Abstract
Purpose: c-MYC has been noted in many tumor types, but its functional significance and clinical utility in oral squamous cell carcinoma (OSCC) are not well known. Here we studied the expression of c-MYC in correlation to clinical outcome in patients with oral squamous cell carcinoma.
Methods: The current study, using immunohistochemical staining, first examined c-MYC expression in OSCC patients and further correlated its expression with clinicopathological parameters.
Results: c-MYC was expressed in the majority of OSCC patients (n=133). The c-MYC expression is associated with histological grade (P=0.0205) of patients with oral squamous cell carcinoma. Multivariate Cox regression analysis revealed that TN stage (P<0.001), American Joint Committee on Cancer (AJCC) stage (P<0.0001), and tumor differentiation (P=0.0025) were independent factors for overall survival in patients with OSCC except for c-MYC expression (P>0.05). Multiplicative-scale interaction between T stage (III/IV) and low c-MYC expression on mortality risk was identified (P=0.0233). Kaplan–Meier survival analysis demonstrated that oral cancer patients (T III/IV stage) with high c-MYC expression had better survival than those with low and medium c-MYC expression (P=0.0270).
Conclusion: Our data indicate that c-MYC is a potential biomarker that can be used as a therapeutic target for treating OSCC patients with T stage (III/IV).
Keywords: T stage, c-MYC, OSCC, biomarker, survival
Introduction
According to WHO, cancer is the second leading cause of mortality worldwide and will soon overcome cardiovascular diseases as the leading cause of death.1 Among the most frequent forms of cancer worldwide, oral squamous cell carcinoma (OSCC) is the most common oral malignancy, and accounts around 2–4% of all cancer cases. In India, it is one of the most common malignancies and has around 50% mortality rate.2
Despite improved therapeutic modalities, clinical and pathological parameters are still insufficient for prognostic characterization of OSSC, and thus the survival of the patients has remained unchanged over the last few decades.3 Therefore, it is important to evaluate the biological prognostic markers that might help in determining the biological diversity of the disease and guide adequate therapeutic procedures. It is proposed that oncogene product has several functions in the prognosis of cancer; these functions are related to cell growth factor, signal transmitter system, growth factor receptor and regulatory factor in the nucleus. Among several oncogenes, myc gene plays an important role not only in the proliferation of the cells but also in control of cell differentiation. Myc is in the family of proto-oncogenes and regulatory genes that code for transcription factor and consists of several members, viz, L-myc, N-myc, and c-myc. C-myc or myc gene was the first to be discovered in this family and is persistently expressed in many different types of cancer, including head and neck squamous cell carcinoma (HNSCC). A number of studies of the molecular mechanisms underlying oncogenesis have shown the emergence of c-Myc expression as a key event, and it is frequently overexpressed in oral cancers as a consequence of gene amplification.4 C-myc overexpression is responsible for poor tumor prognosis5–8 as well as the self-renewal of tumor stem cells.9–12 It not only induces cell proliferation but also apoptosis. For inducing apoptosis it requires p53; however, on phosphorylation of pR6, c-MYC is increased and leads to cell proliferation. The influence of c-MYC in the carcinogenic process has been previously described in many tumors. A study on the progression of pre-neoplastic lesions indicates that the c-myc oncoprotein expression was reported in several stages, including benign keratoses. c-myc expression in OSCC. Eversole and Sapp noted c-MYC expression in both pre-cancerous (keratoses) and early-stage lesions in patients from North America.13 Sakai et al and Waitzberg et al reported the c-MYC positivity in stromal and neoplastic cells of tumors, with nuclear cytoplasmatic or double staining in squamous cell carcinomas.14 Perez-Sayans et al showed higher c-MYC expression in both nuclear and cytoplasmic neoplastic cells.15 Therefore, there is a broad spectrum of c-MYC activation in various head and neck cancers from different parts of the world. Current advances in the understanding of the role of c-MYC expression will allow diagnosis that will be more accurate and prognostic approaches which may lead to novel methods of treatment and prevention. In the present study, we used immunohistochemical (IHC) staining to investigate the expression pattern of c-MYC in OSCCs, as well as to study the prognostic role of c-MYC in oral cancer.
Materials and methods
Participants and clinical tissues
Tissue samples of patients (from January 2000 to December 2008) with oral squamous cell carcinoma were obtained from the Department of Pathology at Changhua Christian Hospital, Taiwan, of which 133 OSCC specimens were used for immunohistochemistry staining. Staging was classified according to the sixth edition of the TNM staging system of AJCC (American Joint Committee on Cancer). The main treatment was tumor removal and radical neck dissection, including post-operation irradiation as well as selective patients treated with 5-FU and cisplatin chemotherapy. This study was approved by the Ethics Committees of Changhua Christian Hospital (Changhua, Taiwan), and we adhered to the guidelines approved by CCH IRB (no. 170143). This study was performed in compliance with the Declaration of Helsinki. Patient information has been decoded, and patient identifiable data were removed. IRB gave approval to use formalin-fixed, paraffin-embedded (FFPE) decoding tissue microarray samples without informed consent.
Tissue microarray and immunohistochemical staining
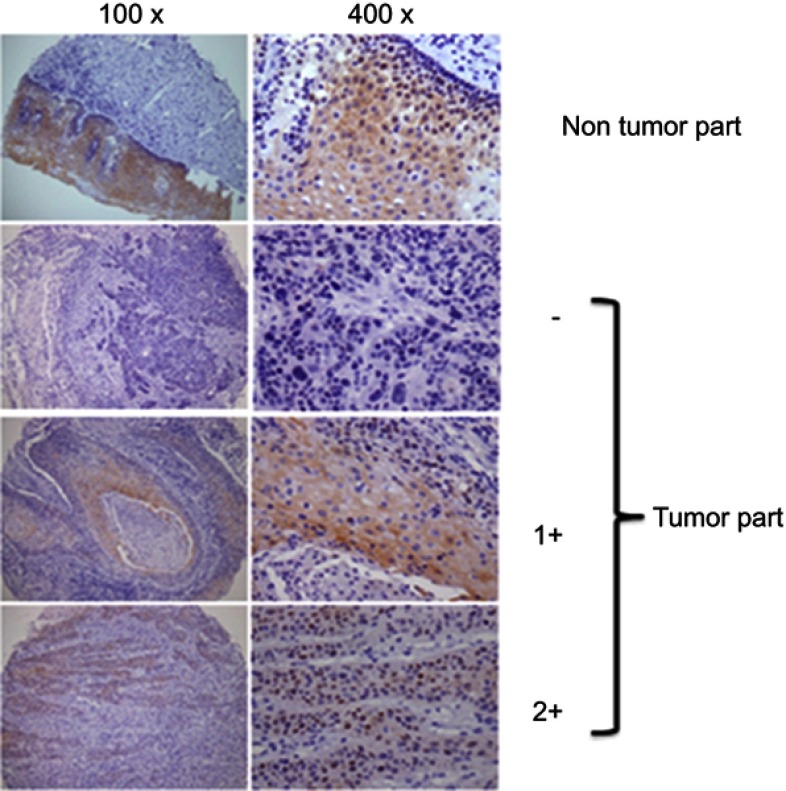
IHC staining for c-MYC (EP121; Bio SB, Santa Barbara, CA, USA) was performed on microarray tissue slides consisting of specimens from 133 OSCC patients. Tissue sections were deparaffinized and rehydrated using routine techniques. Endogenous peroxidase activity was blocked with 3% H2O2 in methanol, hydrated with gradient alcohol and phosphate-buffered saline solution, and incubated in 10 mmol/L citrate buffer (pH 6.0). The sections were incubated with c-MYC antibody in room temperature for 20 min. After washing three times with PBS, the sections were incubated with appropriate peroxidase-labeled secondary antibodies for 30 min at room temperature. The sections were washed three times with PBS and then labeled by diaminobenzidine and counterstained with Mayer’s hematoxylin, dehydrated and mounted. Fixation status, tissue preservation, and IHC technical procedures are followed as per the standard operating procedure in our College of American Pathologists (CAP) accreditation lab. Two experienced pathologists (Kun-Tu Yeh and Chang Wei-Hsiang) independently assessed the results of IHC staining, and a final agreement was obtained for each score at a discussion microscope. We used normal oral buccal mucosa to be an internal control. The staining intensities were scored −, 1+, and 2+ for negative, low, and high signals, respectively.
Statistical analysis
Categorical variables are presented as sample number and percentages. Multivariate logistic regression models were used to evaluate the association between clinicopathologic factors and c-MYC expression. The effects of clinicopathologic factor and c-MYC expression on mortality density among OSCC patients were examined using multivariate Cox’s proportional hazards model with adjustment for age and gender. HRs and CIs were subsequently calculated. Survival outcomes were summarized by the Kaplan–Meier method. P-values were obtained from log-rank tests for the homogeneity of Kaplan–Meier curves between high and low c-MYC expressions in different T stage (III/IV). All analyses were performed using SAS 9.4 Software.
Results
Clinicopathologic characteristics and c-MYC expression in OSCC patients
The patients’ characteristics, including their sex, age, tumor stage, lymph node status, AJCC cancer stage, histological grade, and clinical therapy are listed in Table 1. We evaluated both cytoplasmic and nuclear c-MYC expressions using a semiquantitative scoring system. As shown in Figure 1, c-MYC was found in the cytoplasm and in the nucleus of tumor cells, whereas in most of the cases c-MYC intensity was stronger in the cytoplasm than in the nucleus.
Table 1.
Demographic and characteristics among oral cancer patients
| Factors | No | % |
|---|---|---|
| Gender | ||
| Female | 8 | 6 |
| Male | 125 | 94 |
| Age, year | ||
| ≤49 | 36 | 29.3 |
| 50–59 | 47 | 35.3 |
| 60–69 | 33 | 24.8 |
| ≥70 | 14 | 10.6 |
| T (tumor size) | ||
| I | 38 | 28.6 |
| II | 45 | 33.8 |
| III | 10 | 7.5 |
| IV | 40 | 30.1 |
| N (lymph node) | ||
| N0 | 98 | 73.7 |
| N1 | 14 | 10.5 |
| N2 | 21 | 15.8 |
| N3 | 0 | 0 |
| M (metastasis) | ||
| No | 133 | 100 |
| Yes | 0 | 0 |
| AJCC cancer stage | ||
| I | 34 | 19.8 |
| II | 34 | 20.9 |
| III | 14 | 11.6 |
| IV | 51 | 47.8 |
| Histological grade | ||
| Well | 21 | 15.7 |
| Moderate | 111 | 81.3 |
| Poor | 1 | 3 |
| Clinical therapy | ||
| Surgery | 58 | 43.6 |
| Radiotherapy | 64 | 48.1 |
| Chemotherapy | 11 | 8.2 |
| Chemoradiotherapy | 15 | 11.2 |
Abbreviation: AJCC, American Joint Committee on Cancer
Figure 1.
Expression levels of c-MYC proteins in OSCC clinical specimens. Evaluation for immunohistochemical staining of c-MYC expression. Shown is a representative example of each grade.
Correlation of c-MYC expression with clinical parameters of oral squamous cell carcinoma
Based on the expression level, we further divided the samples into high (n=75) and low (below the median, n=58) c-MYC expression. Clinicopathological features were then evaluated between these two groups Table 2. A positive correlation was observed among c-MYC expression and T stage (P=0.05) and histological grade (P=0.02). No significant correlation between c-MYC expression and other clinicopathologic parameters was found in our study.
Table 2.
Correlation of c-MYC expression with clinical parameters of oral squamous cell carcinoma
| Factors | c-MYC | aaOR (95% CI) | P-value | |
|---|---|---|---|---|
| Low | High | |||
| No. | No. | |||
| (n = 58) | (n=75) | |||
| Tumor size (SD) | 3.16 (1.60) | 2.81 (1.68) | ||
| T classification | ||||
| I/II | 31 | 52 | 1 | |
| III/IV | 27 | 23 | 0.49 (0.24–1.03) | 0.05 |
| N (lymph node) | ||||
| N0/N1 | 48 | 64 | 1 | |
| N2/N3 | 10 | 11 | 0.77 (0.30–1.99) | 0.5 |
| M (metastasis) | ||||
| No | 57 | 75 | 1 | |
| Yes | 1 | 0 | ND | |
| AJCC cancer stage | ||||
| Early stage (I/II) | 25 | 43 | 1 | |
| Advance stage (III/IV) | 33 | 32 | 0.53 (0.26–1.07) | 0.07 |
| Histological grade | ||||
| Well | 4 | 17 | 1 | |
| Moderate/poor | 54 | 58 | 0.25 (0.08–0.81) | 0.02 |
| Death | ||||
| No | 23 | 36 | 1 | |
| Yes | 35 | 39 | 0.55 (0.27–1.15) | 0.11 |
Note: aAdjusted odds ratio (aOR) was controlled for gender and age.
Abbreviation: AJCC, American Joint Committee on Cancer
Clinicopathologic factor and c-MYC expression on mortality density
The effects of clinicopathologic factors and c-MYC expression on mortality in the OSCC patient cohort are shown in Table 3. Adjusted for the effects of age and gender, TN stage, AJCC tumor stage, and moderate/poor tumor differentiation were observed to be associated with a higher mortality risk (aHR =1.9, 2.9, 2.5, 2.9 and 3.7, respectively). The independent mortality risk for patients with high c-MYC expression was non-significant as compared to low c-MYC expression (P>0.05).
Table 3.
The effect of clinicopathologic factor and UNC13C expression on mortality density and adjusted hazard ratio (aHR) among OSCC patients
| Factors | No. of patients | Follow-up | No. of death | Mortality densitya | aHRb | (95% CI) | Interaction |
|---|---|---|---|---|---|---|---|
| (person-year) | P-value | ||||||
| Overall mortality from primary malignancy to death | |||||||
| T classification | |||||||
| I/II | 83 | 554.9 | 22 | 4.0 | 1 | ||
| III/IV | 50 | 242 | 26 | 10.7 | 1.9 | (1.4–2.8) | 0.0002 |
| N classification | |||||||
| N0/N1 | 112 | 736.4 | 33 | 4.5 | 1 | ||
| N2/N3 | 21 | 60.5 | 15 | 24.8 | 2.9 | (2.0–4.1) | <0.0001 |
| AJCC tumor stage | |||||||
| I/II | 68 | 483.1 | 16 | 3.3 | 1 | ||
| III/IV | 65 | 313.7 | 32 | 10.2 | 2.5 | (1.7–3.7) | <0.0001 |
| Tumor differentiation | |||||||
| Well | 21 | 160.1 | 0 | 0.0 | 1 | ||
| Moderate/poor | 112 | 636.7 | 48 | 7.5 | 2.9 | (1.4–5.6) | 0.0025 |
| c-MYC expression | |||||||
| High | 75 | 455.7 | 23 | 5.0 | 1 | ||
| Low | 58 | 341.2 | 25 | 7.3 | 1.4 | (0.82–2.53) | 0.21 |
| Combined effect | |||||||
| T stage/c-MYCc | |||||||
| I/II/High | 52 | 352.5 | 12 | 3.4 | 1 | 0.0233 | |
| I/II/Low | 31 | 202.4 | 10 | 4.9 | 1.4 | (0.6–3.3) | |
| III/IV/High | 23 | 138.8 | 15 | 10.8 | 2.6 | (1.1–5.8) | |
| III/IV/Low | 27 | 103.2 | 11 | 10.7 | 2.9 | (1.4–6.2) | |
| AJCC tumor stage/c-MYCd | |||||||
| I/II/High | 43 | 313.6 | 8 | 2.6 | 1 | 0.0017 | |
| I/II/Low | 25 | 169.45 | 8 | 4.7 | 1.8 | 0.7–4.7 | |
| III/IV/High | 32 | 171.7 | 15 | 8.7 | 3.4 | 1.4–7.7 | |
| III/IV/Low | 33 | 142 | 17 | 12.0 | 6.2 | 1.5–7.8 | |
Note: aMortaility density was displayed as per 100 people-years. baHR was adjusted for gender and age. cSignificant multiplicative-scale interaction between T stage and low expression on mortality risk was identified (P for interaction, 0.0233). dSignificant multiplicative-scale interaction between AJCC stage and low expression on mortality risk was identified (P for interaction, 0.0017).
Abbreviation: AJCC, American Joint Committee on Cancer
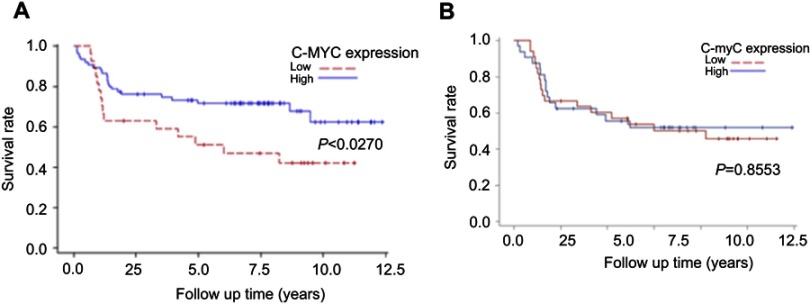
Because c-MYC expression was associated with T stage and AJCC tumor stage, we further evaluated the joint effect of c-MYC expression and the two pathological factors on mortality density. Kaplan–Meier survival analysis (Figure 2A) demonstrated that T (III/IV) stage oral cancer patients with low c-MYC expression showed poor survival rate than those with high c-MYC expression (P=0.0270) and a 2.9 fold higher hazard risk (95% CI, 1.4–6.2) than patients with high c-MYC expression. We further evaluated the combined effect of c-MYC expression and AJCC tumor stage on mortality density. A higher mortality risk was observed to be related to low c-MYC expression and AJCC tumor stage (aHR =6.2, 95% CI, 1.5–7.8; P=0.0017). Surprisingly, Kaplan–Meier analysis did not show any statistical significance of c-MYC (Figure 2B).
Figure 2.
(A) Kaplan–Meier survival curves associated with c-MYC expression among oral cancer patients in T III/IV stage. P-values obtained from log-rank tests for the homogeneity of Kaplan–Meier curves between high and low c-MYC expressions was 0.0270. (B) Kaplan–Meier survival curves associated with c-myC expression among oral cancer patients in AJCC III/IV stage. P-values obtained from log-rank tests for the homogeneity of Kaplan–Meier curves between high and low c-myC expressions was 0.8553.
Discussion
c-MYC expression is a good or poor prognostic marker depending on cancer type, including prostate cancer,16 breast cancer,17 and CRC, including18 OSCC tissues.19 In order to explore the role of c-MYC in OSCC, c-MYC protein expression was tested by IHC staining and c-MYC expression was found in both cytoplasm and nucleus, while c-MYC intensity was stronger in the cytoplasm than in the nucleus. This result is consistent with previous research.15
Cytoplasmic c-MYC expression might indicate a worse prognosis as compared to the typical nuclear pattern;20,21 thus, we stratified OSCC patients based on cytoplasmic c-MYC expression, and further evaluated its relationship with other clinicopathologic characteristics. We found that c-MYC expression was strongly associated with T stage (P=0.05) and histological grade (P=0.02). No significant correlation between c-MYC expression and other clinicopathologic parameters was found in our study. In colorectal cancer (CRC), tongue squamous cell carcinoma (TSCC) and in hepatocellular carcinoma (HCC), high c-MYC expression correlates with poor prognosis.22–24 Bockelman et al found that negative cytoplasmic c-Myc immunoreactivity showed prognostic value in colorectal cancer, while nuclear c-Myc protein expression showed none.21 Multivariate analysis using the Cox proportional hazards model indicated that T, N stage, AJCC tumor stage and tumor differentiation, not cytoplasmic c-MYC expression, were independent prognostic factors for survival in OSCC patients. This result limitation is due to the sample size, the bias in sample scoring21 and the antibody used. Moreover, up to 50% of the tissue specimens were lost in the IHC staining.21 Another important factor for this contradictory result may be due to the types of oral cancer tissues used in c-MYC analysis. For example, Trivedi et al found c-MYC as the most significant independent prognostic factor in tongue SCC (TSCC), but not in buccal SCC (BSCC).22
Previous studies found that c-MYC was more highly expressed in T3/T4 tumors than in T1/T2 tumors and was also correlated with tumor stage and lymphatic permeation.15,25 Based on our univariate analysis we further analyzed the combined effect of clinicopathologic factors; T (III/IV) and AJCC (III/IV) stage and c-MYC expression on mortality risk. A positive correlation was observed between c-MYC expression, T3/T4 and AJCC stage. In our survival analysis, c-MYC expression is associated with poorer OS in T3/T4 stage OSCC patients, whereas OSCC patients with AJCC (III/IV) stage did not have a statistical difference.
In conclusion, low c-MYC expression is observed in oral cancer and more likely to worsen survival in T3/T4 stage oral cancer patients. Thus, c-MYC may be a potential biomarker for diagnosing and treating advance T staged OSCC patients.
Disclosure
The authors have declared that no competing interests exist in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Kumar M, Nanavati R, Modi TG, Dobariya C. Oral cancer: etiology and risk factors: a review. J Cancer Res Ther. 2016;12(2):458–463. doi: 10.4103/0973-1482.186696 [DOI] [PubMed] [Google Scholar]
- 3.Neville BW, Day TA. Oral cancer and precancerous lesions. Ca-Cancer J Clin. 2002;52(4):195–215. [DOI] [PubMed] [Google Scholar]
- 4.Pai R, Pai S, Lalitha R, et al. Over-expression of c-Myc oncoprotein in oral squamous cell carcinoma in the South Indian population. Ecancermedicalscience. 2009;3:128. doi: 10.3332/ecancer.2009.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Field JK, Spandidos DA, Stell PM, Vaughan ED, Evan GI, Moore JP. Elevated expression of the c-myc oncoprotein correlates with poor prognosis in head and neck squamous cell carcinoma. Oncogene. 1989;4(12):1463–1468. [PubMed] [Google Scholar]
- 6.Riou GF. Proto-oncogenes and prognosis in early carcinoma of the uterine cervix. Cancer Surv. 1988;7(3):441–456. [PubMed] [Google Scholar]
- 7.Spandidos DA, Yiagnisis M, Papadimitriou K, Field JK. ras, c-myc and c-erbB-2 oncoproteins in human breast cancer. Anticancer Res. 1989;9(5):1385–1393. [PubMed] [Google Scholar]
- 8.Varley JM, Swallow JE, Brammar WJ, Whittaker JL, Walker RA. Alterations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene. 1987;1(4):423–430. [PubMed] [Google Scholar]
- 9.Adams JM, Kelly PN, Dakic A, Carotta S, Nutt SL, Strasser A. Role of “cancer stem cells” and cell survival in tumor development and maintenance. Cold Spring Harb Symp Quant Biol. 2008;73:451–459. doi: 10.1101/sqb.2008.73.004 [DOI] [PubMed] [Google Scholar]
- 10.Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22(20):2755–2766. doi: 10.1101/gad.1712408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair SK, Burley SK. Structural aspects of interactions within the Myc/Max/Mad network. Curr Top Microbiol Immunol. 2006;302:123–143. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M, Takizawa N, Narita M, Ichisaka T, Yamanaka S. Promotion of direct reprogramming by transformation-deficient Myc. P Natl Acad Sci USA. 2010;107(32):14152–14157. doi: 10.1073/pnas.1009374107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eversole LR, Sapp JP. c-myc oncoprotein expression in oral precancerous and early cancerous lesions. Eur J Cancer B Oral Oncol. 1993;29B(2):131–135. [DOI] [PubMed] [Google Scholar]
- 14.Sakai H, Kawano K, Okamura K, Hashimoto N. Immunohistochemical localization of c-myc oncogene product and EGF receptor in oral squamous-cell carcinoma. J Oral Pathol Med. 1990;19(1):1–4. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Sayans M, Suarez-Penaranda JM, Padin-Iruegas E, et al. Quantitative determination of c-myc facilitates the assessment of prognosis of OSCC patients. Oncol Rep. 2014;31(4):1677–1682. doi: 10.3892/or.2014.3040 [DOI] [PubMed] [Google Scholar]
- 16.Zeng W, Sun H, Meng F, et al. Nuclear C-MYC expression level is associated with disease progression and potentially predictive of two year overall survival in prostate cancer. Int J Clin Exp Pathol. 2015;8(2):1878–1888. [PMC free article] [PubMed] [Google Scholar]
- 17.Elster D, Jaenicke LA, Eilers M, von Eyss B. TEAD activity is restrained by MYC and stratifies human breast cancer subtypes. Cell Cycle (Georgetown, Tex). 2016;15(19):2551–2556. doi: 10.1080/15384101.2016.1207837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KS, Kwak Y, Nam KH, et al. Favorable prognosis in colorectal cancer patients with co-expression of c-MYC and ss-catenin. BMC Cancer. 2016;16(1):730. doi: 10.1186/s12885-016-2770-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YJ, Lin SC, Kao T, et al. Genome-wide profiling of oral squamous cell carcinoma. J Pathol. 2004;204(3):326–332. doi: 10.1002/path.1640 [DOI] [PubMed] [Google Scholar]
- 20.Gong Y, Zhang X, Chen R, Wei Y, Zou Z, Chen X. Cytoplasmic expression of C-MYC protein is associated with risk stratification of mantle cell lymphoma. PeerJ. 2017;5:e3457–e3457. doi: 10.7717/peerj.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bockelman C, Koskensalo S, Hagstrom J, Lundin M, Ristimaki A, Haglund C. CIP2A overexpression is associated with c-Myc expression in colorectal cancer. Cancer Biol Ther. 2012;13(5):289–295. doi: 10.4161/cbt.18922 [DOI] [PubMed] [Google Scholar]
- 22.Trivedi TI, Tankshali RA, Goswami JV, Shukla SN, Shah PM, Shah NG. Identification of site-specific prognostic biomarkers in patients with oral squamous cell carcinoma. Neoplasma. 2011;58(3):217–226. [DOI] [PubMed] [Google Scholar]
- 23.Vora HH, Shah NG, Patel DD, Trivedi TI, Chikhlikar PR. Prognostic significance of biomarkers in squamous cell carcinoma of the tongue: multivariate analysis. J Surg Oncol. 2003;82(1):34–50. doi: 10.1002/jso.10183 [DOI] [PubMed] [Google Scholar]
- 24.Cui J, Dong BW, Liang P, Yu XL, Yu DJ. Effect of c-myc, Ki-67, MMP-2 and VEGF expression on prognosis of hepatocellular carcinoma patients undergoing tumor resection. World J Gastroenterol. 2004;10(10):1533–1536. doi: 10.3748/wjg.v10.i10.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah NG, Trivedi TI, Tankshali RA, et al. Prognostic significance of molecular markers in oral squamous cell carcinoma: a multivariate analysis. Head Neck-J Sci Spec. 2009;31(12):1544–1556. doi: 10.1002/hed.21126 [DOI] [PubMed] [Google Scholar]