Abstract
Many males with FXS meet criteria for ASD. This study was designed to (1) describe ASD symptoms in adolescent and young adult males with FXS (n=44) and (2) evaluate the contributions to ASD severity of cognitive, language, and psychiatric factors, as well as FMRP (the protein deficient in FXS). A few ASD symptoms on the ADOS-2 were universal in the sample. There was less impairment in restricted and repetitive behaviors (RRB) than in the social affective (SA) domain. The best predictor of overall ASD severity and SA severity was expressive syntactic ability. RRB severity was best predicted by the psychiatric factors. Implications for clinical practice and for understanding the ASD comorbidity in FXS are discussed.
Keywords: fragile X syndrome, autism spectrum disorder, language, IQ, psychiatric symptoms, FMRP
Many males with FXS meet criteria for ASD. This study was designed to (1) describe ASD symptoms in adolescent and young adult males with FXS (n=44) and (2) evaluate the contributions to ASD severity of cognitive, language, and psychiatric factors, as well as FMRP (the protein deficient in FXS). A few ASD symptoms on the ADOS-2 were universal in the sample. There was less impairment in restricted and repetitive behaviors (RRB) than in the social affective (SA) domain. The best predictor of overall ASD severity and SA severity was expressive syntactic ability. RRB severity was best predicted by the psychiatric factors. Implications for clinical practice and for understanding the ASD comorbidity in FXS are discussed.
Fragile X syndrome (FXS) is the leading cause of inherited intellectual disability (Crawford, Acuna, and Sherman 2001) and results from a trinucleotide (CGG) expansion in the FMR1 gene on the X chromosome (Oostra and Willemson 2003). This expansion leads to a reduction in or absence of FMRP (fragile X mental retardation protein), which is involved in experience-dependent learning and neural plasticity (Bassell and Warren 2008). The phenotypic consequences are more pronounced in males given the protective presence of an unaffected X and X inactivation in females (Loesch et al. 2004; Tassone et al. 1999; Ligsay and Hagerman 2016; Stembalska et al. 2016). Males with FXS typically have IQs under 70 (Hessl et al. 2009) and experience a range of co-occurring behavioral problems, including symptoms of autism spectrum disorder (ASD; Budimirovic and Kaufmann 2011; Demark, Feldman and Holden 2003; Goodlin-Jones, Nowicki, Bacalman, Tassone, and Hagerman 2005; Harris et al. 2008; Hatton et al. 2006; Kaufmann et al. 2004). The current study was designed to identify the specific ASD symptoms present in males with FXS and to examine the factors contributing to the severity of ASD symptoms during late adolescence and early adulthood, thereby providing insights into the bases of ASD in this population.
ASD in Fragile X Syndrome
More than 90% of males with FXS display behaviors typical for individuals with nonsyndromic ASD (e.g., Harris et al. 2005). These behaviors include perseverative and noncontingent speech (Belser and Sudhalter 2001; Martin, Roberts, Helm-Estabrooks, Sideris, Vanderbilt, and Moskowitz 2012; Murphy and Abbeduto 2007; Sudhalter and Belser 2001; Sudhalter, Cohen, Silverman, and Wolf-Schein 1990), motor stereotypies (e.g., hand flapping; Hagerman 1999), and poor eye contact (Merenstein, Sobesky, Taylor, Riddle, Tran, and Hagerman 1996; Roberts, Weisenfeld, Hatton, and Heath 2007). When using diagnostic instruments developed to evaluate ASD in the general population (e.g., the Autism Diagnostic Observation Schedule, ADOS), 50–60% of males with FXS receive an ASD diagnosis (Budimirovic and Kaufmann 2011; Clifford, Dissanyake, Bui, Huggins, Taylor, and Loesch 2007; Harris et al. 2008; Kaufmann et al. 2004; Kaufmann, Capone, Clarke, and Budimirovic 2008; Kaufmann et al. 2017; Klusek, Martin, and Losh 2014; McDuffie et al. 2010).
There is considerable evidence of the clinical utility of using the ASD diagnosis to characterize individual variation in FXS. In children with FXS, there are differences between those with and without a comorbid ASD diagnosis in (a) reactions to stranger approach (Scherr, Hogan, Hatton, & Roberts, 2017), (b) pragmatic and discourse-level features of language (Estigarrbia, Martin, Roberts, Spencer, Gucwa, and Sideris 2011; Lewis et al. 2006; Martin, Roberts, Helm-Esterbrooks, Sideris, Vanderbilt, and Moskowitz 2012; Roberts, Martin, Moskowitz, Harris, Foreman, and Nelson 2007), and (c) behavioral (e.g., social avoidance) and physiological (e.g., cortisol levels) reactions to socially demands (Roberts et al. 2007; 2009). There is also more overlap in the behavioral and physiological indices of social-communication between individuals with comorbid FXS and ASD and individuals with nonsyndromic ASD than between those with FXS with and without ASD (e.g., Caravella, Ezell, Raque, Hills, and Roberts 2017; Klusek, Martin, and Losh 2014; Lee et al. 2016; Rogers, Wehner, and Hagerman 2001).
Nevertheless, there is evidence that relying solely on a categorical ASD diagnosis in FXS can mask clinically and mechanistically important differences among individuals with FXS and between FXS and nonsyndromic ASD (Abbeduto, McDuffie, and Thurman 2014). For example, young males with FXS and comorbid ASD display less severe ASD symptoms, on average, than do similarly aged males with nonsyndromic ASD (Lee, Martin, Berry-Kravis, and Losh 2016; McDuffie et al. 2015; Thurman et al. 2015). In fact, even after controlling for overall ASD symptom severity, individuals with comorbid FXS and ASD are less impaired in several individual ASD symptoms (e.g., social smiling; McDuffie et al. 2015). Moreover, children with FXS and comorbid ASD exhibit different profiles of impairment in repetitive behaviors (Wolff, Bodfish, Hazlett, Lightbody, Reiss, and Piven et al. 2012) and display structural brain differences on MRI relative to age-matched individuals with nonsyndromic ASD (Hazlett et al. 2009).
Studies of within-syndrome variability have demonstrated that there is specificity in the ASD symptoms that distinguish individuals with FXS who do and do not meet criteria for ASD (Lee et al. 2016). McDuffie et al. (2010), for example, found that differences between 10- to 16-year-olds with FXS who did and did not meet criteria for autistic disorder were largely in restricted and repetitive behaviors (RRB), with few differences on social affective (SA) items.
Together, these findings suggest that a similar categorical diagnosis need not imply identical symptom presentations, which has important implications for understanding the nature of the problems experienced by individuals with FXS, the sources of those problems, and potential approaches to treatment. Indeed, there is controversy more generally within the field of nonsyndromic ASD regarding the value of a categorical approach. Some researchers have argued for abandoning the ASD diagnosis altogether (Waterhouse and Gillberg 2014; Waterhouse, London, and Gillberg 2017), whereas others have argued for recognizing the limitations of the categorical diagnosis and combining it with other symptom-based or dimensional approaches (Mϋller and Amaral 2017). This latter approach would appear to be appropriate for understanding the ASD comorbidity with FXS as well. Moreover, clinical decision-making and determinations about service eligibility still depend on the categorical ASD diagnosis even for FXS. In the present study, we complemented previous categorical approaches and focused on understanding the ASD comorbidity at the symptom level and the relationships between various characteristics of individuals with FXS and their ASD symptoms.
Factors associated with ASD symptoms in FXS
There has been only limited research on the factors associated with ASD symptoms and symptom severity within FXS. Such research is critical for constructing a framework for understanding the nature, bases, and treatment of ASD in FXS.
Chronological age.
ASD symptoms emerge in the first year of life for individuals with FXS and remain stable during the preschool years (Roberts, Tonnsen, McCrary, Caravella, and Shinkareva 2016). As children with FXS move into the school years, however, ASD symptoms begin to change in complex ways. In particular, there is a chronological age-related increase in the severity of at least some ASD symptoms during middle to late childhood (Hatton et al. 2006; Lee et al. 2016; Thurman, McDuffie, Kover, Hagerman, and Abbeduto 2015). It is unclear, however, whether ASD symptoms continue to worsen in adolescence and adulthood. In fact, there is evidence of a lessening of ASD symptoms between early childhood and mid-adolescence for males with FXS, but only in the social communication domain (McDuffie et al. 2010). Neither the trajectory of ASD symptoms in individuals with FXS nor their determinants have been examined during the transition from adolescence to adulthood.
Cognition.
There are differences between individuals with FXS who have comorbid ASD and those with nonsyndromic ASD in cognitive functioning. IQ is lower, on average, in individuals with comorbid FXS and ASD than in individuals with FXS without an ASD diagnosis, and IQ is negatively correlated with ASD symptom severity more generally (Bailey, Hatton, Mesibov, Ament, and Skinner 2000; Bailey, Hatton, Skinner, and Mesibov 2001; Hatton et al. 2006; Hernandez et al. 2009; Kau et al. 2004; Kaufmann et al. 2004; Lewis et al. 2006; McDuffie et al. 2010; Philofsky et al. 2004; Rogers et al. 2001; Thurman et al. 2015). At the same time, however, there seems to be some specificity in the relationship between cognitive ability and ASD symptoms among individuals with FXS, although this relationship may vary with age. Nonverbal cognition has been found to be related to severity of ASD symptoms in both the SA and RRB domains in childhood (Thurman et al. 2015), but only to SA symptoms in the early adolescent years (Lee et al. 2017).
The relationships between cognition and ASD may be particularly complex because cognition also changes with age in FXS, with an age-related decline in IQ that extends into adolescence and, perhaps, beyond (Fisch et al. 2010; Kover et al. 2013; Roberts et al. 2009; Skinner et al. 2005). However, the relationships among cognitive ability and ASD symptoms have not been examined beyond early adolescence in FXS.
Language.
Differences in language profiles are seen between individuals with comorbid FXS and ASD relative to those who have nonsyndromic ASD, and language skills are related to ASD symptoms within FXS (e.g., Philofsky et al. 2004). Lexical learning is less impaired in preschool- and elementary school-age males with FXS, regardless of whether they have comorbid ASD, than in those with nonsyndromic ASD, even after controlling for differences in chronological age, IQ, and ASD symptom severity (Thurman, McDuffie, Hagerman, Josol, and Abbeduto 2017). In contrast, differences between individuals with comorbid FXS and ASD and those with nonsyndromic ASD in pragmatics (i.e., the social uses of language) are minimal after controlling for differences in cognitive ability (Klusek, Martin, and Losh 2014; Lee et al. 2017; Martin, Barstein, Hornickel, Matherly, Durante, and Losh 2017; Martin, Losh, Estgarribia, Sideris, and Roberts 2013). Thus, there is specificity in the relationship of language to ASD in FXS, with the relationship varying across different dimensions of language.
Expressive syntax is an area of considerable impairment in FXS, with several studies documenting delays beyond cognitive-level expectations (Estigarribia et al. 2011; Finestack and Abbeduto 2010; Price et al. 2008; Roberts et al. 2007). Syntactic impairments in FXS also are greater in the expressive than in the receptive domain, with the latter generally found to be consistent with cognitive-level expectations (Abbeduto et al. 2003) or with impairments in specific cognitive skills, such as auditory memory (Oakes et al. 2013). Studies examining expressive syntax in relation to ASD diagnostic status or ASD symptom severity with FXS, however, have yielded inconsistent results (Kover and Abbeduto 2010; Kover et al. 2012; McDuffie et al. 2012; Thurman et al. 2017). Syntax is also delayed relative to age and vocabulary and cognitive-level expectations in many individuals with nonsyndromic ASD (Boucher 2003; Kjelgaard and Tager-Flusberg 2001; Park et al. 2012; Tek et al. 2014). In contrast to FXS, however, the delays in nonsyndromic ASD have been found to be less severe in the expressive than the receptive modality in some studies (Ellis et al. 2010; Luyster 2008; Mitchell et al. 2006).
The different profiles between FXS and nonsyndromic ASD suggest that studies of expressive syntax may be especially useful in clarifying the different bases and correlates of ASD symptoms in FXS. Moreover, impairments in expressive syntax are clinically important, limiting participation in social interaction and thereby possibly contributing to the emergence of ASD symptomatology. There is also some evidence that impairments in expressive syntax may negatively impact aspects of social cognition, such as theory of mind (i.e., the ability to reason about mental states), which is an area of considerable impairment in those with nonsyndromic ASD (Abbeduto, Short-Meyerson, Benson, and Dolish 2004; Benson, Abbeduto, Short, Nuccio and Maas 1993; Shield, Pyers, Martin, and Tager-Flusberg 2016; Tager-Flusberg and Sullivan 1994 2000). Unfortunately, there has been no investigations of expressive syntax in either FXS in older adolescents and young adults (McDuffie et al. 2012; Thurman et al. 2017).
Psychiatric conditions.
Mental health problems and challenging behaviors are frequent in FXS and nonsyndromic ASD. Most notably, symptoms of anxiety are frequent in FXS (Thurman et al. 2014), with almost 70% of males with FXS being diagnosed or treated for an anxiety disorder (Bailey, Raspa, Olmsted, and Holiday 2008; Cordeiro, Ballenger, Hagerman, and Hessl 2011). Studies using gold standard diagnostic measures of anxiety disorders in nonsyndromic ASD converge on a prevalence of 40% to 50% (Simonoff et al. 2008; van Steensel, Bögels, and Perrin 2011; White, Oswald, Ollendick, and Scahill 2009;). Hyperactivity and related problems in attention regulation are also common in FXS (Thurman et al. 2014), as they are in nonsyndromic ASD (Miller, Iosif, Young, Hill, and Ozonoff 2018).
There have been few direct comparisons between FXS and nonsyndromic ASD as regards the prevalence or determinants of mental health problems and challenging behaviors, and existing studies have yielded inconsistent findings. Using parent report, Thurman et al. (2014) found a higher rate of anxiety and manic/hyperactivity symptoms in FXS than in nonsyndromic ASD, even after controlling for nonverbal IQ and ASD severity. In contrast, Ezell et al. (in press) using a structured psychiatric interview (Weller, Weller, Fristad, Rooney, and Schecter 2000) did not find different rates of general anxiety disorder in adolescent and young adult males with FXS relative to nonsyndromic ASD after controlling for IQ. These different results across studies may reflect age-related differences in mental health problems or differences in measurement.
Problems with anxiety and hyperactivity may make social interaction more difficult for affected individuals, thereby interfering with their acquisition of the social competencies reflected in the ASD diagnosis. In fact, anxiety has been found to correlate with measures of socially avoidant behaviors in FXS (Budimirovic and Kaufmann 2011; Kaufmann et al. 2008; Thurman et al. 2014). Unfortunately, there are no data on the contributions of this set of mental health challenges to ASD symptoms in FXS beyond childhood.
FMRP and FMR1 Variation.
FMRP is involved in the translation of numerous genes and signaling pathways and thus, FMRP levels in peripheral blood are correlated with many features of the FXS behavioral phenotype (Hagerman et al. 2017). The translation of several genes that increase risk for ASD is controlled by FMRP, including SHANK, mTOR, and PTEN (Niu et al. 2017). Few studies, however, have examined the relationship between FMRP levels and ASD symptoms in FXS. In the few studies that do exist, FMRP levels have not correlated with ASD symptom severity or ASD diagnosis in FXS samples once IQ is controlled (Cornish et al. 2004; Loesch et al. 2007; McDuffie et al. 2010). This may reflect the fact that FMRP influences ASD symptoms only indirectly through its effects on brain systems underlying cognition (Niu et al. 2017). Studies to date, however, have relied on assays of FMRP that were not strictly quantitative; that is, FMRP levels reflected the proportion of sampled cells expressing FMRP rather than that total amount of FMRP expressed.
Aims of the Present Study
ASD symptoms are prevalent in males with FXS; yet, we lack data on the profile of individual ASD symptoms in late adolescence and early adulthood, which is an important period of transition from school to the demands of adult life. We also lack data on the predictors of ASD symptom severity during this period. Thus, the aims of this study were: (1) to further describe ASD symptoms in adolescent and young adult males with FXS and (2) to evaluate the relative contributions of IQ, expressive syntax, psychiatric factors, and FMRP, to ASD symptom severity overall and to the severity of symptoms in the separate domains of SA and RRB.
Methods
Participants
Participants were males with FXS who participated in a larger longitudinal study of language development in adolescent and young adult males with FXS. Participants were recruited nationally and were tested at one of two university research clinics [deleted for blind review]. Eligibility for the larger study required that participants were 15 and 22 years at enrollment and previously diagnosed as having the FMR1 full mutation with or without mosaicism, according to a medical report provided by the family. Additionally, participants used speech as the primary means of communication and regularly uses three-word or longer phrases, ha no uncorrected sensory/physical impairments that would preclude participation in testing, and resided at home with the biological mother, per parent report. Institutional Review Boards of the participating university sites approved the project. Parental informed consent was obtained. Data for the current study come from the first annual assessment with each participant, with the exception that blood for the genetic analyses reported was drawn at a later annual assessment for a few participants for logistical reasons.
A total of 58 males met inclusionary criteria for the larger project, but the final sample for the present study included 44 participants. Nine participants were excluded because they did not participate in the blood draw. One additional participant was excluded because he did not participate in the ADOS-2 due to scheduling difficulties, and four others were excluded because of errors in administration of one of the standardized tests.
Measures
The measures reported here are a subset of a larger set of measures collected in the longitudinal study. No other publication from this project has had the same focus or combination of measures as the present study.
ASD Symptoms.
The Autism Diagnostic Observation Schedule 2nd edition (ADOS-2; Lord, Rutter, DiLavore, Risi, Gotham, and Bishop 2012) is a semi-structured observational measure in which a trained examiner provides a series of activities and materials to systematically elicit a sample of an individual’s SA and RRB symptoms. Participants received the ADOS-2 module appropriate for their expressive language level and chronological age as specified by the administration manual. Thus, 3, 21, and 20 participants received Modules 1, 2, and 3, respectively. The criterion for administration of Module 1 is “no speech up to an including simple phrases;” for Module 2 it is “phrase speech up to fluent speech;” and for Module 3 it is “fluent speech, approximately equivalent to or higher than the expressive language skills of a typically developing 4-year-old.” Module 4 was not appropriate in this study because it requires “at least a minor level of independence in terms of relationships and goals” (p. 11, Lord et al. 2012), which was not achieved by the participants in the current study given their significant intellectual disabilities (i.e., nonverbal IQs of 46 to 56, see Table 1). Note that because many participants were older than the norming sample of the ADOS-2, we used the upper age limit for each ADOS-2 module to compute the scores included in the present analyses.
Table 1:
Descriptive Characteristics of Participants (n=44)
| Measure | Mean | Range | Standard Deviation | |
|---|---|---|---|---|
| CA | 18.31 | 15.03–22.92 | 2.31 | |
| ASD Overall Severity | 5.57 | 1 – 10 | 2.25 | |
| ASD SA Severity | 5.48 | 1 – 10 | 2.26 | |
| ASD RRB Severity* | 3.75 | 1 – 7 | 1.99 | |
| Nonverbal IQ | 38.68 | 36 – 56 | 4.52 | |
| Expressive Syntax | 42.18 | 40 – 72 | 6.75 | |
| General Anxiety | 6.07 | 0 – 14 | 3.31 | |
| Social Avoidance | 6.98 | 0 – 19 | 4.66 | |
| Manic/Hyperactive | 5.48 | 0 – 12 | 3.11 | |
| FMRP Level | 0.78 | 0 – 10.24 | 1.84 | |
| Frequency | ||||
| Mosaic Status | Full Mutation | Full Mutation Mosaic | ||
| 32 | 12 | |||
Note: This metric represents the original (1 – 7 range severity scale) developed by Hus et al. (2014). Descriptive statistics for the final (1 – 10 rang e severity scale, with scores 2 – 4 unattainable), are as follows: Mean = 6.13, Range: 1 – 10; Standard deviation = 2.98.
The Comparison score, which can range from 1–10, was computed from ADOS-2 algorithm totals and was used as the metric of overall ASD symptom severity. We also used the calibrated severity scores for the SA and RRB symptom subdomains (Hus, Gotham, and Lord 2014). It is important to note that the original RRB severity scores generated by Hus et al. (2014) had a range of 1 – 7. However, due to concerns that different ranges across the two domains would cause confusion, Huss and colleagues transformed this 7-point scale to the same 10-point scale used for both the SA and Comparison score. This goal was achieved by recoding the original scores of 2–7 to a scale of 5 – 10 and making the severity scores of 2, 3 and 4 impossible to obtain. Thus, the final RRB severity scores included scores of 1 and 5 – 10. When using parametric analyses, however, serious methodological concerns arise when utilizing a dependent variable in which multiple scores cannot be obtained (Thurman et al. 2017). In the present study, therefore, the original 1 – 7 scoring metric derived by Hus et al. (2014) was used for the RRB domain.
The Autism Diagnostic Interview-Revised (ADI-R; Rutter et al. 2003) is a caregiver interview that queries the participant’s developmental history and the presence of symptoms of ASD at or near age four years. The biological mother was the caregiver informant in all cases.
In the analysis of individual ASD symptoms, we examined scores on individual ADOS items, with some analyses involving comparisons of those participants who did and did not meet criteria for ASD. We assigned an ASD research diagnosis according to the caseness criteria proposed by Risi et al. (2006), in which ASD is determined by the presence of an ADOS-2 Comparison score of at least 4 along with ADI-R scores that either: (a) meet the autism cutoff for the ADI-R Social Reciprocity Domain and for either the Communication or Repetitive Behavior domains; (b) are within one point of the cutoffs for the Social Reciprocity and Communication domains; or (3) meet the autism cutoff on either the Social Reciprocity or Communication domains and are within two points of the cut-off for the other domain.
Project staff members who had achieved standard research reliability administered the ADOS-2 and ADI-R. For both the ADOS-2 and the ADI-R, 10% of the administrations were randomly selected to assess cross-site reliability (via videotaped administration) across all examiners at both data collection sites. Consensus codes for each reliability administration were achieved through group discussion and the mean percent agreement of each examiner relative to the consensus scores was computed. When considering all items, agreement of examiners with the consensus codes averaged 80% for the ADOS-2 and 91% for the ADI-R.
Nonverbal Cognition.
The Leiter International Performance Scale – Revised (Leiter-R; Roid and Miller 1997) is a standardized measure of nonverbal cognition normed for ages 2–21 years. Administration is nonverbal as is the participant’s mode of response. The subtests comprising the Brief IQ were administered: Figure Ground, Form Completion, Sequential Order, and Repeated Patterns. Standard scores from the Leiter Brief IQ were used as the metric of nonverbal cognition. For the three 22-year-olds in the sample, we used the upper age limit of the Leiter-R norms (i.e., 21) to compute standard scores. One 22-year-old had a Leiter of 56, which was the highest score for the sample and achieved by only one other (17-year-old) participant, whereas the other 22-year-olds fell within the range of scores of the other participants on all other variables of interest.
Expressive Syntax.
The Syntax Construction subtest of the Comprehensive Assessment of Spoken Language (CASL; Carrow-Woolfolk 1995) was used to assess the production of words, phrases, and sentences that require the use of a variety of morphosyntactic rules (e.g., verb tense, plurals, interrogatives, pronouns). Within this task, the participant was instructed to respond to a picture by imitating the examiner, completing a sentence, answering a question designed to elicit a specific syntactic form, formulating a sentence to tell a story, and/or using a model sentence to generate a similar sentence. The advantage of the CASL for this study is that it is highly structured in terms of the participant’s interaction with the examiner and is focused on a well-defined set of stimuli; thus, it is less socially demanding than a conversation or other naturalistic linguistic interaction. Poor performance on the latter types of tasks might well reflect social difficulties as much as syntactic difficulties, thereby producing a misleading correlation with ASD symptoms. The CASL was normed on a sample of 1,700 people representative of the U.S. in terms of the distribution of gender, race, and ethnicity. Internal consistency of the Syntax Construction subtest averaged .80 for adolescents and young adults and a study of test-retest reliability involving a sample of adolescents yielded a correlation of .81. Significant correlations of the Syntax Construction subtest with other standardized measures of language were significant and higher for expressive than receptive measures. Standard scores from this subtest were usedas the metric of expressive language in the current study. Note that for 22-year-olds, we used the upper age limit in the CASL norms (i.e. 21) to compute standard scores.
Psychiatric Symptoms.
The Anxiety, Depression, and Mood Scale (ADAMS, Esbensen et al. 2003) is a 28-item informant report screener for psychiatric disorders in individuals with intellectual disability. Biological mothers were the respondents for the present study. Behaviors are rated on a 4-point Likert scale describing the severity of each problem behavior, with higher scores reflecting behavior that is more problematic. The ADAMS yields five subscale scores: Manic/Hyperactive Behavior, Depressed Mood, Social Avoidance, General Anxiety, and Obsessive/Compulsive Behavior. The measure was normed on a sample of individuals with intellectual disabilities of a wide age range. Raw scores from the General Anxiety, Manic/Hyperactive, and Social Avoidance subscales were used as the metrics of psychiatric symptoms in the current study.
FMR1.
We derived two measures of FMR1 status, using each in separate analyses. Participants provided a peripheral blood sample at the first annual visit at which the participant was able to tolerate the blood draw. Both measures were derived from analyses of these blood samples. Samples were processed at [deleted for anonymous review] and the laboratory staff members who processed the blood were blind to the results of all behavioral testing.
First, we categorized each participant as “full mutation” or “full mutation mosaic.” DNA was eluted from a 3 mm dried blood spot punch and analyzed as described in Adayev et al. (2014). Briefly, FMR1 triplet repeat alleles were amplified by polymerase chain reaction [AmplidexR FMR1 PCR (RUO)] and their sizes were determined by capillary electrophoresis (ABI 3130 Genetic Analyzer, Applied Biosystems, Foster City, CA) interpreted with GeneMapper (Applied Biosystems) software. Samples showing FMR1 repeat alleles with more than 200 triplets were classified as full mutation. Samples showing additional alleles in the premutation size range (55–200 triplets) were classified as full mutation mosaic. Twelve participants fell into this full mutation mosaic category.
Second, the presence of FMRP was quantified using an immunoassay based on a Luminex platform that detects FMRP in dried blood spots using the procedures described in LaFauci et al. (2013). The amount of FMRP in the dried blood spots in this qFMRP assay is reported in concentration as pmol/L (pM). Of the 12 participants who showed evidence of size mosaicism (i.e. PCR products representing alleles in the premutation size range), 10 displayed greater-than-background levels of FMRP, ranging from 0.6 to 10.24 pM. One that had been stored for 220 days had a reading of 0.26 pM, and one stored for 808 days had a reading of 0 and thus, these two were likely low due to prolonged storage (Adayev et al. 2014). Of the remaining full mutation participants, four showed higher-than-background levels of FMRP ranging from 0.8 to 1.52 pM, but no evidence of premutation size alleles. In these four cases, the FMRP could have been due to expression of unmethylated alleles larger than 200 triplets (methylation mosaicism), but that we were unable to assay in DNA from dried blood spots by PCR and so, we classified these participants as full mutation rather than as mosaic. For the remaining 28 full mutation participants included in the present analyses, the qFMRP analysis showed either no FMRP or greater-than-background levels below the lowest standard point (not a limit of detection of the method) in qFMRP assay (0.55 pM).
Statistical Analysis
Descriptive statistics were used to characterize the participants included in the analysis. Graphical approaches were used to illustrate the ASD symptom severity across participants according to Module 1, 2, or 3. Pearson correlations assessed unadjusted cross-sectional associations between variables, both outcomes and predictors, with the exception of the dichotomous variable of mosaic status. The relationship of mosaic status to the other variables was examined through a series of independent sample t tests. Finally, linear regression was used to assess the association between ASD symptom severity (form the ADOS-2) and the predictors. Unless otherwise noted, each regression included as predictors chronological age, nonverbal cognition (Leiter IQ), expressive language (CASL standard score), psychiatric symptoms (ADAMS anxiety, avoidance, or manic/hyperactive score), FMR1 status (FMRP level or FMRP mosaic status). The overall Comparison score and the SA and RRB calibrated severity scores were treated as individual outcomes. For each outcome variable, separate models were fit using a single psychiatric symptom domain as a predictor in combination with the other variables due tothe small sample size, which limited the number of predictors that could be evaluated in a single regression. Thus, there were three regression equations per outcome measure. Due to the large number of models being fit, Benjamini-Hochberg False Discovery Rate (FDR; Benjamini and Hochberg 1995) was applied to the overall model p-values to protect against false discoveries. Diagnostics indicated that model assumptions were reasonably met by the data (i.e., pairs of predictors were no more than moderately correlated). All analyses included the full sample(n=44), and did not distinguish between those who met and did not meet study criteria for ASD.
Results
Descriptive characteristics for the 44 participants are presented in Table 1. Because, as noted previously, a number of participants were excluded due to missing data, we compared the excluded participants to the retained participants on CA, Leiter IQ, CASL standard score, and ADAMS manic/hyperactive, general anxiety, and avoidance scores, as well as on ADOS-2 Comparison scores and the two domain calibrated severity scores, using separate independent sample t tests for each measure. There was a trend for the excluded participants to be more severely affected on average on all variables, although the difference was significant only for CASL standard scores, t(53)=2.14, p=.04 (equal variances not assumed). In part, this pattern likely reflects the fact that gaining compliance for a blood draw, which was the major reason for exclusion from the present analysis, was more difficult for more impaired individuals.
Characterizing ASD symptom severity.
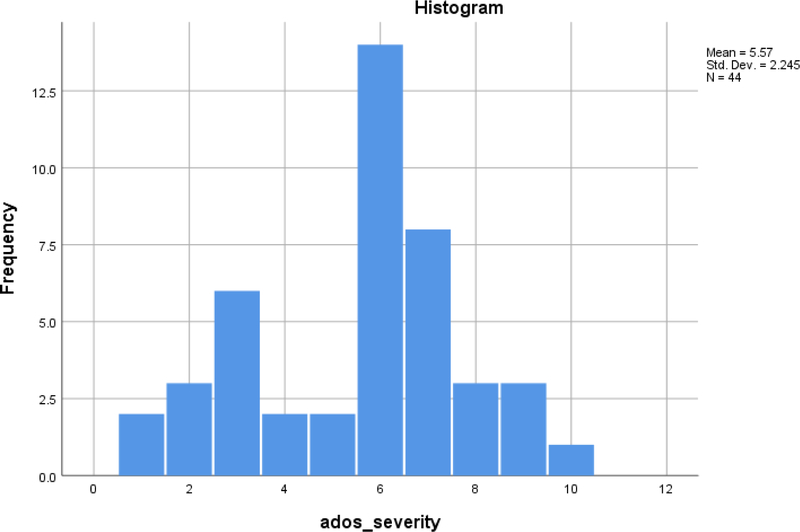
Of the 44 participants, 33 (75%) met the diagnostic classification of ASD derived from the ADOS-2 and ADI-R following Risi et al. (2006). All 3 participants receiving Module 1 met Risi et al. criteria for ASD compared to 18 of 21 receiving Module 2 and 12 of 20 receiving Module 3. The mean ADOS-2 Comparison score for the sample was 5.57, indicating a moderate level of ASD affectedness (see Table 1). The modal ADOS-2 Comparison score (as seen in Figure 1) was 6, which is the upper end of the moderately affected range. Although the ADOS-2 SA calibrated severity scores and the RRB calibrated severity scores were each highly correlated with the overall Comparison score, they were uncorrelated with each other (see Table 2).
Figure 1.
Distribution of ADOS-2 Comparison scores
Table 2.
Concurrent Correlations between Autism Severity and Potential Predictors
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. ASD Overall Severity | -- | |||||||||
| 2. ASD SA Severity | .85***** | -- | ||||||||
| 3. ASD RRB Severity | 54***** | .10 | -- | |||||||
| 4. CA | −.20 | −.11 | −.18 | -- | ||||||
| 5. Nonverbal IQ | −.35* | −.22 | −.29^ | .18 | -- | |||||
| 6. Expressive Syntax | −.44*** | −.38** | −.06 | .30* | .52***** | -- | ||||
| 7. General Anxiety | .10 | .05 | .25^ | .03 | −.07 | .02 | -- | |||
| 8. Avoidance | .23 | .12 | .37* | −.05 | −.04 | −.25 | .45*** | -- | ||
| 9. Manic/Hyperactive | .21 | .08 | .30* | −.08 | −.18 | −.15 | .54***** | .27^ | -- | |
| 10. FMRP Level | −.14 | −.14 | .03 | −.21 | −.05 | .00 | .06 | −.09 | −.13 | -- |
p<.10;
p<.05;
p<.01;
p<.005;
p<.001;
p<.0005 (all tests two-tailed).
We also examined the distribution of scores for individual diagnostic algorithm items separately for each ADOS-2 module. These graphs are presented in Figures 2 through 5 for the entire sample regardless of whether they met criteria for ASD. We did not conduct inferential statistics on these data and present them only for descriptive purposes in light of the sample size and relatively large number of items. Because only three participants received Module 1, item-level data are presented only for Modules 2 and 3.
Figure 2.
Module 2 - Social Affect Algorithm
For each of the SA algorithm items of Module 2, a substantial majority of participants showed some impairment, indicated by a score of 1 or 2 (See Figure 2). Indeed, virtually all participants were scored as showing at least some impairment on eye contact (86%), directed facial expressions (93%), and quality of social overtures items (90%). The one exception to this pattern for the Module 2 SA domain items, was shared enjoyment, for which approximately half the participants showed no impairment (i.e., a score of 0).
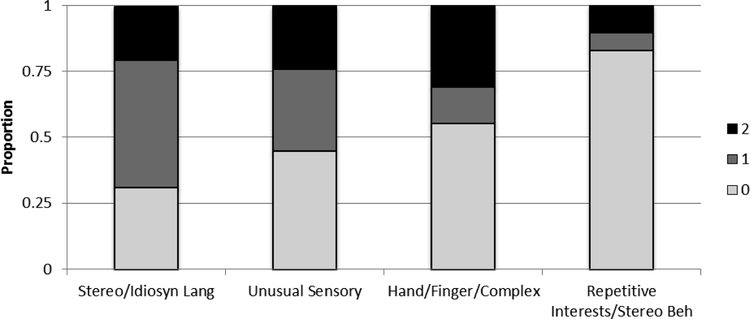
In contrast to the profile for the SA items, relatively large percentages of the participants completing Module 2 received scores of 0 (i.e., no impairment) on the RRB algorithm items (see Figure 3). In fact, almost 80% of the participants received a score of 0 (indicating no impairment) on repetitive interests/stereotyped behavior and approximately half received a score of 0 on unusual sensory interests and hand/finger/complex mannerisms. The exception to this pattern was the stereotyped and idiosyncratic language item, on which nearly three-fourths of participants displayed some impairment.
Figure 3.
Module 2 - Restricted and Repetitive Behavior
For Module 3 SA algorithm items, 70% or more of participants showed some degree of impairment on eye contact, directed facial expressions, and quality of social overture (see Figure 4). Although these three algorithm items were also the most problematic for the Module 2 participants, the absolute percentage of participants showing impairment on these three items was lower for Module 3. In addition, the large majority of participants for the Module 3 SA items of reporting events, using gestures, sharing enjoyment, amount of reciprocal communication, and quality of rapport received a score of 0, indicating no impairment.
Figure 4.
Module 3 - Social Affect
For Module 3 RRB items, the pattern was quite similar to that described for Module 2 (see Figure 5). For three of the four diagnostic items in this domain, half or more of the participants receiving Module 3 were scored as showing no impairment. As for Module 2, the exception was stereotyped and idiosyncratic language.
Figure 5.
Module 3 - Restricted and Repetitive Behavior
Because the ADOS-2 is based on only a single-period of observation and a limited number of items, especially in the RRB domain, we also examined the correspondence between the ADOS-and the ADI-R current scores. First, we found that the ADOS-2 SA calibrated severity score was significantly correlated with scores on both the ADI-R Reciprocal Social Interaction, r = .42, p < .01, and the ADI-R Verbal Communication scores, r = .38, p < .01, and the ADOS-2 RRB calibrated severity score was significantly correlated with the corresponding score on the ADI-R, r = .39, p < .01, Total scores on the two measures were also significantly correlated, r = .52, p < .01 Second, we compared the distributions of scores for those items that were common to both the ADOS-2 and the ADI-R (data not presented here, but available from the authors). The distributions were largely similar, except that parents tended to rate their sons as less impaired (i.e., the ADI-R) relative to the examiners (i.e., the ADOS-2). Thus, the ADOS-2 findings for the adolescents and young adults with FXS are largely corroborated by parental perspectives gleaned from the ADI-R.
Participant factors related to ASD symptom severity.
Bivariate correlations for the ASD symptom severity measures and the continuous predictor variables are presented in Table 2 for the entire sample regardless of whether they met criteria for ASD. In terms of the bivariate correlations, the ADOS-2 Comparison score was significantly negatively correlated with Leiter IQ and CASL standard score. The SA calibrated severity score was significantly negatively correlated with the CASL standard score. The RRB calibrated severity score was marginally negatively correlated with Leiter IQ and significantly (or marginally) positively correlated with each of the ADAMS subscale scores. There were significant correlations among the predictors included in the same regressions; namely, CASL standard score showed a moderate positive correlation with age and with Leiter IQ. We also compared the means for the predictor variables for the mosaic and non-mosaic participants and found significantly higher scores for the non-mosaic participants for ADOS-2 overall Comparison score, t(42)=2.17, p=.04. The non-mosaic participants also displayed marginally higher SA calibrated severity scores, t(42)=1.54, p=.06, and marginally lower Leiter IQs, t(42)=2.17, p=.15 (equal variances not assumed), than the mosaic participants. Because FMRP was not correlated with any dependent measure or predictor (except mosaic status) variable (see Table 2), we do not report regressions that include FMRP, focusing instead on the regressions with mosaic status as a predictor.
The three regressions predicting the ADOS-2 Comparison score were all significant following the Benjamini-Hochberg FDR (see Table 3). The only variables to emerge as unique predictors were CASL standard score and mosaic status, which were either significant or marginally significant depending on which ADAMS subscale score was in the regression. The relationships between the ADOS-2 Comparison score and both the CASL standard score and mosaic status were negative (i.e., less severe ASD symptoms were observed in those with better expressive syntax and in those who were mosaic). Because of the moderate correlation between CASL standard scores and Leiter IQ scores, we also re-ran the regressions with each of these variables separately. The contributions of CASL scores and mosaic status were stronger without Leiter IQ in the equation, whereas Leiter IQ was never a significant predictor when the CASL standard score was omitted. The foregoing regressions were based on the entire sample (n=44).
Table 3.
Linear Regressions Predicting Overall ASD Symptom Severity
| Source | B | SE B | β | p (two-tailed) |
|---|---|---|---|---|
| CA | −.08 | .14 | −.09 | .55 |
| Nonverbal IQ | −.03 | .08 | −..07 | .68 |
| Expressive Syntax | −.11 | .06 | −.33 | .05* |
| Manic/hyperactive | .09 −1.27 |
.10 .72 |
.12 −.25 |
.39 .08^ |
| FMRP Mosaic Status | ||||
| Adjusted R2=.20, F(5,38)= 3.15, p≤.02 | ||||
| CA | −.09 | .14 | −.10 | .51 |
| Nonverbal IQ | −.03 −.12 |
.08 .06 |
−.06 −.35 |
.71 .04* |
| Expressive Syntax | ||||
| Anxiety | .09 | .09 | .13 | .34 |
| FMRP Mosaic Status | −1.38 | .72 | −.28 | .06^ |
| Adjusted R2=.20, F(5,38)= 3.20, p<.02 | ||||
| CA | −.09 | .14 | −.09 | .52 |
| Nonverbal IQ | −.05 −.10 |
.08 .06 |
−.10 −.30 |
.54 .09^ |
| Expressive Syntax | ||||
| Avoidance | .06 | .07 | .13 | .37 |
| FMRP Mosaic Status | −1.22 | .72 | −.24 | .10^ |
| Adjusted R2=20, F(5,38)= 3.17, p<.02 |
The three regressions in which the dependent measure was SA calibrated severity score all approached, but did not reach, statistical significance (see Table 4). Interestingly, however, the variables contributing most to prediction of SA calibrated severity score were CASL standard score and mosaic status, again with negative beta weights (Table 4). When we re-ran the regressions with either CASL standard score or Leiter IQ but not both, the regressions with the former variable were all significant and CASL standard score was a significant unique predictor, whereas none of the regressions with Leiter IQ was significant. These regressions were based on the entire sample (n=44).
Table 4.
Linear Regressions Predicting ASD Calibrated Social Affective Severity Score
| Source | B | SE B | β | p (two-tailed) |
|---|---|---|---|---|
| CA | −.01 | .15 | −.01 | .94 |
| Nonverbal IQ | .03 | .09 | .06 | .74 |
| Expressive Syntax | −.13 | .06 | −.38 | .04* |
| Manic/hyperactive | .01 −1.33 |
.11 .76 |
.02 −.27 |
.92 .09^* |
| FMRP Mosaic Status | ||||
| Adjusted R2=.20, F(5,38)= 2.05, p≤.10 | ||||
| CA | −.02 | .15 | −.02 | .92 |
| Nonverbal IQ | .04 −.13 |
.09 .06 |
.07 −.39 |
.69 .03* |
| Expressive Syntax | ||||
| Anxiety | .07 | .10 | .10 | .51 |
| FMRP Mosaic Status | −1.40 | .76 | −.28 | .07^ |
| Adjusted R2=.12, F(5,38)= 2.16, p≤.08 | ||||
| CA | −.01 | .15 | −.01 | .94 |
| Nonverbal IQ | .03 −.13 |
.09 .06 |
.06 −.38 |
.76 .04* |
| Expressive Syntax | ||||
| Avoidance | .01 | .07 | .01 | .95 |
| FMRP Mosaic Status | −1.32 | .76 | −.26 | .09^ |
| Adjusted R2=.11, F(5,38)= 2.05, p≤.10 |
Of the three regressions in which the calibrated severity score for the RRB domain was the dependent measure, only the model including ADAMS avoidance was significant following the Benjamini-Hochberg FDR (see Table 5). The significant unique predictors in that regression were Leiter IQ (a negative relationship) and ADAMS avoidance (a positive relationship). Similar trends in prediction were observed in the other two regressions for this dependent measure (i.e., Leiter IQ and psychiatric symptoms were the best predictors; see Table 5). When we re-ran the regressions with either CASL standard score or Leiter IQ but not both, none of the regressions with the CASL standard score were significant, whereas those with Leiter IQ approached or were significant with the ADAMS scores being the only significant predictors. These regressions were based on the entire sample (n=44).
Table 5.
Linear Regressions Predicting ASD Calibrated Severity Scores for Restricted and Repetitive Behavior
| Source | B | SE B | β | p (two-tailed) |
|---|---|---|---|---|
| CA | −.14 | .13 | −.16 | .30 |
| Nonverbal IQ | −.12 | .08 | −.28 | .13 |
| Expressive Syntax | .05 | .05 | .18 | .32 |
| Manic/hyperactive | .16 −.47 |
.10 .68 |
.26 −.11 |
.09^ .49 |
| FMRP Mosaic Status | ||||
| Adjusted R2=.09, F(5,38)= 1.82, p≤.13 | ||||
| CA | −.16 | .13 | −.18 | .25 |
| Nonverbal IQ | −.12 .04 |
.08 .05 |
.−.27 .14 |
.14 .43 |
| Expressive Syntax | ||||
| Anxiety | .15 | .09 | .25 | .10^ |
| FMRP Mosaic Status | −.66 | .68 | −.15 | .34 |
| Adjusted R2=.09, F(5,38)= 1.82, p≤.13 | ||||
| CA | −.15 | .12 | −.18 | .22 |
| Nonverbal IQ | −.16 .09 |
.07 .05 |
−.37 .29 |
.03* .10 |
| Expressive Syntax | ||||
| Avoidance | .18 | .06 | .41 | .006* |
| FMRP Mosaic Status | −.32 | .64 | −.07 | .62 |
| Adjusted R2=.19, F(5,38)= 3.06, p≤.02 |
Discussion
Most males with FXS display symptoms of ASD, with a majority meeting diagnostic criteria for ASD. The present study was designed to provide data on the symptom profiles underlying the ASD diagnosis in FXS and the factors associated with the severity of those symptoms for the period spanning the transition from adolescence into adulthood.
ASD Symptoms in FXS
The sample in the present study was not population based, but was recruited without reference to the profile of ASD symptoms or ASD diagnostic history. Nonetheless, we found that three-fourths of the participants met the Risi et al. (2006) criteria for ASD, which rely on both the ADOS-2 and ADI-R. This estimate is higher than most previous studies; however, many previous studies used the DSM-IV diagnosis of Autism rather than the DSM-5 diagnosis of ASD. In fact, our estimate is close to that of Lee et al. (2016), who used DSM-5 criteria. More generally, this high comorbidity is consistent with the notion that studies of FXS, with its known etiology, may offer insights into nonsyndromic ASD, which is etiologically multifactorial.
Although the males with FXS in our sample displayed substantial problems on the SA items of the ADOS, there was variability across symptoms. Independent of the module administered, impairment was observed for a majority of participants for eye contact, directed facial expressions, and quality of social overture. Participants receiving Module 2 also displayed substantial impairment on many other SA items. Thus, these findings reinforce the need for interventions that target the SA domain for individuals with FXS. Interestingly, Module 3 participants displayed impairments on only a few SA items. More research is needed to understand this cross-module difference. Nonetheless, these findings demonstrate the value of looking beyond the categorical diagnosis to the level of individual symptoms.
The present findings suggest that problems in the domain of RRB are not particularly severe in adolescent and young adult males with FXS who have phrase speech; thus, this domain contributes relatively little to their ASD diagnostic classification, at least when considering performance on the ADOS-2. The exception is the use of stereotyped and idiosyncratic language, which was problematic for a majority of participants. The finding of a relatively mild degree of impairment in RRB contrasts with the results of McDuffie et al. (2010), who used the ADI-R and found problems in this domain to be central to distinguishing 10- to 16-year-olds with FXS who did and did not meet criteria for ASD. The different results between studies might reflect the age differences of the samples or the measures used. Future research using multiple methods of assessment on the same sample is needed to clarify this inconsistency. Nonetheless, these item-level analyses again reinforce the point that the ASD categorical diagnosis in FXS can sometimes mask important phenotypic features.
Two of the symptoms that were problematic for most of the males in our sample – lack of eye contact and use of stereotyped and idiosyncratic language, especially in the form of perseveration of a phrase or topic – have long been known to be part of the FXS phenotype (Hagerman et al. 2017). Roberts and colleagues (2007, 2009) utilized examiner-rated experimental measures and documented that avoidance of eye contact is nearly universal in males with FXS. Interestingly, however, prolonged avoidance of eye contact over the course of an interaction, but not initial levels of eye gaze avoidance, was associated with increased severity of ASD features in the Roberts et al. paradigm. These investigators also have suggested that eye gaze avoidance may be a manifestation of anxiety rather than solely a reflection ASD symptomatology in FXS (Roberts et al. under review). Similarly, the repeated use of routinized phrases as well as topic repetition have also been hypothesized to reflect anxiety rather than social impairment per se in FXS (Belser and Sudhalter 1995). Such findings raise concerns about using the ADOS-2 as a diagnostic tool in FXS without some adaptation or recognition that eye contact and use of stereotyped and idiosyncratic language are not useful in discriminating those with FXS who do and do not meet criteria for ASD. The findings also raise the possibility that these symptoms may reflect different underlying problems in FXS and nonsyndromic ASD.
Participant Characteristics Contributing to ASD Symptoms
We examined several possible predictors of ASD symptom severity. We focused on chronological age, nonverbal IQ, expressive syntactic competence, general anxiety, social avoidance, and manic/hyperactive behavior, as well as mosaic status and FMRP level. The present study was unique in examining these constructs for males with FXS in the adolescent to adult transition years, as well as in the focus on expressive syntactic competence.
The most consistent and unique predictor of overall ASD symptom severity was expressive syntactic competence. Our measure of expressive syntax, the CASL Syntax Construction subtest, indexes the ability to generate phrases and sentences exemplifying targeted syntactic features (e.g., past tense marking) in response to various prompts supported by pictures. The format is highly structured and test-like rather than conversational or particularly social in nature, which is in part why we selected it. Nonetheless, scores on this measure predicted both overall ASD symptom severity and SA symptom severity on the ADOS-2, with more advanced expressive syntactic ability associated with less severe ASD symptoms.
The important role of expressive syntax in predicting ASD symptoms is striking given that the ADOS-2 severity scores were constructed to minimize the influence of language ability. Moreover, the prediction of ADOS-2 symptom severity in nonsyndromic ASD samples by at least broad measures of language (e.g., verbal IQ and verbal MA) has been found (Hus et al. 2014; Risi et al. 2006) to be of lesser magnitude than observed in the present study. The present findings, therefore, suggest that (a) ASD symptoms reflect different underlying problems in FXS relative to nonsyndromic ASD and (b) that the ADOS-2 may be less well suited to diagnosing ASD in FXS than in nonsyndromic cases. Our findings also suggest the need to continue developing interventions to improve expressive syntax in individuals with FXS (e.g., McDuffie et al. 2016) and to exercise caution in assuming that problems managing social interaction by individuals with FXS are only “social” in nature.
We also found specificity in the relationship between expressive syntax and ASD symptom severity. In particular, expressive syntax tended to relate to SA symptoms, but not to RRB. It has been hypothesized that limitations in language could be both a cause and consequence of some forms of repetitive behavior (Oakes et al. 2016). The present data do not support this hypothesis, at least for individuals with FXS in the late adolescent and early adult years.
Although nonverbal IQ was significantly negatively correlated with ASD overall symptom severity and marginally negatively correlated with SA symptom severity, it did not make a unique contribution in the regression analyses. It is worth noting that nonverbal IQ was quite low for our sample, with many participants receiving the lowest standard score possible (i.e., 36) and thus, there was a limited range of variation in this construct. These findings contrast with those of previous studies (e.g., Bailey et al. 2000; Bailey et al. 2001; Hatton et al. 2015; Hernandez et al. 2009; Kau et al. 2004; Kaufmann et al. 2004; Lee et al. 2017; Lewis et al. 2006; McDuffie et al. 2010; Philofsky et al. 2004; Rogers et al. 2001; Thurman et al. 2015). This difference in results may reflect age differences in the samples, with the present study being the first to focus exclusively on the late adolescent to early adult period. The discrepant results may also reflect the fact that many of these studies did not use the ADOS-2 to characterize symptom severity. The discrepant results may also be due to the lack of measures of expressive syntax in combination with the cognitive measures in some previous studies.
We also found, however, that nonverbal IQ uniquely contributed to the prediction of RRB severity, although the strength of the association reached a conventional level of significance in only one regression. Greater cognitive impairment was associated with more severe RRB severity, which is consistent with the findings of several other studies using a variety of measures of IQ and RRB and a range of ages (Oakes et al. 2016; Kover et al. 2013), suggesting that it is a robust finding. This relationship has also been found in nonsyndromic ASD (Gabriels et al. 2005; Thurman et al. 2015) and other genetic conditions associated with intellectual disability (Miguel et al. 1997). The mechanism linking these two domains, however, remain to be fully elucidated, which will be critical for developing effective interventions.
Psychiatric symptoms reflective of anxiety, social avoidance, and manic/hyperactive behavior did not make unique contributions to overall ASD symptom severity; however, all three measures from the ADAMS contributed significantly (or approached significance) in the prediction of severity for RRB. Anxiety, social avoidance, and hyperactivity have frequently been found to be areas of special challenge for males with FXS (Bailey et al. 2008; Cordeiro et al. 2011; Roberts et al. 2007 2009, under review; Thurman et al. 2015), and anxiety has been found to be associated with socially avoidant behaviors in FXS (Budimirovic and Kaufmann 2011; Kaufmann et al. 2008; Roberts et al., under review; Thurman et al. 2015). It is likely that these psychiatric symptom clusters contribute to the development of, and serve to maintain, various aspects of RRB (Cordeiro et al. 2011; Talisa, Boyle, Krafa, and Kaufmann 2014). Collectively, these psychiatric problems and the ASD-related symptoms of RRB will serve as substantial barriers to successful transition to an independent adult life for those with FXS. More research on treatment of these problems is needed.
Mosaic status was marginally related to overall ASD symptom severity, with individuals displaying a mosaic pattern less impaired on the ADOS-2 than those with only a full mutation. Importantly, this relationship emerged even after controlling nonverbal IQ and the psychiatric variables. The relationship between ASD symptoms and other indices of FMR1 variation typically reflective of a qualitative measure of FMRP, has not been found in previous studies when IQ is controlled (Cornish et al. 2004; Loesch et al. 2007; McDuffie et al. 2010).
We also found that there was specificity in the relationship between mosaic status and ASD symptom severity, with the relationship emerging for overall severity and SA severity, but not severity of RRB. This pattern of results raises the possibility of different etiologies, neurological determinants, and treatment regimens for these two domains of ASD symptoms in FXS.
Interestingly, we did not observe a relationship between a new quantitative measure of FMRP and any of the ADOS-2 scores (or, for that matter, with any of the predictors). This latter measure of FMRP provides a quantitative metric of the amount of FMRP expressed in blood cells and was thus thought to be a more sensitive index of affectedness at the biological level than the coarser categorical variable of mosaic status. Technical limitations in the quantitative FMRP analysis of the current study were due to variations in collection, storage time and analysis. This still leaves open a possibility for some phenotypic correlation with this biological marker. Peripheral blood FMRP expression, as detected by the method employed in this study, is a function of the leukocyte number. Correction of observed FMRP expression to that variable was not within the scope of this study but should be considered in the future.
CA was not correlated with ASD symptom severity and did not contribute to prediction. This finding contrasts with several previous studies that have documented age-related change in ASD symptoms (e.g., Lee et al. 2016; McDuffie et al. 2010). This discrepancy may reflect our use of the ADOS-2 calibrated severity scores, which provide indices of symptom severity that are normalized relative to CA unlike raw scores used in previous studies. Alternatively, this finding may reflect the fact that a plateau in ASD symptomatology has been reached by late adolescence-early adulthood. Longitudinal studies are needed to verify this possibility.
Limitations and Conclusions
A number of limitations of this study should be noted. First, although several significant relationships emerged in our examination of the predictors of ASD symptom severity, the predictors accounted for only a relatively modest proportion of the variance in severity. Indeed, none of the adjusted R2 values exceeded .20. Thus, there is a need for continued examination of a fuller range of predictors of ASD symptoms in FXS, including a focus on environmental variables. Second, the sample size is relatively small, although it is on a par with most other studies in the FXS field. Moreover, the sample was limited to individuals who have at least some phrase speech and whose families have the ability to travel to one of the testing sites, which limits generalizability of the findings. Third, we included only a single measure of each construct of interest, making it impossible to determine whether the relationships of interest will replicate with a different set of measures of these constructs. Fourth, we have focused largely on the ADOS-2 to characterize ASD symptoms. This instrument provides only a single snapshot of such symptoms and may not provide a sufficient context for soliciting the full range of RRB that characterize an individual, although this limitation is somewhat mitigated by our findings of a reasonable convergence of the ADOS-2 and ADI-R. Fifth, in light of the ages of our participants, we used “out of range” ADOS-2 scores relative to the norming sample in estimating ASD symptom severity, which suggests caution in interpreting the findings. Finally, we examined only concurrently measured relationships, which make interpretation of the direction of causality difficult. Nonetheless, we believe that the interpretations we have offered of the relationships observed are the most parsimonious and have justification in previous studies.
In conclusion, our findings are important in demonstrating the need to supplement studies using the categorical diagnosis of ASD in FXS with an understanding of individual symptoms and symptom domains and the underlying problems that they reflect. Our findings also suggest that problems in expressive syntax, which we know to be quite severe in FXS (Abbeduto et al. 2007), may be a source of limitations in social interaction and even atypical social behaviors and thereby, ASD symptoms. Most importantly, these expressive syntax impairments and the social problems they engender will limit full participation in daily life for young people with ASD. There is thus, a need for efficacious language interventions for this population, targeting among other things, expressive syntax. Although such evidence-based interventions are beginning to emerge (Bullard, McDuffie and Abbeduto 2017; McDuffie 2016a, b), improvements in expressive syntax are proving difficult to achieve (McDuffie et al. 2018). Our findings also clarify the contribution of IQ to ASD, with that contribution being more in the RRB domain than in the SA domain. And finally, the psychiatric problems that are highly comorbid with FXS, including anxiety, social avoidance, and hyperactivity, are likely to create a cascade of problems for the individual with FXS, including the emergence of symptoms of ASD, and thus, should be a focus of interventions.
Funding:
This research was supported by grants R01HD024356 and U54HD079125 from the National Institutes of Health awarded to Leonard Abbeduto.
The authors gratefully acknowledge the families who participated in the project. The authors also thank the many research staff involved in the project, but especially [deleted for anonymous review].
Conflicts of interest: Leonard Abbeduto has received funding to develop and implement outcome measures for clinical trials from F. Hoffman-LaRoche, Ltd., Roche TCRC, Inc., Neuren Pharmaceuticals Limited, and Fulcrum Therapeutics, Inc. W. Ted Brown and Giuseppe LaFauci hold a patent for a measure used in this study (System and Method for Quantifying Fragile X Mental 1 Protein in tissue and blood samples (United States Patent # 8628934), with the assignee for the patent being the Research Foundation for Mental Hygiene, Inc. No other authors have conflicts or financial disclosures to declare.
Footnotes
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All authors obtained review and approval from the Institutional Review Boards of their respective universities/institutions.
This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study or from their legally authorized representatives.
Bibliography
- Abbeduto L, Brady N, & Kover ST (2007). Language development and fragile X syndrome: Profiles, syndrome-specificity, and within-syndrome differences. Mental Retardation and Developmental Disabilities Research Reviews, 13, 36 – 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeduto L, McDuffie A & Thurman AJ (2014). The fragile X syndrome-autism comorbidity: what do we really know? Frontiers in Genetics, 5, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeduto L, Short-Meyerson K, Benson G, & Dolish J (2004). Relation between theory of mind and language ability in children and adolescents with mental retardation. Journal of Intellectual Disability Research, 48, 150–159. [DOI] [PubMed] [Google Scholar]
- Adayev T, LaFauci G, Dobkin C, Caggana M, Wiley V, Field M, et al. (2014). Fragile X 8 protein in newborn dried blood spots. BMC Medical Genetics, 15(1), 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Mesibov GB, Ament N, & Skinner M (2000). Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders, 30, 557–567. [DOI] [PubMed] [Google Scholar]
- Bailey DB Jr., Hatton DD, Skinner M, & Mesibov G (2001). Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders, 31(2), 165–174. [DOI] [PubMed] [Google Scholar]
- Bailey DB Jr., Raspa M, Olmsted M, & Holiday DB. (2008). Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. American Journal of Medication Genetics, A. 15;146A(16), 2060–2069. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, & Warren ST (2008). Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron, 60, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser R & Sudhalter V (1995). Arousal difficulties in males with fragile X syndrome: A preliminary report. Developmental Brain Dysfunction, 8, 270–279. [Google Scholar]
- Belser R, & Sudhalter V (2001). Conversational characteristics of children with fragile X syndrome: Repetitive speech. American Journal on Mental Retardation, 106, 28–38. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 57 (1): 289–300. [Google Scholar]
- Benson G, Abbeduto L, Short K, Nuccio JB, & Maas F (1993). Development of a theory of mind in persons with mental retardation. American Journal on Mental Retardation, 98, 427–433. [PubMed] [Google Scholar]
- Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, & Kaufmann WE (2006). Autism spectrum disorder in fragile X syndrome: differential contribution of adaptive socialization and social withdrawal. American Journal of Medical Genetics, 140A:1814–1826. [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, & Kaufmann WE (2011). What can we learn about autism from studying fragile X syndrome? Developmental Neuroscience, 33, 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard L, McDuffie A, & Abbeduto L (2017). Distance delivery of a parent-implemented language intervention for young boys with fragile X syndrome. Autism & Developmental Language Impairments, 2, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Noyon T, Basuta K, Van de Water J, Tassone F, Hagerman RJ, & Ashwood P (2014). Group I metabotropic glutamate receptor mediated dynamic immune dysfunction in children with fragile X syndrome. Journal of Neuroinflammation, 19, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow-Woolfolk E (1999). Comprehensive Assessment of Spoken Language. Circle Pines: American Guidance Service. [Google Scholar]
- Clifford S, Dissanyake C, Bui QM, Huggins R, Taylor AK, & Loesch DZ (2007). Autism spectrum phenotype in males and females with fragile X full mutation and permutation. Journal of Autism and Developmental Disorders, 37, 738–747. [DOI] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, & Hessl D (2011). Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. Journal of Neurodevelopmental Disorders, 3, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Sudhalter V, Turk J. Attention and language in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10(1):11–16. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, & Sherman SL (2001). FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine, 3, 359 – 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark JL, Feldman MA, & Holden JJ (2003). Behavioral relationship between autism and fragile X syndrome. American Journal on Mental Retardation, 108, 314–326. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, & Ruedrich S (2003). Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. Journal of Autism and Developmental Disorders, 33, 617 – 629. [DOI] [PubMed] [Google Scholar]
- Estigarribia B, Roberts JE, Sideris J, & Price J (2011). Expressive morphosyntax in boys with fragile X syndrome with and without autism spectrum disorder. International Journal of Language & Communication Disorders, 46, 216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezell J et al. (under review). Prevalence and Predictors of Anxiety Disorders in Adolescent and Adult Males with Autism Spectrum Disorder and Fragile X Syndrome. [DOI] [PMC free article] [PubMed]
- Finestack LH, & Abbeduto L (2010). Expressive language profiles of verbally expressive adolescents and young adults with Down syndrome or fragile X syndrome. Journal of Speech, Language, and Hearing Research, 53, 1334–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch GS, Carpenter N, Howard-Peebles PN, Holden JJ, Tarleton J, & Simensen R (2010). The course of cognitive-behavioral development in children with the FMR1 mutation, Williams-Beuren syndrome, and neurofibromatosis type 1: The effect of gender. American Journal of Medical Genetics, 152A(6),1498–509. [DOI] [PubMed] [Google Scholar]
- Gabriels RL, Cuccaro ML, Hill DE, Ivers BJ, & Goldson E (2005). Repetitive behaviors in autism: Relationships with associated clinical features. Research in Developmental Disabilities, 26, 169–181. [DOI] [PubMed] [Google Scholar]
- Gross C, Berry-Kravis EM, & Bassell GJ (2012). Therapeutic strategies in fragile X syndrome: dysregulated mGluR signaling and beyond. Neuropsychopharmacology. 37(1):178–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman R (1999). Neurodevelopmental disorders. Oxford: Oxford University Press. [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB Jr., Moine H, Kooy RF, Tassone F, Gantois I, Sonenberg N, Mandel JL, & Hagerman PJ Fragile X syndrome. (2017) Nat Rev Dis Primers, September 29;3:17065. [DOI] [PubMed] [Google Scholar]
- Harris SW, Goodlin-Jones B, Nowicki S, Bacalman S, Tassone F, and Hagerman RJ (2005). Autism Profiles of Young Males with Fragile X Syndrome. Journal of Developmental and Behavioral Pediatrics, 26(6), 464. [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, et al. (2008). Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation, 113, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Roberts J, and Mirrett P (2006). Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics, 140(17), 1804–1813. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Gerig G, Macfall JR, & Ross AK (2009). Teasing apart the heterogeneity of autism: same behavior, different brains in toddlers with fragile X syndrome and autism. Journal of Neurodevelopmental Disorders, 1:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, and Kaufmann WE (2009). Autism spectrum disorder in fragile X syndrome: A longitudinal evaluation. American Journal of Medical Genetics, 149(6), 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Nguyen D, Green C, Chavez A, Tassone F, Hagerman R, et al. (2009). A solution to limitations of cognitive testing in children with intellectual disabilities: The case of fragile X syndrome. Journal of Neurodevelopmental Disorders, 1, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan AL, Caravella KE, Ezell J, Rague L, Hills K, & Roberts JE (2017). Autism Spectrum Disorder Symptoms in Infants with Fragile X Syndrome: A Prospective Case Series. Journal of Autism and Developmental Disorders, 47(6),1628–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, & Lord C (2014). Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44(10), 2400–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau A, Tierney E, Bukelis I, Stump M, Kates W, Trescher W et al. (2004). Social behavior profile in young males with fragile X syndrome: Characteristics and specificity. American Journal of Medical Genetics, 126A, 9–17. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Capone G, Clarke M Budimirovic DB. (2008). Autism in genetic intellectual disability: Insights into idiopathic autism In Zimmerman AW (Ed.) Current theories and evidence. Totowa, The Humana Press Inc., pp. 81–108. [Google Scholar]
- Kaufmann W, Cortell R, Kau A, Bukelis I, Tierney E, Gray R et al. (2004). Autism spectrum disorder in fragile X syndrome. American Journal of Medical Genetics, 129, 225–234. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, Haas-Givler B, Stackhouse T, Riley C, Peacock G, Sherman SL, Brown WT, & Berry-Kravis E (2017). Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics, 139(Suppl 3):S194–S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M (2014). Consistency between research and clinical diagnoses of autism among boys and girls with fragile X syndrome. Journal of Intellectual Disabilities Research, 58, 940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M (2014). A comparison of pragmatic language in boys with autism and fragile X syndrome. Journal of Speech, Language and Hearing Research, 57, 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover ST, Pierpont E, Kim J-S, Brown WE, and Abbeduto L (2013). A neurodevelopmental perspective on the acquisition of nonverbal cognitive skills in adolescents with fragile X syndrome. Developmental Neuropsychology, 38, 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFauci G, Adayev T, Kascsak R, Kascsak R, Nolin S, Mehta P, Brown WT, & Dobkin C (2013). Fragile X screening by quantification of FMRP in dried blood spots by a Luminex immunoassay. Journal of Molecular Diagnostics,15(4):508–17. [DOI] [PubMed] [Google Scholar]
- Langthorne P, McGill P, O’Reilly MF, Lang R, Machalicek W, Chan JM, & Rispoli M (2011). Examining the function of problem behavior in fragile X syndrome: preliminary experimental analysis. American Journal on Intellectual and Developmental Disabilities. [DOI] [PubMed] [Google Scholar]
- Lee M, Bush L, Martin G, Barstein J, Heckel N, Klusek J, & Losh M (2017). A multi-method investigation of pragmatic development in Down syndrome. American Journal of Intellectual and Developmental Disabilities, 122, 289–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Martin GE, Berry-Kravis E, Losh M (2016). A developmental, longitudinal investigation of autism phenotypic profiles in fragile X syndrome. Journal of Neurodevelopmental Disorders, 30;8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, & Schroeder S (2006). Cognitive language and social-cognitive skills of individuals with fragile X syndrome with and without autism. Journal of Intellectual Disability Research, 50, 532–545. [DOI] [PubMed] [Google Scholar]
- Ligsay A, & Hagerman RJ (2016). Review of targeted treatments in fragile X syndrome. Intractable & Rare Diseases Research, 5(3), 158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, Tassone F, Taylor AK, Hessl D, Hagerman R, & Huggins RM. (2007). Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neurosci Biobehav Rev, 31, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DeLavore P, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I): Modules 1–4. Torrance, CA: Western Psychological Services. [Google Scholar]
- Martin GE, Barstein J, Hornickel J, Matherly S, Durante G, & Losh M (2017). Signaling of noncomprehension in communication breakdowns in fragile X syndrome, Down syndrome, and autism spectrum disorder. Journal of Communication Disorders, 65, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Losh M, Estigarribia B, Sideris J, & Roberts J (2013). Longitudinal profiles of expressive vocabulary, syntax and pragmatic language in boys with fragile X syndrome or Down syndrome. International Journal of Language & Communicative Disorders, 48, 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Roberts JE, Helm-Estabrooks N, Sideris J, Vanderbilt J, & Moskowitz L (2012). Perseveration in the connected speech of boys with Fragile X syndrome with and without autism spectrum disorder. American Journal on Intellectual and Developmental Disabilities,117(5), 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matherly SM, Klusek J, Thurman AJ, McDuffie A, Abbeduto L, & Roberts JE (in press). Cortisol profiles differentiated in adolescents and young adult males with fragile X syndrome versus autism spectrum disorder. Developmental Psychobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Abbeduto L, Lewis P, Kover S, Kim J, Weber A, and Brown W (2010). Autism spectrum disorder in children and adolescents with fragile X syndrome: Within-syndrome differences and age-related changes. American Journal on Intellectual and Developmental Disabilities, 115(4), 307–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Banasik A, Bullard L, Nelson S, Tempero Feigles R, Hagerman RJ, Abbeduto L (2018). Distance delivery of a spoken language intervention for school-aged and adolescent boys with fragile X syndrome. Developmental Neurorehabilitation, 21, 48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Machalicek W, Bullard L, Nelson S, Tempero-Feigles R, Castignetti N, Abbeduto L (2016). A spoken language intervention for school-aged boys with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 121, 236–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Oakes A, Machalicek W, Ma M, Bullard L, Nelson S, & Abbeduto L (2016). Early language intervention using distance video-teleconferencing: A pilot study of young males with fragile X syndrome and their mothers. American Journal of Speech-Language Pathology, 25, 46–66. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Thurman AJ, Hagerman RJ, & Abbeduto L (2015). Symptoms of autism in males with fragile X syndrome: A comparison to nonsyndromic ASD using current ADI-R scores. Journal of Autism and Developmental Disorders, 45, 1925–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein SA, Sobesky WE, Taylor AK, Riddle JE, Tran HX, & Hagerman RJ. (1996). Molecular‐clinical correlations in males with an expanded FMR1 mutation. American Journal of Medical Genetics Part A, 64(2), 388–394. [DOI] [PubMed] [Google Scholar]
- Miguel EC, Baer L, Coffey BJ, Rauch SL, Savage CR, O’Sullivan RL, … & Jenike MA. (1997). Phenomenological differences appearing with repetitive behaviours in obsessive-compulsive disorder and Gilles de la Tourette’s syndrome. The British Journal of Psychiatry, vol. 170, no. 2, pp. 140–145. [DOI] [PubMed] [Google Scholar]
- Miller M, Iosif AM, Young GS, Hill MM, Ozonoff S. (2018). Early Detection of ADHD: Insights From Infant Siblings of Children With Autism. J Clin Child Adolesc Psychol. 2016 October 12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller RA, & Amaral DG (2017). Editorial: Time to give up on Autism Spectrum Disorder? Autism Research,. 10(1), 10–14. [DOI] [PubMed] [Google Scholar]
- Murphy MM, & Abbeduto L (2007). Gender differences in repetitive language in fragile X syndrome. Journal of Intellectual Disability Research, 51, 387–390. [DOI] [PubMed] [Google Scholar]
- Niu M, Han Y, Dy ABC, Du J, Jin H, Qin J, Zhang J, Li Q, & Hagerman RJ (2017). Autism Symptoms in Fragile X Syndrome. J Child Neurol. 2017 September;32(10):903–909. [DOI] [PubMed] [Google Scholar]
- Oakes A, McDuffie A, Thurman AJ, Bullard L, & Abbeduto L (2016). Characterising repetitive behaviours in young males with fragile X syndrome. Journal of Intellectual Disability Research, 60, 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra BA, & Willemson R (2003). A fragile X balance: FMR1 expression levels. Human Molecular Genetics, 12, 249–457. [DOI] [PubMed] [Google Scholar]
- Philofsky A, Hepburn S, Hayes A, Rogers S, & Hagerman R (2004). Linguistic and cognitive functioning and autism symptoms in young children with Fragile X syndrome. American Journal on Mental Retardation, 109(3), 208–218. [DOI] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, … & Pickles A. (2006). Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 45(9), 1094–1103. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Clarke MA, Alcorn K, Carter JC, Long AC, & Kaufmann WE (2009). Autistic behavior in boys with fragile X syndrome: social approach and HPA-axis dysfunction. Journal of Neurodevelopmental Disorders, 1, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Ezell JE, Fairchild AJ, Klusek J, Thurman AJ, McDuffie A, & Abbeduto L (under review). Biobehavioral Composite of Social Aspects of Anxiety in Young Adults with Fragile X contrasted to Autism Spectrum Disorder. [DOI] [PMC free article] [PubMed]
- Roberts JE, Martin G, et al. (2007). Discourse Skills of Boys With Fragile X Syndrome in Comparison to Boys with Down Syndrome. Journal of Speech, Language, and Hearing Research, 50, 475 – 492. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen B, Robinson A, & Shinkareva SV (2012). Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 117(2), 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LA, Hatton DD, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 37, 1748–1760. [DOI] [PubMed] [Google Scholar]
- Roid G, & Miller L (1997). Leiter International Performance Scale—Revised. Wood Dale, IL: Stoelting. [Google Scholar]
- Rogers S, Wehner E, & Hagerman R (2001). The behavioral phenotype in fragile X: Symptomsof autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental and Behavioral Pediatrics, 22, 409–418. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, & Lord C (2008). Autism Diagnostic Interview – Revised. Los Angeles: Western Psychological Services. [Google Scholar]
- Scerif G, Longhi E, Cole V, Karmiloff-Smith A, & Cornish K (2012). Attention across modalities as a longitudinal predictor of early outcomes: The case of fragile X syndrome. Journal of Child Psychology and Psychiatry, 53, 614–650. [DOI] [PubMed] [Google Scholar]
- Scherr JF, Hahn LJ, Hooper SR, Hatton D, Roberts JE (2016). HPA axis function predicts development of working memory in boys with FXS. Brain and Cognition,102, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, & Renner BR (1988). The Childhood Autism Rating Scale (CARS). Los Angeles: Western Psychological Services. [Google Scholar]
- Shattuck P, Seltzer M, Greenberg J, Orsmond G, Bolt D, Kring S, Lounds J, and Lord C (2007). Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. Journal of Autism and Developmental Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield A, Pyers J, Martin A, & Tager-Flusberg H (2016). Relations between language and cognition in native-signing children with autism spectrum disorder. Autism Research, 9(12), 1304–1315.. [DOI] [PubMed] [Google Scholar]
- Skinner M, Hooper S, Hatton D, Roberts J, Mirrett P, Schaaf J, Sullivan K, Wheeler A, and Bailey D (2005). Mapping nonverbal IQ in young boys with fragile X syndrome. American Journal of Medical Genetics A, 132, 25–32. [DOI] [PubMed] [Google Scholar]
- Smith LE, Barker ET, Seltzer MM, Abbeduto L, & Greenberg JS (2012). Behavioral phenotype of fragile X syndrome in adolescence and adulthood. Am J Intellect Dev Disabil, 117(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stembalska A, Łaczmańska I, Gil J, Pesz KA (2016). Fragile X syndrome in females - a familial case report and review of the literature. Dev Period Med. 20(2), 99–104. [PubMed] [Google Scholar]
- Symons FJ, Clark RD, Hatton DD, Skinner M, & Bailey DB jr. (2003). Self-injurious behavior in young boys with fragile X syndrome. American Journal of Medical Genetics, 118A, no. 2, 115–121. [DOI] [PubMed] [Google Scholar]
- Sudhalter V, & Belser RC (2001). Conversational characteristics of children with Fragile X syndrome: Tangential language. American Journal on Mental Retardation, 106, 389–400. [DOI] [PubMed] [Google Scholar]
- Sudhalter V, Cohen I, Silverman W, & Wolf-Schein E (1990). Conversational analysis of males with fragile X, Down syndrome, and autism: Comparison of the emergence of deviant language. American Journal on Mental Retardation, 94, 431–441. [PubMed] [Google Scholar]
- Tager-Flusberg H, & Sullivan K (1994). Predicting and explaining behavior: a comparison of autistic, mentally retarded and normal children. Journal of Child Psychology and Psychiatry. 35(6), 1059–1075. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, & Sullivan K (2000). A componential view of theory of mind: evidence from Williams syndrome. Cognition, 14;76(1), 59–90. [DOI] [PubMed] [Google Scholar]
- Talisa VB, Boyle L, Crafa D, & Kaufmann WE (2014). Autism and anxiety in males with fragile X syndrome: an exploratory analysis of neurobehavioral profiles from a parent survey. American Journal of Medical Genetics, 164A(5), 1198–1203. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Ikle DN, et al. (1999). FMRP expression as a potential prognostic indicator in fragile X syndrome. American Journal of Medical Genetics, 84 (3), 250–261. [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Hagerman RJ, and Abbeduto l. (2014). Psychiatric symptoms in boys with fragile X syndrome: A comparison with nonsyndromic autism spectrum disorder. Research in Developmental Disabilities, 35, 1072–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Josol C, Hagerman RJ, & Abbeduto L (2017). Language skills of males with fragile X syndrome or nonsyndromic autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Kover ST, Hagerman RJ, & Abbeduto L (2015). Autism symptomatology in boys with fragile X syndrome: A cross-sectional developmental trajectories comparison with nonsyndromic ASD. Journal of Autism and Developmental Disorders, 45, 2816 – 2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse L, & Gillberg C (2014). Why autism must be taken apart. Journal of Autism and Developmental Disorders, 44(7), 1788–1792. [DOI] [PubMed] [Google Scholar]
- Waterhouse L, London E, Gillberg C. (2017). The ASD diagnosis has blocked the discovery of valid biological variation in neurodevelopmental social impairment. Autism Research, 10(7),1182. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, & Piven J (2012).Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry, 51, no. 12, 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]