Abstract
In this study, many bacterial strains were screened for the production of minor ginsenosides, but based on conversion competence among the strains, the strain Niabella ginsenosidivorans BS26T has the good ginsenoside-transforming ability. Therefore, the strain BS26T was selected for complete genome sequence analysis to determine the target (glycoside hydrolase) functional genes. Whole genome analysis of strain BS26T showed 43 glycoside hydrolase genes in total. To determine the target functional gene, 12 sets of six different glycoside hydrolases (3 set of β-glucosidase; 3 set of trehalase; 3 set of arabinofuranosidase; 2 set of xylosidase; and one set of each α-galactosidase and α-fucosidase, respectively) were selected and cloned in E. coli BL21 (DE3) using the pGEX4T-1 vector and were characterized. Among these 12 sets of clones, only one, β-glucosidase (BglNg-767), showed ginsenoside conversion ability. The BglNg-767 comprised 767 amino acids and belonged to glycoside hydrolase family 3 (GH3). The recombinant GST-BglNg-767 was capable of altering the ginsenosides Rb1, Rd, and gypenoside XVII (Gyp-XVII) to F2; Rb2 to C–O; Rb3 to C-Mx1, and Rc to C-Mc1. Besides, complete genome sequence analysis of strain BS26T also indicates 30 endopeptidase genes, which may be responsible for self-hydrolysis of the proteins. Therefore, using SDS-PAGE analysis, we predict that the difference between the molecular weight of the expressed protein (around 90 kDa) and the predicted amino-acid sequence (102.7 kDa) is due to self-hydrolysis of the proteins.
Keywords: Niabella ginsenosidivorans, Whole genome sequencing, Bioconversion, Ginsenosides, Recombinant β-glucosidase
Introduction
Ginseng, Panax Ginseng was discovered over 2000 years ago in Korea. Taking up to 6 years to mature, this unique root has a plant that grows from 8 to 15 in. in height. The unique feature of this plant is that it grows in cool moist soil that is frequently watered and that the root attains maturity after up to 6 years with the aerial plant growth reaching 8–15 in. in height (Court 2000). Many accounts of ancient Korean history indicate that ginseng was used as a portion of food among farmers and labours for medicinal purposes and was admired for its strength-giving properties and refreshing powers. Ginseng was and still remains a powerful tonic among herbal medicines. Ginseng currently occupies a dominant position in the list of best-selling herbal medicinal plants worldwide (Yun 2001).
Science and technology have proven the therapeutic power of ginseng and revealed the presence of some valuable major compounds commonly known as saponins or major ginsenosides. These major active compounds of Panax ginseng are categorized as protopanaxadiol (PPD), protopanaxa triol (PPT), and oleanane-type ginsenosides. The Ginseng root contains more than 90% of PPD- and PPT-type major ginsenosides including Rb1, Rb2, Rb3, Rc, Rd, Re, and Rg1 (Christensen 2009). Because of the high molecular weight of these major ginsenosides, it is difficult for the human gastrointestinal tract to absorb them into the bloodstream (Tawab et al. 2003; Xu et al. 2003). Therefore, these macro-compounds are transformed into minor ginsenosides for better pharmacological effects. Compared to major ginsenosides, minor ginsenosides [F2, C–K, Rg3, Rh2, and F1] are present in small amount in the total ginseng extract and also demonstrate high efficacy of anti-oxidative, anti-cancer, anti-aging, anti-osteoporosis, anti-inflammatory, and anti-diabetic effects (Choi et al. 2011a; Kim et al. 2013; Lee et al. 2011; Leung and Wong 2010; Baatar et al. 2018; Siddiqi et al. 2014, 2015), compared to major ginsenosides. To obtain minor ginsenosides from major ginsenosides, three different conversion methods [microbial enzymatic, physical or heat, and chemical (acid or base) treatment] are used to target minor ginsenosides. However, recombinant enzymatic transformation is currently considered a suitable conversion technology for the production of target minor ginsenosides owing to its controlled environmental conditions compared to the physical and chemical conversion methods. Thus, researchers are still trying to determine more suitable and powerful recombinant enzymes for enhanced production (gram unit) of some valuable minor ginsenosides such as Rg3, Rh2, F2, C–K, and others. As a result, our research team succeeded in gram unit production of ginsenoside Rh2-Mix using a recombinant glucoside hydrolase in 2017 [(BglPm_C) Siddiqi et al. 2017a], but this production was only limited to 20(S)-Rh2, 20(R)-Rh2, Rh3, and Rk2. Therefore, in this study, we discovered a novel glucoside hydrolase through complete genome sequencing for the production of many kinds of minor ginsenosides. For the target strain selection and functional gene identification, initial biotransformation was carried out as described previously (Siddiqi et al. 2017b).
During the screening of the conversion of ginsenosides, strain BS26T exhibited efficient conversion of major ginsenosides. The strain was thus subjected to complete genome sequencing to determine the target genes. After complete genome analysis, 12 sets of six (6) different glycoside hydrolases [β, xylosidase, α-galactosidase, α-fucosidase, and β-arabinofuranosidase] were selected for cloning. Thus, among these 12 sets of clones, only one recombinant β-glucosidase (named as BglNg-767) was characterized, which showed the conversion of ginsenosides and belonged to the glycoside hydrolase family 3.
Materials and methods
Materials
The PPD- and PPT-type ginsenoside standards [≥ 98%, Rb1, Rb2, Rb3, Rc, Rd and Gyp-XVII, Re, and Rg1] were purchased from Republic of Korea (AceEMzyme Co., Ltd.) and were used as the primary substrate in this study. Various types of mono-sugar substrates [with the configuration of p-nitrophenyl (pNP) and o-nitrophenyl (oNP)] and other chemicals used in the study were purchased from Sigma.
Screening of β-glucosidase activity of strain BS26T
To determine the β-glucosidase-positive activity of strain BS26, the strain was transferred to R2A agar plate containing 1.0 g/l esculin and 0.5 g/l ferric citrate. The plates were then incubated at 30 °C for 2 days. The release of esculetin (reddish-brown zone) from esculin was selected and used for the bioconversion of ginsenosides as described previously (Siddiqi et al. 2017b).
Identification and cloning of genes involved in ginsenoside metabolism
Primers designing
Based on the ginsenoside conversion activity, the genomic DNA of the strain BS26T was extracted by genomic DNA extraction kit and whole genome sequenced by Macrogen Co., Ltd. (Korea). After the whole genome sequencing, the whole genome sequence was accessioned and annotated by NCBI (https://www.ncbi.nlm.nih.gov; https://img.jgi.doe.gov) under accession number CP015772, as described previously (Lee et al. 2018). Thirty-four (34) target genes for ginsenoside conversion were identified from the WGS by Macrogen Co., Ltd. (Korea) from which six (6) different glycoside hydrolases (trehalase, β-glucosidase, xylosides, α,β-arabinofuranosidase, α-galactosidase, and α-fucosidase) were chosen for cloning and further studies. The oligonucleotide primers designed for cloning of these genes were synthesized with BamHI and XhoI restriction sites by Macrogen Co., Ltd. (Korea) (Table 1). However, in this paper, we focus on the cloning and characterization of a β-glucosidase.
Table 1.
Name of glycoside hydrolases and sets of primers used in this study (sequences 5' → 3')
| No of clones | Names | Total amino acids | Ginsenosides conversion activity | Primers sequences (F, R) | Vector |
|---|---|---|---|---|---|
| 1 | Trehalase-643 | 643 | − |
GGTTCCGCGTGGATCCAGAAAATGTATGATGGGTTTT_F GATGCGGCCGCTCGAGTTAGTTATAATACCCTTGTTC_R |
pGEX 4T-1 |
| 2 | β-Glucosidase-788 | 788 | − |
GGTTCCGCGTGGATCCTTGAAACAGGCGTTTGCGGTGT_F GATGCGGCCGCTCGAGCTATTCCAGCTCGATCTGTTT_R |
|
| 3 | Trehalase-440 | 440 | − |
GGTTCCGCGTGGATCCAAATGGAGGTTTAAAGCGGTA_F GATGCGGCCGCTCGAGTTATTTATTCCTGTCTGCTTC_R |
|
| 4 | β-Glucosidase-784 | 784 | − |
GGTTCCGCGTGGATCCTGTATCTACAATCACCTGATC_F GATGCGGCCGCTCGAGTCAGCGTTGTTTATATTTCAT_R |
|
| 5 | β-Glucosidase-767 | 767 | + |
GGTTCCGCGTGGATCCCGATACAAAAGATTGCTGCTT_F GATGCGGCCGCTCGAGTTACCGTTCCCAATGCACCGT_R |
|
| 6 | Arabinofuranosidase-364 | 364 | − |
GGTTCCGCGTGGATCCCGAAAAATTTTATTATCAGCC_F GATGCGGCCGCTCGAGTCAGCGCGCCCCGGGCATCA_R |
|
| 7 | Arabinofuranosidase-352 | 352 | − |
GGTTCCGCGTGGATCCAACCGTTTTATCTTATGGGCT_F GATGCGGCCGCTCGAGCTATGGCCTGTACTCCTGTTT_R |
|
| 8 | β-Arabinofuranosidase-589 | 589 | − |
GGTTCCGCGTGGATCCCAGGTTGCCGTAGACGGGCTT_F GATGCGGCCGCTCGAGTTAATCAATCTGATTTTGAAA_R |
|
| 9 | Xylosidase-433 | 433 | − |
GGTTCCGCGTGGATCCAAAAGGATTGTTTTAATAGCA_F GATGCGGCCGCTCGAGTCAAACTTTATCCAATCAATA_R |
|
| 10 | Xylosidase-349 | 349 | − |
GGTTCCGCGTGGATCCCCTGAAGACAGTATTGAACAT_F GATGCGGCCGCTCGAGCTATTCCAAAACGTTTAACCC_R |
|
| 11 | α-Galactosidase-352 | 352 | − |
GGTTCCGCGTGGATCCAAAAAAACGATTGTACTATTC_F GATGCGGCCGCTCGAGTTATGGGATCACCCTGGCCGG_R |
|
| 12 | α-Fucosidase-481 | 481 | − |
GGTTCCGCGTGGATCCATTAAGAAATTATTGCTGAGC_F GATGCGGCCGCTCGAGCTAATACACTTCAAAATTTGA_R |
Strain BS26T is available from the host institute (Department of Biotechnology, Hankyong National University, Anseong-si, South Korea) and from two culture collections KACC 16620T (Republic of Korea) and JCM 18199T (Japan).
Cloning of glycosidases, and characterization of novel recombinant BglNg-767
In total, 34 sets of glycoside hydrolases [belonging to different glycoside hydrolase families (GHF 1, 3, 43, and others)] were identified by WGS analysis. Amongst these 34 sets of 6 different glycoside hydrolases, only 12 sets of glycoside hydrolases [containing 3 sets of β-glucosidase (Bgl), 2 sets of trehalase (Thl), 3 sets of arabinofuranosidase (Abf with α and β configuration), 2 sets of xylosidase (Xyl), and 1 set each of galactosidase (Glac) and fucosidase (Fuc)] were nominated for cloning. The selected functional genes were cloned in E. coli DH5-α using the pGEX 4T-1GST fusion vector system. The gene encoding was identified by analyzing the complete genome sequence, and the oligonucleotide primers used for cloning were designed as forward and reverse (as shown in Table 1) by Macrogen Co., Ltd., Korea with BamHI and XhoI restriction sites inserted, respectively. After PCR amplification, the PCR-purified products were inserted into the pGEX 4T-1GST fusion vector using an EzCloning Kit (Enzynomics Co., Ltd., Korea). The resultant recombinants (pGEX-Bgl; pGEX-Thl, pGEX-Abf, pGEX-Xyl, pGEX-Glac, and pGEX-Fuc) were transformed to E. coli BL21 (DE3) and were grown in LB-ampicillin broth at 37 °C, until the OD600 reached 0.4–0.6. After OD600 reached 0.4, the protein was induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and further incubated for 12–18 h at 28 °C. After 12–18 h incubation, the cells (as mentioned above) were harvested through centrifugation and the pellets were suspended in 100 mM sodium phosphate (pH 7.0) for sonication. After 5–10 min sonication (Branson Digital Sonifier 450, CT, Mexico), the ginsenoside conversion activity of each recombinant enzyme was determined by mixing with the PPD-mix type (Rb1, Rb2, Rb3, Rc, and Rd) and incubation at 37 °C for 24 h. Among these 12 sets, only one recombinant enzyme (named as BglNg-767) showed the conversion of PPD-mix-type major ginsenosides to minor ginsenosides and was characterized in this study. The recombinant enzyme (BglNg-767) was purified using a GST·bound agarose resin column (Elpisbiotech Co., Ltd, Korea) and protein uniformity was evaluated by 10% SDS-PAGE. SDS-PAGE analysis showed a difference between the molecular weights of the well-localized proteins in the gel (around 90 kDa) and the full-length sequences (102.7 kDa). After purification of BglNg-767, the molecular weight of the recombinant enzyme was confirmed to be around 95 kDa (Miyake et al. 1978; Ahmad et al. 2005; Rath et al. 2009; Siddiqi et al. 2017b).
Effect of pH, temperature, and metal ions on enzyme activity
To measure the specific activity of BglNg-767, the recombinant protein was mixed with p-nitrophenyl-β-d-glucopyranoside [pNPG, (2 mM as final concentration)] and incubated at 37 °C for 10 min. After 10 min, the reaction was stopped with Na2CO3 (0.5 M, final concentration), and the amount of p-nitrophenol released was measured using a microplate reader at 405 nm (Bio-Rad model 680; Bio-Rad, Hercules, CA). The amount of enzyme required to produce 1 μmol of p-nitrophenol per min is defined as one unit of enzyme activity. The specific activity of the enzyme was expressed as units per milligram of an enzyme. The protein concentration was determined using the Bio-Rad protein assay (Catalogue Number 500-0006) kit. All assays were performed in triplicate.
The effect of pH (pH 2–10) and temperature (4–65 °C) with pH and temperature stability was determined as described previously (Siddiqi et al. 2017b) with slight modifications of substrate concentration. Metals and heavy metals are high atomic weight and high-density elements that occur naturally. Therefore, the availability of heavy metals at various concentrations of disturbs enzyme activity; as a result, high concentrations of heavy metals could inhibit enzyme activity due to toxic effects. Thus, the effects of metals and other chemicals on BglNg-767 activity were determined in the presence of 1 and 10 mM (final concentration) of NaCl, CaCl2, KCl, MgCl2, MnSO4, CoCl2, β-mercaptoethanol, MgSO4, and EDTA for 10 min at 37 °C. The retained activity was determined using pNPG as a substrate and was expressed as a percentage of the activity in the absence of the compound.
In this study, substrate affinity (as shown in Table 2) of BglNg-767 was confirmed using 2.0 mM o-nitrophenyl (oNP) and p-nitrophenyl (pNP) as substrates. The samples were incubated at 37 °C for 5 min, and enzyme activity was determined. The amount of enzyme required to generate 1 μmol of oNP or pNP per min is defined as one unit of enzyme activity (U).
Table 2.
Substrates and ginsenoside conversion activities of 12 sets of clones of strain BS26
| Substrates | Clones of Niabella ginsenosidivorans strain BS26T | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| pNP-β-d-glucopyranoside | − | + | − | − | + | − | − | − | − | − | − | − |
| pNP-β-d-galactopyranoside | − | − | − | − | − | w | − | − | − | − | − | − |
| pNP-β-d-fucopyranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| pNP-β-d-glucosaminide | w | + | w | w | + | w | w | − | − | − | − | − |
| pNP-β-l-arabinofuranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| pNP-β-β-mannopyranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| pNP-β-d-xylopyranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| pNP-α-d-glucopyranoside | − | w | w | w | − | − | w | − | − | − | − | − |
| pNP-α-l-arabinofuranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| pNP-α-d-fucopyranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| pNP-α-l-rhamnopyranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| pNP-α-d-mannopyranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| oNP-β-d-glucopyranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| oNP-β-d-fucopyranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| oNP-β-d-galactopyranoside | − | − | − | − | − | − | − | − | − | − | − | − |
| Bioconversion of ginsenosides | − | − | − | − | + | − | − | − | − | − | − | − |
Clone sets. 1, TrehNg-643; 2, BglNg-788; 3, TrehNg; 4, BglNg-784; 5, BglNg-767; 6, AbfNg-364; 7, AbfNg-352; 8, β-AbfNg-589; 9, XylNg-433; 10, XylNg-349; 11, α-GalNg-352; 12, α-FucNg-481. +, positive; w, weak positive; −, negative
Bgl, β-glucosidase; Treh, trehalase; Abf, arabinofuranosidase; Xyl, xylosidase; Gal, galactosidase; Fuc, fucosidase
Bioconversion of major ginsenosides by recombinant BglNg-767
Primarily, we confirmed the effect of fused GST on the enzyme activity of BglNg-767. Therefore, our analysis shows that BglNg-767 activity was not affected by the fused GST for the biotransformation of PPD-type ginsenosides. Therefore, the specificity and selectivity of the recombinant enzyme (GST-BglNg-767) were used to determine the glucose moieties attached to the C3 and C20 positions in six PPD and two PPT-type [(PPD-type, Rb1, Rb2, Rb3, Rc, Rd, Gyp-XVII) (PPT-type Re and Rg1)] ginsenosides. The crude recombinant enzyme [at a concentration of 0.62 mg/ml in 0.1 M sod-phos buffer (pH 7.0)] was mixed with an equal volume of major ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Gyp-XVII Re, and Rg1 (final concentration of 1000 ppm) at 37 °C for different time intervals. After each specific interval, the samples were collected and the bioconversion of ginsenosides was confirmed by TLC or HPLC analysis.
TLC analysis
Thin layer chromatography (TLC) analysis was performed with CHCl3–CH3OH–H2O (65:35:10, lower phase) as a solvent in 60 F254 silica gel plates (Merck, Germany) and the spots were visualized by spraying 10% (v/v) H2SO4 after heating at 110 °C for 5–10 min. The results were then compared with ginsenoside standards on a TLC plate.
Results and discussion
Genome properties of strain BS26T
The complete genome sequence of strain BS26T shows in total, 43 sets of glycoside hydrolases (14 β-glucosidases; 11 α-glucosidases; 8 β-xylosidase; 8 α-arabinofuranosidase, 1 galactosidase, and 1 fucosidase (https://img.jgi.doe.gov). Interestingly, besides glycoside hydrolases, we also found 30 endopeptidase genes (as shown in Table 3) that may be responsible for the self-hydrolysis of these proteins. Thus, from our SDS-PAGE analysis, we predict that some genes cause self-hydrolysis of the protein. The strain BS26T had a circular chromosome with 5,627,734 bp and the genome sequence has been deposited in GenBank database under the accession number CP015772.
Table 3.
Supplementary information of gene annotated for the BS26T genome
| S. nos | Gene_oid | Locus tag | Source | Cluster/gene Information | Total gene count |
|---|---|---|---|---|---|
| 1 | 2778109959 | Ga0213703_1170 | EC:3.4.24; COG3590 | Hydrolases. Acting on peptide bonds (peptidases). Metalloendopeptidases; Predicted metalloendopeptidase | 8 |
| 2778110699 | Ga0213703_11810 | ||||
| 2778110818 | Ga0213703_11929 | ||||
| 2778111318 | Ga0213703_111430 | ||||
| 2778114241 | Ga0213703_114357 | ||||
| 2778114241 | Ga0213703_114357 | ||||
| 2778113477 | Ga0213703_113591 | ||||
| 2778114612 | Ga0213703_114729 | ||||
| 2 | 2778110061 | Ga0213703_11172 | EC:3.4.21.- | Hydrolases. Acting on peptide bonds (peptidases). Serine endopeptidases. | 1 |
| 3 | 2778110061 | Ga0213703_11172 | KO:K07259 | d-Alanyl-d-alanine-endopeptidase (penicillin-binding protein 4) | 2 |
| 4 | 2778110272 | Ga0213703_11383 | EC:3.4.21.53 | Endopeptidase La. | 2 |
| 5 | 2778110699, 2778114241 | Ga0213703_11810, Ga0213703_114357 | KO:K07386 | Putative endopeptidase [EC:3.4.24] | 3 |
| 6 | 2778110818 | Ga0213703_11929 | COG1164; KO:K08602 | Oligoendopeptidase F | 3 |
| TIGR02289 | Oligoendopeptidase, M3 family | 1 | |||
| 7 | 2778113264 | Ga0213703_113378 | TIGR00382 | Endopeptidase Clp ATP-binding regulatory subunit (clpX) | 1 |
| 2778113265 | Ga0213703_113379 | EC:3.4.21.92 | Endopeptidase Clp. | 3 | |
| 2778113266 | Ga0213703_113380 | ||||
| 2778113857 | Ga0213703_113971 | ||||
| 8 | 2778113231 | Ga0213703_113345 | KO:K17733 | Peptidoglycan l-alanyl-d-glutamate endopeptidase CwlK [EC:3.4] | 2 |
| Product_name | |||||
| 9 | 2778113265 | Ga0213703_113379 | TIGR00493 | ATP-dependent Clp endopeptidase, proteolytic subunit ClpP | 2 |
| 2778113857 | Ga0213703_113971 | ||||
| 10 | 2778114373 | Ga0213703_114490 | ITERM:02952 | O-Sialoglycoprotein endopeptidase (EC 3.4.24.57) | 2 |
Molecular cloning of β-glucosidase gene (BglNg-767) from strain BS26T
After target gene cloning, only two clones (BglNg-788, and BglNg-767) of strain BS26T showed positive activities for pNP-β-d-glucopyranoside. The clones in columns 1–7 were weak positive and negative for pNP-β-d-glucosaminide and pNP-α-d-glucopyranoside, respectively. Similarly, the clones in columns 8–12 [β-AbfNg-589; XylNg-433; XylNg-349; α-GalNg-352; α-FucNg-481] did not show any activity for the 16 types of substrates (as shown in Table 2). Regarding the bioconversion of ginsenosides, only one clone (BglNg-767) was active for the conversion of ginsenosides (Table 2) and was characterized. Amino-acid sequence analysis indicated the predicted molecular weight of BglNg-767 (GenBank accession number: CP015772) as 102.7 kDa with a theoretical pI value of 4.92 (http://web.expasy.org/compute_pi/). The amino-acid BLAST sequence analysis indicated that BglNg-767 was 66.4% identical to the β-glucosidase from Bacteroides thetaiotaomicron VPI-5482 (PUBMED ID: 29131329), which also belongs to glycoside hydrolase family 3 (GH3) and was characterized by Ishiguro et al. 2017. Based on the amino-acid sequence similarity, the glycoside hydrolase families are divided into various groups, which show common structural features of enzymes and substrate specificity (http://www.cazy.org/fam/acc_GH.html) (Henrissat and Davies 1997).
Enzyme expression and bioconversion of ginsenoside by BglNg-767
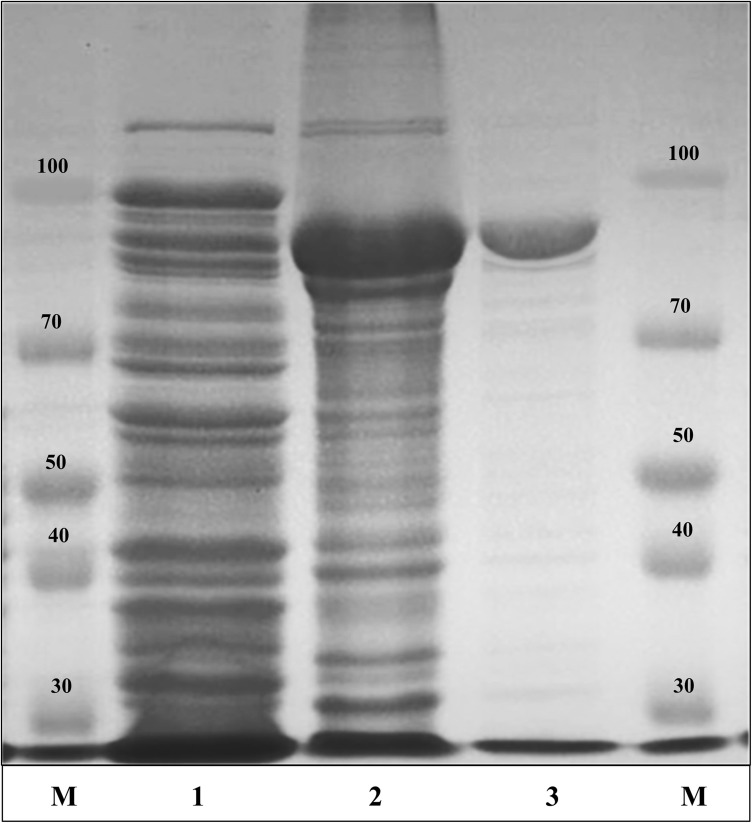
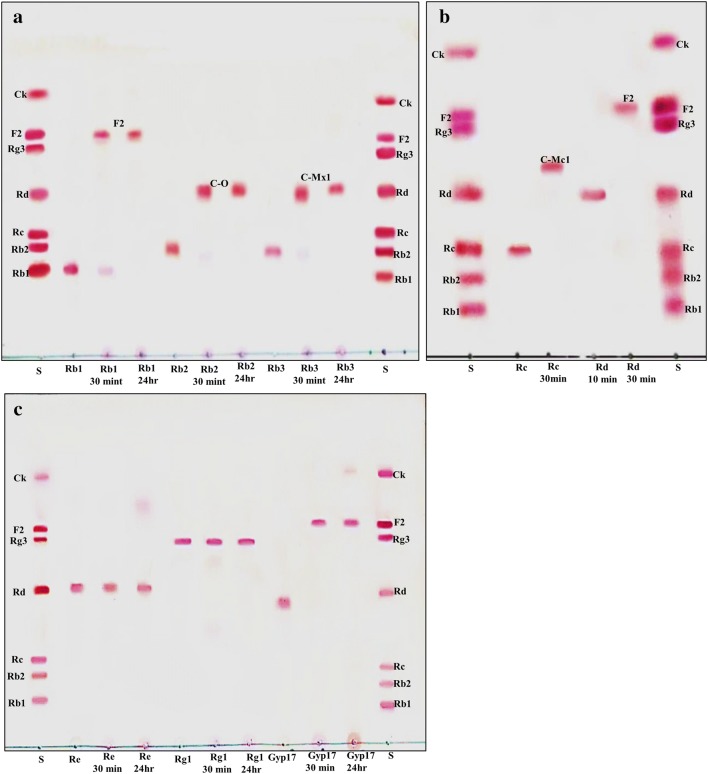
The recombinant pGEX-BglNg-767 was transformed into E. coli BL21 (DE3) and the protein was induced by 0.1 mM IPTG. To maximize the recombinant enzyme yield, the samples [recombinant E. coli BL21 (DE3) in LB + Amp broth] were incubated at various temperatures (18, 22, 25, and 28 °C) with different concentrations of IPTG [0.1, 0.5, and 1.0 mM IPTG (data not shown)]. The maximum level of soluble protein was produced at 28 °C for 18 h with 0.5 mM IPTG induction, as shown in Fig. 1. The bioconversion of PPD- and PPT- type major ginsenosides (Rb1, Rb2, Rb3, Rc, Rd, Gyp-XVII, Re, and Rg1) by BglNg-767 was confirmed by TLC analysis at regular intervals. Based on the Rf values (Fig. 2a–c), BglNg-767 was found to convert only PPD-type major ginsenosides and there was no significant conversion activity for ginsenosides Re and Rg1. Thus, BglNg-767 could efficiently remove the glucose attached at the C3 and C20 positions of PPD-type ginsenosides. The proposed pathways for the bioconversion of PPD-type major ginsenosides (1 mg/ml) are given as; Rb1 → Gyp-XVII → F2; Rb2 → C–O; Rb3 → C-Mx1; Rc → C-Mc1 → C-Mc; Rd → F2; and Gyp-XVII → F2. The biotransformation occurs through stepwise hydrolysis of the outer and inner glucose moieties at the C3 and C20 positions of the aglycon (Fig. 2). However, there was no further hydrolyzing activity for the minor ginsenosides F2, C–O, C-Mx1, and C-Mc1 after a long incubation at 37 °C. The ginsenoside Rd and Gyp-XVII (1 mg/ml) were transformed into F2 within 30 min, indicating that the recombinant BglNg-767 had a preference for the outer glucose attached at the C3 or C20 position. Moreover, the conversion rate was much faster than that of a previously described recombinant enzyme (Cheng et al. 2006 and Chi and Ji 2005), which converted 1 mg/ml of Rb1 to Rd in 24 h.
Fig. 1.
SDS-PAGE analysis of recombinant BglNg-767. Lanes: M, molecular weight standard; 1, un-induced pGEX-BglNg-767; 2, induced pGEX-BglNg-767; 3, GST-BglNg-767 enzyme fraction after purification
Fig. 2.
a–c TLC analyses of time course of ginsenoside bioconversion by BglNg-767. a Bioconversion of ginsenosides Rb1, Rb2, and Rb3. b Transformation of ginsenosides Rc and Rd. c Bioconversion of Re, Rg1, and Gyp-XVII. Lane S, standards (Rb1, Rb2, Rc, Rd, Rg3, F2, and C–K). Developing solvent: CHCl3–CH3OH–H2O (65:35:10, v/v, lower phase)
Purification and characterization of recombinant BglNg-767
The recombinant protein of GST-BglNg-767 was purified using GST•bind agarose resin and the results were confirmed by 10% SDS-PAGE analysis (Fig. 1). From the SDS-PAGE analysis, the difference between the molecular weights of well-localized proteins (around 90 kDa) and the full-length amino-acid sequence (102 kDa) was identified. Therefore, after purifying BglNg-767, the molecular weight of the recombinant enzyme was confirmed to be around 90 kDa, as shown in Fig. 1. The difference between the molecular weight of the recombinant BglNg-767 protein calculated from amino-acid sequence analysis and that calculated after protein purification might be due to self-proteolysis of the bacterial strain protein (Miyake et al. 1978; Ahmad et al. 2005; Rath et al. 2009; Siddiqi et al. 2017b).
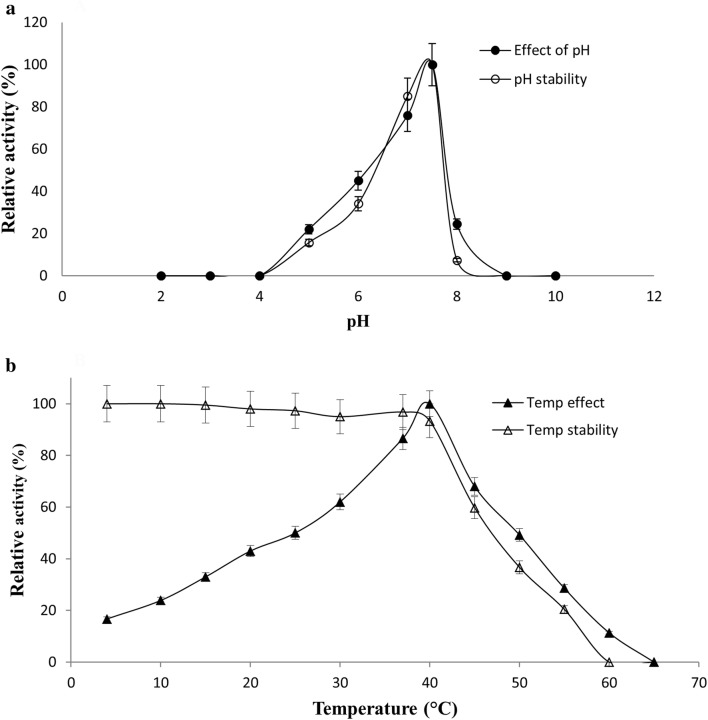
Using purified GST-BglNg-767, the optimum pH and temperature for the bioconversion of ginsenosides were determined. BglNg-767 showed optimal enzyme activity at pH 7.5 (0.1 M sod-phos buffer), and was stable at pH 5.0–7.5 (Fig. 3a). However, at pH less than 5.0 or more than 7.5, the enzyme stability was decreased sharply, as shown in Fig. 3a. The optimal temperature was found to be 40 °C, whereas thermostability was significantly decreased above 37 °C, and no activity was detected when the enzyme was incubated at 60 °C for 10 min (Fig. 3b). BglNg-767 was stable below 37 °C, and about 40% of the activity was lost after incubation at 45 °C for 10–15 min. Thus, the optimal temperature and pH for the activity of BglNg-762 is 37 °C and pH 7.5, respectively. Therefore, the mild optimal temperature and the near-neutral optimal pH of BglNg-767 is similar to that of other described members of the ginsenoside-converting glycoside hydrolase family 3 (GH3) (Siddiqi et al. 2017b; Choi et al. 2011b). Even though the optimum temperature of BglNg-767 for substrate hydrolysis (pNPG) was 40 °C, during a prolonged and constant bioconversion process, the reaction was maintained at 37 °C with an optimum pH of 7–7.5.
Fig. 3.
a, b Effect of pH on the enzyme activity of BglNG-676. The enzyme activities were measured under standard assay conditions. Enzyme solutions containing 2.0 mM pNPGlc were incubated with various buffers at pH 2–10 for 12 h at 4 °C, and for stability, the enzymes were incubated for 10 min at 40 °C in various buffers at pH 2–10 and the residual activities were measured (a). The effect of temperature on the activity and stability of recombinant BglNg-767 was measured under standard assay conditions. The thermo-dependence of BglNg-767 was assayed in 50 mM potassium phosphate buffer (pH 7.5) at varying temperatures ranging from 4 to 60 °C. Thermostability was tested by incubating aliquots of the enzyme in 0.1 M sodium phosphate buffer (pH 7.5) for different periods at various temperatures. After cooling the sample, residual activity was determined (b)
As described in the previous section, different concentrations of heavy metals may affect enzyme activity. Therefore, the effects of metal ions and some chemicals (β-mercaptoethanol and EDTA) were examined on the enzyme activity of BglNg-767 and the results were expressed as a percentage of the activity in the absence of the test compound (Table 4). The analysis showed that enzyme activity of BglNg-767 was not affected by 1 mM of metal ions, but was enhanced as compared to that of the control. In addition, BglNg-767 enzyme activity was decreased by 10 mM of all the tested metal ions, EDTA, and β-mercaptoethanol, as shown in Table 4.
Table 4.
Effects of 1 mM and 10 mM metal ions and other chemicals on the activity of recombinant BglNg-767
| Metal ions or reagents | Relative activity ± SD (%) at | |
|---|---|---|
| 1 mM | 10 mM | |
| NaCl | 126.6 ± 1.3 | 116.1 ± 0.7 |
| KCl | 118.7 ± 2.7 | 105.1 ± 0.6 |
| CaCl2 | 121.9 ± 0.9 | 80.3 ± 0.6 |
| MgCl2 | 119.1 ± 2.7 | 104.1 ± 2.4 |
| CoCl2 | 131.9 ± 2.0 | 60.9 ± 2.7 |
| MnSO4 | 107.7 ± 2.0 | 69.4 ± 1.3 |
| MgSO4 | 103.3 ± 2.7 | 89.8 ± 1.0 |
| β-Mercaptoethanol | 108.4 ± 2.0 | 112.7 ± 0.3 |
| EDTA | 109.7 ± 2.7 | 88.7 ± 1.3 |
| Control | 100.0 ± 2.0 | 100.0 ± 5.1 |
To determine the substrate specificity of BglNg-767, the recombinant enzyme was reacted with α- and β-configurations of 2.0 mM pNP- and oNP-glycosides. The results, summarized in Table 2, showed that BglNg-767 was most active against pNPGlc, followed by oNPGlc, but had no effect on any of the other pNP- and oNP-glycosides.
In this study, we faced a problem with protein BLAST (blastp) in the NCBI for sequence similarity. Using the data of the complete genome sequence of the BS26T strain, we succeeded in cloning 12 sets of six (6) different glycoside hydrolases in E. coli DH5α. However, when we analyzed the sequence similarity for our target functional gene using blastp, the results did not reveal interesting aspects of the conservation of our target functional gene. The NCBI blast analysis showed that our target gene had a sequence similarity of more than 50–60% with different glycoside hydrolases (hypothetical proteins), but after enzyme expression, most of the enzymes did not show specific activity against any substrate, as shown in Table 2. Therefore, the corresponding results for our protein in NCBI blastp were not accurate and the NCBI hypothetical protein will be considered as a putative, uncharacterized protein for strain BS26T.
Ginsenoside transformation and characteristics of recombinant BglNg-767
To confirm the enzymatic transformation pathway, the PPD-type ginsenosides (Rb1, Rb2, Rb3, Rc, Rd, and Gyp-XVII) were treated with BglNg-767 and TLC analyses were performed at regular intervals. GST-BglNg-767 was found to transform six ginsenosides as shown by Rf values from the TLC analysis (Fig. 2a–c). During the biotransformation of Rb1, two types of metabolites (Gyp-XVII and F2) were detected (data not shown). Thus, BglNg-767 successively hydrolyzed the inner and outer glucose moieties at position C3 and C20. It also transformed all PPD-type major ginsenosides into minor ginsenosides within 30 min. During the conversion of all PPD-type ginsenosides, it was confirmed through transformation pathways that BglNg-767 preferred to hydrolyze glucose moieties at C3, rather than those at C20. From TLC analysis, it was observed that BglNg-767 could not hydrolyze the ginsenoside Re and Rg1, indicating that BglNg-767 could not hydrolyze single-glucose molecules attached to C6 and C20 positions.
The suggested pathways for the conversion of ginsenosides by BglNg-767 are given as Rb1 → Gyp-XVII → F2; Rb2 → C–O; Rb3 → C-Mx1; Rc → C-Mc1; Rd → F2, and Gyp-XVII → F2. The biotransformation occurs through stepwise hydrolysis of glucose moieties at the C3 and C20 positions of the aglycon (Fig. 4).
Fig. 4.
Proposed pathway for enzymatic biotransformation of PPD-Mix type ginsenosides by recombinant BglNg-767
Conclusion
Complete genome sequencing (GenBank Accession Number: CP015772) and optical mapping of strain BS26T were performed to identify the genes encoding glycoside hydrolases responsible for ginsenoside transformation. Through complete genome analysis, a novel ginsenoside-transforming β-glucosidase termed as BglNg-767 was identified, which belongs to the glycoside hydrolase superfamily 3 (GH3). The optimum reaction condition for BglNg-767 was 40 °C at pH 7.5. BglNg-767 could convert various types of major ginsenosides such as Rb1, Rb2, Rb3, Rc, Rd, and Gyp-XVII by selective hydrolysis of glucose moieties. During the conversion of PPD-Mix-type ginsenosides, BglNg-767 efficiently hydrolyzed the glucose attached to the C3 and C20 positions.
Besides the 43 glycoside hydrolases, we also found 30 endopeptidase genes. From our SDS-PAGE analysis, we predicted that some genes among these 30 endopeptidases may cause self-hydrolysis of protein.
In this study, we characterized a novel ginsenoside-transforming β-glucosidase (BglNg-767) isolated from Niabella ginsenosidivorans BS26T. Furthermore, due to its quick and efficient reaction with the major ginsenosides Rc, Rd, and Gyp-XVII, BglNg-767 might be useful for mass production of the minor ginsenosides C-Mc1 and F2.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07045774) and by a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR201827103).
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflict of interest.
References
- Ahmad QR, Nguyen DH, Wingerd MA, Church GM, Steffen MA. Molecular weight assessment of proteins in total proteome profiles using 1D-PAGE and LC/MS/MS. Proteome Sci. 2005;1:1. doi: 10.1186/1477-5956-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baatar D, Siddiqi MZ, Im WT, Ul Khaliq N, Hwang SG. Anti-inflammatory effect of ginsenoside Rh2-Mix on lipopolysaccharide-stimulated RAW 264.7 Murine macrophage cells. J Med Food. 2018;21:951–960. doi: 10.1089/jmf.2018.4180. [DOI] [PubMed] [Google Scholar]
- Cheng LQ, Kim MK, Lee JW, Lee YJ, Yang DC. Conversion of major ginsenoside Rb1 to ginsenoside F2 by Caulobacter leidyia. Biotechnol Lett. 2006;28:1121–1127. doi: 10.1007/s10529-006-9059-x. [DOI] [PubMed] [Google Scholar]
- Chi H, Ji GE. Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganism. Biotechnol Lett. 2005;27:765–771. doi: 10.1007/s10529-005-5632-y. [DOI] [PubMed] [Google Scholar]
- Choi SH, Shin TJ, Hwang SH, Lee BH, Kang J, Oh JW, Bae CS, Lee SH, Nah SY. Differential effects of ginsenoside metabolites on HERG k channel currents. J Ginseng Res. 2011;35:191–199. doi: 10.5142/jgr.2011.35.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JR, Hong SW, Kim Y, Jang SE, Kim NJ, Han MJ, Kim DH. Metabolic activities of ginseng and its constituents, ginsenoside Rb1 and Rg1, by human intestinal microflora. J Ginseng Res. 2011;35:301–307. doi: 10.5142/jgr.2011.35.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- Court WE. Ginseng: the genus Panax. Luxembourg: Harwood Academic; 2000. [Google Scholar]
- Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol. 1997;7:637–644. doi: 10.1016/S0959-440X(97)80072-3. [DOI] [PubMed] [Google Scholar]
- Ishiguro R, Tanaka N, Abe K, Nakajima M, Maeda T, Miyanaga A, Takahashi Y, Sugimoto N, Nakai H, Taguchi H. Function and structure relationships of a β-1,2-glucooligosaccharide-degrading β-glucosidase. FEBS Lett. 2017;591:3926–3936. doi: 10.1002/1873-3468.12911. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Yamabe N, Choi P, Lee JW, Ham J, Kang KS. Efficient thermal deglycosylation of ginsenoside Rd and its contribution to the improved anticancer activity of ginseng. J Agric Food Chem. 2013;61:9185–9191. doi: 10.1021/jf402774d. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ahn JY, Shin TJ, Choi SH, Lee BH, Hwang SH, Kang J, Kim HJ, Park CW, Nah SY. Effects of minor ginsenosides, ginsenoside metabolites, and ginsenoside epimers on the growth of Caenorhabditis elegans. J Ginseng Res. 2011;35:375–383. doi: 10.5142/jgr.2011.35.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Siddiqi MZ, Liu QM, Kim DC, Im WT. Complete genome sequence of Niabella ginsenosidivorans BS26T, a ginsenoside-converting bacterium, isolated from compost. Korean J Microbiol. 2018;54:465–467. [Google Scholar]
- Leung KW, Wong AS. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20–22. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake J, Ochiai-Yanagi S, Kasumi T, Takagi T. Isolation of a Membrane protein from R. rubrum Chromatophores and its abnormal behavior in sds-polyacrylamide gel electrophoresis due to a high binding capacity for SDS. J Biochem. 1978;83:1679–1686. doi: 10.1093/oxfordjournals.jbchem.a132080. [DOI] [PubMed] [Google Scholar]
- Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci USA. 2009;106:1760–1765. doi: 10.1073/pnas.0813167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MH, Siddiqi MZ, Ahn S, Kang S, Kim YJ, Veerappan K, Yang DU, Yang DC. Stimulative effect of ginsenosides Rg5: Rk1 on murine osteoblastic MC3T3-E1 cells. Phytother Res. 2014;28:1447–1455. doi: 10.1002/ptr.5146. [DOI] [PubMed] [Google Scholar]
- Siddiqi MZ, Siddiqi MH, Kim YJ, Jin Y, Huq MA, Yang DC. Effect of fermented red ginseng extract enriched in ginsenoside Rg3 on the differentiation and mineralization of preosteoblastic MC3T3-E1 cells. J Med Food. 2015;18:1–7. doi: 10.1089/jmf.2014.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MZ, Cui CH, Park SK, Han NS, Kim SC, Im WT. Comparative analysis of the expression level of recombinant ginsenoside-transforming β-glucosidase in GRAS hosts and mass production of the ginsenoside Rh2-Mix. PLoS ONE. 2017;12(4):e0176098. doi: 10.1371/journal.pone.0176098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MZ, Muhammad Shafi S, Im WT. Complete genome sequencing of Arachidicoccus ginsenosidimutans sp. nov., and its application for production of minor ginsenosides by finding a novel ginsenoside-transforming b-glucosidase. RSC Adv. 2017;7:46745. doi: 10.1039/C7RA02612A. [DOI] [Google Scholar]
- Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/S0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Yun TK. Panax ginseng: a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]