar KZ, Wirth GA, Widgerow AD. Deconstructing negative pressure wound therapy. Int Wound J. 2017;14:649–657. doi: 10.1111/iwj.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orgill DP, Bayer LR. Update on negative-pressure wound therapy. Plast Reconstr Surg. 2011;127 Suppl 1:105S–115S. doi: 10.1097/PRS.0b013e318200a427. [DOI] [PubMed] [Google Scholar]
- 24.Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51:301–331. doi: 10.1067/j.cpsurg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Weidenhagen R, Spelsberg F, Lang R, Jauch KW. New method for sepsis control caused by anastomotic leakage in rectal surgery: the Endo-VAC. Color Dis. 2003;5:1–4. [Google Scholar]
- 26.Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes D, Senninger N, Vowinkel T. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc. 2017;31:2687–2696. doi: 10.1007/s00464-016-5265-3. [DOI] [PubMed] [Google Scholar]
- 27.Berlth F, Bludau M, Plum PS, Herbold T, Christ H, Alakus H, Kleinert R, Bruns CJ, Hölscher AH, Chon SH. Self-Expanding Metal Stents Versus Endoscopic Vacuum Therapy in Anastomotic Leak Treatment After Oncologic Gastroesophageal Surgery. J Gastrointest Surg. 2019;23:67–75. doi: 10.1007/s11605-018-4000-x. [DOI] [PubMed] [Google Scholar]
- 28.Dhayat SA, Schacht R, Mennigen R, Palmes D, Vogel T, Vowinkel T, Senninger N, Laukoetter MG. Long-Term Quality of Life Assessment After Successful Endoscopic Vacuum Therapy of Defects in the Upper Gastrointestinal Tract Quality of Life After EVT. J Gastrointest Surg. 2019;23:280–287. doi: 10.1007/s11605-018-4038-9. [DOI] [PubMed] [Google Scholar]
- 29.Kelm M, Seyfried F, Reimer S, Krajinovic K, Miras AD, Jurowich C, Germer CT, Brand M. Proximal jejunal stoma as ultima ratio in case of traumatic distal duodenal perforation facilitating successful EndoVAC® treatment: A case report. Int J Surg Case Rep. 2017;41:401–403. doi: 10.1016/j.ijscr.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt F, Mennigen R, Vowinkel T, Neumann PA, Senninger N, Palmes D, Laukoetter MG. Endoscopic Vacuum Therapy (EVT)-a New Concept for Complication Management in Bariatric Surgery. Obes Surg. 2017;27:2499–2505. doi: 10.1007/s11695-017-2783-6. [DOI] [PubMed] [Google Scholar]
- 31.Wettstein M, Frieling T, Lüthen R, Heintges T, Niederau C, Oette M, Vogt C, Vom Dahl S. [Endoscopic therapy for leaks in the gastrointestinal tract, the bile ducts and the pancreas] Z Gastroenterol. 2011;49:740–748. doi: 10.1055/s-0031-1273422. [DOI] [PubMed] [Google Scholar]
- 32.Knoop RF, Thimme R, Fischer A. Successful two-sided sponge pull-through treatment of anastomotic leakage following pancreaticoduodenectomy with pancreaticogastrostomy. Endoscopy. 2017;49:1010–1012. doi: 10.1055/s-0043-113552. [DOI] [PubMed] [Google Scholar]
- 33.Borstlap WAA, Musters GD, Stassen LPS, van Westreenen HL, Hess D, van Dieren S, Festen S, van der Zaag EJ, Tanis PJ, Bemelman WA. Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surg Endosc. 2018;32:315–327. doi: 10.1007/s00464-017-5679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manfredi MA, Clark SJ, Staffa SJ, Ngo PD, Smithers CJ, Hamilton TE, Jennings RW. Endoscopic Esophageal Vacuum Therapy: A Novel Therapy for Esophageal Perforations in Pediatric Patients. J Pediatr Gastroenterol Nutr. 2018;67:706–712. doi: 10.1097/MPG.0000000000002073. [DOI] [PubMed] [Google Scholar]
- 35.Panayi AC, Leavitt T, Orgill DP. Evidence based review of negative pressure wound therapy. World J Dermatology. 2017;6:1. [Google Scholar]
- 36.Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117:121S–126S. doi: 10.1097/01.prs.0000225450.12593.12. [DOI] [PubMed] [Google Scholar]
- 37.Kairinos N, Solomons M, Hudson DA. Negative-pressure wound therapy I: the paradox of negative-pressure wound therapy. Plast Reconstr Surg. 2009;123:589–598; discussion 599-600. doi: 10.1097/PRS.0b013e3181956551. [DOI] [PubMed] [Google Scholar]
- 38.Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg. 2008;122:786–797. doi: 10.1097/PRS.0b013e31818237ac. [DOI] [PubMed] [Google Scholar]
- 39.Borgquist O, Ingemansson R, Malmsjö M. The influence of low and high pressure levels during negative-pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg. 2011;127:551–559. doi: 10.1097/PRS.0b013e3181fed52a. [DOI] [PubMed] [Google Scholar]
- 40.Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086–1096; discussion 1097-1098. doi: 10.1097/01.prs.0000135330.51408.97. [DOI] [PubMed] [Google Scholar]
- 41.Erba P, Ogawa R, Ackermann M, Adini A, Miele LF, Dastouri P, Helm D, Mentzer SJ, D'Amato RJ, Murphy GF, Konerding MA, Orgill DP. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg. 2011;253:402–409. doi: 10.1097/SLA.0b013e31820563a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, Orgill DP. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg. 2006;56:418–422. doi: 10.1097/01.sap.0000202831.43294.02. [DOI] [PubMed] [Google Scholar]
- 43.Timmers MS, Le Cessie S, Banwell P, Jukema GN. The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55:665–671. doi: 10.1097/01.sap.0000187182.90907.3d. [DOI] [PubMed] [Google Scholar]
- 44.Malsiner CC, Schmitz M, Horch RE, Keller AK, Leffler M. Vessel transformation in chronic wounds under topical negative pressure therapy: an immunohistochemical analysis. Int Wound J. 2015;12:501–509. doi: 10.1111/iwj.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–76; discussion 577. [PubMed] [Google Scholar]
- 46.Lancerotto L, Bayer LR, Orgill DP. Mechanisms of action of microdeformational wound therapy. Semin Cell Dev Biol. 2012;23:987–992. doi: 10.1016/j.semcdb.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Mouës CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 48.Saadi A, Perentes JY, Gonzalez M, Tempia AC, Wang Y, Demartines N, Ris HB, Krueger T. Vacuum-assisted closure device: a useful tool in the management of severe intrathoracic infections. Ann Thorac Surg. 2011;91:1582–1589. doi: 10.1016/j.athoracsur.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 49.Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg. 2006;118:390–397; discussion 398-400. doi: 10.1097/01.prs.0000227675.63744.af. [DOI] [PubMed] [Google Scholar]
- 50.Patmo AS, Krijnen P, Tuinebreijer WE, Breederveld RS. The Effect of Vacuum-Assisted Closure on the Bacterial Load and Type of Bacteria: A Systematic Review. Adv Wound Care (New Rochelle) 2014;3:383–389. doi: 10.1089/wound.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virgilio E, Ceci D, Cavallini M. Surgical Endoscopic Vacuum-assisted Closure Therapy (EVAC) in Treating Anastomotic Leakages After Major Resective Surgery of Esophageal and Gastric Cancer. Anticancer Res. 2018;38:5581–5587. doi: 10.21873/anticanres.12892. [DOI] [PubMed] [Google Scholar]
- 52.Leeds SG, Mencio M, Ontiveros E, Ward MA. Endoluminal Vacuum Therapy: How I Do It. J Gastrointest Surg. 2019;23:1037–1043. doi: 10.1007/s11605-018-04082-z. [DOI] [PubMed] [Google Scholar]
- 53.Loske G. Endoscopic negative pressure therapy of the upper gastrointestinal tract. Chirurg. 2019;90:1–6. doi: 10.1007/s00104-018-0727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kondo A, de Moura EG, Bernardo WM, Yagi OK, de Moura DT, de Moura ET, Bravo JG, Yamazaki K, Sakai P. Endoscopy vs surgery in the treatment of early gastric cancer: Systematic review. World J Gastroenterol. 2015;21:13177–13187. doi: 10.3748/wjg.v21.i46.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann PA, Mennigen R, Palmes D, Senninger N, Vowinkel T, Laukoetter MG. Pre-emptive endoscopic vacuum therapy for treatment of anastomotic ischemia after esophageal resections. Endoscopy. 2017;49:498–503. doi: 10.1055/s-0042-123188. [DOI] [PubMed] [Google Scholar]
- 56.Loske G, Schorsch T, Schmidt-Seithe H, Müller C. Intraluminal endoscopic vacuum therapy in a case of ischemia of the blind end of the jejunal loop after Roux-en-Y gastrectomy. Endoscopy. 2014;46 Suppl 1 UCTN:E575–E576. doi: 10.1055/s-0034-1390913. [DOI] [PubMed] [Google Scholar]
- 57.Ooi G, Burton P, Packiyanathan A, Loh D, Chen R, Shaw K, Brown W, Nottle P. Indications and efficacy of endoscopic vacuum-assisted closure therapy for upper gastrointestinal perforations. ANZ J Surg. 2018;88:E257–E263. doi: 10.1111/ans.13837. [DOI] [PubMed] [Google Scholar]
- 58.Loske G, Schorsch T, Müller C. Intraluminal and intracavitary vacuum therapy for esophageal leakage: a new endoscopic minimally invasive approach. Endoscopy. 2011;43:540–544. doi: 10.1055/s-0030-1256345. [DOI] [PubMed] [Google Scholar]
- 59.Kuehn F, Schiffmann L, Janisch F, Schwandner F, Alsfasser G, Gock M, Klar E. Surgical Endoscopic Vacuum Therapy for Defects of the Upper Gastrointestinal Tract. J Gastrointest Surg. 2016;20:237–243. doi: 10.1007/s11605-015-3044-4. [DOI] [PubMed] [Google Scholar]
- 60.Ahrens M, Schulte T, Egberts J, Schafmayer C, Hampe J, Fritscher-Ravens A, Broering DC, Schniewind B. Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy. 2010;42:693–698. doi: 10.1055/s-0030-1255688. [DOI] [PubMed] [Google Scholar]
- 61.Bludau M, Hölscher AH, Herbold T, Leers JM, Gutschow C, Fuchs H, Schröder W. Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC) Surg Endosc. 2014;28:896–901. doi: 10.1007/s00464-013-3244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pournaras DJ, Hardwick RH, Safranek PM, Sujendran V, Bennett J, Macaulay GD, Hindmarsh A. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J Surg. 2018;42:2507–2511. doi: 10.1007/s00268-018-4463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischer A, Thimme R, Hopt UT, Richter-Schrag HJ. Two-sided sponge (TSS) treatment: Description of a novel device and technique for endoscopic vacuum treatment (EVT) in the upper gastrointestinal tract. Endosc Int Open. 2016;4:E937–E940. doi: 10.1055/s-0042-111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loske G, Liedke M, Schlöricke E, Herrmann T, Rucktaeschel F. Endoscopic negative-pressure therapy for duodenal leakage using new open-pore film and polyurethane foam drains with the pull-through technique. Endoscopy. 2017;49:E300–E302. doi: 10.1055/s-0043-119346. [DOI] [PubMed] [Google Scholar]
- 65.Loske G, Schorsch T, Müller CT. Prevention of reflux after esophagectomy with endoscopic negative pressure therapy using a new double-lumen open-pore film drainage with an intestinal feeding tube. Endoscopy. 2017;49:E294–E295. doi: 10.1055/s-0043-118211. [DOI] [PubMed] [Google Scholar]
- 66.Wallstabe I, Tiedemann A, Schiefke I. Endoscopic vacuum-assisted therapy of infected pancreatic pseudocyst using a coated sponge. Endoscopy. 2012;44 Suppl 2 UCTN:E49–E50. doi: 10.1055/s-0031-1291525. [DOI] [PubMed] [Google Scholar]
- 67.Loske G, Aumiller J, Rucktäschel F, Schorsch T. Spontaneous perforation of an intramural esophageal pseudodiverticulosis treated with intraluminal endoscopic vacuum therapy using a double-lumen vacuum drainage with intestinal feeding tube. Endoscopy. 2016;48 Suppl 1:E154–E155. doi: 10.1055/s-0042-105364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SY, Kim KW, Lee JI, Park DK, Park KY, Park CH, Son KH. Esophageal Endoscopic Vacuum Therapy with Enteral Feeding Using a Sengstaken-Blakemore Tube. Korean J Thorac Cardiovasc Surg. 2018;51:76–80. doi: 10.5090/kjtcs.2018.51.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamamoto F, Lima ALM, Rezende MR, Mattar-Junior R, Leonhardt MC, Kojima KE, Santos CCD. A new low-cost negative-pressure wound therapy versus a commercially available therapy device widely used to treat complex traumatic injuries: a prospective, randomized, non-inferiority trial. Clinics (Sao Paulo) 2017;72:737–742. doi: 10.6061/clinics/2017(12)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wedemeyer J, Schneider A, Manns MP, Jackobs S. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc. 2008;67:708–711. doi: 10.1016/j.gie.2007.10.064. [DOI] [PubMed] [Google Scholar]
- 71.Noh SM, Ahn JY, Lee JH, Jung HY, AlGhamdi Z, Kim HR, Kim YH. Endoscopic Vacuum-Assisted Closure Therapy in Patients with Anastomotic Leakage after Esophagectomy: A Single-Center Experience. Gastroenterol Res Pract. 2018;2018:1697968. doi: 10.1155/2018/1697968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Still S, Mencio M, Ontiveros E, Burdick J, Leeds SG. Primary and Rescue Endoluminal Vacuum Therapy in the Management of Esophageal Perforations and Leaks. Ann Thorac Cardiovasc Surg. 2018;24:173–179. doi: 10.5761/atcs.oa.17-00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wedemeyer J, Brangewitz M, Kubicka S, Jackobs S, Winkler M, Neipp M, Klempnauer J, Manns MP, Schneider AS. Management of major postsurgical gastroesophageal intrathoracic leaks with an endoscopic vacuum-assisted closure system. Gastrointest Endosc. 2010;71:382–386. doi: 10.1016/j.gie.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 74.Loske G, Schorsch T, Müller C. Endoscopic vacuum sponge therapy for esophageal defects. Surg Endosc. 2010;24:2531–2535. doi: 10.1007/s00464-010-0998-x. [DOI] [PubMed] [Google Scholar]
- 75.Loske G, Schorsch T, Dahm C, Martens E, Müller C. Iatrogenic perforation of esophagus successfully treated with Endoscopic Vacuum Therapy (EVT) Endosc Int Open. 2015;3:E547–E551. doi: 10.1055/s-0034-1392566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heits N, Stapel L, Reichert B, Schafmayer C, Schniewind B, Becker T, Hampe J, Egberts JH. Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann Thorac Surg. 2014;97:1029–1035. doi: 10.1016/j.athoracsur.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Weidenhagen R, Hartl WH, Gruetzner KU, Eichhorn ME, Spelsberg F, Jauch KW. Anastomotic leakage after esophageal resection: new treatment options by endoluminal vacuum therapy. Ann Thorac Surg. 2010;90:1674–1681. doi: 10.1016/j.athoracsur.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Mencio MA, Ontiveros E, Burdick JS, Leeds SG. Use of a novel technique to manage gastrointestinal leaks with endoluminal negative pressure: a single institution experience. Surg Endosc. 2018;32:3349–3356. doi: 10.1007/s00464-018-6055-x. [DOI] [PubMed] [Google Scholar]
- 79.Brangewitz M, Voigtländer T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer J, Manns MP, Schneider AS, Wedemeyer J. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy. 2013;45:433–438. doi: 10.1055/s-0032-1326435. [DOI] [PubMed] [Google Scholar]
- 80.Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, Kurdow R, Arlt A, Ellrichmann M, Jürgensen C, Schreiber S, Becker T, Hampe J. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc. 2013;27:3883–3890. doi: 10.1007/s00464-013-2998-0. [DOI] [PubMed] [Google Scholar]
- 81.Hwang JJ, Jeong YS, Park YS, Yoon H, Shin CM, Kim N, Lee DH. Comparison of Endoscopic Vacuum Therapy and Endoscopic Stent Implantation With Self-Expandable Metal Stent in Treating Postsurgical Gastroesophageal Leakage. Medicine (Baltimore) 2016;95:e3416. doi: 10.1097/MD.0000000000003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mennigen R, Harting C, Lindner K, Vowinkel T, Rijcken E, Palmes D, Senninger N, Laukoetter MG. Comparison of Endoscopic Vacuum Therapy Versus Stent for Anastomotic Leak After Esophagectomy. J Gastrointest Surg. 2015;19:1229–1235. doi: 10.1007/s11605-015-2847-7. [DOI] [PubMed] [Google Scholar]
- 83.Rausa E, Asti E, Aiolfi A, Bianco F, Bonitta G, Bonavina L. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus. 2018:31. doi: 10.1093/dote/doy060. [DOI] [PubMed] [Google Scholar]
- 84.de Moura EGH, Orso IRB, Aurélio EF, de Moura ETH, de Moura DTH, Santo MA. Factors associated with complications or failure of endoscopic balloon dilation of anastomotic stricture secondary to Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016;12:582–586. doi: 10.1016/j.soard.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 85.Moura D, Oliveira J, De Moura EG, Bernardo W, Galvão Neto M, Campos J, Popov VB, Thompson C. Effectiveness of intragastric balloon for obesity: A systematic review and meta-analysis based on randomized control trials. Surg Obes Relat Dis. 2016;12:420–429. doi: 10.1016/j.soard.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 86.Brunaldi VO, Jirapinyo P, de Moura DTH, Okazaki O, Bernardo WM, Galvão Neto M, Campos JM, Santo MA, de Moura EGH. Endoscopic Treatment of Weight Regain Following Roux-en-Y Gastric Bypass: a Systematic Review and Meta-analysis. Obes Surg. 2018;28:266–276. doi: 10.1007/s11695-017-2986-x. [DOI] [PubMed] [Google Scholar]
- 87.de Moura EGH, Ribeiro IB, Frazão MSV, Mestieri LHM, de Moura DTH, Dal Bó CMR, Brunaldi VO, de Moura ETH, Nunes GC, Bustamante FAC, Dos Passos Galvão Neto M, Matuguma SE, Bernardo WM, Santo MA. EUS-Guided Intragastric Injection of Botulinum Toxin A in the Preoperative Treatment of Super-Obese Patients: a Randomized Clinical Trial. Obes Surg. 2019;29:32–39. doi: 10.1007/s11695-018-3470-y. [DOI] [PubMed] [Google Scholar]
- 88.Madruga-Neto AC, Bernardo WM, de Moura DTH, Brunaldi VO, Martins RK, Josino IR, de Moura ETH, de Souza TF, Santo MA, de Moura EGH. The Effectiveness of Endoscopic Gastroplasty for Obesity Treatment According to FDA Thresholds: Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. Obes Surg. 2018;28:2932–2940. doi: 10.1007/s11695-018-3335-4. [DOI] [PubMed] [Google Scholar]
- 89.Barrichello S, Minata MK, García Ruiz de Gordejuela A, Bernardo WM, de Souza TF, Galvão Neto M, Hourneaux de Moura DT, Santo MA, Hourneaux de Moura EG. Laparoscopic Greater Curvature Plication and Laparoscopic Sleeve Gastrectomy Treatments for Obesity: Systematic Review and Meta-Analysis of Short- and Mid-Term Results. Obes Surg. 2018;28:3199–3212. doi: 10.1007/s11695-018-3330-9. [DOI] [PubMed] [Google Scholar]
- 90.de Moura DTH, de Moura EGH, Neto MG, Thompson CC. To the Editor. Surg Obes Relat Dis. 2019;15:155–157. doi: 10.1016/j.soard.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 91.Nedelcu M, Manos T, Cotirlet A, Noel P, Gagner M. Outcome of leaks after sleeve gastrectomy based on a new algorithm adressing leak size and gastric stenosis. Obes Surg. 2015;25:559–563. doi: 10.1007/s11695-014-1561-y. [DOI] [PubMed] [Google Scholar]
- 92.Nimeri A, Ibrahim M, Maasher A, Al Hadad M. Management Algorithm for Leaks Following Laparoscopic Sleeve Gastrectomy. Obes Surg. 2016;26:21–25. doi: 10.1007/s11695-015-1751-2. [DOI] [PubMed] [Google Scholar]
- 93.Valli PV, Gubler C. Review article including treatment algorithm: endoscopic treatment of luminal complications after bariatric surgery. Clin Obes. 2017;7:115–122. doi: 10.1111/cob.12182. [DOI] [PubMed] [Google Scholar]
- 94.Morell B, Murray F, Vetter D, Bueter M, Gubler C. Endoscopic vacuum therapy (EVT) for early infradiaphragmal leakage after bariatric surgery-outcomes of six consecutive cases in a single institution. Langenbecks Arch Surg. 2019;404:115–121. doi: 10.1007/s00423-019-01750-9. [DOI] [PubMed] [Google Scholar]
- 95.Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis. 2016;12:1278–1285. doi: 10.1016/j.soard.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 96.Cuadrado Ayuso M, Franco Herrera R, Lago Oliver J. Succesful Management of Laparoscopic Sleeve Gastrectomy Leak with Negative Pressure Therapy. Obes Surg. 2017;27:2452–2453. doi: 10.1007/s11695-017-2752-0. [DOI] [PubMed] [Google Scholar]
- 97.Szymanski K, Ontiveros E, Burdick JS, Davis D, Leeds SG. Endolumenal Vacuum Therapy and Fistulojejunostomy in the Management of Sleeve Gastrectomy Staple Line Leaks. Case Rep Surg. 2018;2018:2494069. doi: 10.1155/2018/2494069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott RB, Ritter LA, Shada AL, Feldman SH, Kleiner DE. Endoluminal vacuum therapy for gastrojejunal anastomotic leaks after Roux-en-Y gastric bypass: a pilot study in a swine model. Surg Endosc. 2016;30:5147–5152. doi: 10.1007/s00464-016-4823-z. [DOI] [PubMed] [Google Scholar]
- 99.Seyfried F, Reimer S, Miras AD, Kenn W, Germer CT, Scheurlen M, Jurowich C. Successful treatment of a gastric leak after bariatric surgery using endoluminal vacuum therapy. Endoscopy. 2013;45 Suppl 2 UCTN:E267–E268. doi: 10.1055/s-0033-1344569. [DOI] [PubMed] [Google Scholar]
- 100.Loske G, Rucktäschel F, Schorsch T, van Ackeren V, Stark B, Müller CT. Successful endoscopic vacuum therapy with new open-pore film drainage in a case of iatrogenic duodenal perforation during ERCP. Endoscopy. 2015;47:E577–E578. doi: 10.1055/s-0034-1393388. [DOI] [PubMed] [Google Scholar]
- 101.Loske G, Schorsch T, Mueller CT. Endoscopic intraluminal vacuum therapy of duodenal perforation. Endoscopy. 2010;42 Suppl 2:E109. doi: 10.1055/s-0029-1243947. [DOI] [PubMed] [Google Scholar]
- 102.Glatz T, Fischer A, Hoeppner J, Thimme R, Walker C, Richter-Schrag HJ. Vacuum sponge therapy using the pull-through technique via a percutaneous endoscopic gastrostomy to treat iatrogenic duodenal perforation. Endoscopy. 2015;47 Suppl 1:E567–E568. doi: 10.1055/s-0034-1393369. [DOI] [PubMed] [Google Scholar]
- 103.Yoo T, Hou LA, Reicher S, Chen KT, Eysselein VE. Successful repair of duodenal perforation with endoscopic vacuum therapy. Gastrointest Endosc. 2018;87:1363–1364. doi: 10.1016/j.gie.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 104.Bravo JG, Ide E, Kondo A, de Moura DT, de Moura ET, Sakai P, Bernardo WM, de Moura EG. Percutaneous endoscopic versus surgical gastrostomy in patients with benign and malignant diseases: a systematic review and meta-analysis. Clinics (Sao Paulo) 2016;71:169–178. doi: 10.6061/clinics/2016(03)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loske G, Schorsch T, Gobrecht O, Martens E, Rucktäschel F. Transgastric endoscopic vacuum therapy with a new open-pore film drainage device in a case of infective pancreatic necrosis. Endoscopy. 2016;48 Suppl 1:E148–E149. doi: 10.1055/s-0042-106576. [DOI] [PubMed] [Google Scholar]
- 106.Schorsch T, Müller C, Loske G. Pancreatico-gastric anastomotic insufficiency successfully treated with endoscopic vacuum therapy. Endoscopy. 2013;45 Suppl 2 UCTN:E141–E142. doi: 10.1055/s-0032-1326260. [DOI] [PubMed] [Google Scholar]
- 107.Loske G, Strauss T, Riefel B, Mueller CT, Schorsch T. Endoscopic vacuum therapy in the management of anastomotic insufficiency after pancreaticoduodenectomy. Endoscopy. 2012;44 Suppl 2 UCTN:E94–E95. doi: 10.1055/s-0031-1291698. [DOI] [PubMed] [Google Scholar]
- 108.DE-Moura DTH, Farias GFA, Brunaldi VO, Tranquillini CV, Dos-Santos MEL, Matuguma SE, Jukemura J, DE-Moura EGH. Lumen-apposing metal stent and eletrocautery enhanced delivery system (hot axiostm) for drainage of walled-off necrosis: the first brazilian case report. Arq Bras Cir Dig. 2019;32:e1430. doi: 10.1590/0102-672020180001e1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oliveira JF, Moura DTH, Moura ETGH Guedes HG, Otoch JP AE. Ultrasound-guided biliary drainage: a new era of endoscopic surgery. Revista do Colégio Brasileiro de Cirurgiões. 2016;43:198–208. [Google Scholar]
- 110.Farias GFA, Bernardo WM, De Moura DTH, Guedes HG, Brunaldi VO, Visconti TAC, Gonçalves CVT, Sakai CM, Matuguma SE, Santos MELD, Sakai P, De Moura EGH. Endoscopic versus surgical treatment for pancreatic pseudocysts: Systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14255. doi: 10.1097/MD.0000000000014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blumetti J, Abcarian H. Management of low colorectal anastomotic leak: Preserving the anastomosis. World J Gastrointest Surg. 2015;7:378–383. doi: 10.4240/wjgs.v7.i12.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clifford RE, Fowler H, Govindarajah N, Vimalachandran D, Sutton PA. Early anastomotic complications in colorectal surgery: a systematic review of techniques for endoscopic salvage. Surg Endosc. 2019;33:1049–1065. doi: 10.1007/s00464-019-06670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008;22:1818–1825. doi: 10.1007/s00464-007-9706-x. [DOI] [PubMed] [Google Scholar]
- 114.Arezzo A, Bini R, Lo Secco G, Verra M, Passera R. The role of stents in the management of colorectal complications: a systematic review. Surg Endosc. 2017;31:2720–2730. doi: 10.1007/s00464-016-5315-x. [DOI] [PubMed] [Google Scholar]
- 115.Lippert E, Klebl FH, Schweller F, Ott C, Gelbmann CM, Schölmerich J, Endlicher E, Kullmann F. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis. 2011;26:303–311. doi: 10.1007/s00384-010-1104-5. [DOI] [PubMed] [Google Scholar]
- 116.Manta R, Caruso A, Cellini C, Sica M, Zullo A, Mirante VG, Bertani H, Frazzoni M, Mutignani M, Galloro G, Conigliaro R. Endoscopic management of patients with post-surgical leaks involving the gastrointestinal tract: A large case series. United European Gastroenterol J. 2016;4:770–777. doi: 10.1177/2050640615626051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blot C, Sabbagh C, Rebibo L, Brazier F, Chivot C, Fumery M, Regimbeau JM. Use of transanastomotic double-pigtail stents in the management of grade B colorectal leakage: a pilot feasibility study. Surg Endosc. 2016;30:1869–1875. doi: 10.1007/s00464-015-4404-6. [DOI] [PubMed] [Google Scholar]
- 118.Kuehn F, Janisch F, Schwandner F, Alsfasser G, Schiffmann L, Gock M, Klar E. Endoscopic Vacuum Therapy in Colorectal Surgery. J Gastrointest Surg. 2016;20:328–334. doi: 10.1007/s11605-015-3017-7. [DOI] [PubMed] [Google Scholar]
- 119.von Bernstorff W, Glitsch A, Schreiber A, Partecke LI, Heidecke CD. ETVARD (endoscopic transanal vacuum-assisted rectal drainage) leads to complete but delayed closure of extraperitoneal rectal anastomotic leakage cavities following neoadjuvant radiochemotherapy. Int J Colorectal Dis. 2009;24:819–825. doi: 10.1007/s00384-009-0673-7. [DOI] [PubMed] [Google Scholar]
- 120.van Koperen PJ, van Berge Henegouwen MI, Rosman C, Bakker CM, Heres P, Slors JF, Bemelman WA. The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc. 2009;23:1379–1383. doi: 10.1007/s00464-008-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Glitsch A, von Bernstorff W, Seltrecht U, Partecke I, Paul H, Heidecke CD. Endoscopic transanal vacuum-assisted rectal drainage (ETVARD): an optimized therapy for major leaks from extraperitoneal rectal anastomoses. Endoscopy. 2008;40:192–199. doi: 10.1055/s-2007-995384. [DOI] [PubMed] [Google Scholar]
- 122.Nagell CF, Holte K. Treatment of anastomotic leakage after rectal resection with transrectal vacuum-assisted drainage (VAC). A method for rapid control of pelvic sepsis and healing. Int J Colorectal Dis. 2006;21:657–660. doi: 10.1007/s00384-005-0083-4. [DOI] [PubMed] [Google Scholar]
- 123.Nerup N, Johansen JL, Alkhefagie GA, Maina P, Jensen KH. Promising results after endoscopic vacuum treatment of anastomotic leakage following resection of rectal cancer with ileostomy. Dan Med J. 2013;60:A4604. [PubMed] [Google Scholar]
- 124.Riss S, Stift A, Kienbacher C, Dauser B, Haunold I, Kriwanek S, Radlsboek W, Bergmann M. Recurrent abscess after primary successful endo-sponge treatment of anastomotic leakage following rectal surgery. World J Gastroenterol. 2010;16:4570–4574. doi: 10.3748/wjg.v16.i36.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Moura DT, Guedes H, Tortoretto V, Arataque TP, de Moura EG, Román JP, Rodela GL, Artifon EL. [Comparison of colon-cleansing methods in preparation for colonoscopy-comparative of solutions of mannitol and sodium picosulfate] Rev Gastroenterol Peru. 2016;36:293–297. [PubMed] [Google Scholar]
- 126.Rocha RSP, Ribeiro IB, de Moura DTH, Bernardo WM, Minata MK, Morita FHA, Aquino JCM, Baba ER, Miyajima NT, de Moura EGH. Sodium picosulphate or polyethylene glycol before elective colonoscopy in outpatients? A systematic review and meta-analysis. World J Gastrointest Endosc. 2018;10:422–441. doi: 10.4253/wjge.v10.i12.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strangio G, Zullo A, Ferrara EC, Anderloni A, Carlino A, Jovani M, Ciscato C, Hassan C, Repici A. Endo-sponge therapy for management of anastomotic leakages after colorectal surgery: A case series and review of literature. Dig Liver Dis. 2015;47:465–469. doi: 10.1016/j.dld.2015.02.007. [DOI] [PubMed] [Google Scholar]


