Abstract
Male chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is represented by a heterogeneous group of symptoms that can cause an important impairment of daily quality of life for patients. Diagnosis of CP/CPPS is often not clear and treatment can be challenging, as it varies according to the different causative factors and derived symptoms. Differently from approaches used in the past, the diagnosis and subsequent treatment rely on separating this entity from chronic bacterial prostatitis and considering it as a multifactorial disease. Autoimmunity and inflammation, myofascial tenderness, neuroinflammation, and psychological causes have been clearly related to this disease, and therefore CPPS should not only be considered as related to benign prostatic enlargement. A multitude of different symptoms related to urinary, genital, rectal, and perineal areas can be attributed to this condition and therefore should be routinely investigated in patients, as well as possible differential diagnoses which can cause the same symptoms, such as pudendal nerve entrapment syndrome. The aim of this narrative review is to focus on CPPS after an infectious cause has been excluded.
Keywords: chronic prostatitis, chronic pelvic pain syndrome, pudendal neuralgia, physical therapy, pharmacological treatment
Introduction
The term “chronic prostatitis” is used to define and include many different symptomatological patterns, and its understanding is still enigmatic for many physicians and patients. Overall, it is estimated that the prevalence of chronic prostatitis among the male population is about 4.5–9%, with recurrence rates increasing up to 50% with increasing age.1–3 Therefore, it has a similar prevalence to that of ischemic heart disease and higher than diabetes.4 Although idiopathic urogenital and anorectal pain syndromes are not uncommon, effective treatments remain elusive for this patient group. Pain and functional disorders in these parts of the body can be embarrassing, limiting the desire to discuss the symptoms with the physician; similarly, clinicians may not be familiar enough with these syndromes, leading to misdiagnosis. Moreover, most of the involved patients usually complain of many different symptoms, not limited to “pure” prostatodynia but also presenting with: lower urinary tract symptoms (LUTS) with pollakiuria, dysuria, nocturia, urinary dribbling, or weak urinary stream; symptoms related to the anorectal area, such as constipation, sensation of foreign body in the rectus, and rectal pain during and after defecation; symptoms related to the external genitalia, represented by genital pain or burning and premature ejaculation, spontaneous sexual stimulation, or alteration of orgasms;5,6 patients can also refer to an associated low back pain, worsened in the sitting position. These symptoms can appear simultaneously or progressively, generating an increasing sensation of discomfort and anxiety for the patients, who often do not feel completely understood by their referring physician, with a progressive impairment of their quality of life (QoL).
Following the definition provided by the US National Institutes of Health (NIH) classification,7 chronic prostatitis is divided into different categories: a combination of chronic bacterial prostatitis (CBP), chronic pelvic pain syndrome (CPPS), or asymptomatic prostatitis. The overall NIH classification of prostatitis syndromes includes:
Category I: Acute bacterial prostatitis (ABP), due to acute bacterial infection determining prostatitis symptoms, systemic infection, and acute bacterial UTI.
Category II: Chronic bacterial prostatitis (CBP), with a demonstrated chronic bacterial prostatic infection with or without prostatitis symptoms.
Category III: Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), where an infective agent is absent, and the disease is led by chronic pelvic pain symptoms and voiding symptoms in the absence of UTI. Those patients present with urological pain or discomfort in the pelvic region, associated with urinary symptoms and/or sexual dysfunction, lasting for at least 3 of the previous 6 months This category is further divided into IIIA, inflammatory, and IIIB, non-inflammatory.
Category IV: Asymptomatic inflammatory prostatitis (AIP) due to prostate inflammation in the absence of genitourinary tract symptoms, always associated with CPPS.
Categories III and IV prostatitis cover a wide range of multifaceted chronic pain syndromes with varied clinical patterns, diagnostic pathways, and treatments. This field of research has rapidly evolved during the past few years, aiming at a wider understanding of the involved pathophysiological patterns. The physician should not only be focused on “usual” urological symptoms and complaints but also investigate all possible related symptoms in the anorectal, urinary, and genital area, as well as neurological, immunological, and psychological factors that may be related and influence the disease process.
Moreover, symptoms may also be due to other pathophysiology not related to prostatic inflammation, such as pudendal neuralgia due to pudendal nerve compression, which is often misdiagnosed and treated as a CPPS, with limited results.8,9
Recent studies suggest that many chronic pain conditions, particularly fibromyalgia (FM), chronic fatigue syndrome (CFS), and irritable bowel syndrome (IBS), share several demographic, clinical, and psychosocial aspects, as well as pain-related subjective and objective features, with CPPS, perhaps reflecting a mutual primary pathophysiology.10,11 Temporomandibular joint dysfunction, postural anomalies, asymmetry of body axis, and muscle disorders contribute to the long-term development of severe pain syndromes that can also reflect in the urogenital area.12–14
Given the diverse range of symptoms and possible etiologies, it is evident that a “one size fits all” diagnostic investigation and therapeutic approach is not possible, and the treatment can represent a real challenge for the involved physician, who has to evaluate the patient as a whole, taking into account not only aspects directly related to the reported symptoms, as stated in a recent paper on the European Association of Urology guidelines.15 The aim of this narrative review is to focus on CPPS after an infectiou/CPPS after an infectious cause has been excluded. The principal studies considered in this work and discussed below are listed in Table 1.
Table 1.
Summary of the most relevant studies included in this review
| Studies | Study type | Domain analyzed | Treatment(s) | No. of patients | p |
|---|---|---|---|---|---|
| Zhao et al29 | RCT | • Pain • Quality of life |
Celecoxib vs placebo | 32 vs 32 |
<0.006 <0.032 |
| Bates et al30 | RCT | • NIH-CPSI | Oral prednisolone vs placebo | 18 | 0.48 |
| Wagenlehner et al32 | RCT | • NIH-CPSI • Pain • Quality of life |
Pollen extract vs placebo | 70 vs 69 |
0.0126 0.0086 0.0250 |
| Cai et al35 | RCT | • NIH-CPSI • Pain • Quality of life |
Pollen extract vs ibuprofen | 41 vs 46 |
0.002 <0.001 0.002 |
| Pontari et al36 | RCT | • NIH-CPSI • ≥6 points NIH-CPSI score decrease |
Pregabalin vs placebo | 218 vs 106 |
<0.05 0.07 |
| Nickel et al48 | RCT | • NIH-CPSI | Alfuzosin vs placebo | 136 vs 136 | 0.90 |
| Kaplan et al54 | RCT | • NIH-CPSI group 1 • NIH-CPSI group 2 |
Saw palmetto vs finasteride | 32 vs 32 | 0.41 <0.003 |
| Fitzgerald et al59 | RCT | Patient global response assessment | Myofascial physical therapy vs global massage | 24 vs 23 | 0.03 |
| Lee et al62 | RCT | Predefined clinical response criterion | Acupuncture vs sham procedures | 44 vs 45 | 0.017 |
| Schneider et al63 | Prospective series | • Pain • Quality of life |
TENS | 60 |
<0.001 <0.001 |
Note: Significant values (p<0.05) are shown in bold.Abbreviations: RCT, randomized controlled trial; NIH-CPSI, National Institutes of Health Chronic Prostatitis Symptom Index; TENS, posterior tibial nerve stimulation/transcutaneous electrical nerve stimulation.
The UPOINT clinical phenotyping system for CPPS
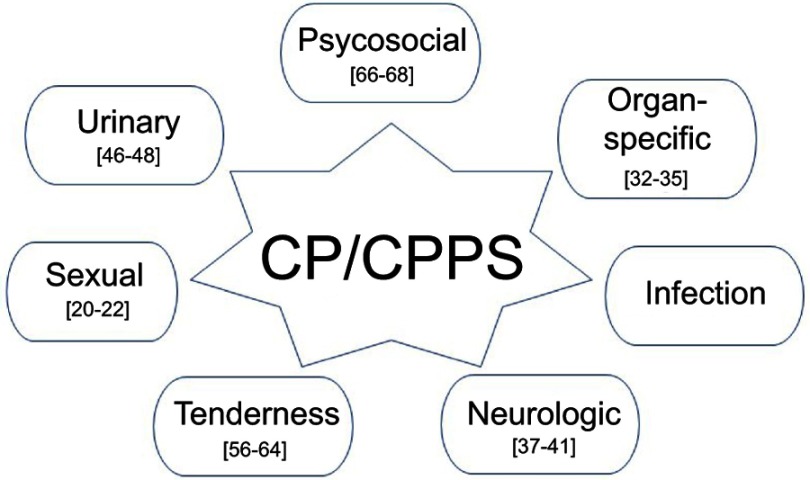
The most widely adopted questionnaire for clinical evaluation of CPPS is the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI).16 This tool, validated in 1999, is composed of nine different questions investigating pain, urinary symptoms, and QoL related to CPPS. In 2009, Shoskes et al17 proposed a dedicated clinical classification of CPPS, to separately identify the different possible reported symptoms. It considers: Urinary symptoms (mostly regarding the storage phase but also the voiding phase); Psychosocial dysfunction (depression or catastrophizing thoughts); Organ-specific findings (prostate tenderness or swelling, leukocytosis in prostatic fluid, hematospermia, prostatic calcifications); Infection (exclusion of infective etiology or bowel contamination); Neurological/systemic (presence of abdominal and/or pelvic pain, IBS, FM, CFS); and Tenderness of muscles (presence of palpable muscle spasm or trigger points in abdomen and pelvic floor).
Classifying patients according to the clinical phenotype can allow for exploration of the relative contribution of each domain to the severity of symptoms and, ultimately, to the treatment response by focusing on the main complaints from the individual patient. The authors found that patients identify their complaints in three main areas and only 22% of them reported a single positive domain. Several works in the literature confirm and validate the clinical utility of this approach.18,19 This evidence demonstrates that CPPS originates from different patterns of pathology and that the therapeutic approach is wide and differs in relation to the reported symptoms. Moreover, the resolution of one “aspect” can lead to the resolution of others, even if not strictly related. Magri et al,20 applying the UPOINT classification system on 1,227 patients in a multicentric European series, noticed that many of these patients also complained about sexual dysfunction. Ejaculatory pain is also specifically reported in the NIH-CPSI symptom index (question 2b). Therefore, an implementation of the UPOINT classification has been proposed, adding the domain of sexual dysfunction (erectile dysfunction, impaired sexual desire, and orgasmic dysfunction) as a clinical pattern related to CPPS. Consistent with the study by Magri et al,20 in a US study on 162 men, those with a positive sexual dysfunction domain were found to report a lower QoL.21 A 2016 meta-analysis estimated the overall prevalence of sexual dysfunction in men with CP/CPPS as up to 0.62 (95% CI 0.48–0.75), while the prevalence of erectile dysfunction and premature ejaculation was 0.29 (95% CI 0.24–0.33) and 0.40 (95% CI 0.30–0.50), respectively.22
The understanding of this clinical phenotyping system for CP/CPPS (Figure 1) can explain why this disease has a wide multifactorial genesis, potentially different in each patient, therefore generating an individual multifaceted complex of symptoms for every patient diagnosed with CP/CPPS. A review by Magistro et al23 analyzed 28 randomized controlled trials (RCTs) evaluating various treatments for CP/CPPS, and underlined that monotherapy is never enough to achieve symptom relief and that the therapeutic approach should focus on the different symptomatic pattern presented by the patient in a multimodal setting.
Figure 1.
Different phenotypes of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) according to the UPOINT classification system,17,20 with the most relevant research work cited in the text.
Modulation of inflammatory process
Since the early 2000s, the immunological mechanisms responsible for chronic inflammation in CP/CPPS have been exhaustively explored. Not surprisingly, many different factors seem to be involved. In vivo and in vitro CP/CPPS studies24,25 showed a sort of autoimmunity against prostate cells inducted by the inflammation, with the recruiting leukocytes including Th1 cells and mast cells, which enhance and trigger the development of CPPS,26 in a similar way to that described for rheumatoid arthritis, multiple sclerosis, and inflammatory bowel diseases.27,28
Thus, medications able to interrupt this molecular mechanism can have a primary therapeutic role.
An RCT by Zhao et al29 demonstrated the efficacy of celecoxib (dosage 200 mg four times per day, administered for 6 weeks) over placebo for pain modulation; however, symptoms suddenly re-presented after treatment discontinuation. Another trial failed to demonstrate a benefit from oral prednisolone administration over 4 weeks.30 Similarly, studies involving monoclonal antibodies did not show significant clinical CPPS improvement.31 Common anti-inflammatory drugs (ie, NSAIDs) seem to give rapid relief of symptoms but only for a short period (2–4 weeks). This limits the application of these drugs to acute phases of the disease, without the possibility for long-term administration. Moreover, their well-known side effects further limit their applicability for pain reduction in CP/CPPS.
A multicentric RCT by Wagenlehner et al32 investigated the therapeutic benefit of 12 weeks of treatment with pollen extract (Cernilton) in men with CP/CPPS. Participants were evaluated using the NIH-CPSI questionnaire, the number of leukocytes in post-prostatic massage urine, the International Prostate Symptom Score (IPSS), and the sexuality domain of a life satisfaction questionnaire at baseline and after 6 and 12 weeks of treatment. Overall, a decrease in the NIH-CPSI total score by at least 25% or at least 6 points was noticed in the 70.6% of patients undergoing this treatment compared to placebo. Therefore, several studies demonstrated the anti-inflammatory and anti-proliferative role of pollen extract in CP/CPPS due to the inhibition of prostaglandin and leukotriene synthesis in both molecular and clinical trials. Moreover, flower pollen extracts inhibit 5α-reductase after prolonged therapy and this can also contribute to the reported amelioration of LUTS reported in clinical trials. No major adverse events have been reported in the literature and this treatment is active part of daily urological practice.33,34 An RCT by Cai et al35 investigated the anti-inflammatory properties of oral administration of pollen extract in association with vitamins in CPPS. Patients were equally divided into two homogeneous groups, receiving oral capsules of DEPROX 500® (two capsules every 24 hours) or ibuprofen (600 mg, one tablet three times a day) for 4 weeks. At the final evaluation, a greater and statistically significant improvement in urinary function and QoL was noticed in the DEPROX 500 group compared with the ibuprofen group. Therefore, these therapeutic agents can improve total symptoms, pain, and QoL in CP/CPPS patients.
Neuromodulatory therapies
Neuromodulating medications have a widespread application in the treatment of neuropathic pain, and studies have begun to define their role in the treatment of CP/CPPS.
The proposal of an analgesic neuromodulation of CP/CPPS derives from some studies concerning the effects of a neuromodulatory drug, pregabalin, in increasing dosages (from 150 mg to 600 mg daily) over 6 weeks of treatment, which showed an amelioration of pain symptoms. This is probably due to a chronic activation of sensitive terminations in CP/CPPS that lead to the transmission of nociceptive signals. However, patients did not report symptom resolution but mostly a modest improvement in pain symptoms. Therefore, this effect has not been clearly demonstrated and pregabalin should only be prescribed as a second-line treatment.36
A modification of prostate innervation during age and with the development of benign prostatic hyperplasia (BPH) has been demonstrated,37 with an increase in α1-adrenoceptor density leading to an augmented basal tone of the urethral sphincter, and a subsequent increase in urine pressure inside the bladder that can contribute towards increasing the inflammatory response.38
Therefore, neuromodulator therapy can have a consistent role in this pathophysiological pattern in combination with other drugs (ie, α-blockers).
Cordaro et al39 investigated the role of the fatty acid amide-signaling molecule palmitoylethanolamide (PEA) in combination with a biological precursor of resveratrol with antioxidant properties named polydatin, for the reduction of BPH-induced neural-mediated inflammation and prostate growth as well as for CPPS symptom modulation. PEA is an endogenous fatty acid amide, belonging to the class of nuclear factor agonists with affinity to cannabinoid-like G-coupled receptors GPR55 and GPR119, with neuromodulatory and anti-inflammatory properties.40,41 PEA’s mechanism of action, depicted for the first time in 1993 by the Nobel Prize winner Rita Levi-Montalcini, is described as autacoid local injury antagonism,42,43 and acts as a regulator of mast cell-induced inflammation at the level of sensory nerve endings. Therefore, PEA can reduce neuropathic pain and disorders based on glial cell overactivation, such as in diabetes and glaucoma.44 Histological findings in CP/CPPS patients' prostates often show infiltrating lymphocytes and macrophages around glandular elements, and therefore PEA could have similar neuronal and immune-modulating effects on prostatic cells. Although not yet validated for CP/CPPS through RCTs, such medications are already in clinical use and have shown a beneficial effect in other clinical applications, for example in reducing the vascular inflammation and oxidative stress responsible for atherosclerosis.45
Alpha-blockers, phosphodiesterase type 5 inhibitors and 5α-reductase inhibitors
The pharmacological influence of selective α-blocking agents (such as silodosine or terazosine) is directed against the smooth muscle in the prostatic gland and capsule. Moreover, alpha-lytic agents are active on the region of the bladder neck in patients with BPH, which is frequently associated with CP. A reduction of voiding pressures and of voiding flow patterns can significantly reduce the discomfort of the patient with CP/CPPS and could be considered in a multimodal therapeutic regimen.46,47
A multicenter, randomized, double-blind, placebo-controlled trial involving 292 patients evaluated the possible efficacy of the α-blocker alfuzosin in reducing overall symptoms in CP/CPPS patients, with the primary outcome being a reduction of at least 4 points in the NIH-CPSI score after 12 weeks of therapy compared to placebo.48 The results were discouraging, with similar response rates in the two groups (34.8% vs 36%, p=0.9). Other studies reported the relief of symptoms by α-blockers, but always limited to patients with bladder outlet obstruction.49–52 Evidently, those drugs proivde relief from voiding symptoms rather than from pain. So, alpha-blockers should not be prescribed for patients without voiding issues and attention must be paid when prescribing them to young patients owing to the known negative effect on ejaculation.
Another effective type of drug with the potential to reduce LUTS, erectile dysfunction, and symptoms of CP/CPPS is the phosphodiesterase-5 inhibitor (PDE5-I). A study by Oelke et al.53 demonstrated a similar efficacy of PDE5-I compared to common α-blockers in terms of amelioration of LUTS and restoration of urinary flow rate, with the known additional positive effect on erectile function.
As known for BPH treatment, 5α-reductase inhibitors (5-ARIs) reduce prostate volume by inhibiting the conversion of testosterone to dihydrotestosterone, the metabolically active form responsible for prostatic growth. An RCT by Kaplan et al54 provided better amelioration of symptoms compared to saw palmetto over a 1-year period. In another study considering a group of men enrolled in a prostate cancer risk reduction study, the NIH-CPSI scores decreased significantly for those under dutasteride compared to placebo.55 Therefore, although a clear action on CP/CPPS has not been demonstrated, the reported amelioration of CPSI scores could encourage 5-ARI therapy in selected patients.
Physiotherapy and pelvic floor muscle relaxation
Prostatic massage, perineal or pelvic floor massage, and myofascial trigger-point release have been proposed as a beneficial treatment modality for patients who complain of perineal soreness and difficulty in bladder/rectal evacuation. Muscle tenderness is clearly an important cause of chronic pain and the derived increase in intrapelvic pressure can also lead to worsening of bladder/prostatic symptoms in addition to possible effects in developing chronic orchialgia or low back pain.56,57
Anderson et al, in a series of 74 patients, demonstrated the relationship between specific areas of pelvic pain and specific myofascial trigger points, showing a clear relationship of visceral pain to particular muscular areas.58 Many studies have reported pain relief after muscular massage or physical therapy. One of the most relevant is a study by Fitzgerald et al,59 which demonstrated better symptom relief for CPPS patients undergoing specific myofascial therapy over global massage, with global response assessment response rates of 57% vs 21% (p=0.03). It is thus important to refer patients to highly specialized physiotherapy centers.60
Other physical therapy interventions include electromagnetic therapy, microwave thermotherapy, extracorporeal shockwave therapy, acupuncture, and posterior tibial nerve stimulation/transcutaneous electrical nerve stimulation (TENS). Some publications have shown a clear beneficial effect of acupuncture, probably by reducing the neuropathic pain.61 Lee et al, in a controlled trial, demonstrated its efficacy for CPPS symptom reduction compared to placebo.62 Attention has also been focused on TENS, owing to its proven efficacy on musculoskeletal pain. The advantages of this treatment are the possibility to deliver it at the patient's home and the absence of side effects. Schneider et al,63 in a series of 60 patients with CPPS refractory to α-blockers and common analgesics, described symptom relief in 50% of subjects after 12 weeks of TENS treatment, with perceived amelioration in 70%. Even if TENS is effective, there are no RCTs proving its efficacy on chronic pain relief.64
Psychological aspects
An important aspect of CP/CPPS is the long-term duration and evolution of the symptoms. These can be frustrating and disabling, with consequent limitations on the patient’s daily activities and QoL. Psychological support has been proposed as an integrative therapy in the vision of a multimodal approach.65 This is focused on perceiving patients’ internal beliefs in relation to chronic pain, which are often causative of depression or catastrophic thinking. Moreover, there is often a variable perception of pain, which can increase owing to the person focusing on the symptoms. Therefore, patients should be investigated regarding their social support and interactions, daily and family life, and relationship and sexual issues, to orient them towards healthy coping strategies.66
The importance of multidisciplinary treatment is emphasized by several reviews, where the need for high-quality psychological treatment evaluation is underlined.67,68
Therefore, a mental distress evaluation should always be carried out by a dedicated psychologist or psychiatric team, with the aim of addressing susceptible patients.
Differential diagnosis with pudendal nerve entrapment syndrome
Originating from the sacral branches S2, S3, and S4, the pudendal nerve (PN) carries sensation from the external genitalia and the skin of the perineum and anus regions, as well as the motor supply to various pelvic muscles, including the male or female external urethral sphincter and the external anal sphincter. Its main branches are the superficial branch, with a sensitive innervation from the perineum and scrotum/labia region, and the deep branch, with a motor supply for bulbocavernosus, ischiocavernosus, superficial and deep transverse perineus, and sphincter urethrae muscles. The terminal branch of the PN is the dorsal nerve of the penis or clitoris. Therefore, this nerve is related to the entire pelvic and anogenital area, and its compression or irritation can lead to a wide variety of symptoms.
Pudendal neuralgia, also known as Alcock’s canal syndrome, was first described by Amarenco et al in 1987, in a French paper.69 It may be related to or be secondary to childbirth, pelvic surgery, intense cycling, sacroiliac skeletal abnormalities, or age-related changes. The most well-known diagnostic criteria for PN are the Nantes criteria, first published in 2008.70 The diagnosis is mainly clinical, and its five main signs according to these criteria are: 1) pain in the anatomical territory of the PN (ie, between the anus and the penis/clitoris area), which is 2) worsened by sitting; 3) symptoms do not wake the patient during the night; 4) no objective sensory loss on clinical examination; and 5) positive anesthetic PN block.
As can be seen from the Nantes criteria, the diagnosis is mainly clinical. Pereira et al related the possible different symptoms to the site of PN compression, with four different sites:71 sacral root emergency; infrapiriform below the sacroischiatic ligament; Alcock’s canal; and terminal branches (ie, perineal nerve, dorsal clitoris, and inferior anal nerve).
Symptoms are extremely variable. Pain is probably the last symptom experienced and patients often report a long history of functional problems. The symptoms are often increased in the seated position and became worse during the daytime, with relief during the night; a cutaneous allodynia is typical and due to PN irritation, the symptoms can increase after sexual intercourse, with a delay of 24–48 hours, or after defecation. Women describe worsening of the symptoms when they have sexual intercourse associated with an orgasm. Similarly, men may report changes in the quality of orgasms and premature ejaculation.
The coexistence of pain in the perineum and lower limbs/pseudosciatica is indicative of a disorder of the second segment of the PN. The PN can be entrapped under the sacroischiatic ligament simultaneously with the sciatic and inferior gluteal nerves. PN compression in this area is often the cause of pudendal neuralgia and can be treated with conservative measures or with surgical decompression.72–75
Conservative measures include muscle stretches and exercises to correct spasms and imbalances, as well as electrical stimulation and biofeedback. Postural anomalies also have to be investigated and corrected.9
Anesthetic infiltration of the PN provides the definitive diagnostic test for PN entrapment, providing a temporary resolution of reported symptoms, and should be performed before any attempt at surgical decompression.76
Owing to the highly variable differences in symptom presentation, pudendal neuralgia is often underestimated and probably often misdiagnosed and confused with CPPS. This condition should always be ruled out in patients presenting with apparent CPPS, particularly at a young age.
Conclusions
CP/CPPS is a multivariate and complex disease, often presenting a difficult diagnostic framework. This pathology has a consistent impact on patients’ QoL as it can last for years or a lifetime if not correctly identified and treated. Moreover, owing to its high prevalence, it imposes a significant economic burden on the health-care system. Practitioners need to move away from old-fashioned habits such as endless courses of empiric antibiotics or underestimation of the patients’ reported problems in the absence of an objective finding, and embrace a multimodal approach to CPPS. Moreover, differential diagnoses such as PN entrapment syndrome should always be considered and investigated.
Abbreviations list
ABP, acute bacterial prostatitis; AIP, asymptomatic inflammatory prostatitis; 5-ARI, 5α-reductase inhibitor; BPH, benign prostatic hyperplasia; CBP, chronic bacterial prostatitis; CFS, chronic fatigue syndrome; CP/CPPS, chronic prostatitis/chronic pelvic pain syndrome; FM, fibromyalgia; IBS, irritable bowel syndrome; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; NIH, National Institutes of Health; NIH-CPSI, National Institutes of Health Chronic Prostatitis Symptom Index; PDE5-I, phosphodiesterase-5 inhibitor; PEA, palmitoylethanolamide; PN, pudendal nerve; QoL, quality of life; TENS, transcutaneous electrical nerve stimulation.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Clemens JQ, Meenan RT, O’Keeffe Rosetti MC, Kimes T, Calhoun EA. Prevalence of and risk factors for prostatitis: population based assessment using physician assigned diagnoses. J Urol. 2007;178(4 Pt 1):1333–1337. doi: 10.1016/j.juro.2007.05.140 [DOI] [PubMed] [Google Scholar]
- 2.Roberts RO, Lieber MM, Rhodes T, Girman CJ, Bostwick DG, Jacobsen SJ. Prevalence of a physician-assigned diagnosis of prostatitis: the Olmsted county study of urinary symptoms and health status among men. Urology. 1998;51(4):578–584. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo M, Marchetti F, Travaglini F, Trinchieri A, Nickel JC. Prevalence, diagnosis and treatment of prostatitis in Italy: a prospective urology outpatient practice study. BJU Int. 2003;92(9):955–959. [DOI] [PubMed] [Google Scholar]
- 4.Adams PF, Marano MA. Current estimates from the National health interview survey, 1994. Vital Health Stat. 1995;10(193 Pt 2):261–520. [PubMed] [Google Scholar]
- 5.Roberts RO, Jacobson DJ, Girman CJ, Rhodes T, Lieber MM, Jacobsen SJ. Prevalence of prostatitis-like symptoms in a community based cohort of older men. J Urol. 2002;168(6):2467–2471. doi: 10.1097/01.ju.0000036433.07079.ce [DOI] [PubMed] [Google Scholar]
- 6.Kunishima Y, Mori M, Kitamura H, Satoh H, Tsukamoto T. Prevalence of prostatitis-like symptoms in Japanese men: population-based study in a town in Hokkaido. Int J Urol. 2006;13(10):1286–1289. doi: 10.1111/j.1442-2042.2004.01556.x [DOI] [PubMed] [Google Scholar]
- 7.Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282(3):236–237. [DOI] [PubMed] [Google Scholar]
- 8.Ramsden CE, McDaniel MC, Harmon RL, et al. Pudendal nerve entrapment as source of intractable perineal pain. Am J Phys Med Rehabil. 2003;82(6):479–484. doi: 10.1097/01.PHM.0000069196.15353.7D [DOI] [PubMed] [Google Scholar]
- 9.Benson JT, Griffis K. Pudendal neuralgia, a severe pain syndrome. Am J Obstet Gynecol. 2005;192(5):1663–1668. doi: 10.1016/j.ajog.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182(5):2123–2131. doi: 10.1016/j.juro.2009.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bullones Rodríguez MÁ, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2013;189(1 Suppl):S66–S74. doi: 10.1016/j.juro.2012.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korszun A, Papadopoulos E, Demitrack M, Engleberg C, Crofford L. The relationship between temporomandibular disorders and stress-associated syndromes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(4):416–420. [DOI] [PubMed] [Google Scholar]
- 13.Krieger JN, Stephens AJ, Landis JR, et al.; MAPP Research Network. Relationship between chronic nonurological associated somatic syndromes and symptom severity in urological chronic pelvic pain syndromes: baseline evaluation of the MAPP study. J Urol. 2015;193(4):1254–1262. doi: 10.1016/j.juro.2014.10.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet. 1999;354(9182):936–939. doi: 10.1016/S0140-6736(98)08320-2 [DOI] [PubMed] [Google Scholar]
- 15.Engeler DS, Baranowski AP, Dinis-Oliveira P, et al.; European Association of Urology. The 2013 EAU guidelines on chronic pelvic pain: is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development. Eur Urol. 2013;64(3):431–439. doi: 10.1016/j.eururo.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Litwin MS, McNaughton-Collins M, Fowler FJ Jr, et al. The National institutes of health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic prostatitis collaborative research network. J Urol. 1999;162(2):369–375. [DOI] [PubMed] [Google Scholar]
- 17.Shoskes DA, Nickel JC, Dolinga R, Prots D. Clinical phenotyping of patients with chronic prostatitis/chronic pelvic pain syndrome and correlation with symptom severity. Urology. 2009;73(3):538–542. discussion 542-3 DOI: 10.1016/j.urology.2008.09.074 [DOI] [PubMed] [Google Scholar]
- 18.Tran CN, Li J, Shoskes DA. An online UPOINT tool for phenotyping patients with chronic prostatitis. Can J Urol. 2014;21(2):7195–7200. [PubMed] [Google Scholar]
- 19.Zhao Z, Zhang J, He J, Zeng G. Clinical utility of the UPOINT phenotype system in Chinese males with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a prospective study. PLoS One. 2013;8(1):e52044. doi: 10.1371/journal.pone.0052044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magri V, Wagenlehner F, Perletti G, et al. Use of the UPOINT chronic prostatitis/chronic pelvic pain syndrome classification in European patient cohorts: sexual function domain improves correlations. J Urol. 2010;184(6):2339–2345. doi: 10.1016/j.juro.2010.08.025 [DOI] [PubMed] [Google Scholar]
- 21.Davis SN, Binik YM, Amsel R, Carrier S. Is a sexual dysfunction domain important for quality of life in men with urological chronic pelvic pain syndrome? Signs “UPOINT” to yes. J Urol. 2013;189(1):146–151. doi: 10.1016/j.juro.2012.08.083 [DOI] [PubMed] [Google Scholar]
- 22.Li HJ, Kang DY. Prevalence of sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome: a meta-analysis. World J Urol. 2016;34(7):1009–1017. doi: 10.1007/s00345-015-1720-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magistro G, Wagenlehner FM, Grabe M, Weidner W, Stief CG, Nickel JC. Contemporary management of chronic prostatitis/chronic pelvic pain syndrome. Eur Urol. 2016;69(2):286–297. doi: 10.1016/j.eururo.2015.08.061 [DOI] [PubMed] [Google Scholar]
- 24.Dunphy EJ, Eickhoff JC, Muller CH, Berger RE, Mcneel DG. Identification of antigen-specific IgG in sera from patients with chronic prostatitis. J Clin Immunol. 2004;24:492–502. doi: 10.1023/B:JOCI.0000040920.96065.5a [DOI] [PubMed] [Google Scholar]
- 25.John H, Maake C, Barghorn A, Zbinden R, Hauri D, Joller-Jemelka HI. Immunological alterations in the ejaculate of chronic prostatitis patients: clues for autoimmunity. Andrologia. 2003;35(5):294–299. [PubMed] [Google Scholar]
- 26.Breser ML, Salazar FC, Rivero VE, Motrich RD. Immunological mechanisms underlying chronic pelvic pain and prostate inflammation in chronic pelvic pain syndrome. Front Immunol. 2017;8:898. doi: 10.3389/fimmu.2017.00898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149(3):660–672. doi: 10.1016/j.neuroscience.2007.07.053 [DOI] [PubMed] [Google Scholar]
- 28.Walker ME, Hatfield JK, Brown MA. New insights into the role of mast cells in autoimmunity: evidence for a common mechanism of action? Biochim Biophys Acta. 2012;1822(1):57–65. doi: 10.1016/j.bbadis.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao WP, Zhang ZG, Li XD, et al. Celecoxib reduces symptoms in men with difficult chronic pelvic pain syndrome (category IIIA). Braz J Med Biol Res. 2009;42:963–967. doi: 10.1590/S0100-879X2009005000021 [DOI] [PubMed] [Google Scholar]
- 30.Bates SM, Hill VA, Anderson JB, et al. A prospective, randomized, double-blind trial to evaluate the role of a short reducing course of oral corticosteroid therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome. BJU Int. 2007;99:355–359. doi: 10.1111/j.1464-410X.2007.06667.x [DOI] [PubMed] [Google Scholar]
- 31.Goldmeier D, Madden P, McKenna M, Tamm N. Treatment of category III A prostatitis with zafirlukast: a randomized controlled feasibility study. Int J STD AIDS. 2005;16:196–200. doi: 10.1258/0956462053420239 [DOI] [PubMed] [Google Scholar]
- 32.Wagenlehner FM, Schneider H, Ludwig M, Schnitker J, Brahler E, Weidner W. A pollen extract (Cernilton) in patients with inflammatory chronic prostatitis-chronic pelvic pain syndrome: a multicentre, randomised, prospective, double-blind, placebo-controlled phase 3 study. Eur Urol. 2009;56:544–551. doi: 10.1016/j.eururo.2009.05.046 [DOI] [PubMed] [Google Scholar]
- 33.Cai T, Verze P, La Rocca R, Anceschi U, De Nunzio C, Mirone V. The role of flower pollen extract in managing patients affected by chronic prostatitis/chronic pelvic pain syndrome: a comprehensive analysis of all published clinical trials. BMC Urol. 2017;17(1):32. doi: 10.1186/s12894-017-0223-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pirola GM, Puliatti S, Bocchialini T, Martorana E, Micali S, Bianchi G. Efficacy of pollen extract in association with group B vitamins for pain relief in chronic prostatitis/chronic pelvic pain syndrome: a survey of urologists‘ knowledge about its clinical application. Arch Ital Urol Androl. 2017;89(1):22–25. doi: 10.4081/aiua.2017.1.22 [DOI] [PubMed] [Google Scholar]
- 35.Cai T, Wagenlehner FM, Luciani LG, et al. Pollen extract in association with vitamins provides early pain relief in patients affected by chronic prostatitis/chronic pelvic pain syndrome. Exp Ther Med. 2014;8(4):1032–1038. doi: 10.3892/etm.2014.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pontari MA, Krieger JN, Litwin MS, et al. Pregabalin for the treatment of men with chronic prostatitis/chronic pelvic pain syndrome: a randomized controlled trial. Arch Intern Med. 2010;170:1586–1593. doi: 10.1001/archinternmed.2010.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White CW, Xie JH, Ventura S. Age-related changes in the innervation of the prostate gland: implications for prostate cancer initiation and progression. Organogenesis. 2013;9(3):206–215. doi: 10.4161/org.24843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada S, Ashizawa N, Ushijima H, Nakayama K, Hayashi E, Honda K. Alpha-1 adrenoceptors in human prostate: characterization and alteration in benign prostatic hypertrophy. J Pharmacol Exp Ther. 1987;242:326–330. [PubMed] [Google Scholar]
- 39.Cordaro M, Impellizzeri D, Siracusa R, et al. Effects of a co-micronized composite containing palmitoylethanolamide and polydatin in an experimental model of benign prostatic hyperplasia. Toxicol Appl Pharmacol. 2017;329(329):231–240. doi: 10.1016/j.taap.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 40.Lo Verme J, Fu J, Astarita G, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67(1):15–19;Epub 2004 Oct 1.doi: 10.1124/mol.104.006353 [DOI] [PubMed] [Google Scholar]
- 41.Lambert DM, Vandevoorde S, Jonsson KO, Fowler CJ. The palmitoylethanolamide family: a new class of anti-inflammatory agents? Curr Med Chem. 2002;9(6):663–674. Review DOI: 10.2174/0929867023370707 [DOI] [PubMed] [Google Scholar]
- 42.Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci U S A. 1995;92(8):3376–3380. doi: 10.1073/pnas.92.8.3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aloe L, Leon A, Levi-Montalcini R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions. 1993;39:C145–C147. doi: 10.1007/BF01972748 [DOI] [PubMed] [Google Scholar]
- 44.Donvito G, Bettoni I, Comelli F, Colombo A, Costa B. Palmitoylethanolamide relieves pain and preserves pancreatic islet cells in a murine model of diabetes. CNS Neurol Disord Drug Targets. 2015;14(4):452–462. [DOI] [PubMed] [Google Scholar]
- 45.Gugliandolo E, Fusco R, Biundo F, et al. Palmitoylethanolamide and polydatin combination reduces inflammation and oxidative stress in vascular injury. Pharmacol Res. 2017;123:83–92. doi: 10.1016/j.phrs.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 46.Barbalias GA, Nikiforidis G, Liatsikos EN. Alpha-blockers for the treatment of chronic prostatitis in combination with antibiotics. J Urol. 1998;159:883–887. [PubMed] [Google Scholar]
- 47.Mehik A, Alas P, Nickel JC, Sarpola A, Helström PJ. Alfuzosin treatment for chronic prostatitis/chronic pelvic pain syndrome: a prospective, randomized, double-blind, placebo-controlled, pilot study. Urology. 2003;62(3):425–429. [DOI] [PubMed] [Google Scholar]
- 48.Nickel JC, Krieger JN, McNaughton-Collins M, et al.; Chronic Prostatitis Collaborative Research Network. Alfuzosin and symptoms of chronic prostatitis-chronic pelvic pain syndrome. N Engl J Med. 2008;359(25):2663–2673. doi: 10.1056/NEJMoa0803240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuğcu V, Taşçi AI, Fazlioğlu A, et al. A placebo-controlled comparison of the efficiency of triple- and monotherapy in category III B chronic pelvic pain syndrome (CPPS). Eur Urol. 2007;51(4):1113–1117; discussion 1118. doi: 10.1016/j.eururo.2006.09.036 [DOI] [PubMed] [Google Scholar]
- 50.Nickel JC, O’Leary MP, Lepor H, et al. Silodosin for men with chronic prostatitis/chronic pelvic pain syndrome: results of a phase II multicenter, double-blind, placebo-controlled study. J Urol. 2011;186:125–131. doi: 10.1016/j.juro.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 51.Cheah PY, Liong ML, Yuen KH, et al. Terazosin therapy for chronic prostatitis/chronic pelvic pain syndrome: a randomized, placebo-controlled trial. J Urol. 2003;169:592–596. doi: 10.1097/01.ju.0000042767.95019.76 [DOI] [PubMed] [Google Scholar]
- 52.Krieger JN, McNaughton-Collins M, Anderson RU, et al. Alfuzosin and symptoms of chronic prostatitis–chronic pelvic pain syndrome. N Engl J Med. 2008;359:2663. doi: 10.1056/NEJMoa0801936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oelke M, Giuliano F, Mirone V, et al. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61:917–925. doi: 10.1016/j.eururo.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 54.Kaplan SA, Volpe MA, Te AE. A prospective, 1-year trial using saw palmetto versus finasteride in the treatment of category III prostatitis/chronic pelvic pain syndrome. J Urol. 2004;171(1):284–288. doi: 10.1097/01.ju.0000101487.83730.80 [DOI] [PubMed] [Google Scholar]
- 55.Nickel JC, Roehrborn C, Montorsi F, Wilson TH, Rittmaster RS. Dutasteride reduces prostatitis symptoms compared with placebo in men enrolled in the REDUCE study. J Urol. 2011;186(4):1313–1318. doi: 10.1016/j.juro.2011.05.071 [DOI] [PubMed] [Google Scholar]
- 56.Potts JM, O’Dougherty E. Pelvic floor physical therapy for patients with prostatitis. Curr Urol Rep. 2000;1:155. doi: 10.1007/s11934-000-0051-z [DOI] [PubMed] [Google Scholar]
- 57.Anderson RU, Wise D, Sawyer T, et al. Integration of myofascial trigger point release and paradoxical relaxation training for treatment of chronic pelvic pain in men. J Urol. 2005;174:155. doi: 10.1097/01.ju.0000161609.31185.d5 [DOI] [PubMed] [Google Scholar]
- 58.Anderson RU, Sawyer T, Wise D, Morey A, Nathanson BH. Painful myofascial trigger points and pain sites in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2009;182(6):2753–2758. doi: 10.1016/j.juro.2009.08.033 [DOI] [PubMed] [Google Scholar]
- 59.Fitzgerald MP, Anderson RU, Potts J, et al. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urol. 2013;189:S75–S85. doi: 10.1016/j.juro.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson RU, Wise D, Nathanson BH. Chronic prostatitis and/or chronic pelvic pain as a psychoneuromuscular disorder-a meta-analysis. Urology. 2018;120:23–29. doi: 10.1016/j.urology.2018.07.022 [DOI] [PubMed] [Google Scholar]
- 61.Sikiru L, Shmaila H, Muhammed SA. Transcutaneous electrical nerve stimulation (TENS) in the symptomatic management of chronic prostatitis/chronic pelvic pain syndrome: a placebo control randomized trial. Int Braz J Urol. 2008;34(6):708–713; discussion 714. [DOI] [PubMed] [Google Scholar]
- 62.Lee SW, Liong ML, Yuen KH, et al. Validation of a sham acupuncture procedure in a randomised, controlled clinical trial of chronic pelvic pain treatment. Acupunct Med. 2011;29:40–46. doi: 10.1136/aim.2010.003137 [DOI] [PubMed] [Google Scholar]
- 63.Schneider MP, Tellenbach M, Mordasini L, Thalmann GN, Kessler TM. Refractory chronic pelvic pain syndrome in men: can transcutaneous electrical nerve stimulation help? BJU Int. 2013;112(2):E159–E163. doi: 10.1111/bju.12005 [DOI] [PubMed] [Google Scholar]
- 64.Nnoaham KE, Kumbang J. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst Rev. 2008;16(3):CD003222. [DOI] [PubMed] [Google Scholar]
- 65.Nickel JC, Mullins C, Tripp DA. Development of an evidence-based cognitive behavioural treatment program for men with chronic prostatitis/chronic pelvic pain syndrome. World J Urol. 2008;26:167–172. doi: 10.1007/s00345-008-0235-6 [DOI] [PubMed] [Google Scholar]
- 66.Ginting JV, Tripp DA, Nickel JC. Self-reported spousal support modifies the negative impact of pain on disability in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2011;78:1136–1141. doi: 10.1016/j.urology.2011.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenbaum TY. How well is the multidisciplinary model working? J Sex Med. 2011;8(11):2957–2958. doi: 10.1111/j.1743-6109.2011.02527.x [DOI] [PubMed] [Google Scholar]
- 68.Macea DD, Gajos K, Daglia Calil YA, Fregni F. The efficacy of web-based cognitive behavioral interventions for chronic pain: a systematic review and meta-analysis. J Pain. 2010;11(10):917–929. doi: 10.1016/j.jpain.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 69.Amarenco G, Lanoe Y, Perrigor M. Un nouveau syndrome canalaire: la compression du nerf honteux interne dans le canal d’Alcock ou paralysie périnéale du cycliste. Presse Med. 1987;16:399. [PubMed] [Google Scholar]
- 70.Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol Urodyn. 2008;27(4):306–310. doi: 10.1002/nau.20505 [DOI] [PubMed] [Google Scholar]
- 71.Pereira A, Pérez-Medina T, Rodríguez-Tapia A, Chiverto Y, Lizarraga S. Correlation between anatomical segments of the pudendal nerve and clinical findings of the patient with pudendal neuralgia. Gynecol Obstet Invest. 2018;83(6):593–599. doi: 10.1159/000489497 [DOI] [PubMed] [Google Scholar]
- 72.Popeney C, Ansell V, Renney K. Pudendal entrapment as an etiology of chronic perineal pain: diagnosis and treatment. Neurourol Urodyn. 2007;26(6):820–827. Erratum in: Neurourol Urodyn. 2008;27(4):360 DOI: 10.1002/nau.20421 [DOI] [PubMed] [Google Scholar]
- 73.Robert R, Labat JJ, Bensignor M, et al. Decompression and transposition of the pudendal nerve in pudendal neuralgia: a randomized controlled trial and long-term evaluation. Eur Urol. 2005;47(3):403–408. doi: 10.1016/j.eururo.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 74.Possover M. Laparoscopic management of endopelvic etiologies of pudendal pain in 134 consecutive patients. J Urol. 2009;181(4):1732–1736. doi: 10.1016/j.juro.2008.11.096 [DOI] [PubMed] [Google Scholar]
- 75.Erdogru T, Avci E, Akand M. Laparoscopic pudendal nerve decompression and transposition combined with omental flap protection of the nerve (Istanbul technique): technical description and feasibility analysis. Surg Endosc. 2014;28(3):925–932. doi: 10.1007/s00464-013-3248-1 [DOI] [PubMed] [Google Scholar]
- 76.Fanucci E, Manenti G, Ursone A, et al. Role of interventional radiology in pudendal neuralgia: a description of techniques and review of the literature. Radiol Med. 2009;114(3):425–436. doi: 10.1007/s11547-009-0371-0 [DOI] [PubMed] [Google Scholar]