Abstract
Introduction: Curcuma wenyujin is a plant which belongs to the family of Zingiberaceae, found in South Asia and China. C. wenyujin is a major constituent in Chinese traditional medicine and is used to treat liver diseases, blood clots, and is also prescribed as a painkiller. C. wenyujin possesses antioxidant, antiproliferative, and antitumorogenic properties, and many researchers have proved the efficacy of C. wenyujin against various types of cancer. The major drawback of this historical drug is it's low bioavailability.
Methods: This study synthesized gold nanoparticles using C. wenyujin and assessed its potency against in vitro renal cancer cells. The biosynthesized C. wenyujin gold nanoparticles (CWAuNPs) were characterized using UV-Spec, DLS, FTIR, SAED, TEM, EDAX, and Atomic Force analysis. The cytotoxicity of CWAuNPs against renal cancer cell lines A498 and SW-156 was assessed with MTT assay. The induction of apoptosis by CWAuNPs in A498 cell was measured using apoptotic staining DAPI, Rhodamine 123, and H2DCFDA. The apoptotic activity of CWAuNPs was further confirmed with flow cytometric analysis. The molecular mechanism of CWAuNPs was analyzed with qPCR and immunoblotting analysis of caspases, proapoptotic, and antiapoptotic proteins.
Results: The characterization of results of synthesized CWAuNPs satisfy the distinctive properties of a potent nanodrug. The results of apoptotic staining techniques confirm the induction of CWAuNPs in A498 by increasing the apoptotic Caspase 3,9, Bid, and Bad, and decreasing the antiapoptotic protein Bcl-2, Bcl-xl expressions, which is authentically proven by the qPCR and immunoblotting analysis.
Conclusion: In conclusion, these results confirmed that biosynthesized CWAuNPs is a potent anticancer agent which induces apoptosis in the A498 renal carcinoma cell line.
Keywords: Curcuma wenyujin, CWAuNPs, renal cancer, A498, anticancer agent, apoptosis
Introduction
One of the most debilitating health problems of today’s human population is cancer, which ranks as the second cause of death worldwide.1,2 Pharmaceutical industries are effectively working on developing a potent cost-effective anticancer drug with fewer side-effects. Between 2005 and 2018, over 1,100 anticancer drugs were discovered, most of which are in clinical trials, and a few drugs were waiting for the approval of the Food and Drug administration. The average amount to be spent for cancer therapies and supportive care increased to 133 billion dollars in 2017.3 Anticancer drugs mostly target the growth factors, tumor suppressor proteins, apoptotic proteins, and transcription factors.4 Even though the anticancer drugs in the market effectively inhibit the cancer progression, the recurrence of cancer and the side-effects induced by these drugs are inevitable. This decreases the quality-of-life of the cancer-surviving patients. Therefore, it is necessary to discover a herbal-based drug which is a potent anticancer drug with little or no side-effects, which is at the same time cost-effective.
Native medicines or traditional folk medicines play a vital role in treating various chronic diseases: asthma, arthritis, cancer, diabetes, dementia, etc. Globally, traditional medicines are classified as Ayurvedic therapy, Arab therapy, Chinese therapy, and Western therapy.5 Using folk medicinal herbs in cancer treatment has proven to be efficient. Hence, in the present study, we analyzed the efficacy of one such drug, Curcuma wenyujin, a Chinese herb which belongs to the Zingiberaceae family. Ezhu is one of the traditional Chinese medicines to treat various diseases such as liver disorder, blood clots, pain, arthiritis, etc.6 Curcuma wenyujin is one of the ingredients in Ezhu. It possesses anti-inflammatory, antimicrobial, antifungal, anticancer, antiarthiritic, and anitproliferative properties.7,8 Anticancer activity of C. wenyujin against various types of cancers such as gastric, prostate, ovary, and uterine has been proven,9,10 but the efficacy of the drug is less than what is expected due to its low bioavailability. Hence, in this present study, we biosynthesized gold nanoparticle using C. wenyujin and analyzed its anticancer potential.
The usage of nanomaterials in the field of medicine is increasing by the day, but the materials used for synthesis of nanomaterials should be taken into consideration. Nanoparticles synthesized by physical and chemical methods, are either less potent or causes toxic side-effect, hence using ecofriendly substances in the synthesis of nanoparticles is more biologically significant. Among all nanomaterials, gold nanoparticles are more potent due to their remarkable plasmonresonant optical property and bioconjugation property with biomolecular probes11,12 and, as a result, they are used to treat various diseases in traditional medicine. In this present study, we synthesized gold nanoparticles using green technology with C. wenyujin, a medicinal herb.
The major pathway in which cancer develops is by apoptosis. Apoptosis the programmed cell death and it is regulated by various apoptotic and anti-apoptotic protein. Deregulation in the apoptotic pathway is induced by the imbalance in oxidant and antioxidants levels.13 Hence, in this present study, we biosynthesized ecofriendly gold nanoparticles with the traditional Chinese medicinal herb C. weyujin and characterized their morphology, size, nature, and functional groups using various techniques. Then, we investigated the anticancer potential of synthesized gold nanoparticles in vitro using renal cancer cell lines.
Materials and methods
Chloroauric acid trihydrate (HAuCl4·3H2O) (99.9%), Eagle Minimum Essential Medium, Leibovitz’s L-15 medium, Fetal Bovine Serum, Trypsin, Antibiotic-Antimycotic solutions, DMSO, MTT, and all other chemicals of high quality were purchased from Sigma Aldrich Co. (St. Louis, MO, USA). Curcuma wenyujin was freshly purchased from the local market.
Biosynthesis of Curcuma wenyujin gold nanoparticle (CWAuNP)
To the freshly ground 60 mg of fresh C. wenyujim rhizome, 4 ml of 10 mM sodium hydroxide was added, and it was made up to 10 ml with sterile distilled water. The solution was filtered and used for further synthesis of gold nanoparticles. A solution of 1 mM HAuCl4 was prepared with distilled water and, to 8 ml of 1 mM HAuCl4, 1 ml of C. wenyujin extract was added dropwise. The solution was kept in a stirrer for 90 minutes for the synthesis of gold nanoparticle. The color change from yellow to ruby red indicated the synthesis of gold nanoparticles.14
Characterization of CWAuNPs
Uv-visible spectroscopy analysis of CWAuNPs
CWAuNPs were subjected to UV-Vis spectroscopic analysis at the spectrum range of 300–700 nm to check the bioreduction property. The procedure was carried out from 24 hours to 30 days to measure the stability of the compound (Lambda 25, Perkin Elmer, Waltham, MA, USA).
Dynamic light scattering measurements of CWAuNPs
The average particle size, zeta potential, and the distribution of the synthesized CWAuNPs was measured using zeta sizer (Malvern, USA).
Fourier transform infrared spectroscopy analysis of CWAuNP
The functional groups present in the biosynthesized CWAuNP were assessed by Fourier Transform Infrared Spectroscopy. 2.5 mg of dried CWAuNP powder was mixed with 250 mg of potassium bromide, and the mixture was placed in a FTIR sample holder. The mixture was then subjected to FTIR analysis at a range of 4000–500 cm−1 spectra using FTIR Spectrum 2000 (Perkin Elmer) . About 50 scans at a resolution of 4 cm per scan was performed, and the data obtained were assessed using the software WINFIRST (Mattson, USA).
Selected area diffraction pattern of the synthesized CWAuNP
The structural crystallinity of synthesized CWAuNP was analyzed by Selected Area Diffraction Pattern using the Malvern Zetasizer instrument. The samples for SAED analysis were prepared with Millipore filtered water to avoid possible contaminant reactions. The distance between the planes was calculated based on the patterns obtained after the SAED analysis using the formula λL Rd=(1), where λL is a constant of the microscope, R is the ring radius, and d is the interplanar distance.
Atomic force microscopic analysis of CWAuNP
The sizes of the biosynthesized CWAuNPs were further determined by Atomic Force Microscopic Analysis. CWAuNP powder was placed on the probe of AFM made up of silica, and the sample was dried with nitrogen gas before analyzing it with the contact mode of a Perkin Elmer atomic force microscope.
Transmission electron microscopic analysis and EDAX of CWAuNPs
The size and the morphology of the biosynthesized CWAuNP were assessed through Transmission Electron Microscopic Analysis, and the presence of gold particles in the compound was confirmed using EDAX analysis. The biosynthesized CWAuNP were coated with the copper grip and dried overnight. The dried samples were assessed using a high resolution transmission electron microscopy instrument (JEOL, Japan) at an acceleration voltage of 120 kV, and the images were analyzed using Image J software.
Biosynthesized CWAuNP anticancer activity against renal cancer in vitro
Culturing of renal carcinoma cell line
The human renal carcinoma cell lines A498 and SW-156 were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). The A498 cell lines were cultured in Eagle’s Minimal Essential medium along with 10% fetal bovine serum, whereas SW-156 cell lines were cultured in Leibovitz’s L-15 Medium along with 10% fetal bovine serum. 1% of antibiotic-antimycotic solution was added to the culture medium to avoid bacterial and fungal contamination. The cell lines were incubated at 37°C with 5% CO2, and the media was replaced after every 48 hours or when the color of the media changes to yellow. Upon attaining 80% of confluency, the cells were trypsinized for subculturing using the mixture of 0.25% trypsin and 0.02% ethyldiaminetetaacetic acid (EDTA).
Cytotoxicity assay
The CC50 dose of CWAuNP against two different human renal carcinoma cell lines A498 and SW-156 were assessed by MTT assay.15 105 numbers of cells were plated in each well (200 μl well−1) of 96-well microtiter plates and incubated with different concentrations of CWAuNPs ranging from 0–50 µg/ml at 37°C in a CO2 incubator for 24 hours. After 24 hours, the cell viability was measured by MTT assay. To each well of the culture plate, 20 µl of MTT solution was added and incubated in the dark for 4 hours. Mitochondrial succinic dehyrogenase enzyme secreted by the metabolically active cells reduces MTT to form insoluble blue formazan crystals. After 4 hours incubation, the supernatant was discarded and the formazan crystals were dissolved with 100 µl of DMSO. The OD value of the control and treated cells were measured at 570 nm using a microplate reader to calculate the CC50 value of CWAuNPs.
Apoptotic activity of CWAuNps against renal cancer in vitro
Reactive oxygen species analysis
The effect of CWAuNps on the generation of intracellular ROS was assessed by fluroscent H2DCFDA stain. 3×104 A498 cells were seeded on a 6-well plate and incubated with 25 µg/ml and 50 µg/ml of CWAuNP for 24 hours at 37ºC, 5% CO2. The control and treated cells were the incubated with 5 mM of 2’,7’-dichlorodihydrofluoresceindiacetate (H2DCFDA, Invitrogen, Waltham, MA, USA) for 15 minutes in the dark. Then the stained cells were viewed under under a NIKON Eclipse 80i fluorescent microscope (Japan) with an excitation at 488 nm and emission at 528 nm wavelengths, respectively.16 The stained images captured were then assessed using ImageJ software.
Mitochondrial membrane potential analysis
The hallmark change in apoptotic cells was mitochondrial dysfunction; the increased mitochondrial membrane permeability led to the release of cytochrome C, thereby initiating apoptosis. The permeability of the mitochondrial membrane was assessed using Rhodamine 123 staining. 3×104 renal carcinoma cell A498 were seeded into the 6-well plate and incubated with 25 µg/ml and 50 µg/ml of CWAuNP for 24 hours at 37ºC, 5% CO2. The control and treated cells were then stained with 1 mM Rhodamine 123 stain (Invitrogen) for 15 minutes.17 The cells were washed with PBS and then viewed under a NIKON Eclipse 80i fluorescent microscope. The images were captured and assessed using ImageJ software
Nuclear morphology analysis
The nuclear morphology of the control and CWAuNP treated A498 renal carcinoma cells was assessed to detect the induction of apoptosis by CWAuNP. The induction of apoptosis by biosynthesized CWAuNPs was analyzed using DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) staining (Sigma Aldrich, USA). 3×104 A498 cells were inoculated in 6-well plates and incubated with 25 µg/ml and 50 µg/ml of CWAuNP for 24 hours at 37ºC with 5% CO2. After the incubation period, the medium was removed and the cells were washed with ice cold PBS. Then cells were fixed with 3% paraformaldehyde for 10 minutes at room temperature. The fixed cells were permeabilized with 0.2% Triton X−100 for 10 minutes at room temperature. The cells were then stained with 0.5 µg/ml DAPI stain for 5 minutes at 37ºC; the stained cells were viewed under a NIKON Eclipse 80i fluorescent microscope, with an excitation at 359 nm and emission at 461 nm wavelengths, respectively.18
Flow cytometry analysis
The induction of apoptosis by biosynthesized CWAuNP was assessed by flow cytometry after staining with phycoerythrin-fluorescein isothiocynate (Apoptosis detection kit) and propidium iodide. The treated cells were centrifuged at 800 g for 5 minutes at 4ºC, and the pellet was collected, washed with ice cold PBS, and centrifuged again. The pellet was resuspended with PBS and 10 µl of PE Annexin V, and 5 µl of FITC was added and incubated for 15 minutes in the dark. After incubation, the cells were stained with 5 µl propidium iodide for 5 minutes. The cells were then assessed with fluorescence activated cell sorter (FACScan, BD) to detect the apoptotic and necrotic cells.
Apoptotic gene expression analysis
The mRNA expression levels of apoptotic genes Caspase 3, Caspase9, Bax, Bcl-2, and Bid were measured using real time PCR analysis. The total RNA from the control and with 25 µg/ml and 50 µg/ml of CWAuNPs treated A498 renal cancer cells were isolated using TRI Reagent by the method of Chomczynski and Sacchi.19 Two micrograms of total RNA isolated from the control and treated cells were reverse-transcribed to cDNA according to the manufacturer’s protocol using the Superscript III first strand cDNA synthesis kit (Invitrogen). The real time PCR reaction for the above genes was carried out in an MX3000p PCR system (Stratagene, Europe) using the primers sequence:
Caspase-3-F:TGACTGGAAAGCCGAAACTC; R:AGCCTCCACCGGTATCTTCT,
Caspase-9-F:CGAACTAACAGGCAAGCAGC; R:ACCTCACCAAATCCTCCAGAAC,
Bid-F:CCTTGCTCCGTGATGTCTTTC; R:GTAGGTGCGTAGGTTCTGG,
Bax-F: GCTGGACATTGGACTTCCTC; R:CTCAGCCCATCTTCTTCCAG,
Bcl-2-F:ATTGGGAAGTTTCAAATCAGC R:TGCATTCTTGGACGAGGG.
Using MESA Green PCR Master Mix, the specific genes were amplified and the specificity was determined by melting curve analysis. The data were analyzed by comparative CT method and the fold change is calculated by the 2−CT method described by Schmittgen and Livak17 using CFX Manager Version 2.1 (Bio Rad, Hercules, CA, USA).
Immunoblotting analysis of apoptotic protein expression
The expression of apoptotic protein Caspase 3, 9, Bax, Bid, Bcl-2, and Bcl-xl were assessed in the control and two different concentrations of CWAuNPs treated A498 renal cancer cell line using immunoblotting technique. The control and the CWAuNPs treated cells were washed with PBS and lyzed by sonicating using 200 µl of ice cold lysis buffer. The sonicated cells were then subjected to centrifugation for 30 minutes at 4°C, 10,000×g. Supernatant was collected and quantified for protein using Bradford reagent; 40 µg of protein from each group was mixed with sample buffer, boiled for 5 minutes at the temperature of 95°C, and subjected to electrophoresis with 10% SDS-PAGE. The electrophoresed samples were then transferred to PVDF membrane blocked with 5% skimmed milk for 1 hour, and the membranes were incubated with mouse monoclonal anti-human Caspase 3 (sc7272), Caspase 9 (sc-56076), Bax (sc-7480), Bid (sc-56025), Bcl-2 (sc-7382), and Bcl-xl (sc-8392). The secondary antimouse HRP labeled antibodies were added to the membrane after a wash with PBS. The bands of the protein were then viewed using ECL kit (Perkin Elmer).
Statistical analysis
The data were expressed as the mean±standard deviation (SD) of three independent observations. Data were subjected to statistical analysis by performing one-way ANOVA followed by Student’s Newman Keul’s test using Graph Pad prism 4 statistical software. P-values<0.05 were considered significant.
Results
Characterization of CWAuNPs
UV-visible spectroscopic and DLS analysis of CWAuNPs
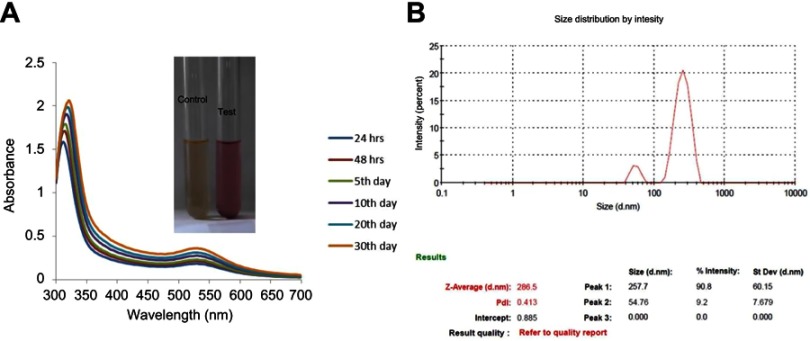
The biosynthesized CWAuNPs at different time periods were confirmed with UV spectroscopic analysis. The maximum surface plasmon resonance peak of the CWAuNPs was observed at the wavelength of 530 nm, which is consistent with the increase in time in the period of incubation, and no difference was observed in surface plasmon resonance at different durations of time (Figure 1A). The DLS analysis of CWAuNPs confirms the size of the CWAuNP as 286.5 nm diameter, and the polydispersity value of CWAuNP is reported to be 0.413 (Figure 1B).
Figure 1.
The UV–visible spectrum absorption pattern at different time period durations (A) and dynamic light scattering (B) of biosynthesized gold nanoparticles from Chinese medicinal herb Curcuma wenyujin (CWAuNPs).
Abbreviations: PDI, polydispersity index; ST.DEV, standard deviation.
FTIR and SAED analysis of CWAuNPs
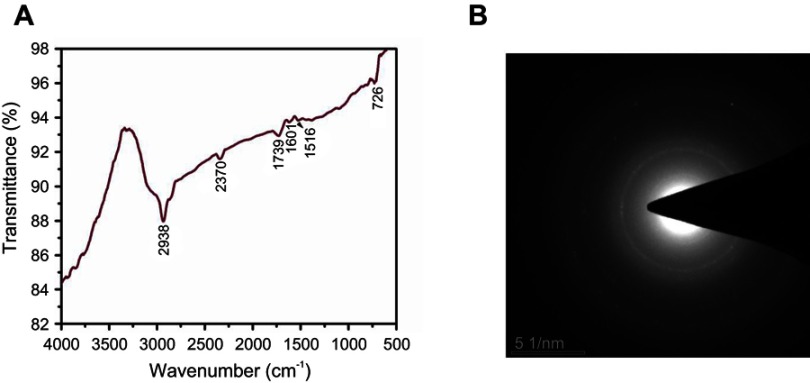
The active biological components present in biosynthesized CWAuNPs were assessed by FTIR analysis and are represented in Figure 2A. The maximum spectroscopic peaks were measured between 700–3000 cm−1. The peaks were observed at 726 cm−1, 1516 cm−1, 1601 cm−1, 1739cm−1, 2370cm−1, and 2938 cm−1. The peaks at 726 cm−1 and 1,516 cm−1 signify the olefnic bending vibrations of long-chain linear aliphatic structures of the heptadiene chain present in curcumin. The absorption peaks measured, 1,601cm−1 and 1,739cm−1, were due to the stretching of N-H and carbonyl groups present in amide groups of gold nanoparticles synthesized. The peak at 2,938 cm−1 may be due to the methylene symmetric and antisymmetric vibrations of hydrocarbons of proteins. The SAED analysis of CWAuNPs confirms that the synthesized gold nanoparticels are spherical in shape, and the reflection patterns of CWAuNPs indicates the crystalline structure (Figure 2B).
Figure 2.
Fourier-transform infrared spectroscopy (A) and selection area diffraction pattern analysis of biosynthesized gold nanoparticles from Chinese medicinal herb Curcuma wenyujin (CWAuNPs).
TEM and EDAX analysis of CWAuNPs
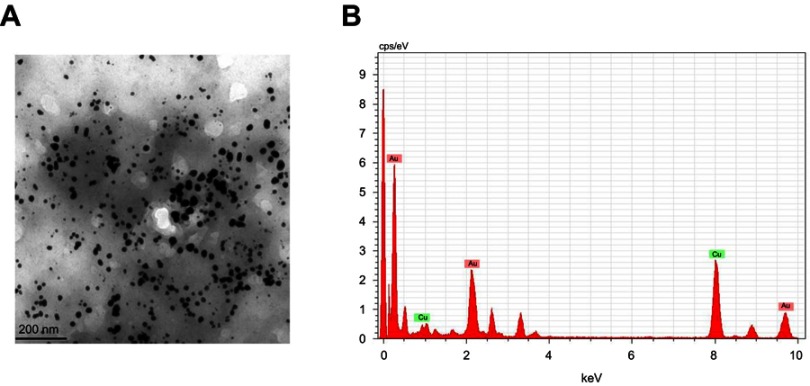
The morphology and presence of gold ions in the biosynthesized nanoparticle was confirmed with TEM and EDAX analysis. The TEM images of CWAuNPs confirm the spherical shape, which correlates with the results of the SAED pattern (Figure 3A). The size of the gold nanoparticles was measured to be 200 nm. The EDAX results of CWAuNPs are shown in Figure 3B. This shows a strong peak around 6 KeV, designating the presence of gold ions in the biosynthesized CWAuNPs, and a few peaks were observed at 3 KeV, exhibiting the presence of Cu atoms, these could be from the copper grids which are used to support the filament.
Figure 3.
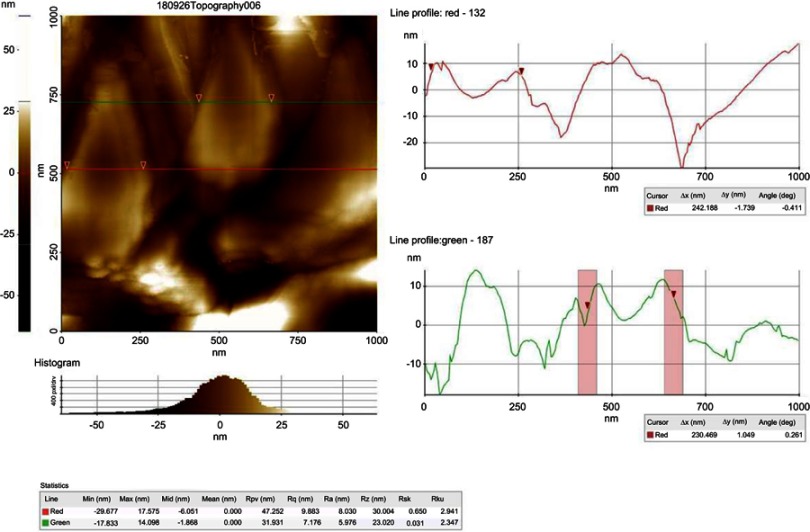
Atomic force microscopy analysis of biosynthesized gold nanoparticles from Chinese medicinal herb Curcuma wenyujin (CWAuNPs).
Abbreviation: nm, nanometer.
Atomic force microscopy analysis of CWAuNPs
The atomic force microscopic image of the biosynthesized CWAuNPs are shown in Figure 4. The size of the particle was found to be 230 nm, which correlates with the results of TEM and DLS analysis.
Figure 4.
High resolution transmission electron microscopy (TEM) (A) and energy dispersive X-ray analysis (EDAX) (B) of biosynthesized gold nanoparticles from Chinese medicinal herb Curcuma wenyujin (CWAuNPs).
Anticancer activity of CWAuNPs
CWAuNPs cytotoxicity effect on renal carcinoma cells
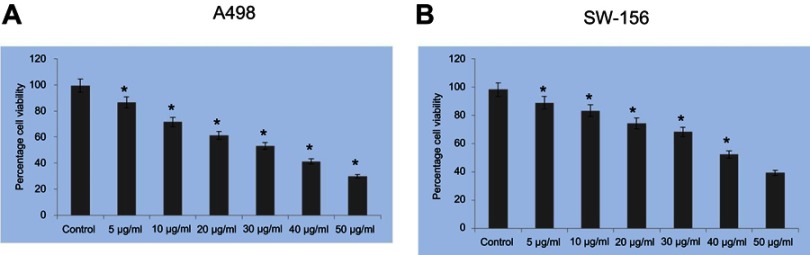
The cytotoxicity efficacy of CWAuNPs against renal cancer in vitro was assessed with two different renal carcinoma cell lines, A498 and Sw-156, and the values are shown in Figure 5. MTT assay was performed to evaluate the CC50 dose of CWAuNP against the renal cancer cell line. Both cell lines were treated with different concentrations of CWAuNP, ranging from 5 µg/ml to 50 µg/ml for 24 hours. Compared to SW-156, the A498 cell lines were more sensitive to CWAuNPs, the CC50 value was obtained at 25 µg/ml for the A498 cell line, while it was obtained at 40 µg/ml for the SW-156 cell line. Hence, to further analyze the anticancer activity of CWAuNPs 25 and 50 µg/ml, a lower and higher dose than the CC50 obtained was chosen. Since the A498 renal carcinoma cell line was more sensitive to CWAuNPs, it was chosen for further analysis by other synthesized gold nanoparticles.
Figure 5.
Cytotoxic effects of biosynthesized gold nanoparticle from Chinese medicinal herb Curcuma wenyujin (CWAuNPs) against renal carcinoma cell lines A498 (A) and SW-156 (B). Both A498 and SW-156 cells were treated with six different concentrations of CWAuNPs, ranging from 5–50 µg/ml for 24 hours and assessed for CC50 value with MTT assay. Each bar represents the mean±SEM of three independent observations. p*<0.05 is considered as statistically significant.
CWAuNPs-induced apoptotic effect against in vitro renal cancer
Accumulation of reactive oxygen species in CWAuNPs treated A498 cells
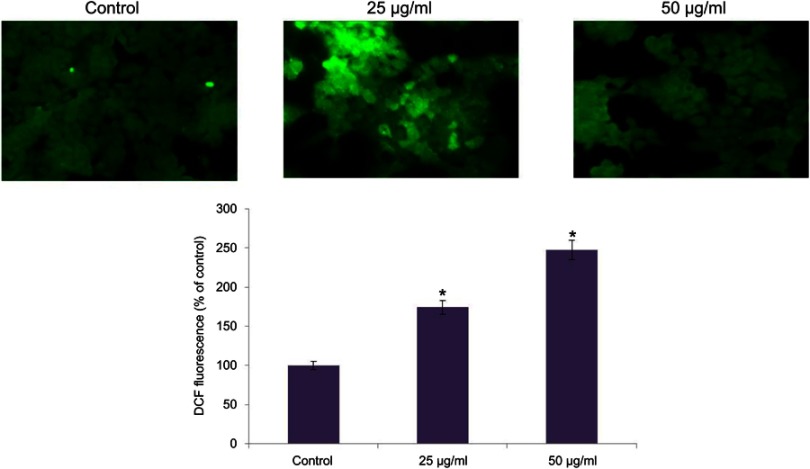
The imbalance in reactive oxygen species and antioxidant levels plays a critical role in tumor initiation and progression. The induction of ROS by CWAuNPs in A498 cell line was assessed by H2DCFDA staining, and is shown in Figure 6. Compared to the control, increased green fluroscence was observed in 25 µg/ml CWAuNPs treated cells, indicating the initiation of apoptosis by CWAuNPs in A498 cell line
Figure 6.
Apoptotic effect of biosynthesized gold nanoparticle from Chinese medicinal herb C. wenyujin (CWAuNPs) on ROS induction in renal carcinoma cell line A498. A498 cells were treated with 25 µg/ml and 50 µg/ml of CWAuNPs for 24 hours and after 24 hours of treatment, the control and treated cells were stained with 2’,7’-dichlorodihydrofluoresceindiacetate (H2DCFDA) for 15 minutes. The experiment was performed three times, and the representative images of each group are presented. *P<0.05.
Mitochondrial dysfunction in CWAuNPs treated A498 cells
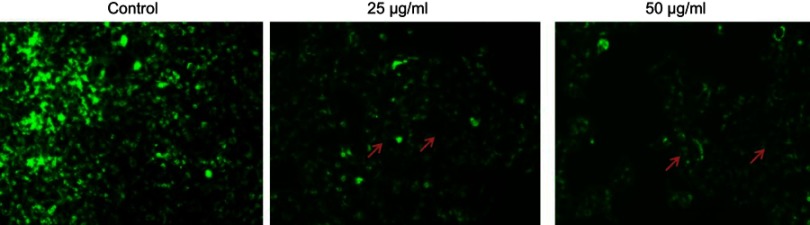
The efficacy of CWAuNPs to increase oxidative stress and induce rupture of the mitochondrial membrane was assessed. It was found to increase the release of cyctochrome C enzyme and initiate apoptosis. The induction of mitochondrial dysfunction by CWAuNPs in the A498 cell line was measured by Rhodamine 123 staining technique and is shown in Figure 7. The results of Rhodamine 123 staining of the control and CWAuNPs treated A498 cell line correlates with the results of the ROS staining technique. The decreased green fluroscence in CWAuNPs treated cells indicates the decreased mitochondrial membrane permeability and increased mitochondrial dysfunction leading to apoptosis.
Figure 7.
Apoptotic effect of biosynthesized gold nanoparticle from Chinese medicinal herb C. wenyujin (CWAuNPs) on mitochondrial membrane permeability in renal carcinoma cell line A498. A498 cells were treated with 25 µg/ml and 50 µg/ml of CWAuNPs for 24 hours and after 24 hours of treatment, the control and treated cells were stained with 1 mM Rhodamine 123 for 15 minutes. The experiment was performed thrice, and the representative images of each group are presented.
Note: arrow: apoptotic cells.
Nuclear morphological changes in CWAuNPs treated A498 cells
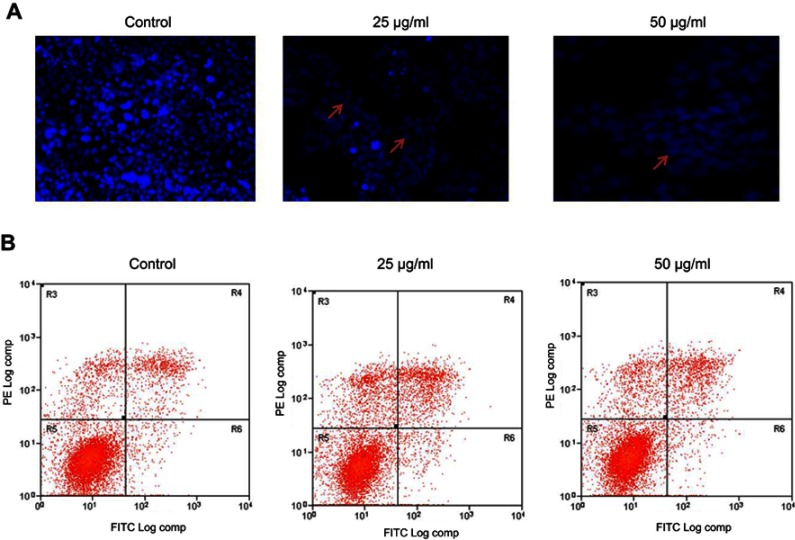
The morphological changes induced by CWAuNPs in the A498 cell line were assessed by DAPI staining, and the results are shown in Figure 8A. The DAPI staining of the control and CWAuNPs clearly depicts the induction of apoptosis by CWAuNPs in the 498 cell line. Compared to the control, the CWAuNPs treated A498 cell line showed a decreased intensity of blue fluroscence, which is decreased in a dose-dependent manner. This indicates the CWAuNPs induced DNA nick which is the initiation of apoptosis.
Figure 8.
Apoptotic effect of biosynthesized gold nanoparticle from Chinese medicinal herb C. wenyujin (CWAuNPs) on nuclear morphology of renal carcinoma cell line A498. (A) DAPI staining: A498 cells were treated with 25 µg/ml and 50 µg/ml of CWAuNPs for 24 hours and after 24 hours of treatment, the control and treated cells were stained with 4′,6-diamidino-2-phenylindole dihydrochloride for 5 minutes. The experiment was performed thrice, and the representative images of each group are presented. (B) Flow cytometric analysis with Annexin FITC and Phycoerythrin. A498 cells were treated with 25 µg/ml and 50 µg/ml of CWAuNP’s for 24 hours and after 24 hours of treatment, the control and treated cells were stained with10 µl of PE Annexin V and 5 µl of FITC for 15 minutes in the dark. After incubation, the cells were stained with 5 µl propidium iodide for 5 minutes, and assessed with fluorescence activated cell sorter (FACScan, BD) to detect the apoptotic and necrotic cells.
Note: Arrow: apoptotic cells.
Abbreviations: FITC-Fluorescein isothiocyanate; DAPI -4′,6-diamidino-2-phenylindole.
The induction of apoptosis by CWAuNPs was confirmed with FLOW cytometry assay using PE FITC staining (Figure 8B). An increased number of early and late apoptotic cells were found in 50 µg/ml CWAuNPs treated A498 cells. Compared to the control, the number of early and late apoptotic cells were increased in 25 µg/ml CWAuNPs treated A498 cells. Few necrotic cells were observed in both the control and the CWAuNPs treated A498 cells. This supports the results of DAPI staining and induction of apoptosis by CWAuNPs.
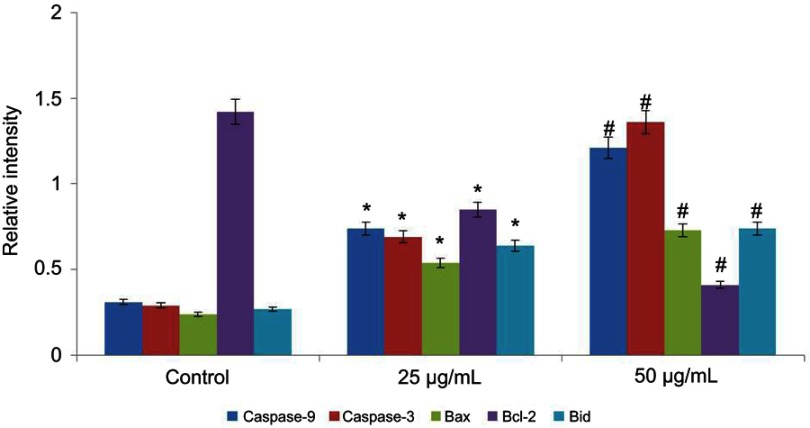
Effect of CWAuNPs on apoptotic gene expressions in A498 cell line
The effect of biosynthesized CWAuNPs on apoptotic gene expressions in the A498 cell line was assessed by real time PCR analysis (Figure 9). Both caspases, caspase 3 and caspase 9, were increased in the CWAuNPs treated A498 cell line compared to the control cells. The apoptotic genes Bid and Bax also significantly increased in a dose-dependent manner in the CWAuNPs treated A498 cell line, whereas the antiapoptotic gene Bcl2 expression was decreased in the CWAuNPs treated A498 cell line.
Figure 9.
Anticancer effect of biosynthesized gold nanoparticle from Chinese medicinal herb C. wenyujin (CWAuNPs) on apoptotic gene expression in renal carcinoma cell line A498. A498 cells were treated with 25 µg/ml and 50 µg/ml of CWAuNPs for 24 hours and after 24 hours of treatment, the control and treated cells were subjected to total RNA isolation and cDNA conversion. The cDNA of the control and treated groups were subjected to qPCR analysis with specific apoptotic genes Caspase 3, Caspase 9, Bid, Bax, and Bcl-2. Each bar represents the mean±SEM of three independent observations. P<0.05 is considered as statistically significant. *P<0.05; #P<0.01.
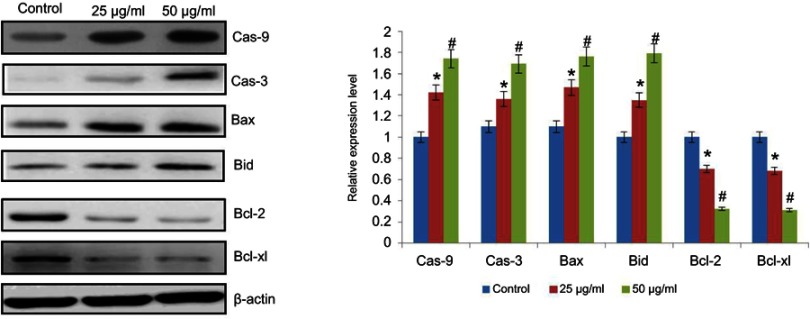
Effect of CWAuNPs on apoptotic protein expressions in A498 cell line
Figure 10 shows the effect of CWAuNPs on apoptotic protein expressions in the A498 cell line. The results of apoptotic protein expression correlates with the gene expression results of the control and the CWAuNPs treated A498 renal carcinoma cell line. The expression of apoptotic proteins Bid, Bax, Caspase 3, and Caspase 9 were significantly increased in a dose-dependent manner in CWAuNPs treated cells compared to the control untreated A498 cells. The antiapoptotic proteins Bcl2 and Bcl-xl were increased in the control untreated A498, whereas they were significantly decreased in 25 µg/ml and 50 µg/ml CWAuNPs treated cells.
Figure 10.
Anticancer effect of biosynthesized gold nanoparticle from Chinese medicinal herb C. wenyujin (CWAuNPs) on apoptotic protein expression in renal carcinoma cell line A498. A498 cells were treated with 25 µg/ml and 50 µg/ml of CWAuNPs for 24 hours and after 24 hours of treatment, the control and treated cells were subjected to total protein isolation. The 50 µg of total protein from the control and treated groups were subjected to electrophoresis and immublotting analysis with specific apoptotic proteins Caspase 3 (Cas-3), Caspase 9 (Cas-9), Bid, Bax, Bcl2, and Bclxl. Each bar represents the mean±SEM of three independent observations. p<0.05 is considered as statistically significant. *p<0.05; #p<0.01.
Discussion
Phytomedicine, a persuasive alternative to allopathic medicine, is blooming today for treating various diseases.20 It overcomes most of the serious side-effects induced by allopathic medicines, and it is also effective in curing diseases. Cancer is one of the major problems of today’s world and a huge amount has been spent on the treatment and supportive care for cancer patients. Recently, the usage of phytochemicals in treating cancer is drastically increasing. Combination therapy, alongside allopathic drugs, has been used to reduce the side-effects and to increase the efficacy.21
Nanomedicine is also a thriving field in developing various drugs to treat chronic diseases. Gold nanoparticles possess unique physicochemical properties which qualify them as an efficient drug in the field of Nanomedicine.22 They target the cells accurately. Therefore,, they are used in gene targeted therapy, and various drugs are being coated with gold nanoparticles to improve efficacy.23 Hence, in this present study, we developed a nanodrug using a Chinese medicinal herb, C. wenyujin, and assessed its anticancer potential against in vitro renal cancer.
The optical properties of synthesized gold nanoparticle CWAuNPs were characterised using UV-Vis spectroscopy The absorbance was observed at 530 nm due to the surface plasmon resonance property of gold, which shifts to longer wavelengths.24 This also correlates with the theory of Mie,25 which states that nanoparticles of spherical shape will show a strong absorption at 520 nm, which diminishes after 600 nm. We have also assessed the stability of the CWAuNPs, and this tends to be stable for more than a month. This may be due to the fact that the polyphenols present in C. wenyujin have acted as a capping agent and stabilized the nanoparticles.14
FTIR analysis of CWAuNPs showed significant peaks at 726 cm−1, 1516 cm−1, 1601 cm−1, 1739 cm−1, 2370 cm−1, and 2938 cm−1. The olefinic bending of the long chain linear aliphatic structure and the aromatic double bond stretching, N-H, and carbonyl groups present in amide groups confirmed the presence of curcumin moiety in biosynthesized gold nanoparticle, CWAuNPs. This correlates with previous literature.26,27 The spherical reflection pattern obtained in SAED analysis was confirmed with TEM images. A previous study by Sreelakshmi et al14 also reported that a spherical gold nanoparticle of size 200 nm was synthesized using rhizome extract of C. longa. The shape of the synthesized CW-AuNPs was observed through TEM observation, which clearly depicts the spherical shape of nanoparticles, as it is the unique optical and electronic property of gold nanoparticles.28 The EDAX analysis showed the presence of gold particles, which confirmed the formation of gold nanoparticles. Furthermore, it was also confirmed with the Atomic Force microscopic analysis.
The biosynthesized CWAuNPs were subjected to cytotoxicity analysis with two different renal cancer cell lines, A498 and SW-156, before assessing its anticancer activity. The aqueous gold nanoparticles synthesized using curcumin polymer conjugates was reported to be cytotoxic to various cancer cell lines like CaCo2, glioma, and HeLa.29 This correlates with our present study. The biosynthesized CWAuNPs have potentially inhibited both the renal cancer cells, A498, and the SW-156. However, A498 cells are more sensitive to CWAuNP than the CC50 cell line. The value was obtained at 25 µg/ml, as opposed to the 40 µg/ml required for the SW-156 cell line. The reason for this is not clear, but incubating the cell lines with different time durations and assessing with MTT assay may provide clear results.
Targeting the apoptotic pathway with anticancer drug may be an effective tool to inhibit the cancer progression. Therefore, in this present study, we assessed the effectiveness of CWAuNPs (our drug) on the apoptotic protein expression in the renal cancer cell line A498. Reactive oxygen species plays a vital role in cancerous cells. It increases during mitochondrial dysfunction, increased peroxisome, oxidases activity, and when there is increased metabolic function.29,30 Macrophage produces reactive oxygen and nitrogen species during inflammatory conditions which target the tumor cells and induce apoptosis.31 In this present study, we assessed the ROS generation and mitochondrial membrane potential of CWAuNPs with H2DCFDA and Rhodamine 123 staining. Our results clearly depict that CWAuNPs have increased the generation of reactive oxygen species and decreased the mitochondrial membrane potential of A498 renal cancer cells.
Apoptotic cells exhibit hallmark characteristic features such as condensed chromatin, cell shrinkage, plasma membrane budding, and the membrane exposes phosphatidylserine to the extracellular surface.32 To assess whether our drug induces these morphological features in A498 cells, we performed DAPI staining and FLOW cytometric analysis. The results confirmed that CWAuNPs induced apoptosis and increased the apoptotic cells in a dose-dependent manner.
Apoptosis can be initiated via two different pathways; intrinsic pathway and extrinsic pathway. Both intrinsic and extrinsic pathways are executed by caspases, which belong to the proteases family.33 Caspases are classified into initiator caspases and executioner caspases. Deregulation of these caspases leads to proliferation of cancer; hence, drug targeting these caspases will be more effective. Both our gene and protein expression analysis of initiator caspase 9 and executioner caspase 3 were increased in CWAuNPs treated cells, and this confirms the induction of apoptosis by CWAuNPs.
The activation of proapoptotic proteins and inactivation of antiapoptotic proteins lead to the disruption in mitochondrial outer membrane permeability, resulting in apoptosis.34 In this present study, CWAuNPs increased the proapoptotic proteins Bid and Bax, and decreased the antiapoptotic proteins Bcl-2 and Bcl-xl expressions, which subsequently triggered apoptosis in A498 renal cancer cells. The disruption of mitochondrial membrane potential by CWAuNPs was also confirmed with Rhodamine 123 stain. Therefore, it clearly signifies our biosynthesized CWAuNPs increased the ROS and triggered apoptosis via activation of proapoptotic proteins and inhibiting antiapoptotic proteins.
Conclusion
In conclusion, we synthesized an ecofriendly herbal-based gold nanoparticle with the traditional medicinal herb C. wenyujin and it effectively induced apoptosis in the renal cancer cell line A498. The physical and chemical analysis of our gold nanoparticle confirmed that it as a persuasive anticancer drug. The synthesis of CWAuNPs and its stability was confirmed with UV-Spec, DLS, FTIR, SAED, TEM, EDAX, and AFM analysis. The cytotoxicity of CWAuNPs against renal cancer cell lines A498 and SW-156 was assessed with MTT assay. The generation of reactive oxygen species by CWAuNPs in A498 cells was assessed with H2DCFDA stain and ROS induced mitochondrial membrane damage. Nuclear damage was confirmed with Rhodamine 123 and DAPI staining. The results of our FLOW cytometric analysis on the control and the treated cells authentically proved the induction apoptosis by CWAuNPs. The qPCR and immunoblotting analysis gives insight that CWAuNPs have activated the proapoptotic proteins and inhibited antiapoptotic, thereby inducing apoptosis in A498 renal cancer cells. In summary, our results confirmed CWAuNPs is a potent anticancer drug against renal cancer.
Acknowledgment
This work was supported by the Technology Talent and Platform Plan, Engineering Technology Research Center, Construction and Capacity Enhancement Project: kidney cancer diagnosis and clinical translational medicine platform (Project No: 2016DH001).
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.May M. Statistics: attacking an epidemic. Nature. 2014;509(7502):S50–S51. doi: 10.1038/509S50a [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21294 [DOI] [PubMed] [Google Scholar]
- 3.Aitken M, Kleinrock M, Simorellis A, Nass D. Global oncology trends 2018, innovation, expansion and disruption. USA: IQVIA Institute for Human Data Science; 2018. [Google Scholar]
- 4.Millimouno FM, Dong J, Yang L, Li J, Li X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev Res. 2014;7(11):1081–1107. doi: 10.1158/1940-6207.CAPR-14-0136 [DOI] [PubMed] [Google Scholar]
- 5.Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R, Darwiche N. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis. 2015;20(12):1531–1562. doi: 10.1007/s10495-015-1169-2 [DOI] [PubMed] [Google Scholar]
- 6.Boxin G. Traditional Chinese Medicine Is An Intangible Science: My Medical Practice And Reflections Of Tcm. Singapore: World Scientific; 2018. August 7. [Google Scholar]
- 7.Lai EY, Chyau CC, Mau JL, et al. Antimicrobial activity and cytotoxicity of the essential oil of Curcuma zedoaria. Am J Chin Med. 2004;32(2):281–290. doi: 10.1142/S0192415X0400193X [DOI] [PubMed] [Google Scholar]
- 8.Tohda C, Nakayama N, Hatanaka F, Komatsu K. Comparison of anti-inflammatory activities of six curcuma rhizomes: a possible curcuminoid-independent pathway mediated by Curcuma phaeocaulis extract. Evid Based Complement Alternat Med. 2006;3:255–260. doi: 10.1093/ecam/nel008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong Z, Dang Y, Yuan X, et al. Furanodiene, a natural product, inhibits breast cancer growth both in vitro and in vivo. Cell Physiol Biochem. 2010;30:778–790. doi: 10.1159/000341457 [DOI] [PubMed] [Google Scholar]
- 10.Zhou R, Xu L, Ye M, Liao M, Du D, Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res. 2014;46:753–760. doi: 10.1055/s-0034-1376977 [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya R, Murkherjee P. Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev. 2008;60:1289–1306. doi: 10.1016/j.addr.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 12.Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24:1415–1426. doi: 10.1007/s11095-007-9271-y [DOI] [PubMed] [Google Scholar]
- 13.Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D‘Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY). 2016;8(4):603–619. doi: 10.18632/aging.100934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sreelakshmi NG, Datta KKR, Addlagatta A, Ummanni R, Subba Reddy BV. Green synthesis of Curcumin capped gold nanoparticles and evaluation of their Cytotoxicity. Nanosci Nanotechnol Lett. 2013;5:1–8. doi: 10.1166/nnl.2013.1678 [DOI] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. [DOI] [PubMed] [Google Scholar]
- 16.Elumalai P, Gunadharini DN, Senthilkumar K, et al. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett. 2012;215(2):131–142. doi: 10.1016/j.toxlet.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, Zhang Y, Liu K, et al. Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Prolif. 2018;4:e12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Zong M, Xu W, et al. Natural pyrethrins induces apoptosis in human hepatocyte cells via Bax- and Bcl-2-mediated mitochondrial pathway. Chem Biol Interact. 2017;262:38–45. doi: 10.1016/j.cbi.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999 [DOI] [PubMed] [Google Scholar]
- 20.Chaudhary T, Chahar A, Sharma JK, Kaur K, Dang A. Phytomedicine in the Treatment of Cancer: A Health Technology Assessment. J Clin Diagn Res. 2015;9(12):XC04–XC09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apaya MK, Chang MT, Shyur LF. Phytomedicine polypharmacology: cancer therapy through modulating the tumor microenvironment and oxylipin dynamics, Pharmacol. Ther. 2016;162:58–68. [DOI] [PubMed] [Google Scholar]
- 22.Chanda N, Kan P, Watkinson LD, et al. Radioactive gold nanoparticles in cancer therapy: therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing mice. Nanomedicine. 2010;6(2):201–209. doi: 10.1016/j.nano.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 23.Tiwari PM, Vig K, Dennis VA, Singh SR. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 2011;1:31–63. doi: 10.3390/nano1010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brause R, Moeltgen H, Kleinermanns K. Characterization of laser-ablated and chemically reduced silver colloids in aqueous solution by UV/VIS spectroscopy and STM/SEM microscopy. Appl Phys B. 2002;75(6–7):711–716. doi: 10.1007/s00340-002-1024-3 [DOI] [Google Scholar]
- 25.Mie G. Articles on the optical characteristics of turbid tubes, especially colloidal metal solutions. Ann Phys. 1908;25(3):377–445. [Google Scholar]
- 26.Coates JP. The interpretation of infrared spectra: published reference sources. Appl Spectrosc Rev. 1996;31:179–192. doi: 10.1080/05704929608000568 [DOI] [Google Scholar]
- 27.Sindhu K, Rajaram A, Sreeram K, Rajaram R. Curcumin conjugated gold nanoparticle synthesis and its biocompatibility. RSC Adv. 2014;4:1808–1818. doi: 10.1039/C3RA45345F [DOI] [Google Scholar]
- 28.Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22(2):577–583. doi: 10.1021/bp0501423 [DOI] [PubMed] [Google Scholar]
- 29.Manju S, Sreenivasan K. Gold nanoparticles generated and stabilized by water soluble curcumin–polymer conjugate: blood compatibility evaluation and targeted drug delivery onto cancer cells. J Colloid Interface Sci. 2012;368:144–151. doi: 10.1016/j.jcis.2011.11.024 [DOI] [PubMed] [Google Scholar]
- 30.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. Jeccr. 2011;30:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–537. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297 [DOI] [PubMed] [Google Scholar]
- 34.Giam M, Huang DC, Bouillet P. BH3-only proteins and their roles in programmed cell death. Oncogene. 2008;27:S128–36. doi: 10.1038/onc.2009.50 [DOI] [PubMed] [Google Scholar]