Abstract
Background
Colorectal cancer is the second leading cause of cancer death in the U.S. and third-most common cancer in both men and women. Colorectal cancer screening (CRCS) rates remain low, particularly among vulnerable patients receiving care at federally qualified health centers. Through its Value Transformation Framework, the National Association of Community Health Centers provides a systematic approach to improving CRCS by transforming health center infrastructure, care delivery, and people systems—to improve health outcomes, patient and staff experiences, and lower costs (Quadruple Aim).
Methods
We combined the Value Transformation Framework, evidence-based CRCS interventions, and the Learning Community Model to drive system improvements and implement evidence-based practices. Multi-disciplinary teams at 8 health centers in Georgia and Iowa participated for 1-year with Primary Care Association support.
Results
Pre−/post- 1-year-intervention data showed, within health centers, raw percentage of eligible patients screened for CRC increased from 33.2% (13.5%–61.7%) in January 2017 to 46.5% (14.2%–81.5%) in December 2017, with an overall 13.3 percentage point average increase. This translates into an average increase of 3.3 (95% CI: 1.7, 5.0) eligible patients screened per month per health center over the year or 317 additional patients meeting CRCS guidelines. Specific interventions associated with higher CRCS rates included standing orders, sharing performance data, and electronic health record alerts.
Conclusion
Findings support a three-pronged approach for improving CRCS: The Value Transformation Framework's evidence-based recommendations, with actionable CRC interventions, offered in a learning community. These results guide methodological approaches to improving CRCS in health centers through a multi-level, multi-modality quality improvement and transformation approach.
Keywords: Colorectal Cancer, Screening, Health centers, Quality Improvement, Transformation, Evidence-base, Quadruple Aim
1. Background
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States and the third most common cancer in both men and women (U.S. Cancer Statistics Working Group, 2017). In 2014, 51,651 people in the United States died from CRC (U.S. Cancer Statistics Working Group, 2017). Screening programs aimed at early diagnosis and removal of precancerous polyps reduce both CRC incidence and mortality (Pignone et al., 2002). For average-risk individuals 50–75 years, the U.S. Preventive Services Task Force (USPSTF) gives colorectal cancer screening (CRCS) a grade ‘A' recommendation, its highest endorsement and one that indicates that the net benefit of this activity is substantial (U.S. Preventive Services Task Force, 2016).
Despite the benefits of early cancer detection and the availability of effective screening tests, rates of CRCS remain low. While strategies such as patient and provider reminders, provider assessment and feedback, and reduction of structural barriers have shown to be effective in increasing CRCS (Joseph et al., 2016; Green et al., 2013; Brouwers et al., 2011b, p. 111; Brouwers et al., 2011a, p. 112; Baron et al., 2010), overall screening rates are 63% among adults 50 years and older, below the Healthy People 2020 Goal of 70.5% (Office of Disease Prevention and Health Promotion, 2020). The most current national health center data show the percent of health center patients 50–75 years who had an appropriate screening test for CRC is 39.89% (HRSA, 2018). While data from the 2015 National Health Interview Survey indicate that there are disparities in CRCS based on race/ethnicity, a lack of health insurance or a lack of a usual source of care are the most common factors associated with lower screening rates (White et al., 2017; Knight et al., 2015; Klabunde et al., 2011; Ayanian et al., 1993). Data also show that higher education and income levels are associated with a greater proportion of persons receiving CRCS (Solbak et al., 2018; White et al., 2017; Klabunde et al., 2011).
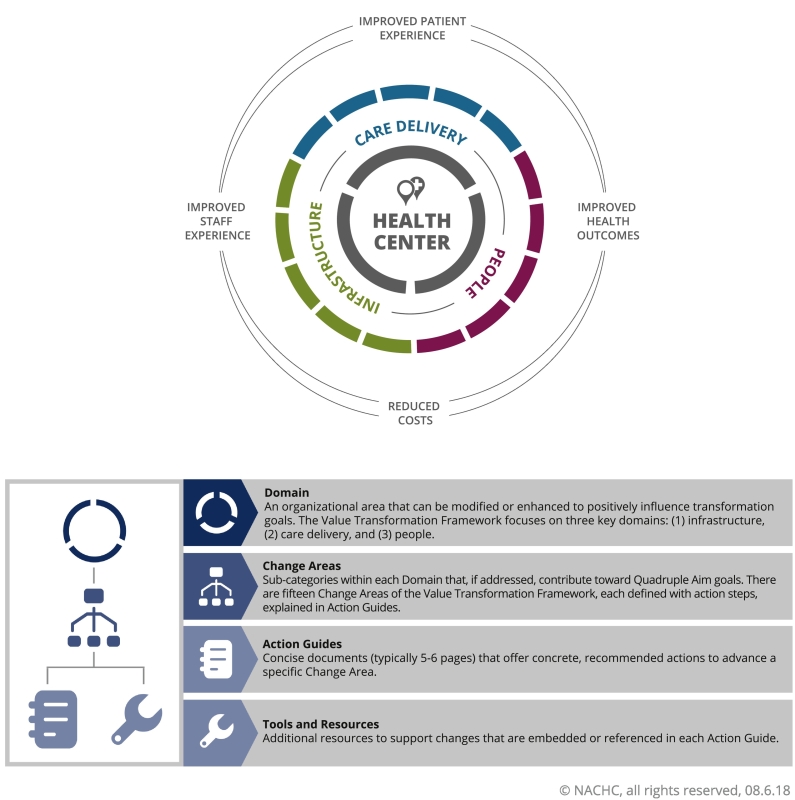
While CRC is a critical health issue, providers must also attend to other consequential health matters that impact the health and well-being of their patients, including hypertension, diabetes, obesity, and depression. With such competing priorities, we took a different approach in this project for improving CRCS. The National Association of Community Health Centers' (NACHC) Quality Center applied its Value Transformation Framework in nesting health center CRCS improvement efforts within a larger health systems change effort. The Value Transformation Framework (VTF) seeks to influence clinical conditions and performance metrics across the system, including CRCS rates, by providing pathways to modifying health center infrastructure, care delivery, and people systems that can lead to improved health outcomes, improved patient and staff experience, and reduced costs (Quadruple Aim).
The NACHC Quality Center developed the Value Transformation Framework for federally qualified health centers (FQHCs) and the primary care associations (PCAs) and health center controlled networks (HCCNs) that work with them. Below are descriptions of each of these organizations engaged in this project:
-
•
NACHC: The national health care organization dedicated to expanding health care access for the medically underserved through the Community Health Center model.
-
•
FQHCs: Community-based health care providers that receive funds from the Health Resources and Services Administration (HRSA) Health Center Program to provide primary care to medically underserved populations regardless of insurance status or ability to pay.
-
•
PCAs: State or regional health center membership organizations that offer training and technical assistance to safety net providers.
-
•
HCCNs: Groups of health centers working together to support and enhance the use of health information technology to improve access and quality and lower costs.
The majority of patients served by health centers live below the federal poverty level and face social and environmental risk factors that affect their ability to access health care. HRSA's Health Center Program serves nearly 1400 organizations that provide primary care services to approximately 26 million patients at over 10,000 delivery sites (HRSA, 2016). Despite the complexity of serving at-risk patients with high burdens of chronic disease, health centers provide high quality chronic care that meets or exceeds national practice standards (Goldman et al., 2012), and improves outcomes and reduces complications (Chin, 2011) at lower costs (Ku et al., 2009). Overall, health centers save the health care system $24 billion annually (Ku et al., 2009).
1.1. Multicomponent interventions to increase CRCS rates
The Community Prevention Services Task Force (CPSTF) recommends multicomponent interventions to increase CRCS (Community Prevention Services Task Force, 2016). CPSTF defines multi-component interventions as a combination of two or more interventions, each falling within one of three strategies: increasing community demand (e.g., patient reminders), increasing community access (e.g., appointment scheduling assistance), and increasing provider delivery of screening services (e.g., provider reminders). CPSTF based its recommendations on a systematic review of 56 studies (2004–2013) evaluating the effect of interventions on CRCS rates (Community Prevention Services Task Force, 2016). Other studies in safety-net practices have found improvements in CRCS rates using approaches with multiple interventions (Serra et al., 2017; Hendren et al., 2014; Green et al., 2013; Fiscella et al., 2011; Green et al., 2010).
1.2. The Value Transformation Framework
The Value Transformation Framework (Fig. 1) (NACHC, 2019), the conceptual model developed by the NACHC Quality Center, identifies and organizes evidence-based interventions to transform health center systems. These value-driven, evidence-based interventions can lead to success in achieving the “Quadruple Aim” (Institute for Healthcare Improvement, 2017):
-
1.
Improved health outcomes;
-
2.
Improved patient experiences;
-
3.
Improved staff experiences; and
-
4.
Reduced costs.
Fig. 1.
Value Transformation Framework.
The transition from a volume-driven to value-driven model requires that health centers focus on improving quality and outcomes, while reducing costs. This is difficult to do. Therefore, to support health centers that are otherwise overwhelmed by voluminous amounts of information, recommendations, and improvement efforts, we created the Value Transformation Framework to provide a succinct evidenced-based model that guides value-driven systems change. The Value Transformation Framework addresses the challenges of competing priorities by translating proven and promising research, solutions, and practices into manageable steps that health centers can apply to their Quadruple Aims efforts.
For this paper, we examined the results of nesting CRCS within a larger approach to systems-level transformation centered on the Value Transformation Framework.
1.3. The Learning Community Model
The Learning Community Model (based on the Institute for Healthcare Improvement's Breakthrough Series and HRSA's Health Disparities Collaborative) (Haggstrom et al., 2010; Kilo, 1998; Wagner et al., 2001) includes the core components of a cross-disciplinary health center team, regular project calls and training, and active coaching. The learning model for this study included an initial in-person training led by the NACHC team, supplemented by face-to-face coaching and support by state PCA staff, and project-wide virtual learning modalities including webinars, conference calls, and telephonic technical assistance and coaching. Change activities were framed within health center quality improvement (QI) efforts that include the Model for Improvement's Plan-Do-Study-Act (PDSA) approach (Langley et al., 2009), offering concrete action steps staff could readily implement. This learning community approach was structured yet flexible to meet the local health center organizational culture and approach to QI.
The project reported in this paper aimed to influence clinical and performance metrics across the health center system by applying the Value Transformation Framework—a conceptual framework that guides changes in health center infrastructure, care delivery, and people systems—in ways that can lead to improved health outcomes, improved patient and staff experience, and reduced costs (Quadruple Aim). Our hypothesis was that the Value Transformation Framework, when combined with evidence-based CRCS interventions in a collaborative learning approach, can improve the percent of health center patients who receive CRCS within guidelines.
2. Methods
We combined the Value Transformation Framework, evidence-based CRCS interventions, and the Learning Community Model to drive health center system improvements and implement evidence-based practices. The project embedded the work of cancer screening and prevention within a systems transformation approach guided by the NACHC Quality Center's Value Transformation Framework.
In 2017, serving as the project lead, NACHC selected PCAs to participate in the Cancer Transformation Project through a competitive application process. The PCAs then selected participant health centers sites (hereafter “health centers”). NACHC required that the PCA also serve as an HCCN or demonstrate a commitment to partner with a HCCN. The final selected organizations included four health centers each in Georgia and Iowa (Table 1) (6 urban, 2 rural) (U.S. Census Bureau, 2010) with the Georgia and Iowa Primary Care Associations serving as PCA/HCCN partners. Each center selected a target intervention site. The analyses and results reported in this paper pertain to the target intervention sites.
Table 1.
Profile of the 8 participating health centers (6 urban, 2 rural).
| 2016 UDS (uniform data systems) data elementa | Average | Range |
|---|---|---|
| Number of patient visits | 17,496 | 2500–38,000 |
| % Racial/ethnic minorities | 49% | 5%–77% |
| % Best served in another language | 11% | <1%–25% |
| % Uninsured | 30% | 5%–54% |
| Baseline colorectal cancer screening | 39% | 31%–56% |
Organizational-level UDS data rounded to the nearly whole % or hundreds.
This quality improvement project was submitted to the A.T. Still University (ATSU, Arizona) Institutional Review Board and deemed to not fall under the jurisdiction of the Board.
2.1. Quality improvement and transformation approach
The Cancer Transformation Project centered on a core transformation and QI approach. The project team guided all health centers through a subset of the Value Transformation Framework's Change Areas. This subset included: (1) Population Health Management (specifically Risk Stratification and Models of Care), (2) Care Management, (3) Care Teams, (4) Patient Engagement, and (5) Leadership. Additionally, project staff provided all teams with a focused set of evidence-based cancer screening interventions organized by the Framework's three domains: infrastructure, care delivery, and people.
The project team embedded evidence-based cancer screening interventions within the project Learning Community Model that included:
-
•
Multi-disciplinary teams;
-
•
Transformation coaching;
-
•
Evidence-based transformation “action steps” outlined in the Action Guides of the Value Transformation Framework; and
-
•
Iterative change processes built on Plan-Do-Study-Act (PDSA) (Langley et al., 2009).
Based on Action Guides to direct change, the project team directed health center teams to begin their work by first completing a risk stratification process to divide their patient population by risk category (Action Guide: Risk Stratification). They then defined the models of care to be delivered to each risk category (Action Guides: Models of Care). Throughout the project year, the project team guided and coached health center staff in evidenced-based action steps outlined in additional Action Guides (Care Management, Care Teams, Patient Engagement, and Leadership).
We held initial in-person project launch meetings with health center teams and PCA/HCCN QI staff in each state (January 31, 2017, Iowa; March 2, 2017, Georgia). At these meetings, the NACHC Principal Investigator (PI) presented the project model and approach and reviewed expectations and requirements. Specifically, the PI explained QI fundamentals, the Value Transformation Framework, interactive workflow mapping, clinical outcome measures for CRCS, evidence-based interventions for CRCS, population health approaches using risk stratification and models of care, and reporting requirements. The project team guided health centers in the selection of an initial set of evidence-based interventions, then coached them throughout the year on how to implement additional cancer screening and system-level interventions at their sites. Health center priorities, resources, and staffing influenced decisions related to which interventions to apply.
2.2. Study setting
Each of the 8 participating health centers identified a team of individuals to lead project efforts that included, at a minimum, a nurse care manager, a provider, and QI, health information technology (HIT), and finance representatives. Health centers received funding to support the hiring of a nurse care manager. Nurse care managers, who had varied training and experience in care management, supported project efforts. These included using population health approaches, developing a care management model, managing abnormal screening results, and coordinating referral, tracking, and follow-up. All health centers filled this role with a Registered Nurse care manager except for one health center that hired a foreign-trained medical doctor who provided patient education and care coordination similar to the role played by the RN care managers.
2.3. Intervention
Year 1 interventions took place at 8 health centers between January 2017 and December 2017. At the launch meetings, each health center team mapped current patient visit workflows and CRCS processes. They outlined possible areas of system and CRCS interventions to pursue during the intervention period. During the intervention year, all teams received core content in six transformation areas via the Value Transformation Framework's Action Guides: Population Health—Risk Stratification; Population Health—Models of Care; Care Management; Care Teams; Patient Engagement; and Leadership. NACHC also used the Value Transformation Framework to organize CRCS-specific interventions within the model's three change areas: infrastructure, care delivery, and people. Through a collaborative dialogue process, each health center selected its own final number and type of evidence-based interventions to implement. Table 4 summarizes the frequency with which 10 key CRCS evidence-based interventions were implemented across the 8 health centers.
Table 4.
Number of months each intervention was in place by site.
| Health center intervention site |
||||||||
|---|---|---|---|---|---|---|---|---|
| Type of year 1 intervention | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 1. Written policy for CRCS (formal/approved) | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 12 |
| 2. Written procedure/graphic workflow for CRCS | 12 | 1 | 1 | 1 | 11 | 4 | 8 | 12 |
| 3. Standing orders for CRCS | 12 | 12 | 2 | 0 | 0 | 0 | 0 | 12 |
| 4. Clinical champion for CRCS | 12 | 5 | 0 | 10 | 11 | 0 | 0 | 0 |
| 5. Patient outreach/recall for CRCS | 12 | 7 | 8 | 8 | 1 | 12 | 4 | 12 |
| 6. Patient incentive for CRCS | 0 | 5 | 0 | 8 | 10 | 12 | 8 | 0 |
| 7. Performance data shared at provider/team-level | 12 | 12 | 0 | 0 | 1 | 12 | 12 | 12 |
| 8. Performance data shared at site/health center- level | 12 | 12 | 12 | 12 | 5 | 12 | 12 | 12 |
| 9. Provider alert in EHR that CRCS needed | 12 | 12 | 12 | 5 | 12 | 12 | 12 | 12 |
| 10. Pre-visit chart review for CRCS | 12 | 9 | 8 | 5 | 0 | 12 | 12 | 12 |
| Mean # Interventions over 12 months | 9.2 | 6.8 | 3.8 | 4.6 | 3.5 | 6.3 | 5.5 | 9.2 |
Abbreviations: CRCS: colorectal cancer screening; EHR: electronic health record;
FOBT/FIT: fecal occult blood testing/fecal immunochemical test.
2.4. Data collection
Prior to project launch, and again at the conclusion of Year 1, we administered organizational assessments at each participating health center. To corroborate the data reported, multiple members of each health center team participated in these assessments. Before the in-person project launch sessions, we administered a pre-intervention assessment comprised of 75 items. Post-intervention, we administered the same 75-items and added 20 new items. These assessments, organized by the three Value Transformation Framework domains, captured data on organizational factors as well as interventions undertaken by the health centers. We classified interventions as ‘present’ or ‘absent’ for any given month during the project's intervention year.
We collected CRCS data using guidelines for health center federal reporting under the Uniform Data Systems (2017) (Table 2) (HRSA, 2017). While FQHCs report UDS metrics on an annual basis, participating health centers tracked and reported data to the project team monthly. The project team provided coaching and support in data validation and collection and reporting of measures. Teams also reported qualitative data on implementation of interventions via a monthly narrative report.
Table 2.
Monthly colorectal cancer screening reporting using Uniform Data System (UDS) instructions. Source: https://bphc.hrsa.gov/sites/default/files/bphc/datareporting/reporting/2017udsreportingmanual.pdf
| Measure | Measure definition | Numerator | Denominator |
|---|---|---|---|
| Colorectal cancer screening | The percentage of patients ages 50–75 years with the appropriate screening for colorectal cancer. | Number of patients with a documented CRCS based on the following criteria: Colonoscopy during the past 10 years OR Flexible sigmoidoscopy during the past 5 years OR Fecal occult blood test (FOBT), including the fecal immunochemical (FIT) test during the past 12 months. |
Number of patients 51–75 years who had at least one medical visit during the past month. Excludes patients with a diagnosis of colorectal cancer or evidence of colectomy. |
2.5. Analysis
Summary statistics are provided as means (standard deviations) and counts (percentages), as appropriate. A generalized linear mixed models approach with a negative binomial link was used to model the percent of patients who met CRCS criteria (Table 2) each month across the 1-year intervention period. Data were clustered by site, using an auto-regressive covariance matrix for month. CRCS rate per month was calculated by offsetting the count of eligible patients who met criteria each month by the count of patients who were eligible for screening. To evaluate the relationship of specific interventions with CRCS rates, data were coded as “0” = no intervention and “1” = presence of intervention for each month and each site. This variable was added to the analysis outlined above, and the main effect of “intervention present/absent” was interpreted. Each of the 10 interventions of interest was tested in a separate model. Alpha = 0.05 (two-tailed) was used as the criterion for statistical significance. No adjustments were made for multiplicity. Analyses were conducted using SPSS version 24 (IBM Corporation, Armonk, New York).
3. Results
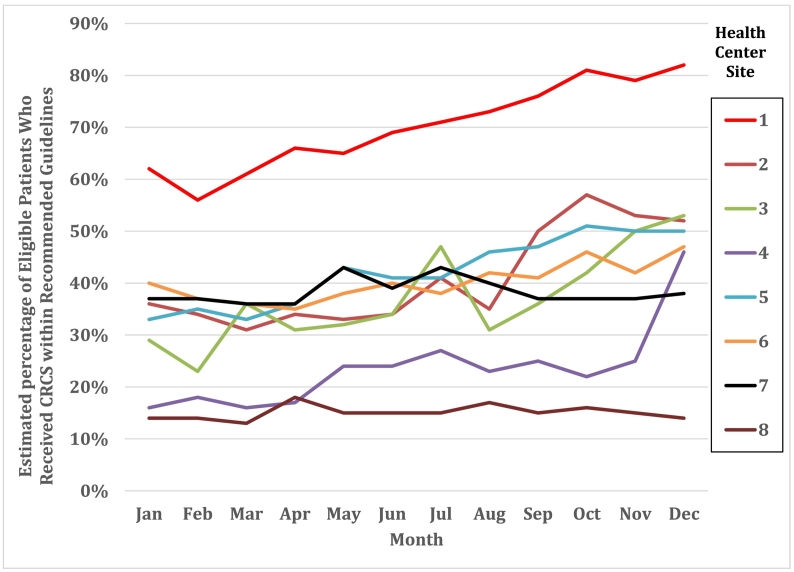
At baseline (January 2017), the percent of eligible patients who received CRCS within recommended guidelines ranged from 13.5% to 61.7%; by December 2017, the percentages ranged from 14.2% to 81.5%. Across health centers, the average raw percentage of patients who received CRCS within guidelines (95% confidence interval) in January 2017 was 33.2% (24.7, 44.6). In December 2017, the average percentage of patients up-to-date with CRCS as per UDS guidelines increased to 46.5% (35.9, 60.2). This represents an overall increase of 13.3 percentage points or a relative change of 40.1% across health centers. When weighted by monthly health center patient census, this translates into an average increase of 3.3 (95% CI: 1.7, 5.0) eligible patients screened per month, per health center, over the course of the intervention year or 317 additional patients meeting CRCS guidelines. Table 3 provides the raw percentages of CRCS for the 8 health centers during the month of program inception (January 2017), the final intervention month (December 2017), and the average for the 12-month intervention period. Fig. 2 provides the month-by-month percentage of patients who received CRCS per UDS guidelines graphically for each center.
Table 3.
Percent of patients up-to-date with CRCS for each health center, Georgia and Iowa, during January 2017, December 2017, and the 12-month intervention period.
| Health center site | January 2017 | December 2017 | 12 Months |
|---|---|---|---|
| 1 | 61.7% | 81.5% | 70.2% |
| 2 | 35.6% | 51.9% | 40.8% |
| 3 | 29.2% | 52.7% | 37.0% |
| 4 | 15.6% | 45.8% | 23.5% |
| Georgia | 35.6% | 58.0% | 42.9% |
| 5 | 33.0% | 50.0% | 42.2% |
| 6 | 39.9% | 46.6% | 40.2% |
| 7 | 37.1% | 37.7% | 38.4% |
| 8 | 13.5% | 14.2% | 15.0% |
| Iowa | 30.9% | 37.1% | 34.0% |
| Overall | 33.2% | 47.6% | 38.4% |
Fig. 2.
Percent of patients up-to-date with CRCS for each month and each health center site.
Table 4 shows the number of months that the 8 health centers implemented each of 10 key interventions. The mean number of interventions in place per health center over the 12-month intervention period ranged from 3.5 to 9.2.
Individual interventions (Table 5) that were associated with a greater percentage of patients receiving CRCS included: standing orders for CRCS, sharing of CRCS performance data at the site and/or health center level, and electronic health record (EHR) provider alerts for CRCS.
Table 5.
Census-weighted means (95% CI) for percent of eligible patients up-to-date with CRCS guidelines when interventions absent and present.
| Intervention | Absent | Present | p-value |
|---|---|---|---|
| 1. Written policy for CRCS (formal/approved) | 40.8 (32.3, 51.5) | 31.1 (22.4, 43.2) | 0.049 |
| 2. Written procedure/graphic workflow for CRCS | 36.2 (27.3, 48.2) | 41.2 (32.1, 52.9) | 0.121 |
| 3. Standing orders for CRCS | 36.5 (30.2, 44.2) | 43.4 (31.6, 59.6) | 0.026a |
| 4. Clinical champion for CRCS | 39.2 (30.4, 50.6) | 39.7 (29.8, 52.9) | 0.814 |
| 5. Patient outreach/recall for CRCS | 37.9 (29.1, 49.5) | 40.4 (31.2, 52.3) | 0.180 |
| 6. Patient incentive for CRCS | 38.5 (28.6, 51.8) | 40.7 (31.7, 52.3) | 0.412 |
| 7. Performance data shared at provider/team-level | 38.5 (30.1, 48.1) | 39.9 (30.1, 52.9) | 0.534 |
| 8. Performance data shared at site and/or organization level | 35.2 (27.6, 45.0) | 39.8 (30.5, 51.8) | <0.0001a |
| 9. Provider alert in EHR that CRCS is needed | 31.7 (25.1, 40.1) | 40.3 (31.4, 51.9) | <0.001a |
| 10. Pre-visit chart review for CRCS | 34.1 (25.7, 45.2) | 40.0 (28.7, 55.6) | 0.447 |
Significantly favor intervention.
Interventions that appeared to be associated with greater CRCS, but that did not achieve statistical significance, included written procedures or workflows for CRCS, pre-visit chart reviews for CRCS, patient outreach and recall, patient incentives, performance shared at the provider and/or team level, and having a CRCS clinical champion.
4. Discussion
Through its Value Transformation Framework, NACHC's Quality Center seeks to provide a conceptual framework to advance the Quadruple Aim goals of improved health outcomes, improved patient experience, improved staff experience, and reduced costs. This project was unique because it combined three concurrent and complementary approaches: (1) the Value Transformation Framework, (2) evidence-based CRCS interventions, and (3) the Learning Community Model to improve systems and implement evidence-based practices. Through the application of these three approaches, the project improved CRCS.
On average, the pre−/post- 1-year-intervention data showed the overall percent of health center patients who received CRCS per UDS guidelines increased by 13.3% percentage points or a 40.1% relative change over the intervention year. This is an average increase of 3.3 eligible patients screened per month per health center over the course of the intervention year, or 317 additional patients meeting CRCS guidelines.
Interventions that proved statistically significant included standing orders for CRCS, sharing of CRCS performance data at the site and/or health center level, and EHR provider alerts for CRCS. Although certain interventions that we expected to be associated with improvements did not prove statistically significant (i.e., performance feedback at the provider and team level, CRCS clinical champion, written policy for CRCS), health centers may not have had enough experience to date with these interventions to show impact. During Year 2 of the project, we will monitor and explore additional analyses on these and other factors. Although some of the improvements seen in this project might be expected due to temporal trends and other influences promoting CRCS, the improvements in CRCS rates for the specific sites where this project was implemented showed an average 13.3 percentage point increase over the 2017 year. At baseline (January 2017), the percent of eligible patients who received CRCS within recommended guidelines ranged from 13.5% to 61.7%; by December 2017, the percentages ranged from 14.2% to 81.5%. This contrasts to the national average percentage point increase of 2.1% from 2016 to 2017 (HRSA, 2017). While a statistical comparison is not advised given the marked difference in clinic venues and populations, the observed difference is worthy of note. See Fig. 2 for the range of change in CRCS among the 8 participating health centers.
These results support previous findings and recommendations for the use of multicomponent interventions to increase CRCS rates (Serra et al., 2017; Community Prevention Services Task Force, 2016; Hendren et al., 2014; Green et al., 2013; Fiscella et al., 2011; Green et al., 2010; White et al., 2017). Given the multi-faceted and complex nature of health delivery processes and systems of care, we expected that various and concurrent interventions would be needed to influence outcomes. The one aberrant observation was Site 8, which reported a large number of interventions but low levels of patients with CRCS per UDS guidelines. Site 8 had lower levels of engagement in the project. This was evidenced by less participation in project calls, incomplete reporting, and low levels of participation with coaching sessions. We suspect this site may not have applied the data validation guidance to the degree followed by the other sites and could have overestimated the number of interventions completed.
Despite the one aberrant observation, the overall findings were positive. These findings support a three-pronged approach to improving CRCS: (1) the Value Transformation Framework's evidence-based changes, (2) actionable CRCS interventions, and (3) a Learning Community Model. The results of this work guide methodological approaches to improving CRCS in health centers, with a clear method for multi-level, multi-modality quality improvement and transformation. The findings illustrate the impact of the Value Transformation Framework as part of a multi-layered approach towards improving health outcomes.
This project suggests to health center providers, care teams, and researchers what may work with respect to systems change and application of evidence-based interventions. Future research can consider assessing the degree to which organizational characteristics such as leadership support and readiness for change affect the implementation of interventions and outcomes.
4.1. Limitations
This project was limited to 8 health centers in 2 states. The results may therefore not be generalizable to other health centers, particularly those that differ in size, geography, or patient populations served. While we provided a standardized conceptual framework for systems change, each health center was able to operationalize evidence-based interventions in ways that matched their organizational culture, QI systems, and resources—therefore, the unique methods used by each health center may not be generalizable. However, the overall structure, three-pronged approach, and methods can be applied when collaborating with health centers in the future.
Health centers self-reported data. While there is a possibility of over- or under- estimating, this was managed by the process used to corroborate reported results. Further, health centers used the same methods for reporting data for this project as the methods they use to report annual required HRSA UDS measures.
We reported the numerator for the rate calculation as the number of patients with documented CRCS tests. Dividing this number by the denominator (patients seen at the health center who should have a history of CRCS or should be screened) provided an estimate of the percentage of patients seen each month who were screened for CRCS. A patient who was seen, for example, in February and again in July during the 12-month intervention period was counted twice and included in the denominator for both of those months. Caution should thus be exercised in the interpretation of these data—which represent the percentage of age-appropriate patients seen at a health center each month who had CRCS.
5. Conclusions
Despite the availability of effective screening tests for early detection and treatment, CRC remains one of the most common cancers. When not detected early, CRC is associated with high patient mortality. This project points to interventions that other health centers can adopt and adapt, which are associated with improved CRCS rates. This work suggests that a conceptual model focused on systems transformation (the Value Transformation Framework), coupled with condition-specific interventions and deployed in a Learning Community, can result in improved health outcomes.
Acknowledgments
NACHC has a Cooperative Agreement with the CDC: Grant Number: 6 NU38OT000223-04-02.
Contributor Information
Cheryl Modica, Email: cmodica@nachc.org.
Joy H. Lewis, Email: jhlewis@atsu.edu.
Curt Bay, Email: cbay@atsu.edu.
References
- Ayanian J.Z., Kohler B.A., Abe T., Epstein A.M. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N. Engl. J. Med. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. Jul 29. [DOI] [PubMed] [Google Scholar]
- Baron R.C., Melillo S., Rimer B.K. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers: a systematic review of provider reminders. Am. J. Prev. Med. 2010;38(1):110–117. doi: 10.1016/j.amepre.2009.09.031. Jan. [DOI] [PubMed] [Google Scholar]
- Brouwers M.C., De Vito C., Bahirathan L. Effective interventions to facilitate the uptake of breast, cervical and colorectal cancer screening: an implementation guideline. Implement. Sci. 2011;6:112. doi: 10.1186/1748-5908-6-112. Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers M.C., De Vito C., Bahirathan L. What implementation interventions increase cancer screening rates? A systematic review. Implement. Sci. 2011 Sep 29;6:111. doi: 10.1186/1748-5908-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin M. Quality improvement implementation and disparities: the case of the health disparities collaboratives. Med. Care. 2011;49:S65–S71. doi: 10.1097/MLR.0b013e31823ea0da. Suppl. Dec. [DOI] [PubMed] [Google Scholar]
- Community Prevention Services Task Force Increasing cancer screening multicomponent interventions. 2016. https://www.thecommunityguide.org/sites/default/files/assets/Cancer-Screening-Multicomponent-Interventions.pdf August.
- Fiscella K., Humiston S., Hendren S. A multimodal intervention to promote mammography and colorectal cancer screening in a safety-net practice. J. Natl. Med. Assoc. 2011;103(8):762–768. doi: 10.1016/s0027-9684(15)30417-x. Aug. [DOI] [PubMed] [Google Scholar]
- Goldman L.E., Chu P.W., Tran H., Romano M.J., Stafford R.S. Federally Qualified Health Centers and private practice performance on ambulatory care measures. Am. J. Prev. Med. 2012;43(2):142–149. doi: 10.1016/j.amepre.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B.B., Wang C.Y., Horner K. Systems of support to increase colorectal cancer screening and follow-up rates (SOS): design, challenges, and baseline characteristics of trial participants. Contemp Clin Trials. 2010;31(6):589–603. doi: 10.1016/j.cct.2010.07.012. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B.B., Wang C.Y., Anderson M.L. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann. Intern. Med. 2013;158(5 Pt 1):301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggstrom D.A., Clauser S.B., Taplin S.H. The health disparities cancer collaborative: a case study of practice registry measurement in a quality improvement collaborative. Implement. Sci. 2010;5:42. doi: 10.1186/1748-5908-5-42. Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Resources and Services Administration (HRSA) National Health Center Data. UDS Data Comparisons. 2017. https://bphc.hrsa.gov/uds/datacenter.aspx
- Health Resources and Services Administration (HRSA), Health Center Program National Health Center Data. 2016. https://bphc.hrsa.gov/uds/datacenter.aspx
- Hendren S., Winters P., Humiston S. Randomized, controlled trial of a multimodal intervention to improve cancer screening rates in a safety-net primary care practice. J. Gen. Intern. Med. 2014;29(1):41–49. doi: 10.1007/s11606-013-2506-1. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Resources and Services Administration (HRSA) 2018. Health Center Program, Quality of Care Measures, National Data.https://bphc.hrsa.gov/uds/datacenter.aspx?q=t6b&year=2016&state= [Google Scholar]
- Institute for Healthcare Improvement The Triple Aim or Quadruple Aim? Four points to help set your strategy. 2017. http://www.ihi.org/communities/blogs/the-triple-aim-or-the-quadruple-aim-four-points-to-help-set-your-strategy
- Joseph D.A., Redwood D., DeGroff A., Butler E.L. Use of evidence-based interventions to address disparities in colorectal cancer screening. MMWR Suppl. 2016;65(1):21–28. doi: 10.15585/mmwr.su6501a5. Feb 12. [DOI] [PubMed] [Google Scholar]
- Kilo C.M. A framework for collaborative improvement: lessons from the Institute for Healthcare Improvement's Breakthrough Series. Qual Manag Health Care. 1998;6(4):1–13. doi: 10.1097/00019514-199806040-00001. Sep. [DOI] [PubMed] [Google Scholar]
- Klabunde C.N., Cronin K.A., Breen N., Waldron W.R., Ambs A.H., Nadel M.R. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol. Biomark. Prev. 2011;20(8):1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J.R., Kanotra S., Siameh S., Jones J., Thompson B., Thomas-Cox S. Understanding barriers to colorectal cancer screening in Kentucky. Prev. Chronic Dis. 2015;12:E95. doi: 10.5888/pcd12.140586. Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku L. Geiger Gibson/RCHN Community Health Foundation at the George Washington University; 2009. Using Primary Care to Bend the Curve: Estimating the Impact of a Health Center Expansion on Health Care Costs. (Policy Research Brief No. 14). September. [Google Scholar]
- Langley G.L., Moen R., Nolan K.M., Nolan T.W., Norman C.L., Provost L.P. 2nd edition. Jossey-Bass Publishers; San Francisco: 2009. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. [Google Scholar]
- National Association of Community Health Centers . 2019. Value Transformation Framework.http://www.nachc.org/clinical-matters/value-transformation-framework/ [Google Scholar]
- Office of Disease Prevention and Health Promotion, U.S. Department of Health and Human Services Healthy People 2020. Colorectal Cancer Screening. https://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives
- Pignone M., Rich M., Teutsch S.M., Berg A.O., Lohr K.N. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. preventive services task force. Ann. Intern. Med. 2002;137(2):132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. Jul 16. [DOI] [PubMed] [Google Scholar]
- Serra Y.A., Colón-López V., Savas L.S. Using intervention mapping to develop health education components to increase colorectal cancer screening in Puerto Rico. Front in Public Health. 2017;5:324. doi: 10.3389/fpubh.2017.00324. Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbak N.M., Xu J.Y., Vena J.E. Patterns and predictors of adherence to colorectal cancer screening recommendations in Alberta's tomorrow project participants stratified by risk. BMC Public Health. 2018;18(1):177. doi: 10.1186/s12889-018-5095-4. Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Cancer Statistics Working Group . Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Atlanta (GA): 2017. United States Cancer Statistics: 1999–2014 Cancer Incidence and Mortality Web-Based Report.http://nccd.cdc.gov/uscs [Google Scholar]
- U.S. Census Bureau . Census Urban Area Criteria. 2010. Urban and Rural. 2010 Census Urban and Area Classification.https://www.census.gov/geo/reference/urban-rural.html [Google Scholar]
- U.S. Preventive Services Task Force Published final recommendations. Colorectal Cancer: screening. 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2 June.
- Uniform Data System . Health Resources and Services Administration (HRSA). Bureau of Primary Health Care; Rockville, MD: 2017. Reporting Instructions for 2017 Health Center Data (2017 UDS Manual)https://www.bphc.hrsa.gov/datareporting/reporting/2017udsreportingmanual.pdf August 31. [Google Scholar]
- Wagner E.H., Glasgow R.E., Davis C. Quality improvement in chronic illness care: a collaborative approach. Jt Comm. J. Qual. Improv. 2001;27(2):63–80. doi: 10.1016/s1070-3241(01)27007-2. Feb. [DOI] [PubMed] [Google Scholar]
- White A., Thompson T.D., White M.C. Cancer screening test use–United States, 2015. MMWR Morb. Mortal. Wkly Rep. 2017;66(8):201–206. doi: 10.15585/mmwr.mm6608a1. Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]