Highlights
-
•
Solving nervous system evolution requires cross-species comparison of neuronal types.
-
•
Neuronal types are commonly defined by their specific structure and function.
-
•
We provide an operational definition of cell types that allows evolutionary comparison.
-
•
The identity of neuronal types is best reflected by specifying transcription factors.
-
•
Families of related neuronal types are conserved across large evolutionary distances.
Abstract
Major questions in the evolution of neurons and nervous systems remain unsolved, such as the origin of the first neuron, the possible convergent evolution of neuronal phenotypes, and the transition from a relatively simple decentralized nerve net to the complex, centralized nervous systems found in modern bilaterian animals. In recent years, comparative single-cell transcriptomics has opened up new research avenues addressing these issues. Here, we review recent conceptual progress toward an evolutionary definition of cell types, and how it facilitates the identification and large-scale comparison of neuronal types and neuron type families from single-cell data — with the family of GABAergic neurons in distinct parts of the vertebrate forebrain as prime example. We also highlight strategies to infer cell type-specific innovation, so-called apomeres, from single-cell data.
Current Opinion in Neurobiology 2019, 56:144–152
This review comes from a themed issue on Neuronal identity
Edited by Sacha Nelson and Oliver Hobert
For a complete overview see the Issue and the Editorial
Available online 1st March 2019
https://doi.org/10.1016/j.conb.2019.01.022
0959-4388/© 2019 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
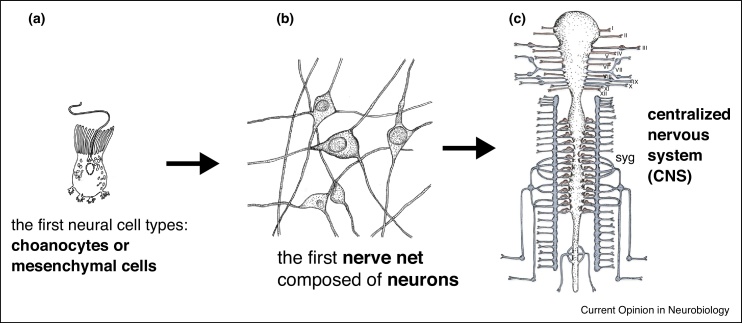
The evolution of neurons and the nervous system is one of the remaining great mysteries of animal evolution. Recent studies have addressed different aspects of nervous system evolution, from its very origins in ancestral metazoans to the acquisition of its most complex forms in extant bilaterian animals [1, 2, 3] (Figure 1). One of the most exciting questions is when and in what form the first neuron emerged, with choanocyte-like or mesenchymal protrusive cells representing possible variants (Figure 1a). Relating to this is the question whether neurons evolved only once or have different evolutionary origins — as suggested for example by the major differences in transmitter usage, synapse architecture and neuronal morphology observed in ctenophore, cnidarian and bilaterian nervous systems [4]. Independent of this, it is increasingly clear that the first manifestation of a nervous system was in the form of a nerve net (Figure 1b). Composed of distributed neurons with neurites in a mesh-like arrangement that cover large parts of the body, nerve nets are found in today’s ctenophores and in cnidarians [5, 6, 7,8•]. Comparative neurodevelopmental studies indicate homology of nerve net neurons with those of bilaterian centralized nervous systems [2,9], and the expression of transcription factors of the NK homeodomain family involved in neuroectodermal mediolateral patterning, such as Nkx2, Nkx6, Msx, and Dlx, in the cnidarian nerve net indicates that distinct subsets of nerve net neurons were incorporated into distinct parts of the centralized and peripheral systems in bilaterian evolution [10] (Figure 1c).
Figure 1.
From neural precursors to nerve net to nerve cords: evolution of the nervous system. The first neural cell types may have resembled choanocyte-like cells, reminiscent of unicellular choanoflagellates — polarized cells with an apical cilium surrounded by an apical microvillar collar, as frequently observed in today’s sensory neuronal morphologies [4]. Another candidate precursor are mesenchymal cells with cellular protrusions, resembling today’s interneuron and motoneuron morphologies. The nerve net is depicted as described for cnidarians, and the centralized nervous system is drawn to represent a prototype vertebrate system.
These hypotheses come with clear predictions of how neuronal cell types originated and diversified. For example, the hypothesis of multiple (convergent) origins of neurons predicts that neurons will not form a single, united family in the animal cell type tree, but rather belong to distinct families [11]. Likewise, the nerve net hypothesis predicts that neuronal cell types in distinct parts of the bilaterian central nervous system should relate to different elements of the nerve net still found in cnidarians and ctenophores [12]. In recent years, the promises of single-cell transcriptomics have raised expectations that the evolution of neurons, neuronal types, and, ultimately, nervous systems may be solvable by a comparative approach, via the in toto molecular characterization of cell types in a broad range of animals. In this review, we will discuss recent progress in methodology and some case studies illustrating this new approach. While most studies have focused on neuronal cell types in mammalian systems [13, 14, 15, 16, 17,18••,19], others have pioneered cell type comparison in other vertebrates [20••], chordates [21•], other bilaterians [22•,23•,24,25,26•], and several lower metazoans [27,28••].
Pivotal to testing these hypotheses is resolving what a cell type actually is. That is, what are we to identify and compare with single cell data and why? The comparison of neuronal types requires a definition of cell types that enables recognizing and comparing cell types across species and phyla. Reviewing previous efforts to conceptualize cell types in an evolutionary framework, we present an operational definition of cell types that prioritizes shared transcriptional regulatory machinery over shared functional effector genes. This is useful for detecting homologous neuronal types that have evolved phenotypic differences and also sheds light on the distinction between cell type and state. Next, we discuss how diversification of cell type regulatory mechanisms has produced hierarchical cell type families in modern animals. Using GABAergic neurons as example, we show how related neuron types can be detected from the comparison of single-cell transcriptomes. Finally, we will focus on the evolution of novelty in structure and function, with the synapse as prime example. Ultimately, new comparative single-cell datasets may enable us to pinpoint all major diversification events that occurred in the evolution of neuronal types, and how they contributed to the step-wise emergence of neural characteristics that led to the diversity of nervous systems that exist today.
Phenotypic classifications of neuronal types
Conventional classification schemes for neuronal cell types were principally focused on structure and function; that is, neuronal morphology and physiology. Most prominently, Ramón y Cajal classified neurons according to morphological criteria [29], and various physiological and structural parameters gained from recordings, pharmacology, imaging approaches, and extensive molecular parts lists have meanwhile added to such classifications, focusing for example on mammalian retina and cortex (reviewed in Refs. [30,31]). More recently, Zeng and Sanes have suggested that a neuronal cell type is “a population of neurons with properties that are homogeneous within the population but differ from those of other neurons” [31]). However, while these approaches can provide useful classifications for neuron types within one organism, they are problematic for comparing across species. In particular, phenotypic definitions fail to distinguish two key types of evolutionary changes: the phenotypic alteration of the same cell type existing in two compared species and the origination of entirely new cell types.
An evolutionary definition of cell types
To test hypotheses on neuronal origins and interrelationships, a general concept is needed of how cell types originate and evolve, and how they are related within and between species. To this end, considerable conceptual progress has been made in the past years by several authors, converging toward common solutions. Most importantly, an evolutionary definition of a cell type has emerged that allows identifying the same (homologous) cell type in different species regardless of its different morphological and physiological manifestations [20••,32, 33, 34, 35], and investigating the evolutionary emergence of new cell types.
To illustrate the value in considering evolutionary history, a view on the very beginnings of animal multicellularity is most helpful. Before cell types came into existence, the cells that constituted our unicellular ancestors used all information available in the genome, at least over their life time. At a given timepoint some genes were off and others only selectively used due to phenotypic plasticity, but the overall accessible genomic information per cell equaled the genome. Importantly, this changed in multicellular animals with the emergence of cell types. In an evolutionary process driven by division of labor and cellular specialization, animals started to partition their genomic information, so that different sets of cells had access to specific parts of the genome only, resulting in the stable expression of distinct expression programs. Mechanistically this required the evolution of new combinations of specifying transcription factors, or terminal selectors, [34] capable of mediating differential expression and implementing distinct cellular fates. In consequence, transcription factor families underwent major radiations in early metazoans [36,37•]. In extant animals, the small set of terminal selectors that direct cell type-specific expression are often found to physically interact, forming a ‘core regulatory complex’ [32], and are now widely recognized as key to cellular, and in particular neuronal identity [30,32, 33, 34,38].
The existence of a cell type-specific differentiation program is directly linked to a cell type’s ability to evolve its expression program in a specific manner. With increasing evolutionary time, cell type terminal selectors can increase the number of genes under their control, resulting in an ever greater number of cell type-specific differences — a process referred to as genetic individuation [32]. Concomitantly, expression of some effector genes will be lost, or gained anew, and coding sequences will evolve — to the effect that cellular phenotypes can vary even while the terminal selector-driven, regulatory program remains constant [32,39].
Here, we thus advocate an operational definition of a cell type as ‘a set of cells accessing the same regulatory program driving differentiation’. Following this definition, how do we recognize neural cells – sensory neurons, interneurons, motor neurons – that belong to the same type? Within a species, we would require that cells of the same type implement the same hard-wired differentiation program, using the same transcription factors, regulatory elements, microRNAs, and so on. For across species comparisons, although specific cis-regulatory elements may differ, homologous cell types will share a conserved set of specific terminal selectors. The advent of single-cell RNAseq has enabled us to efficiently and exhaustively detect the diversity of such programs within whole brain parts [13, 14, 15, 16, 17,18••,19], and entire animals [20••,21•,22•,23•,24,25,26•,27,28••]. In particular, these studies deliver close-to-complete combinations of transcription factors in an unbiased manner, including factors unique to a given type, which should be a good proxy for the distinct regulatory programs governing cellular differentiation. Likewise, these studies have unraveled elaborate lists of effector genes unique for specific cell types, reflecting the output of these programs, and thus qualify for testing hypotheses on the evolution of neuronal types as outlined above.
Cell types versus cell states
Complicating matters, however, some authors report continuous variation in expression for some genes within such defined types, with graded transcriptomic heterogeneity being present including transcription factors [40], highlighting difficulties in drawing the line between cell type and cell state. A more recent work has thus focused on the distinction of cell types versus cell states — the latter representing the various manifestations of the same type found in actual single cell data. For example, Tasic and coauthors distinguish cell types and states based on reversibility: “states (e.g., circadian changes in transcription) are reversibly accessible to a cell, while a type is a region of a multidimensional space that encompasses the states and is constant within a certain time window” [33]. Likewise, Poulin et al. define cell state “by genes that are reversibly regulated by extracellular cues or transitory stimuli, … in contrast to cell type” [30]. In line with this, continuous variation with graded transcriptomic and functional heterogeneity can be found within neuron types [40], and some neurons change through neuronal plasticity, which presents itself in a graded fashion. Both neuronal and non-neuronal types display activity-dependent transcriptional changes that differ at the cell type level [33].
The evolutionary process itself also plays a part in blurring the lines between cell state and cell type. In a particularly well-studied example, the origin of a novel type of fibroblast in the uterus of placental mammals was instigated initially by environmental variation (in this case pregnancy), followed by the evolution of a new regulatory complex that locked in its unique expression program [41••]. These findings are consistent with a scenario that a hard-wired cell type-specific differentiation program establishes a multidimensional space of possible cell states, with which cells of a given type respond to the environment, and which may serve in the future as fuel for further cell type diversification. Consequently, the cellular heterogeneity apparent from single-cell profiling is a combination of discrete and continuous variation, and represents both cell types and states [33]. Understanding the extent to which a regulatory program can vary transiently, and the types of variation that can be environmentally induced in cells expressing the same combination of terminal selectors, is a key challenge for future comparative single-cell studies. As a practical matter, we suggest that bona fide cell types should be distinguished by their capability to sustain expression autonomously, without reliance on constant external or environmental input to maintain their distinct state.
Cell type families
A key observation about cell types is that they are not equally dissimilar from each other, but rather form a transcriptional hierarchy within the organism [42]. For the nervous system, the hierarchical nature of cell type classifications has been emphasized by Tasic [33] and Zeng and Sanes [31]. The prevalence of hierarchical organization among cell type transcriptomes is perhaps surprising. For instance, why should variation across cell types be an inter-nested hierarchy when the underlying regulatory interactions that drive development and differentiation often form complex networks? We have proposed that this hierarchy of cell type expression programs results from an evolutionary diversification process in which the evolution of two distinct, hard-wired regulatory programs arise from a single precursor program [35]. The expected result of this sequential splitting of cell type expression programs is the formation of a ‘cell type family’ that is composed of evolutionarily related cell types, each of which expresses cell type-specific genes alongside gene expression shared due to their origin from a common ancestral cell type [22•].
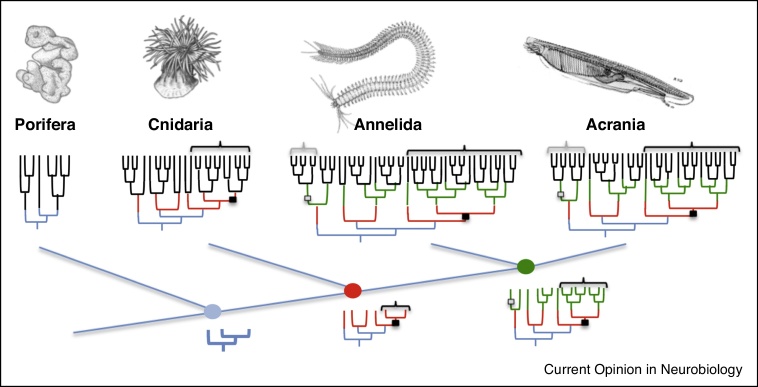
If the diversification of cell types has indeed occurred in a hierarchical manner in animal evolution, the fascinating hypothesis emerges that cell type families should be shared between animal groups; and the most ancient and biggest cell type families should be shared between all animals, provided that the respective split of regulatory programs predated the last common metazoan ancestor. In a hypothetical scheme, Figure 2 illustrates the progressive diversification of cell types in the lines of evolution leading to extant animal phyla. In this scheme, the earliest diversifications (blue lines) predated the last common metazoan ancestor, leading to cell type families that should be shared between all animals. Other diversifications predated the cnidarian-bilaterian ancestor (red lines), producing additional cell type families that should be shared between cnidarians and bilaterians, and so on. Importantly, this scheme predicts that at the higher family level, evolutionary cell type trees of different species should be largely congruent, even at the phylum level. That means, one could use the cell type family tree of one group to validate that of others, until finally a consensus picture of cell type evolution in animals will emerge.
Figure 2.
Hypothetical scheme illustrating cell type diversification in animal evolution. For each tree, the cell type branching events that had already occurred in a given ancestor are drawn in the color of that ancestor. Older cell type families are shared between remote phyla, whereas younger families are phylum-specific, or specific to smaller taxonomic units. The birth of the first neuron is indicated by a black box and the family of neurons demarcated by brackets, the (hypothetical) emergence of a second, neuron-like cell type family in stem bilaterians by grey box and brackets. For simplicity, other possible modes of cell type evolution such as fusion are not represented here.
Branching events in the cell type tree represent the birth of sister cell types, accompanied by cellular innovations (gains, modifications, or losses of structure/function, see below) that make them distinct. For example, we can map the birth of the first neuron onto such a hypothetical tree — which later diversified into a family of neuron types that exists in cnidarians and bilaterians (black box and brackets in Figure 2). This family might comprise, for example, the interneurons and motor neurons of the bilaterian trunk. Our hypothetical tree also illustrates that, at least in most cases, individual neuronal cell types should often be species-specific and unlikely to be specifically shared between two compared species. True 1:1 homologies of cell types will only apply if no further diversification events have occurred since the species split. In most cases, homology will be apparent at the family level (colored lines in the figure). This has practical implications for comparative datasets, as comparisons between species must be made not only at the level of individual cell types, but among all levels of the cell type hierarchy.
Building evolutionary cell type trees
The construction and interpretation of evolutionary cell type trees has received growing attention [42, 43, 44, 45]. Taking into account these studies, there are several important points to consider when building cell type trees from single-cell data. First, tree reconstruction should be performed on metacells, rather than on individual cell transcriptomes. The use of metacells, representing small sets of highly similar individual cells, reduces the noise inherent in sparse single-cell data, and helps alleviate problems with tree reconstruction that would occur with very large datasets. Second, tree construction programs should be used that explicitly model the evolution process or which minimize artifacts, such as maximum likelihood or parsimony-based methods for qualitative expression data, or the neighbor-joining method for quantitative expression data. These methods can and should be used with tools such as bootstrapping, to determine statistical support for cell type clades. Third, clusters that do not represent differentiated cell types, such as transitional multipotent progenitor states, should be excluded to ensure that tree-building relies on evolutionary and not on developmental signal [46]. Mixing developmental states into hierarchical clustering can cloud evolutionary relationships inferred between differentiation programs. On the one hand, the same developmental precursor can give rise to evolutionarily distinct cell types, as observed in the retina [32]. On the other hand, cell types that are evolutionarily closely related (as shown by overall similarity of expression and transcriptional regulators), often arise from distant developmental precursors. This is observed for example for vertebrate osteocytes, arising from the ectodermal neural crest or from the mesodermal lineage — as well as for the vertebrate retina [32]. Fourth, beyond development, other non-evolutionary components also shape the expression profile of cell types and likewise generate conflicting clustering. For example, cellular states such as stress response may preferentially occur in some cells and not in others; conflicting signal may also arise from shared organ-specific adaptations; or from the co-option of effector gene cassettes by otherwise unrelated cell types. Approaches designed to dissect expression variation into distinct components, such as independent component analysis and weighted correlation network analysis, are valuable for identifying both hierarchical and non-hierarchical expression. Finally, it can be preferable to limit tree building to the differential expression of transcription factors, as best proxy for the ‘regulatory program driving differentiation’ that underlies the evolutionary individuation of cell types (see above).
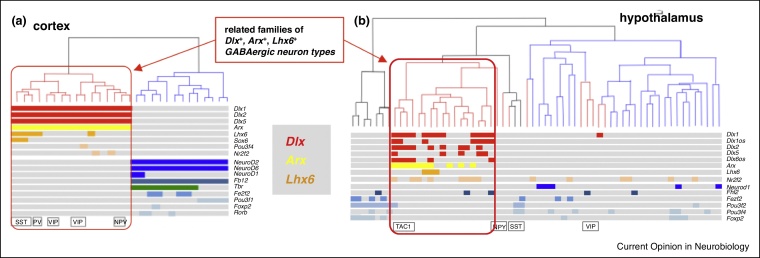
Illustrating the concept of tree building and cell type families, the multitude of neural cell types identified for cortex, striatum and hypothalamus have been found to assemble into superclusters [13, 14, 15,19,47]. Importantly, and in line with the above evolutionary definition of cell types, specific combinations of transcription factors characterize each of these candidate cell type families, and can be used for cross-comparison. One important example points to the families of GABAergic neurons detected both in telencephalic cortex [15,19] and in diencephalic hypothalamus [14] (Figure 3). These families specifically express the transcription factors Dlx1,2,-5 and Arx (as opposed to other types of GABAergic neurons specified by Gata and Tal transcription factors, which are found in the midbrain [48], hindbrain and spinal cord). Corroborating this, Zeisel et al. [18••] examined and compared neuron types across the entire mouse nervous system, and validated the specific presence of Dlx+, Arx+ GABAergic neuron type families in cortex, thalamus and hypothalamus. This work highlights the unique regulatory profile of Dlx+, Arx+ GABAergic neurons compared to all other neurons, which also comprises Lhx6 and Six3 transcription factors [18••]. The shared regulatory profile of GABAergic neurons in cortex and hypothalamus suggests that the common origin of these related families predated the evolutionary divergence of diencephalon and telencephalon in the vertebrate forebrain. In future work, this hypothesis can be tested via the construction of rigorous cell type trees following the guidelines above, limited for example to transcription factor expression.
Figure 3.
GABA-ergic cell types express Dlx, Arx and Lhx6 transcription factors: (a) in the mouse cortex [15] and (b) in hypothalamus [14]. Red branches in the cell type trees indicate GABAergic neurons; blue branches indicate glutamatergic neurons. Transcription factor expression in the hypothalamus has been plotted by us according to Supplementary Table 6 in Ref. [14].
Importantly, to uncover truly specific combinations of cell type- or family gene expression, and to build comprehensive cell type trees, it is necessary to sequence and compare cells for entire specimens — referred to as whole-body scRNA-seq. We and others have pioneered whole-body scRNA-seq, for L2 larval nematode [25], 2dpf Platynereis larvae [22•], the sea anemone Nematostella [28••] and other basal metazoans [27], and the planarian Schmidtea [23•,24]. Only in the whole-body context can cell type and cell type family-specific combinations of transcription factors and effectors be comprehensively determined, and trees be built and compared as schematized in Figure 2. With the current state-of-the-art single-cell technology, this is currently possible for various smaller invertebrates, but remains a challenge for insects and, in particular, vertebrates, in which cells count in millions or billions at differentiation stages [49].
Famous apomeres: nematocysts, opsins, and synapses
If it is not for primary classification — what is the significance of cellular structure and function in the context of an evolutionary cell type definition? Here, an entirely new perspective emerges, which focuses on the structural/functional innovation characteristic for a given cell type. Once a new cell type comes into existence, its new and unique regulatory program will support the differential expression of cell type-specific genes. This will make it phenotypically distinct from other cell types, and, if advantageous, guarantee the cell type’s evolutionary survival. In this process, the de novo acquisition and modification of existing cell type-specific effector genes is driving functional divergence. Such new cell type-specific modules, or new variants of modules, representing structural/functional innovation, have been termed apomeres (analogous to apomorphies at the species level) [32].
Apomeres can arise in manifold ways. New structure/function often emerges via the birth of new proteins, triggering neofunctionalization. For this, the evolution of the cnidocytes in cnidarians is a prime example, with thousands of novel proteins setting up the stinging organelle [50]. Cnidocytes might be sister to sensory cells, and their genetic individuation appears to have started with the birth of the paired-type homeodomain duplicate PaxA, and of Mef2 and Jun duplicates [28••,51,52], as a part of their specific regulatory differentiation program. Not surprisingly, cnidocytes appear genetically highly individuated when compared to other cell types [28••]. The invention of the light-sensitive opsin, by covalently linking the retinal chromophore to the protein through a Schiff base, represents another striking innovation that occurred in basal Metazoa [53]. In this case, the Schiff base transformed a pre-existing G-protein-coupled retinal receptor into the first opsin, enabling novel functionality. Interestingly, however, the cell type that first benefitted from this apomere has not yet been identified – but single-cell transcriptome data for placozoans – a basal metazoan group that possesses the pre-existing G-protein-coupled retinal receptor but no bona fide opsin — may contribute to solving this issue [27].
Another important apomere for nervous system evolution is the occurrence of the first synapse. This event must have been tightly linked to the evolution of the first neuron [11,32]; yet, so far remains entirely enigmatic. Neither is it clear what exactly the innovation was, nor in what cell types it initially took place and contributed to their individuation. From the data available, we can infer that most components of the presynapse and postsynapse predated metazoans and exist in sponges and placozoans devoid of nervous systems [1,54]. Yet, sponges, unlike bilaterians, do not co-regulate synaptic gene expression [55], and synaptic proteins do not appear to interact with cell junction machinery [56], indicating that the synaptic apomere might have arisen by new combinations of effector genes that were not co-expressed before. Again, in depth single-cell transciptomics of sponges and placozoans should enable us to identify the cell types expressing presynaptic and/or postsynaptic proteins, and subsequent subcellular localization of specifically expressed, presynaptic and postsynaptic proteins may reveal synapse-like organelles in candidate protoneurons.
Taken together, the quest for apomeres, or cell type-specific innovation, has only just begun, and represents one of the most exciting follow-ups now possible with the manifold comparative single-cell transcriptome datasets. Several remarks appear warranted. First, whole-body single-cell sequencing, as explained above, besides its benefits for detecting cell type-specific regulatory programs, is also pivotal for identifying apomeres, as effector genes are compared across entire bodies and their specificity more easily detectable. Second, there is an urgent need to establish cellular localization protocols for these species, via antibodies or via tagged proteins. In combination with mass spectroscopy, this will allow investigating the assembly of cell-type specific proteins into macromolecular complexes, their subcellular localization within complexes, and function. Third, there are cases where much of the cellular innovation will involve loss rather than gain of structure/function, triggering division of labor, or subfunctionalization, across sister cell types [35]. Division of labor must be prevalent, as indicated by the surprisingly rich protein repertoire of choanoflagellates, our unicellular cousins, that is shared with Metazoa [57].
Conclusions
To unravel the real, historic tree of neuronal types, we require comparative data that must be informative about (1) the distinct complements of cell types in today’s animal phyla; (2) how they assemble into cell type families and how these families are interrelated; (3) the regulatory changes underlying the birth of new sister cell types; and (4), the cellular innovations characteristic for diversifying cell type families. Comparative single-cell sequencing data, in conjunction with experimental validation via functional genetics and imaging approaches, satisfy these demands and will open up a new understanding of nervous system evolution. For selected families, family composition and key innovations have already been partially established, for example, for cnidocytes or for ciliary photoreceptors, which first required integrating ciliary opsin with guanylyl cyclase-based signaling in the ciliary membrane [58]. However, the functional and regulatory innovations for the first and most subsequently diverging neuron type families remain obscure. While it is plausible that the synapse was a key innovation in the first neurons, it is not clear in which cells it first evolved, what components were initially necessary, and in what order other presynaptic or postsynaptic components such as transmitter-synthesizing enzymes, receptors, or matrix molecules were added in the bilaterian lineage. The key to resolving these debates requires a clear definition of cell types that distinguishes phenotypic evolution from the origination of new cell types. Only with this in hand can the centralization, cell type diversification, and other key innovations, of animal nervous systems be fully understood.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank all members of the Arendt lab for enthusiastic discussions and support. The work was supported by the European Research Council ‘NeuralCellTypeEvo’ Advanced grant (DA, PYB, JM), the European Molecular Biology Laboratory (KA).
References
- 1.Burkhardt P., Gronborg M., McDonald K., Sulur T., Wang Q., King N. Evolutionary insights into premetazoan functions of the neuronal protein homer. Mol Biol Evol. 2014;31:2342–2355. doi: 10.1093/molbev/msu178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendt D., Tosches M.A., Marlow H. From nerve net to nerve ring, nerve cord and brain - evolution of the nervous system. Nat Rev Neurosci. 2015;17:61–72. doi: 10.1038/nrn.2015.15. [DOI] [PubMed] [Google Scholar]
- 3.Liebeskind B.J., Hillis D.M., Zakon H.H., Hofmann H.A. Complex homology and the evolution of nervous systems. Trends Ecol Evol. 2016;31:127–135. doi: 10.1016/j.tree.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moroz L.L., Kocot K.M., Citarella M.R., Dosung S., Norekian T.P., Povolotskaya I.S., Grigorenko A.P., Dailey C., Berezikov E., Buckley K.M. The ctenophore genome and the evolutionary origins of neural systems. Nature. 2014;510:109–114. doi: 10.1038/nature13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jager M., Chiori R., Alie A., Dayraud C., Queinnec E., Manuel M. New insights on ctenophore neural anatomy: immunofluorescence study in Pleurobrachia pileus (Muller, 1776) J Exp Zool B Mol Dev Evol. 2010;316B:171–187. doi: 10.1002/jez.b.21386. [DOI] [PubMed] [Google Scholar]
- 6.Arendt D., Benito-Gutierrez E., Brunet T., Marlow H. Gastric pouches and the mucociliary sole: setting the stage for nervous system evolution. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2015.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch T.C., Klimovich A., Domazet-Loso T., Grunder S., Holstein T.W., Jekely G., Miller D.J., Murillo-Rincon A.P., Rentzsch F., Richards G.S. Back to the basics: cnidarians start to fire. Trends Neurosci. 2017;40:92–105. doi: 10.1016/j.tins.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Dupre C., Yuste R. Non-overlapping neural networks in hydra vulgaris. Curr Biol. 2017;27:1085–1097. doi: 10.1016/j.cub.2017.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first calcium imaging activity recording of selected neurons in a cnidarian opens up an entirely new perspective on the functioning of a nerve net.
- 9.Richards G.S., Rentzsch F. Regulation of nematostella neural progenitors by SoxB, Notch and bHLH genes. Development. 2015;142:3332–3342. doi: 10.1242/dev.123745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arendt D. Animal evolution: convergent nerve cords? Curr Biol. 2018;28:R225–R227. doi: 10.1016/j.cub.2018.01.056. [DOI] [PubMed] [Google Scholar]
- 11.Moroz L.L. Convergent evolution of neural systems in ctenophores. J Exp Biol. 2015;218:598–611. doi: 10.1242/jeb.110692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tosches M.A., Arendt D. The bilaterian forebrain: an evolutionary chimera. Curr Opin Neurobiol. 2013;23:1080–1089. doi: 10.1016/j.conb.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 13.La Manno G., Gyllborg D., Codeluppi S., Nishimura K., Salto C., Zeisel A., Borm L.E., Stott S.R., Toledo E.M., Villaescusa J.C. Molecular diversity of midbrain development in mouse, human, and stem cells. Cell. 2016;167:566–580. doi: 10.1016/j.cell.2016.09.027. e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romanov R.A., Zeisel A., Bakker J., Girach F., Hellysaz A., Tomer R., Alpar A., Mulder J., Clotman F., Keimpema E. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci. 2017;20:176–188. doi: 10.1038/nn.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeisel A., Munoz-Manchado A.B., Codeluppi S., Lonnerberg P., La Manno G., Jureus A., Marques S., Munguba H., He L., Betsholtz C. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 16.Haring M., Zeisel A., Hochgerner H., Rinwa P., Jakobsson J.E.T., Lonnerberg P., La Manno G., Sharma N., Borgius L., Kiehn O. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci. 2018;21:869–880. doi: 10.1038/s41593-018-0141-1. [DOI] [PubMed] [Google Scholar]
- 17.Munoz-Manchado A.B., Bengtsson Gonzales C., Zeisel A., Munguba H., Bekkouche B., Skene N.G., Lonnerberg P., Ryge J., Harris K.D., Linnarsson S. Diversity of interneurons in the dorsal striatum revealed by single-cell RNA sequencing and PatchSeq. Cell Rep. 2018;24:2179–2190. doi: 10.1016/j.celrep.2018.07.053. e2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Zeisel A., Hochgerner H., Lonnerberg P., Johnsson A., Memic F., van der Zwan J., Haring M., Braun E., Borm L.E., La Manno G. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014. doi: 10.1016/j.cell.2018.06.021. e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first comprehensive single-cell transcriptomics study for the entire mouse central nervous system with several cell type trees, as an entry into working out the interrelationships of vertebrate neuronal families.
- 19.Tasic B., Menon V., Nguyen T.N., Kim T.K., Jarsky T., Yao Z., Levi B., Gray L.T., Sorensen S.A., Dolbeare T. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19:335–346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Tosches M.A., Yamawaki T.M., Naumann R.K., Jacobi A.A., Tushev G., Laurent G. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science. 2018;360:881–888. doi: 10.1126/science.aar4237. [DOI] [PubMed] [Google Scholar]; A pioneer comparative single-cell transcriptome study elucidating interrelationships between cortical neuron types in mice and reptiles, challenging previous hypotheses on cortex evolution. This study also introduces new methodology for comparing cell types across distantly related species.
- 21•.Horie R., Hazbun A., Chen K., Cao C., Levine M., Horie T. Shared evolutionary origin of vertebrate neural crest and cranial placodes. Nature. 2018;560:228–232. doi: 10.1038/s41586-018-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; A fascinating new perspective on neural crest and placode evolution using a combination of lineage tracing, gene disruption, and single-cell RNA-sequencing.
- 22•.Achim K., Eling N., Vergara H.M., Bertucci P., Musser J.M., Vopalensky P., Brunet T., Collier P., Benes V., Marioni J.C. Whole-body single-cell sequencing reveals transcriptional domains in the annelid larval body. Mol Biol Evol. 2018;35:1047–1062. doi: 10.1093/molbev/msx336. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pioneer whole-body single-cell transcriptome study for an entire annelid larva. Dissects spatial organization of transcriptional domains in an entire annelid larva, showing that cell type families do not always align with apparent morphological boundaries.
- 23•.Fincher C.T., Wurtzel O., de Hoog T., Kravarik K.M., Reddien P.W. Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science. 2018;360 doi: 10.1126/science.aaq1736. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pioneer whole-body single-cell transcriptome study for an entire planarian specimen delivers a comprehensive account of cell type and cell type families, as a starting point for exploring spiralian cell type evolution.
- 24.Plass M., Solana J., Wolf F.A., Ayoub S., Misios A., Glazar P., Obermayer B., Theis F.J., Kocks C., Rajewsky N. Cell type atlas and lineage tree of a whole complex animal by single-cell transcriptomics. Science. 2018;360 doi: 10.1126/science.aaq1723. [DOI] [PubMed] [Google Scholar]
- 25.Cao J., Packer J.S., Ramani V., Cusanovich D.A., Huynh C., Daza R., Qiu X., Lee C., Furlan S.N., Steemers F.J. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science. 2017;357:661–667. doi: 10.1126/science.aam8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Konstantinides N., Kapuralin K., Fadil C., Barboza L., Satija R., Desplan C. Phenotypic convergence: distinct transcription factors regulate common terminal features. Cell. 2018;174:622–635. doi: 10.1016/j.cell.2018.05.021. e613. [DOI] [PMC free article] [PubMed] [Google Scholar]; Single-cell transcriptomics of the Drosophila optic lobe unravel distinct identities of glutamatergic neuron types, unraveling convergent evolution toward the same transmitter system.
- 27.Sebe-Pedros A., Chomsky E., Pang K., Lara-Astiaso D., Gaiti F., Mukamel Z., Amit I., Hejnol A., Degnan B.M., Tanay A. Early metazoan cell type diversity and the evolution of multicellular gene regulation. Nat Ecol Evol. 2018;2:1176–1188. doi: 10.1038/s41559-018-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Sebe-Pedros A., Saudemont B., Chomsky E., Plessier F., Mailhe M.P., Renno J., Loe-Mie Y., Lifshitz A., Mukamel Z., Schmutz S. Cnidarian cell type diversity and regulation revealed by whole-organism single-cell RNA-Seq. Cell. 2018;173:1520–1534. doi: 10.1016/j.cell.2018.05.019. e1520. [DOI] [PubMed] [Google Scholar]; A whole-body atlas of cell types in the cnidarian Nematostella that reveals complex homology relationships between neuron types across metazoa.
- 29.Ramón y Cajal S. Maloine, A.; Paris: 1909. Histologie Du Système Nerveux de L’homme & Des Vertébrés. [Google Scholar]
- 30.Poulin J.F., Tasic B., Hjerling-Leffler J., Trimarchi J.M., Awatramani R. Disentangling neural cell diversity using single-cell transcriptomics. Nat Neurosci. 2016;19:1131–1141. doi: 10.1038/nn.4366. [DOI] [PubMed] [Google Scholar]
- 31.Zeng H., Sanes J.R. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci. 2017;18:530–546. doi: 10.1038/nrn.2017.85. [DOI] [PubMed] [Google Scholar]
- 32.Arendt D., Musser J.M., Baker C.V., Bergman A., Cepko C.L., Erwin D.H., Pavlicev M., Schlosser G., Widder S., Laubichler M. Evolution of sister cell types by individuation. Nat Rev Genet. 2016;17:744–757. doi: 10.1038/nrg.2016.127. [DOI] [PubMed] [Google Scholar]
- 33.Tasic B. Single cell transcriptomics in neuroscience: cell classification and beyond. Curr Opin Neurobiol. 2018;50:242–249. doi: 10.1016/j.conb.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Hobert O. Terminal selectors of neuronal identity. Curr Top Dev Biol. 2016;116:455–475. doi: 10.1016/bs.ctdb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Arendt D. The evolution of cell types in animals: emerging principles from molecular studies. Nat Rev Genet. 2008;9:868–882. doi: 10.1038/nrg2416. [DOI] [PubMed] [Google Scholar]
- 36.Paps J., Holland P.W.H. Reconstruction of the ancestral metazoan genome reveals an increase in genomic novelty. Nat Commun. 2018;9 doi: 10.1038/s41467-018-04136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Richter D.J., Fozouni P., Eisen M.B., King N. Gene family innovation, conservation and loss on the animal stem lineage. eLife. 2018;7 doi: 10.7554/eLife.34226. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides a detailed portrait of gene gain and loss in early animals, finding that origination of many transcriptional regulatory and signaling gene families occurred in the first animals.
- 38.Paul A., Crow M., Raudales R., He M., Gillis J., Huang Z.J. Transcriptional architecture of synaptic communication delineates GABAergic neuron identity. Cell. 2017;171:522–539. doi: 10.1016/j.cell.2017.08.032. e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serrano-Saiz E., Poole R.J., Felton T., Zhang F., De La Cruz E.D., Hobert O. Modular control of glutamatergic neuronal identity in C. elegans by distinct homeodomain proteins. Cell. 2015;155:659–673. doi: 10.1016/j.cell.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cembrowski M.S., Menon V. Continuous variation within cell types of the nervous system. Trends Neurosci. 2018;41:337–348. doi: 10.1016/j.tins.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 41••.Erkenbrack E.M., Maziarz J.D., Griffith O.W., Liang C., Chavan A.R., Nnamani M.C., Wagner G.P. The mammalian decidual cell evolved from a cellular stress response. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2005594. [DOI] [PMC free article] [PubMed] [Google Scholar]; An investigation of the decidual stromal cell, a novel fibroblast cell type in placental mammals, finds it arose by rewiring an ancestral stress response, highlighting how transitory cell states can pave the way for new cell types in evolution.
- 42.the FANTOM Consortium. Liang C., Forrest A.R., Wagner G.P. The statistical geometry of transcriptome divergence in cell-type evolution and cancer. Nat Commun. 2015;6 doi: 10.1038/ncomms7066. [DOI] [PubMed] [Google Scholar]
- 43.Kin K. Inferring cell type innovations by phylogenetic methods-concepts, methods, and limitations. J Exp Zool B Mol Dev Evol. 2015;324:653–661. doi: 10.1002/jez.b.22657. [DOI] [PubMed] [Google Scholar]
- 44.Kin K., Nnamani M.C., Lynch V.J., Michaelides E., Wagner G.P. Cell-type phylogenetics and the origin of endometrial stromal cells. Cell Rep. 2015;10:1398–1409. doi: 10.1016/j.celrep.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 45.Liang C., Musser J.M., Cloutier A., Prum R.O., Wagner G.P. Pervasive correlated evolution in gene expression shapes cell and tissue type transcriptomes. Genome Biol Evol. 2018;10:538–552. doi: 10.1093/gbe/evy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marioni J.C., Arendt D. How single-cell genomics is changing evolutionary and developmental biology. Annu Rev Cell Dev Biol. 2017;33:23.1–23.17. doi: 10.1146/annurev-cellbio-100616-060818. [DOI] [PubMed] [Google Scholar]
- 47.Kee N., Volakakis N., Kirkeby A., Dahl L., Storvall H., Nolbrant S., Lahti L., Bjorklund A.K., Gillberg L., Joodmardi E. Single-cell analysis reveals a close relationship between differentiating dopamine and subthalamic nucleus neuronal lineages. Cell Stem Cell. 2017;20:29–40. doi: 10.1016/j.stem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Achim K., Peltopuro P., Lahti L., Tsai H.H., Zachariah A., Astrand M., Salminen M., Rowitch D., Partanen J. The role of Tal2 and Tal1 in the differentiation of midbrain GABAergic neuron precursors. Biol Open. 2013;2:990–997. doi: 10.1242/bio.20135041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briggs J.A., Weinreb C., Wagner D.E., Megason S., Peshkin L., Kirschner M.W., Klein A.M. The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution. Science. 2018;360 doi: 10.1126/science.aar5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beckmann A., Xiao S., Muller J.P., Mercadante D., Nuchter T., Kroger N., Langhojer F., Petrich W., Holstein T.W., Benoit M. A fast recoiling silk-like elastomer facilitates nanosecond nematocyst discharge. BMC Biol. 2015;13:3. doi: 10.1186/s12915-014-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Babonis L.S., Martindale M.Q. PaxA, but not PaxC, is required for cnidocyte development in the sea anemone Nematostella vectensis. Evodevo. 2017;8 doi: 10.1186/s13227-017-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sunagar K., Columbus-Shenkar Y.Y., Fridrich A., Gutkovich N., Aharoni R., Moran Y. Cell type-specific expression profiling unravels the development and evolution of stinging cells in sea anemone. BMC Biol. 2018;16 doi: 10.1186/s12915-018-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feuda R., Hamilton S.C., McInerney J.O., Pisani D. Metazoan opsin evolution reveals a simple route to animal vision. Proc Natl Acad Sci U S A. 2012;109:18868–18872. doi: 10.1073/pnas.1204609109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burkhardt P. The origin and evolution of synaptic proteins - choanoflagellates lead the way. J Exp Biol. 2015;218:506–514. doi: 10.1242/jeb.110247. [DOI] [PubMed] [Google Scholar]
- 55.Conaco C., Bassett D.S., Zhou H., Arcila M.L., Degnan S.M., Degnan B.M., Kosik K.S. Functionalization of a protosynaptic gene expression network. Proc Natl Acad Sci U S A. 2012;109(Suppl. 1):10612–10618. doi: 10.1073/pnas.1201890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schippers K.J., Nichols S.A. Evidence of signaling and adhesion roles for beta-catenin in the sponge ephydatia muelleri. Mol Biol Evol. 2018;35:1407–1421. doi: 10.1093/molbev/msy033. [DOI] [PubMed] [Google Scholar]
- 57.King N., Westbrook M.J., Young S.L., Kuo A., Abedin M., Chapman J., Fairclough S., Hellsten U., Isogai Y., Letunic I. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamb T.D., Arendt D., Collin S.P. The evolution of phototransduction and eyes. Philos Trans R Soc Lond B Biol Sci. 2009;364:2791–2793. doi: 10.1098/rstb.2009.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]