Abstract
The misfolding and aggregation of α-synuclein (αsyn) in the central nervous system is associated with a group of neurodegenerative disorders referred to as the synucleinopathies. In addition to being a pathological hallmark of disease, it is now well-established that upon misfolding, αsyn acquires pathogenic properties, such as neurotoxicity, that can contribute to disease development. The mechanisms that produce αsyn misfolding and the molecular events underlying the neuronal damage caused by these misfolded species are not well-defined. A consistent observation that may be relevant to αsyn's pathogenicity is its ability to associate with lipids. This appears important not only to how αsyn aggregates, but also to the mechanism by which the misfolded protein causes intracellular damage. This review discusses the current literature reporting a role of lipids in αsyn misfolding and neurotoxicity in various synucleinopathy disorders and provides an overview of current methods to assess protein misfolding and pathogenicity both in vitro and in vivo.
Keywords: alpha-synuclein, protein misfolding, neurodegenerative disease, Parkinson disease, lipid, neurotoxicity, neurodegeneration, amyloid, synucleinopathy, Lewy body, mitochondrial function
Introduction
The deposition of misfolded α-synuclein (αsyn)3 in the central nervous system occurs in a group of neurodegenerative disorders referred to as the synucleinopathies. They include Parkinson's disease (PD), multiple-system atrophy (MSA), and dementia with Lewy body (DLB), among others. Largely age-related disorders that are overwhelmingly sporadic in origin, little is known about the mechanisms that underlie disease pathogenesis. It is clear, however, that αsyn can directly contribute to pathogenic mechanisms associated with disease. Evidence to support this comes from the finding that several point mutations in the protein's encoding gene, SNCA, cause early onset familial disease (1–7), and SNPs in SNCA increase susceptibility to sporadic disease (8, 9). Also, there is now substantial evidence that the protein adopts pathogenic features upon misfolding, including the ability to seed normal protein to misfold and be neurotoxic (reviewed in Ref. 10). In this regard, understanding how this protein misfolds and contributes to disease pathogenesis is an important avenue of research that requires further attention.
Underpinning our incomplete knowledge on the pathogenesis of these disorders is the complex nature of αsyn. The protein normally exists as an intrinsically disordered monomer (11–16); however, it is reported to be capable of existing as a dynamic or folded helical tetramer under certain native environments (17–19). αsyn can also undergo α-helical folding upon associating with lipid membranes (19–22), a feature that is thought to be pertinent to the normal functioning of the protein. This is particularly relevant for roles it may have at the presynaptic terminal, where it is found highly enriched (23, 24). Specifically, some of the strongest evidence reports that the protein plays an important role in the regulation of synaptic vesicles. These are lipid-rich membranous structures that contain neurotransmitters, and their release at the synapse allows the propagation of nerve impulses between neurons. αsyn has been shown to bind to synaptic vesicles (25) and is essential to soluble N-ethylmaleimide–sensitive factor attachment receptor (SNARE) complex assembly: a multimeric protein unit that is involved in the docking and fusion of synaptic vesicles with the presynaptic membrane in neurons (26–29). However, there is no consensus on the primary function of αsyn, with numerous other functions being proposed in various and diverse biological processes. These include roles in the following: regulation of glucose levels (30–33), antioxidant activity (34–36), neuronal differentiation (37, 38), suppression of apoptosis (39), and regulation of dopamine synthesis (40, 41).
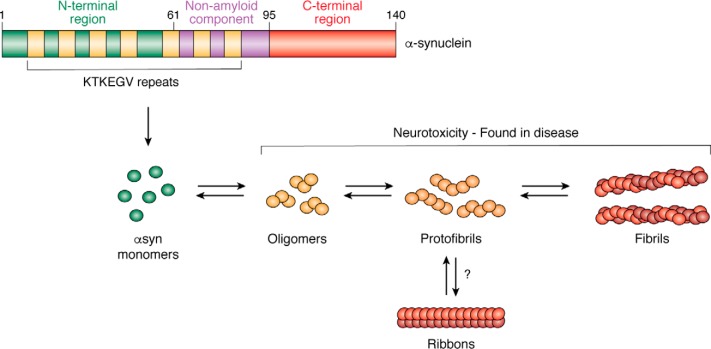
αsyn contains three core regions, which span its entire 140-amino acid length: an unstructured N terminus (amino acids 1–60), a central non-amyloid component (NAC) region (amino acids 61–95), and a C terminus (amino acids 96–140) (Fig. 1). Here, the amphipathic N terminus and NAC region contain seven repeat regions composed of imperfect KTKEGV hexameric motifs, whereas the C terminus contains 10 Glu and 5 Asp residues and hence has a high net negative charge. The central hydrophobic NAC region was appropriately named following its identification within plaques of Alzheimer's disease (AD) patients. AD is a common neurodegenerative disorder notably associated with the abnormal accumulation of β-amyloid (Aβ) in the brain; however, misfolded asyn can also be present. In this regard, its name was coined to describe a component of AD-associated plaques that was distinct from the previously identified Aβ protein (42, 43). NAC peptides are capable of self-aggregation as well as seeding Aβ aggregation (44–46), and these misfolded species are toxic to immortalized neuronal cells (46). As such, the NAC region is considered to be the highly pathogenic region of the protein. The N terminus of αsyn is the region that has been shown to strongly associate with lipid vesicles (47–51); however, a recent study demonstrates that the C terminus also exhibits a high affinity to lipid vesicles in the presence of calcium (25).
Figure 1.
α-Synuclein (αsyn) and the various conformations that can form upon misfolding. αsyn is a 14-kDa protein that contains several core regions and has seven imperfect KTKEGV repeats. In disease, monomeric protein aggregates, forming soluble misfolded oligomers. These oligomers can extend into protofibrils and mature species, such as fibrils or ribbons. Whereas there are limited studies on the biophysical properties of ribbons and their formation, the other misfolded structures exist in equilibrium with each other and can both expand and contract to higher- or lower-order conformations.
The relationship between αsyn and lipids has long been a point of interest for the synucleinopathy field. A few years following αsyn's identification in 1988 (52), it was noted that the hexameric motifs on SNCA share a high degree of sequence homology with apolipoproteins, which bind and transport lipid molecules (53). This observation suggested that lipids may be a binding target of αsyn, which was later confirmed by a study showing that WT αsyn undergoes structural rearrangement upon interacting with synthetic lipid vesicles (20). Subsequent work demonstrating that this association can be lost in preparations of protein-harboring disease-associated mutations (50) gave evidence for lipids being relevant to the pathogenic mechanisms of the synucleinopathies. Today, many studies have focused on understanding their association. However, despite the now strong evidence reporting the ability of lipids and αsyn to interact together, a concern with these data is the somewhat contradictory results relating to the mechanism of binding, lipid class preference, and activation of downstream pathways within a cell. Indeed, while it is generally accepted that their interaction is relevant for the protein's normal functioning, numerous studies show that lipids can induce and/or accelerate the disease-associated misfolding of αsyn, producing species that harbor neurotoxic properties. In this regard, lipids appear able to influence both the normal functioning and pathogenic features of αsyn; however, the context in which either influence occurs in a biological setting remains poorly defined.
An important factor governing the conflicting results seen is the dynamic nature of lipids, which are complex proteins that often exert their function in association with proteins and other lipids. Given that their functional properties are strongly dictated by their microenvironment, caveats exist in the ability of in vitro studies to accurately model in vivo interactions. Currently, the field would benefit from planned processes to define limits of experimental methodologies and establish the centrality of these interactions using well-defined system networks and models. This review gives an overview on our current understanding of the role αsyn plays in the synucleinopathies and how lipids may modulate the protein's misfolding and neurotoxicity. Furthermore, appropriate considerations when extrapolating laboratory data to the human condition are described, including suggestions on how the field can best work toward elucidating the importance of αsyn:lipid interactions to the various synucleinopathy disorders.
αsyn in the synucleinopathies
The synucleinopathies are distinguished by the cell type and brain region sensitive to the deposition of misfolded αsyn (54). Here, intraneuronal deposits of αsyn called Lewy bodies (LBs) or Lewy neurites are features of PD and DLB (55–59), whereas MSA-associated αsyn is principally found aggregated in a type of glial cell called oligodendroglia and are called glial cytoplasmic inclusions (60–62). Concomitant neuronal loss is likewise disease type–specific, where, although dopaminergic neurons of the substantia nigra are particularly vulnerable in PD, the profile of neuronal loss is more widespread in MSA and DLB. Despite this, the generation of neuronal loss and its associated clinical presentation is highly variable (54). While this diversification is most obvious between the categorical subtypes of synucleinopathies (e.g. PD versus MSA), variation is also found within a given disorder. Indeed, in the case of PD, the clinical guidelines used to diagnose disease carry a high degree of error, with a recent systematic review and meta-analysis reporting a pooled diagnostic accuracy of 80.6% in specialized clinics (63). This broad spectrum of clinical and pathological profiles associated with synucleinopathies supports the idea that numerous mechanisms may underlie αsyn misfolding and neurotoxicity, depending on its structure and/or locality.
While the detection of αsyn-positive aggregates in the central nervous system in disease indicates the presence of β-sheet–rich mature fibrils, numerous smaller species are known to present in the brain in disease. These include small soluble oligomers and protofibrils (64, 65) (Fig. 1). Consistent with the growth of fibrils from smaller misfolded units that expand by recruiting monomeric protein into the growing aggregate, these species exist in equilibrium with each other and harbor the ability to both expand and contract into higher- or lower-order conformations (66). Additionally, numerous conformations of mature species have been reported to exist, with the protein capable of forming cylindrical, elongated fibril structures as well as ribbons that exhibit a flatter, shorter morphology (67, 68). To date, ribbons have only been produced and studied from inducing misfolding in recombinant protein, and therefore their relevance in human disorders, including any interspecies interactions, is unclear (Fig. 1).
Changes to the post-translational modifications of αsyn are another striking feature of the synucleinopathies. Under normal conditions, αsyn may undergo various post-translational modifications, such as serine/threonine and tyrosine phosphorylation (69–73), N-terminal acetylation (69), ubiquitination (69, 74, 75), sumoylation (76), tyrosine nitration (77), transglutamination (78–80), and methionine oxidation (81). In disease, the abundance of post-translational modifications is altered with high levels of αsyn phosphorylated at the serine residue at position 129 (pSer-129) found in both LBs and glial cytoplasmic inclusions (82). Under normal physiological conditions, pSer-129 accounts for a low abundance in the overall pool of αsyn; however, within LBs, it accounts for over 90% of the total protein (69). The relevance of pSer-129 to αsyn biology is still a debated topic, and accordingly, the biochemical processes that lead to elevated phosphorylation of αsyn in disease and the consequence of such an alteration are unknown. Recently, a study showed that, compared with WT αsyn, the expression of mutants that do not undergo phosphorylation (S129A and S129G) is more toxic to cells and produces the formation of αsyn aggregates that are larger (83). Hence, although further analysis is required, it is intriguing to speculate that the phosphorylation of αsyn may be a protective mechanism to remove protein aggregates from the cell.
As mentioned previously, the expression of αsyn protein is associated with disease as various point mutations cause early onset familial PD. To date, five mutations in αsyn have been found associated with PD: A53T (1), A30P (2), E46K (3), H50Q (4, 5), and G51D (6). The mutation A53E also causes disease but is distinguished clinically with atypical PD and a mixed PD and MSA pathological profile (7). Duplications (84) and triplications (85) in SNCA can cause familial PD; however, consistent with a dose-dependent response of the translated protein, duplications in SNCA are not completely penetrant (86).
Similar to the human condition, the expression of disease-associated mutant αsyn or the overexpression of WT protein in experimental animals (such as Drosophila and mice) causes protein aggregation and clinical disease associated with neurological dysfunction (87–90). This is one of the most well-used models to study the pathogenicity of misfolded αsyn. Depending on the research question being addressed, alternate transgenic systems are used to study other features of disease, such as motor impairment and loss of dopaminergic neurons in PD (91). Nontransgenic mice have also been used to model disease, whereby the inoculation of misfolded αsyn can cause endogenous protein to misfold, causing the aggregation of protein in association with neurological dysfunction (92, 93). While each of these models has been useful to study certain features of disease, to date no model system is capable of recapitulating all relevant biochemical and neuropathological features consistent with the development of a given synucleinopathy disorder in the human condition.
The association of αsyn with lipids
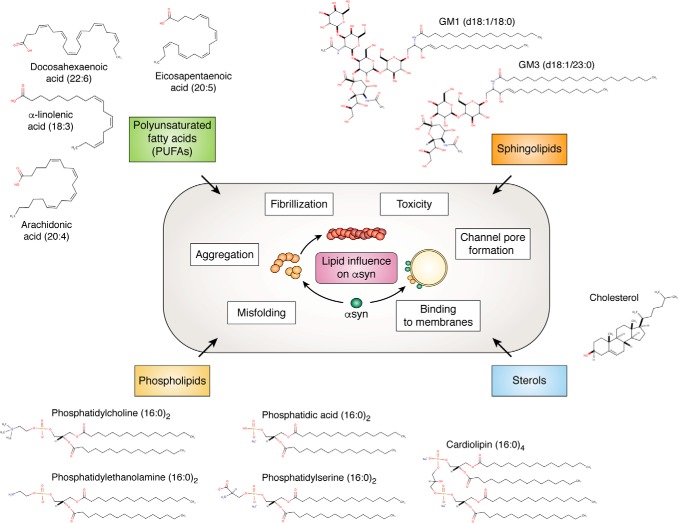
Lipids are a heterogeneous collection of molecules defined generally as any group of organic compounds that is insoluble in water but soluble in organic solvents. They play essential roles in a diverse range of cellular processes. Most notably, phospholipids are the main component of lipid bilayers that form the membranes that compartmentalize organelles and encase the cell from the extracellular space. Lipids are also important sources of heat and energy, can act as signaling molecules, and can be protein recruitment platforms. Although broadly classified by their structure, a large degree of diversity can exist within lipid subclasses; for example, phospholipids contain hydrocarbon chains that can vary in fatty acid chain length, double bond number, and position. Composition diversity in the ratio of lipids also presents within membranes between organelles, an observation that in many circumstances can reflect the unique functioning of the organelle (reviewed in Ref. 94). Several lipids described in this review are shown in Fig. 2.
Figure 2.
Structures of the lipid molecules reported to influence αsyn misfolding and/or toxicity. Lipid structures were generated using MarvinSketch version 19.4.0.
Many studies have demonstrated an interaction of αsyn with the polyunsaturated fatty acids (PUFAs) α-linolenic acid, docosahexaenoic acid (DHA), and eicosapentaenoic acid. Recombinant αsyn harbors an increased propensity to aggregate when exposed to both free forms of PUFA and those esterified with phospholipids (95, 96). Treating cultured neurons with α-linolenic acid or eicosapentaenoic acid causes an elevation in the formation of αsyn oligomers (97), which go on to form higher-order aggregates. This finding is specific to the class of lipid, given that a similar effect could not be achieved upon treatment with either monounsaturated or saturated fatty acids (97). Critically, in this system, the formation of oligomers by α-linolenic acid is also associated with cytotoxicity (98). These findings in cultured cells are supported by studies using recombinant protein, where the chronic treatment of αsyn with DHA induces α-helical folding in the protein prior to its conversion to fibrillar species (96, 99). PUFAs may also be regulated by αsyn in disease. Elevated levels of PUFAs are observed in soluble brain fractions in PD and DLB brain (100). In αsyn knockout mice, the PUFAs DHA and α-linolenic acid are down-regulated (100). Taken together, these studies suggest that an association of αsyn with PUFAs may contribute to both healthy normal function and disease pathogenesis.
PUFAs are also particularly sensitive to lipid peroxidation, which is a feature of PD (101). A product of lipid peroxidation, 4-hydroxy-2-nonenal has been implicated in various detrimental processes in disease; it can generate protein adducts within LBs in neurons (102) and alter dopamine transport, which contributes to the PD-associated feature of reduced dopamine levels (103). Hence, peroxidation of PUFAs may directly augment disease pathogenesis. However, a recent study reveals a protective role of PUFAs by chemical modulation of αsyn. In the presence of DHA, αsyn is modified at position His-50, forming a covalent adduct (104). This suggests a role of the protein in sequestering free radicals, and hence an association of αsyn with PUFAs may be neuroprotective. While any connection remains not well-defined, this finding aligns with other published works that suggest a neuroprotective role of αsyn (28, 39, 105).
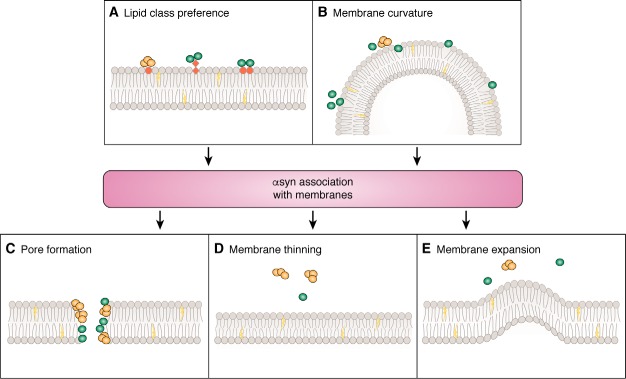
In addition to PUFAs, several types of phospholipids have been shown to associate with αsyn. αsyn has a greater affinity for synthetic vesicles containing phosphatidylethanolamine (PE) compared with those that are phosphatidylcholine (PC)-rich (106), and several studies report no or weak binding of αsyn to preparations containing solely PC (21, 107–111). In preparations of recombinant αsyn mixed with synthetic vesicles composed of PE and phosphatidylserine (PS) (1:1, w/w), the concentration of vesicles dose-dependently increased the abundance of dimeric αsyn species in the pelleted insoluble fraction compared with the lipid-free supernatant (106), and hence this may suggest that dimers are the relevant species that interacts with these lipids. Numerous studies show that monomeric WT or mutant αsyn preferentially binds to vesicles made partially of phosphatidic acid (PA) (107, 112, 113). Specifically, αsyn has higher affinity for PA than PS (20, 47, 107, 112), and the binding of αsyn to PA-rich membranes stabilizes the secondary structure and increases the α-helix content of the protein (20). The reasons for these differential binding affinities of αsyn to the various lipid classes are likely multifaceted; however, lipid structure is considered to be important (Fig. 3A). In the case of PS, its bulky headgroup is thought to sterically interfere with αsyn binding to other lipids, and hence mechanisms of binding at the molecular level may be divergent among the lipid classes or mixed populations of lipids. Competition binding may also be a contributing factor in mixed lipid populations, a notion that is supported by the finding that the binding of αsyn to PA may be augmented upon incubation with PE (113). Lipid charge is also considered relevant, where generally αsyn has a preference to associate with acidic phospholipids (e.g. PS and PA) compared with those with a neutral charge (e.g. PE and PC); however, it is not the sole contributing factor, given that αsyn binding to acidic phospholipids cannot be totally abolished under conditions of high ionic strength (20).
Figure 3.
Potential mechanisms that influence the association of αsyn with membranes and neurotoxic pathways formed. Several factors are thought to influence the ability of αsyn to associate with membranes, including lipid class preference (A) and/or membrane curvature (B). Such interactions can cause damage to membrane integrity via pore formation (C), membrane thinning (D), and membrane expansion (E).
Cardiolipin (CA) is a diphosphatidylglycerol lipid that has also been shown to bind to αsyn, with several studies showing that preparations of monomeric protein interact with synthetic vesicles containing CA with higher affinity than those lacking the lipid (114, 115). CA-only vesicles have the highest affinity for oligomeric αsyn (115), and hence these species may be the most relevant to this interaction; however, investigations into the ability of CA-containing synthetic lipid vesicles to modulate αsyn fibrillization have reported no effect (116). The sterol lipid, cholesterol, may also associate with αsyn given that a peptide fragment of αsyn (67–78) binds to cholesterol and is highly toxic to cultured neurons (117). Recently, cholesterol has been shown to facilitate the binding of oligomeric αsyn with physiologically relevant membranes (109); however, its precise mechanism for this is unknown.
The monosialogangliosides (GMs) are another group of lipids shown to associate with αsyn (118–121); however, discrepancies exist in which subclass of GM αsyn has the greatest affinity for. Monomeric preparations of αsyn strongly bind to GM1, where it induces α-helical folding in the protein that inhibits its fibrillization (118, 122). In cultured cells, the action of inhibitors that impede the internalization of αsyn may be reversed upon exposure to GM1 (120), and this system could be prevented upon disruption of lipid raft structures (120). Hence, GM1 is considered to be a vehicle of αsyn internalization within lipid domains. Others show a stronger interaction of αsyn with GM3, demonstrated by its ability to modulate the pathogenicity of disease-associated αsyn. Misfolded forms of both WT and mutant αsyn can produce pores in model membranes (123, 124), which is a feature ascribed to aggregated αsyn at membranes that cause membrane permeability and toxicity. The finding that GM3 inhibits channel pore formation caused by WT misfolded αsyn (119, 121) suggests a direct interaction with this lipid class at the cell membrane.
It is clear that αsyn has a high affinity toward various lipid species. Upon binding, some lipids are able to accelerate folding in αsyn, which in some cases leads to increased pathogenicity of the protein. Many studies investigating such interactions use recombinant monomeric protein; however, it is important to note that in the studies that do not fully characterize the structure of the protein, it is possible that the protein associating with the lipid is actually small oligomers that have formed via spontaneous aggregation in solution. This highlights an important consideration in interpreting which species of αsyn interacts with lipids. Further investigations will be important to define the structural properties of misfolded αsyn that associates with lipids within a biological environment and the consequence this interaction has for αsyn-related disorders.
Furthermore, while many lipid classes have been implicated to associate with αsyn, variations exist between the ways these interactions are studied. Most studies investigate protein:lipid interactions using small unilamellar vesicles (SUVs) or planar lipid bilayers. Membrane curvature is a major contributor to the affinity αsyn has to membranes, with enhanced membrane binding being observed in membranes with increased curvature (112, 125–127) (Fig. 3B). This is presumably due to the smaller size creating an increase in the number of “packing defects” (128–130), random protein binding sites that arise on the membrane due to the exposure of the hydrophobic acyl chain interior. Because this is likely to induce a degree of artificial error, SUVs may only be an appropriate tool to study potential biological interactions that αsyn has with highly curved membranes. A potential candidate for this type of interaction is an association of αsyn with synaptic vesicles. Studies showing support of this interaction in vivo report the two in close proximity in human brain (88, 131), and αsyn associates with synaptic vesicles isolated from rodent brain (25). However, it is likely that this is not the only type of lipid structure αsyn associates with within a cell. In particular, aside from being packed within lipid bilayers, free forms of lipids are found within a cell, and hence systems modeling biological membranes may not appropriately reflect or identify all possible interactions αsyn has with lipids within a cellular environment. This is an important consideration when interpreting experimental data reporting how lipids contribute to αsyn misfolding.
The preparation of lipids is also relevant to the mechanism of αsyn:lipid interaction at a molecular level. The association of αsyn with lipid membranes sees a proportion of the protein's first 98 residues undergo structural rearrangement, increasing the α-helical content as either a pair of anti-parallel α-helices or a single extended α-helix (21, 22, 132–135). The precise nature of this interaction appears to depend on the lipid membrane (architecture or composition), lipid/protein ratio, or αsyn sequence in modulating one or several of the distinct modes of αsyn:lipid binding that have been observed (25, 136–140). Nonetheless, in the case of SUVs composed of a mixed lipid population (PE, PS, and PC) used in ratios that mimic the lipid composition of synaptic vesicles, it has been shown that residues 1–12 are responsible for anchoring the protein onto the lipid surface and partially insert into the hydrophobic acyl chain region (139). Taken together, these studies demonstrate that the precise molecular interactions that occur are highly specific and are modulated by various αsyn- and lipid-specific factors.
Neurotoxic mechanisms of αsyn in association with lipids
Aside from contributing to the misfolding of αsyn, lipids may also directly contribute to the neurotoxicity of the protein. This may occur by favoring the production of a neurotoxic form and/or by dictating the locality of misfolded protein within a cell, which leads to damaging interactions with lipid-rich organelles. The latter notion is supported by the observation that αsyn associates with lipid-rich organelles and, in many of these contexts, results in dramatic consequences to the functioning of the cell.
One of the most common intracellular targets reported for αsyn-induced damage is the mitochondrion. This is the organelle responsible for producing the majority of the cell's energy in the form of ATP and deficits in mitochondrial functioning is a central feature of PD. Many studies have implicated αsyn in directly modulating mitochondrial readouts in various animal (141–146) and cell culture models of disease (147–149). Transgenic mice overexpressing WT αsyn have reduced ATP production and associated elevations in reactive oxygen species (ROS) and oxidative mitochondrial damage (141, 142, 148), while primary cultures derived from transgenic mice expressing human mutant A53T αsyn have impaired mitochondrial membrane potential and reduced maximum respiration (144). The expression of A53T exclusively in dopaminergic neurons shows reductions in substrate-specific respiration (146).
A major component to mitochondrial respiration is oxidative phosphorylation, which involves five complexes (Complexes I–IV and ATP synthase) that act to shuttle electrons between Complexes I–IV within the inner mitochondrial membrane, ultimately producing a proton gradient between the mitochondrial matrix and intermembrane space that drives the generation of ATP by ATP synthase. In addition to overall decreases in mitochondrial respiration, αsyn has been implicated in causing functional deficits, particularly at the level of Complex I. Cells expressing WT or mutant αsyn have deficits in Complex I (146, 147), and comparing respiration in neuronal cells that harbor deficits in specific complexes implicates Complex I in misfolded αsyn-induced reductions to respiration (148). Reductions in Complex I activity are also observed in post-mortem PD brain (150–152). Critically, evidence that these mitochondrial deficits may be due to a direct interaction of αsyn with the organelle comes from the finding that both mutant and WT αsyn associate with isolated mitochondria (149, 153) and mitochondria in vivo (146, 147, 149, 154, 155). In addition, αsyn has been shown to bind to membranes mimicking mitochondrial membranes, with a preference for those containing CA (114, 115), a lipid that is almost exclusively localized to the inner mitochondrial membrane, where it is also biosynthesized. As such, a direct interaction of αsyn with mitochondria may be a pathogenic mechanism relevant to synucleinopathies that present with mitochondrial deficits.
There is also evidence that αsyn is neurotoxic following an association with the cell membrane. When expressed in yeast, both WT and A53T αsyn localize to the cell membrane, and in this system, moderate expression of the proteins is toxic in conjunction with 20S proteasome dysfunction (156). In human neuronal cells, the expression of A53T or A30P exhibits elevations in intracellular calcium, depolarization of the membrane, and elevated cell death compared with cells expressing WT protein (157). Similar changes to membrane properties are found following the application of exogenous misfolded αsyn to cultured cells in association with caspase-dependent cell death (158). These observations are thought to be due to morphological changes to the cell membrane. As described previously, misfolded αsyn may elicit these toxic signals by producing pore-like structures on membranes (123, 124); however, it may not be the only mechanism of αsyn toxicity of membranes, given that several studies also report membrane expansion, and increased membrane curvature and/or membrane thinning occurs in association with perturbations in membrane integrity (159–162) (Fig. 3, C–E). Collectively, these studies support the notion that pathogenic processes associated with αsyn at the cellular membrane likely contribute to the generation of neurotoxic pathways; however, its precise mechanism of action remains unclear. A relevant point to the ability of αsyn to cause damage at the cell membrane is whether this damage is driven by interactions within the extracellular space or intracellularly from the cytosol. This is important, given that certain lipids are preferentially located on either the inner or outer leaflet of the cell membrane; for example, in a normally functioning cell, PS and PE are typically largely expressed on the inner membrane, whereas the majority of PC is on the outer membrane (163).
Adverse effects may also arise from alterations of αsyn at the synapse. Although largely conjectural, support for this as a relevant theory comes from studies showing that αsyn plays an important role at the synapse; αsyn has been shown to directly bind the essential SNARE protein, VAMP2, and enhance SNARE complex assembly in vitro and in vivo (27), and ablation of the protein causes age-dependent impairment of complex assembly (164). The involvement of αsyn at the SNARE complex requires the presence of the PUFA arachidonic acid (164), and interestingly, it has been shown that αsyn may modulate synaptic transmission by cross-bridging the lipid PS to VAMP2 to facilitate SNARE-dependent vesicle docking (165). Hence, it could be expected that a loss of αsyn in these areas would cause drastic effects on neuronal transmission and health. In the context of disease, this may be triggered by the presence of misfolded αsyn in this area of the cell and/or localization of αsyn away from the synapse upon misfolding.
An important consideration to the studies reporting neurotoxic mechanisms associated with αsyn:lipid interactions is whether different conformations of αsyn harbor different toxicities. Although outside the scope of this review, differential toxicity has been reported among the various conformations of misfolded αsyn (reviewed in Ref. 10). Accordingly, it will be interesting to determine whether lipids favor the production of any given conformation that exhibits a certain neurotoxic property or if a type of species favors an association with lipid-rich organelles. A recent study has reported the ability of a certain oligomer type to insert into the membrane of SUVs, causing a loss of membrane integrity (166). This may represent a pathogenic mechanism distinct from other misfolded conformations that cause damage; however, further investigations into the effect of different conformational species on various lipid-rich organelles will be required to determine this.
The notion of misfolded structure dictating toxicity is also particularly relevant to distinguish the pathogenic mechanisms among the various synucleinopathy disorders. For example, while mitochondrial dysfunction is a feature of PD, it does not routinely present in the other synucleinopathy disorders. Because PD is also distinguished by the selective loss of dopaminergic neurons of the substantia nigra and dopamine enhances the production of β-sheet–negative, oligomeric αsyn (167–169), if this is the pathogenic conformation that modulates mitochondrial respiration, it may generate a larger quantity of the pathogenic species that target the mitochondrion in this disorder. Likewise, MSA and DLB may be more susceptible to synaptic dysfunction, given that dementia is a frequent symptom of MSA and DLB (54). Various synaptic proteins predict cognitive decline in DLB (170), and the loss of VAMP2 and monomeric αsyn correlate with the duration of dementia in DLB and a subset of PD that presents with dementia (171). Hence, synapse loss is likely an important component to synucleinopathies that present with this symptom. Although interesting to speculate, these hypotheses should be examined in controlled experiments using well-characterized misfolded conformations.
Experimental considerations and future prospects for studying αsyn:lipid interactions
While it is well-established that αsyn and lipids interact with each other, the mechanisms by which this occurs are complex, with features of both molecules being capable of drastically influencing their association. The resulting effect this interaction can have on αsyn is multifaceted. As such, the intricate balance that dictates the ability of lipids to contribute to its normal functioning in an organism, versus driving pathogenic mechanisms associated with disease, is not well-defined.
It is highly possible that the vast range of described mechanisms of αsyn:lipid interactions in vitro are relevant in some capacity to the human condition. However, in the absence of unified results, caveats to the relevance of protein:lipid interactions observed in an artificial environment should be considered. Basic interaction studies are unable to reflect the diverse range of cellular processes and biochemical changes that occur within an organism. For example, whereas numerous in vitro data report that the C terminus of αsyn associates with various lipid membranes only weakly or not at all (21, 48, 140, 172), it has recently been observed to have a strong binding affinity to synaptic vesicles in the presence of calcium (25). This is an important observation given the large degree of calcium fluctuations that occur at the synapse (which can reach concentrations in the hundreds of μm range (173, 174)), and highly implicates the C terminus as relevant to the ability of αsyn to interact with synaptic vesicles and potentially other lipid-rich structures within the presynapse. Such studies also demonstrate the importance of using biologically derived vesicles that are involved in normal cellular functioning and harbor lipid: protein compositions in biologically relevant ratios. In addition to phospholipids, lipid membranes are enriched with various other lipids species, as well as peripheral and integral membrane proteins, which are not represented in synthetic preparations. As in the case of cholesterol facilitating the binding of oligomeric αsyn to membranes (109), such molecules could foreseeably be important drivers or cofactors in αsyn:lipid interactions. Recently, several studies have investigated the association of recombinant αsyn with isolated biological lipid vesicles, including synaptic vesicles (25) and exosomes (116), which are membranous extracellular vesicles of endosomal origin. The use of such samples is important to further our understanding of the importance of membrane composition to αsyn misfolding and/or neurotoxicity.
Additionally, to determine the biological relevance of αsyn:lipid interactions, experimental methodologies distinct from in vitro analyses should also be pursued. Here, the implementation of systems biology approaches to studying protein networks and systematic, unbiased biochemical screens to study protein:lipid interactions could provide additional information. This should include global analysis of the lipidome and proteome using human tissue, where possible. Such findings could then be integrated with in vitro studies using defined lipids and solute conditions to enable artificial systems to act more as complementary assays rather than experiments to uncover primary leads. While lipid research has previously been limited by technical aspects of standard techniques, such as mass accuracy and resolution in MS-based approaches (reviewed in Ref. 175), new advancements in the field will no doubt reveal important additional information. For instance, the use of a combination of cutting-edge structural, biochemical, and computational approaches has recently been shown as a powerful tool to study previously challenging protein:lipid interactions (176), which may be relevant in the context of αsyn.
In addition to improvements in tools to study lipids, the field would also greatly benefit from advanced tools to model the synucleinopathy disorders. Many studies investigating the pathogenicity of αsyn use fibrillar species that have been produced from recombinant protein, which may be easily generated by exposing αsyn in neutral buffer to continuous shaking at an ambient temperature. This method produces large quantities of homogeneous fibrillar protein after several days. Variations in the preparation of αsyn, the buffer it is reconstituted in, or the addition of compounds produces other defined species (such as oligomers or ribbons), with several studies characterizing the properties of well-defined αsyn structural species (67, 68). Although these studies are useful to attribute pathogenic properties to specific conformations of misfolded αsyn, a caveat to these systems is that these techniques only produce one type of misfolded species. These homogeneous populations are unlikely to reflect the numerous conformations found in human disease, and hence the ability to model in vivo disease, including any interspecies interactions that occur, is insufficient. New methods of protein fibrillization may alleviate these technical issues. The protein misfolding cyclic amplification assay is a system used traditionally to study the misfolding of PrPC into PrPSc, which is a misfolded protein that is associated with the neurodegenerative prion disorders (177), and it has also been shown to produce misfolded αsyn species of various sizes (178). An additional consideration in experiments using recombinant protein is that often they do not exhibit disease-associated post-translational modifications of the protein, such as phosphorylation of αsyn at position Ser-129. However, several recent studies have produced phosphorylated recombinant misfolded αsyn and studied their pathogenicity in various disease systems (179–181). Experimental findings from these types of studies will be useful to provide further insight into the misfolding and pathogenicity of αsyn and identify tools that best model human disease-associated αsyn.
Ultimately, the greatest advancements in the field will require collaborative efforts by researchers from a range of backgrounds with specialist technical skills in areas such as computational and structural biology, lipid characterization and transport, and protein misfolding. In doing so, aspects relevant to studying αsyn:lipid interactions may be handled by those with expertise in a given area to help piece together a global picture of how they interact and the nuances pertinent to αsyn's functioning in both health and disease.
The authors declare that they have no conflicts of interest with the contents of this article.
- αsyn
- α-synuclein
- PD
- Parkinson's disease
- AD
- Alzheimer's disease
- MSA
- multiple-system atrophy
- DLB
- dementia with Lewy body
- SNARE
- soluble N-ethylmaleimide–sensitive factor attachment receptor
- NAC
- non-amyloid component
- Aβ
- β-amyloid
- LB
- Lewy body
- PUFA
- polyunsaturated fatty acid
- DHA
- docosahexaenoic acid
- PE
- phosphatidylethanolamine
- PC
- phosphatidylcholine
- PS
- phosphatidylserine
- PA
- phosphatidic acid
- SUV
- small unilamellar vesicle
- ROS
- reactive oxygen species
- GM1
- monosialotetrahexosylganglioside
- GM3
- monosialodihexosylganglioside.
References
- 1. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., et al. (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- 2. Krüger R., Kuhn W., Muller T., Woitalla D., Graeber M., Kosel S., Kösel S., Przuntek H., Epplen J. T., Schöls L., and Riess O. (1998) Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat. Genet. 18, 106–108 10.1038/ng0298-106 [DOI] [PubMed] [Google Scholar]
- 3. Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarës B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D. G., and de Yebenes J. G. (2004) The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 10.1002/ana.10795 [DOI] [PubMed] [Google Scholar]
- 4. Appel-Cresswell S., Vilarino-Guell C., Encarnacion M., Sherman H., Yu I., Shah B., Weir D., Thompson C., Szu-Tu C., Trinh J., Aasly J. O., Rajput A., Rajput A. H., Jon Stoessl A., and Farrer M. J. (2013) α-Synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Movement Disord. 28, 811–813 10.1002/mds.25421 [DOI] [PubMed] [Google Scholar]
- 5. Proukakis C., Dudzik C. G., Brier T., MacKay D. S., Cooper J. M., Millhauser G. L., Houlden H., and Schapira A. H. (2013) A novel α-synuclein missense mutation in Parkinson disease. Neurology 80, 1062–1064 10.1212/WNL.0b013e31828727ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lesage S., Anheim M., Letournel F., Bousset L., Honoré A., Rozas N., Pieri L., Madiona K., Dürr A., Melki R., Verny C., Brice A., and French Parkinson's Disease Genetics Study Group (2013) G51D α-synuclein mutation causes a novel Parkinsonian-pyramidal syndrome. Ann. Neurol. 73, 459–471 10.1002/ana.23894 [DOI] [PubMed] [Google Scholar]
- 7. Pasanen P., Myllykangas L., Siitonen M., Raunio A., Kaakkola S., Lyytinen J., Tienari P. J., Pöyhönen M., and Paetau A. (2014) Novel α-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson's disease-type pathology. Neurobiol. Aging 35, 2180.e1–5 10.1016/j.neurobiolaging.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 8. Simón-Sánchez J., Schulte C., Bras J. M., Sharma M., Gibbs J. R., Berg D., Paisan-Ruiz C, Lichtner P., Scholz S. W., Hernandez D. G., Krüger R., Federoff M., Klein C., Goate A., Perlmutter J., et al. (2009) Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat. Genet. 41, 1308–1312 10.1038/ng.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nalls M. A., Pankratz N., Lill C. M., Do C. B., Hernandez D. G., Saad M., DeStefano A. L., Kara E., Bras J., Sharma M., Schulte C., Keller M. F., Arepalli S., Letson C., Edsall C., et al. (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat. Genet. 46, 989–993 10.1038/ng.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ugalde C. L., Finkelstein D. I., Lawson V. A., and Hill A. F. (2016) Pathogenic mechanisms of prion protein, amyloid-β and α-synuclein misfolding: the prion concept and neurotoxicity of protein oligomers. J. Neurochem. 139, 162–180 10.1111/jnc.13772 [DOI] [PubMed] [Google Scholar]
- 11. Weinreb P. H., Zhen W., Poon A. W., Conway K. A., and Lansbury P. T. Jr. (1996) NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry 35, 13709–13715 10.1021/bi961799n [DOI] [PubMed] [Google Scholar]
- 12. Uversky V. N., Li J., and Fink A. L. (2001) Evidence for a partially folded intermediate in α-synuclein fibril formation. J. Biol. Chem. 276, 10737–10744 10.1074/jbc.M010907200 [DOI] [PubMed] [Google Scholar]
- 13. Dedmon M. M., Lindorff-Larsen K., Christodoulou J., Vendruscolo M., and Dobson C. M. (2005) Mapping long-range interactions in α-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J. Am. Chem. Soc. 127, 476–477 10.1021/ja044834j [DOI] [PubMed] [Google Scholar]
- 14. Wu K. P., Kim S., Fela D. A., and Baum J. (2008) Characterization of conformational and dynamic properties of natively unfolded human and mouse α-synuclein ensembles by NMR: implication for aggregation. J. Mol. Biol. 378, 1104–1115 10.1016/j.jmb.2008.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maiti N. C., Apetri M. M., Zagorski M. G., Carey P. R., and Anderson V. E. (2004) Raman spectroscopic characterization of secondary structure in natively unfolded proteins: α-synuclein. J. Am. Chem. Soc. 126, 2399–2408 10.1021/ja0356176 [DOI] [PubMed] [Google Scholar]
- 16. Fauvet B., Mbefo M. K., Fares M. B., Desobry C., Michael S., Ardah M. T., Tsika E., Coune P., Prudent M., Lion N., Eliezer D., Moore D. J., Schneider B., Aebischer P., El-Agnaf O. M., et al. (2012) α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J. Biol. Chem. 287, 15345–15364 10.1074/jbc.M111.318949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartels T., Choi J. G., and Selkoe D. J. (2011) α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477, 107–110 10.1038/nature10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang W., Perovic I., Chittuluru J., Kaganovich A., Nguyen L. T. T., Liao J. L., Auclair J. R., Johnson D., Landeru A., Simorellis A. K., Ju S., Cookson M. R., Asturias F. J., Agar J. N., Webb B. N., et al. (2011) A soluble α-synuclein construct forms a dynamic tetramer. Proc. Natl. Acad. Sci. U.S.A. 108, 17797–17802 10.1073/pnas.1113260108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burré J., Vivona S., Diao J., Sharma M., Brunger A. T., and Südhof T. C. (2013) Properties of native brain α-synuclein. Nature 498, E4–E6; discussion E6–E7 10.1038/nature12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davidson W. S., Jonas A., Clayton D. F., and George J. M. (1998) Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273, 9443–9449 10.1074/jbc.273.16.9443 [DOI] [PubMed] [Google Scholar]
- 21. Chandra S., Chen X., Rizo J., Jahn R., and Südhof T. C. (2003) A broken α-helix in folded α-synuclein. J. Biol. Chem. 278, 15313–15318 10.1074/jbc.M213128200 [DOI] [PubMed] [Google Scholar]
- 22. Jao C. C., Hegde B. G., Chen J., Haworth I. S., and Langen R. (2008) Structure of membrane-bound α-synuclein from site-directed spin labeling and computational refinement. Proc. Natl. Acad. Sci. U.S.A. 105, 19666–19671 10.1073/pnas.0807826105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy D. D., Rueter S. M., Trojanowski J. Q., and Lee V. M. (2000) Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 20, 3214–3220 10.1523/JNEUROSCI.20-09-03214.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jakes R., Spillantini M. G., and Goedert M. (1994) Identification of 2 distinct synucleins from human brain. FEBS Lett. 345, 27–32 10.1016/0014-5793(94)00395-5 [DOI] [PubMed] [Google Scholar]
- 25. Lautenschläger J., Stephens A. D., Fusco G., Ströhl F., Curry N., Zacharopoulou M., Michel C. H., Laine R., Nespovitaya N., Fantham M., Pinotsi D., Zago W., Fraser P., Tandon A., St George-Hyslop P., et al. (2018) C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction. Nat. Commun. 9, 712 10.1038/s41467-018-03111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burré J., Sharma M., and Südhof T. C. (2014) α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl. Acad. Sci. U.S.A. 111, E4274–E4283 10.1073/pnas.1416598111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burré J., Sharma M., Tsetsenis T., Buchman V., Etherton M. R., and Südhof T. C. (2010) α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663–1667 10.1126/science.1195227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chandra S., Gallardo G., Fernández-Chacón R., Schlüter O. M., and Südhof T. C. (2005) α-Synuclein cooperates with CSPα in preventing neurodegeneration. Cell 123, 383–396 10.1016/j.cell.2005.09.028 [DOI] [PubMed] [Google Scholar]
- 29. Choi B. K., Choi M. G., Kim J. Y., Yang Y., Lai Y., Kweon D. H., Lee N. K., and Shin Y. K. (2013) Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc. Natl. Acad. Sci. U.S.A. 110, 4087–4092 10.1073/pnas.1218424110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geng X., Lou H., Wang J., Li L., Swanson A. L., Sun M., Beers-Stolz D., Watkins S., Perez R. G., and Drain P. (2011) α-Synuclein binds the K-ATP channel at insulin-secretory granules and inhibits insulin secretion. Am. J. Physiol. Endocrinol. Metab. 300, E276–E286 10.1152/ajpendo.00262.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma S. K., Chorell E., Steneberg P., Vernersson-Lindahl E., Edlund H., and Wittung-Stafshede P. (2015) Insulin-degrading enzyme prevents α-synuclein fibril formation in a nonproteolytical manner. Sci. Rep. 5, 12531 10.1038/srep12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodriguez-Araujo G., Nakagami H., Hayashi H., Mori M., Shiuchi T., Minokoshi Y., Nakaoka Y., Takami Y., Komuro I., Morishita R., and Kaneda Y. (2013) α-Synuclein elicits glucose uptake and utilization in adipocytes through the Gab1/PI3K/Akt transduction pathway. Cell. Mol. Life Sci. 70, 1123–1133 10.1007/s00018-012-1198-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodriguez-Araujo G., Nakagami H., Takami Y., Katsuya T., Akasaka H., Saitoh S., Shimamoto K., Morishita R., Rakugi H., and Kaneda Y. (2015) Low α-synuclein levels in the blood are associated with insulin resistance. Sci. Rep. 5, 12081 10.1038/srep12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu M., Qin Z. J., Hu D., Munishkina L. A., and Fink A. L. (2006) α-Synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles. Biochemistry 45, 8135–8142 10.1021/bi052584t [DOI] [PubMed] [Google Scholar]
- 35. Latchoumycandane C., Anantharam V., Kitazawa M., Yang Y., Kanthasamy A., and Kanthasamy A. G. (2005) Protein kinase Cδ is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J. Pharmacol. Exp. Ther. 313, 46–55 10.1124/jpet.104.078469 [DOI] [PubMed] [Google Scholar]
- 36. Hashimoto M., Hsu L. J., Rockenstein E., Takenouchi T., Mallory M., and Masliah E. (2002) α-Synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J. Biol. Chem. 277, 11465–11472 10.1074/jbc.M111428200 [DOI] [PubMed] [Google Scholar]
- 37. Ostrerova N., Petrucelli L., Farrer M., Mehta N., Choi P., Hardy J., and Wolozin B. (1999) α-Synuclein shares physical and functional homology with 14-3-3 proteins. J. Neurosci. 19, 5782–5791 10.1523/JNEUROSCI.19-14-05782.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen R. H. C., Wislet-Gendebien S., Samuel F., Visanji N. P., Zhang G., Marsilio D., Langman T., Fraser P. E., and Tandon A. (2013) α-Synuclein membrane association is regulated by the Rab3a recycling machinery and presynaptic activity. J. Biol. Chem. 288, 7438–7449 10.1074/jbc.M112.439497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jin H., Kanthasamy A., Ghosh A., Yang Y., Anantharam V., and Kanthasamy A. G. (2011) α-Synuclein negatively regulates protein kinase Cδ expression to suppress apoptosis in dopaminergic neurons by reducing p300 histone acetyltransferase activity. J. Neurosci. 31, 2035–2051 10.1523/JNEUROSCI.5634-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peng X., Tehranian R., Dietrich P., Stefanis L., and Perez R. G. (2005) α-Synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J. Cell Sci. 118, 3523–3530 10.1242/jcs.02481 [DOI] [PubMed] [Google Scholar]
- 41. Yang W., Wang X., Duan C., Lu L., and Yang H. (2013) α-Synuclein overexpression increases phospho-protein phosphatase 2A levels via formation of calmodulin/Src complex. Neurochem. Int. 63, 180–194 10.1016/j.neuint.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 42. Uéda K., Fukushima H., Masliah E., Xia Y., Iwai A., Yoshimoto M., Otero D. A., Kondo J., Ihara Y., and Saitoh T. (1993) Molecular-cloning of cDNA-encoding an unrecognized component of amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 11282–11286 10.1073/pnas.90.23.11282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Masliah E., Iwai A., Mallory M., Uéda K., and Saitoh T. (1996) Altered presynaptic protein NACP is associated with plaque formation and neurodegeneration in Alzheimer's disease. Am. J. Pathol. 148, 201–210 [PMC free article] [PubMed] [Google Scholar]
- 44. Han H., Weinreb P. H., and Lansbury P. T. Jr. (1995) The core Alzheimer's peptide NAC forms amyloid firbils which seed and are seeded by β-amyloid: is NAC a common trigger or target in neurodegenerative disease? Chem. Biol. 2, 163–169 10.1016/1074-5521(95)90071-3 [DOI] [PubMed] [Google Scholar]
- 45. Iwai A., Yoshimoto M., Masliah E., and Saitoh T. (1995) Non-Aβ component of Alzheimer's disease amyloid (NAC) is amyloidogenic. Biochemistry 34, 10139–10145 10.1021/bi00032a006 [DOI] [PubMed] [Google Scholar]
- 46. El-Agnaf O. M. A., Jakes R., Curran M. D., Middleton D., Ingenito R., Bianchi E., Pessi A., Neill D., and Wallace A. (1998) Aggregates from mutant and wild-type α-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of β-sheet and amyloid-like filaments. FEBS Lett. 440, 71–75 10.1016/S0014-5793(98)01418-5 [DOI] [PubMed] [Google Scholar]
- 47. Perrin R. J., Woods W. S., Clayton D. F., and George J. M. (2000) Interaction of human α-synuclein and Parkinson's disease variants with phospholipids: structural analysis using site-directed mutagenesis. J. Biol. Chem. 275, 34393–34398 10.1074/jbc.M004851200 [DOI] [PubMed] [Google Scholar]
- 48. Eliezer D., Kutluay E., Bussell R. Jr., and Browne G. (2001) Conformational properties of α-synuclein in its free and lipid-associated states. J. Mol. Biol. 307, 1061–1073 10.1006/jmbi.2001.4538 [DOI] [PubMed] [Google Scholar]
- 49. Jao C. C., Der-Sarkissian A., Chen J., and Langen R. (2004) Structure of membrane-bound α-synuclein studied by site-directed spin labeling. Proc. Natl. Acad. Sci. U.S.A. 101, 8331–8336 10.1073/pnas.0400553101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jensen P. H., Nielsen M. S., Jakes R., Dotti C. G., and Goedert M. (1998) Binding of α-synuclein to brain vesicles is abolished by familial Parkinson's disease mutation. J. Biol. Chem. 273, 26292–26294 10.1074/jbc.273.41.26292 [DOI] [PubMed] [Google Scholar]
- 51. Bartels T., Ahlstrom L. S., Leftin A., Kamp F., Haass C., Brown M. F., and Beyer K. (2010) The N-terminus of the intrinsically disordered protein α-synuclein triggers membrane binding and helix folding. Biophys. J. 99, 2116–2124 10.1016/j.bpj.2010.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maroteaux L., Campanelli J. T., and Scheller R. H. (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 8, 2804–2815 10.1523/JNEUROSCI.08-08-02804.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. George J. M., Jin H., Woods W. S., and Clayton D. F. (1995) Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 15, 361–372 10.1016/0896-6273(95)90040-3 [DOI] [PubMed] [Google Scholar]
- 54. McCann H., Stevens C. H., Cartwright H., Halliday G. M. (2014) α-Synucleinopathy phenotypes. Parkinsonism Rel. Disord. 20, S62–S67 10.1016/S1353-8020(13)70017-8 [DOI] [PubMed] [Google Scholar]
- 55. Spillantini M. G., Schmidt M. L., Lee V. M. Y., Trojanowski J. Q., Jakes R., and Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- 56. Baba M., Nakajo S., Tu P. H., Tomita T., Nakaya K., Lee V. M. Y., Lee V. M., Trojanowski J. Q., and Iwatsubo T. (1998) Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am. J. Pathol. 152, 879–884 [PMC free article] [PubMed] [Google Scholar]
- 57. Irizarry M. C., Growdon W., Gomez-Isla T., Newell K., George J. M., Clayton D. F., and Hyman B. T. (1998) Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain α-synuclein immunoreactivity. J. Neuropathol. Exp. Neurol. 57, 334–337 10.1097/00005072-199804000-00005 [DOI] [PubMed] [Google Scholar]
- 58. Wakabayashi K., Matsumoto K., Takayama K., Yoshimoto M., and Takahashi H. (1997) NACP, a presynaptic protein, immunoreactivity in Lewy bodies in Parkinson's disease. Neurosci. Lett. 239, 45–48 10.1016/S0304-3940(97)00891-4 [DOI] [PubMed] [Google Scholar]
- 59. Spillantini M. G., Crowther R. A., Jakes R., Hasegawa M., and Goedert M. (1998) α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473 10.1073/pnas.95.11.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tu P. H., Galvin J. E., Baba M., Giasson B., Tomita T., Leight S., Nakajo S., Iwatsubo T., Trojanowski J. Q., and Lee V. M. (1998) Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann. Neurol. 44, 415–422 10.1002/ana.410440324 [DOI] [PubMed] [Google Scholar]
- 61. Wakabayashi K., Yoshimoto M., Tsuji S., and Takahashi H. (1998) α-Synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci. Lett. 249, 180–182 10.1016/S0304-3940(98)00407-8 [DOI] [PubMed] [Google Scholar]
- 62. Spillantini M. G., Crowther R. A., Jakes R., Cairns N. J., Lantos P. L., and Goedert M. (1998) Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci. Lett. 251, 205–208 10.1016/S0304-3940(98)00504-7 [DOI] [PubMed] [Google Scholar]
- 63. Rizzo G., Copetti M., Arcuti S., Martino D., Fontana A., and Logroscino G. (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86, 566–576 10.1212/WNL.0000000000002350 [DOI] [PubMed] [Google Scholar]
- 64. Kramer M. L., and Schulz-Schaeffer W. J. (2007) Presynaptic α-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J. Neurosci. 27, 1405–1410 10.1523/JNEUROSCI.4564-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Garcia-Esparcia P., Hernández-Ortega K., Koneti A., Gil L., Delgado-Morales R., Castaño E., Carmona M., and Ferrer I. (2015) Altered machinery of protein synthesis is region- and stage-dependent and is associated with α-synuclein oligomers in Parkinson's disease. Acta Neuropathol. Commun. 3, 76 10.1186/s40478-015-0257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cremades N., Cohen S. I., Deas E., Abramov A. Y., Chen A. Y., Orte A., Sandal M., Clarke R. W., Dunne P., Aprile F. A., Bertoncini C. W., Wood N. W., Knowles T. P., Dobson C. M., and Klenerman D. (2012) Direct observation of the interconversion of normal and toxic forms of α-synuclein. Cell 149, 1048–1059 10.1016/j.cell.2012.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bousset L., Pieri L., Ruiz-Arlandis G., Gath J., Jensen P. H., Habenstein B., Madiona K., Olieric V., Böckmann A., Meier B. H., and Melki R. (2013) Structural and functional characterization of two α-synuclein strains. Nat. Commun. 4, 2575 10.1038/ncomms3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peelaerts W., Bousset L., Van der Perren A., Moskalyuk A., Pulizzi R., Giugliano M., Van den Haute C., Melki R., and Baekelandt V. (2015) α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 10.1038/nature14547 [DOI] [PubMed] [Google Scholar]
- 69. Anderson J. P., Walker D. E., Goldstein J. M., de Laat R., Banducci K., Caccavello R. J., Barbour R., Huang J., Kling K., Lee M., Diep L., Keim P. S., Shen X., Chataway T., Schlossmacher M. G., et al. (2006) Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 281, 29739–29752 10.1074/jbc.M600933200 [DOI] [PubMed] [Google Scholar]
- 70. Okochi M., Walter J., Koyama A., Nakajo S., Baba M., Iwatsubo T., Meijer L., Kahle P. J., and Haass C. (2000) Constitutive phosphorylation of the Parkinson's disease associated α-synuclein. J. Biol. Chem. 275, 390–397 10.1074/jbc.275.1.390 [DOI] [PubMed] [Google Scholar]
- 71. Pronin A. N., Morris A. J., Surguchov A., and Benovic J. L. (2000) Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J. Biol. Chem. 275, 26515–26522 10.1074/jbc.M003542200 [DOI] [PubMed] [Google Scholar]
- 72. Ellis C. E., Schwartzberg P. L., Grider T. L., Fink D. W., and Nussbaum R. L. (2001) α-Synuclein is phosphorylated by members of the Src family of protein-tyrosine kinases. J. Biol. Chem. 276, 3879–3884 10.1074/jbc.M010316200 [DOI] [PubMed] [Google Scholar]
- 73. Nakamura T., Yamashita H., Takahashi T., and Nakamura S. (2001) Activated Fyn phosphorylates α-synuclein at tyrosine residue 125. Biochem. Biophys. Res. Commun. 280, 1085–1092 10.1006/bbrc.2000.4253 [DOI] [PubMed] [Google Scholar]
- 74. Hasegawa M., Fujiwara H., Nonaka T., Wakabayashi K., Takahashi H., Lee V. M. Y., Trojanowski J. Q., Mann D., and Iwatsubo T. (2002) Phosphorylated α-synuclein is ubiquitinated in α-synucleinopathy lesions. J. Biol. Chem. 277, 49071–49076 10.1074/jbc.M208046200 [DOI] [PubMed] [Google Scholar]
- 75. Tofaris G. K., Razzaq A., Ghetti B., Lilley K. S., and Spillantini M. G. (2003) Ubiquitination of α-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J. Biol. Chem. 278, 44405–44411 10.1074/jbc.M308041200 [DOI] [PubMed] [Google Scholar]
- 76. Dorval V., and Fraser P. E. (2006) Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and α-synuclein. J. Biol. Chem. 281, 9919–9924 10.1074/jbc.M510127200 [DOI] [PubMed] [Google Scholar]
- 77. Giasson B. I., Duda J. E., Murray I. V. J., Chen Q., Souza J. M., Hurtig H. I., Ischiropoulos H., Trojanowski J. Q., and Lee V. M. (2000) Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science 290, 985–989 10.1126/science.290.5493.985 [DOI] [PubMed] [Google Scholar]
- 78. Citron B. A., Suo Z., SantaCruz K., Davies P. J. A., Qin F., and Festoff B. W. (2002) Protein crosslinking, tissue transglutaminase, alternative splicing and neurodegeneration. Neurochem. Int. 40, 69–78 10.1016/S0197-0186(01)00062-6 [DOI] [PubMed] [Google Scholar]
- 79. Junn E., Ronchetti R. D., Quezado M. M., Kim S. Y., and Mouradian M. M. (2003) Tissue transglutaminase-induced aggregation of α-synuclein: implications for Lewy body formation in Parkinson's disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 100, 2047–2052 10.1073/pnas.0438021100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Andringa G., Lam K. Y., Chegary M., Wang X., Chase T. N., and Bennett M. C. (2004) Tissue trans glutaminase catalyzes the formation of α-synuclein crosslinks in Parkinson's disease. FASEB J. 18, 932–934 10.1096/fj.03-0829fje [DOI] [PubMed] [Google Scholar]
- 81. Uversky V. N., Yamin G., Souillac P. O., Goers J., Glaser C. B., and Fink A. L. (2002) Methionine oxidation inhibits fibrillation of human α-synuclein in vitro. FEBS Lett. 517, 239–244 10.1016/S0014-5793(02)02638-8 [DOI] [PubMed] [Google Scholar]
- 82. Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M. S., Shen J., Takio K., and Iwatsubo T. (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 10.1038/ncb748 [DOI] [PubMed] [Google Scholar]
- 83. Tenreiro S., Reimão-Pinto M. M., Antas P., Rino J., Wawrzycka D., Macedo D., Rosado-Ramos R., Amen T., Waiss M., Magalhães F., Gomes A., Santos C. N., Kaganovich D., and Outeiro T. F. (2014) Phosphorylation modulates clearance of α-synuclein inclusions in a yeast model of Parkinson's disease. PLoS Genet. 10, e1004302 10.1371/journal.pgen.1004302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chartier-Harlin M. C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., Levecque C., Larvor L., Andrieux J., Hulihan M., Waucquier N., Defebvre L., Amouyel P., Farrer M., and Destée A. (2004) α-Synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169 10.1016/S0140-6736(04)17103-1 [DOI] [PubMed] [Google Scholar]
- 85. Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., et al. (2003) α-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 10.1126/science.1090278 [DOI] [PubMed] [Google Scholar]
- 86. Itokawa K., Sekine T., Funayama M., Tomiyama H., Fukui M., Yamamoto T., Tamura N., Matsuda H., Hattori N., and Araki N. (2013) A case of α-synuclein gene duplication presenting with head-shaking movements. Mov. Disord. 28, 384–387 10.1002/mds.25243 [DOI] [PubMed] [Google Scholar]
- 87. Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., and Mucke L. (2000) Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269 10.1126/science.287.5456.1265 [DOI] [PubMed] [Google Scholar]
- 88. Kahle P. J., Neumann M., Ozmen L., Muller V., Jacobsen H., Schindzielorz A., Okochi M., Leimer U., van Der Putten H., Probst A., Kremmer E., Kretzschmar H. A., and Haass C. (2000) Subcellular localization of wild-type and Parkinson's disease-associated mutant α-synuclein in human and transgenic mouse brain. J. Neurosci. 20, 6365–6373 10.1523/JNEUROSCI.20-17-06365.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Feany M. B., and Bender W. W. (2000) A Drosophila model of Parkinson's disease. Nature 404, 394–398 10.1038/35006074 [DOI] [PubMed] [Google Scholar]
- 90. van der Putten H., Wiederhold K. H., Probst A., Barbieri S., Mistl C., Danner S., Kauffmann S., Hofele K., Spooren W. P., Ruegg M. A., Lin S., Caroni P., Sommer B., Tolnay M., and Bilbe G. (2000) Neuropathology in mice expressing human α-synuclein. J. Neurosci. 20, 6021–6029 10.1523/JNEUROSCI.20-16-06021.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chesselet M. F., and Richter F. (2011) Modelling of Parkinson's disease in mice. Lancet Neurol. 10, 1108–1118 10.1016/S1474-4422(11)70227-7 [DOI] [PubMed] [Google Scholar]
- 92. Luk K. C., Kehm V., Carroll J., Zhang B., O'Brien P., Trojanowski J. Q., and Lee V. M. (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tran H. T., Chung C. H. Y., Iba M., Zhang B., Trojanowski J. Q., Luk K. C., and Lee V. M. (2014) α-Synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 7, 2054–2065 10.1016/j.celrep.2014.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Horvath S. E., and Daum G. (2013) Lipids of mitochondria. Prog. Lipid Res. 52, 590–614 10.1016/j.plipres.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 95. Perrin R. J., Woods W. S., Clayton D. F., and George J. M. (2001) Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J. Biol. Chem. 276, 41958–41962 10.1074/jbc.M105022200 [DOI] [PubMed] [Google Scholar]
- 96. Broersen K., van den Brink D., Fraser G., Goedert M., and Davletov B. (2006) α-Synuclein adopts an α-helical conformation in the presence of polyunsaturated fatty acids to hinder micelle formation. Biochemistry 45, 15610–15616 10.1021/bi061743l [DOI] [PubMed] [Google Scholar]
- 97. Sharon R., Bar-Joseph I., Frosch M. P., Walsh D. M., Hamilton J. A., and Selkoe D. J. (2003) The formation of highly soluble oligomers of α-synuclein is regulated by fatty acids and enhanced in Parkinson's disease. Neuron 37, 583–595 10.1016/S0896-6273(03)00024-2 [DOI] [PubMed] [Google Scholar]
- 98. Assayag K., Yakunin E., Loeb V., Selkoe D. J., and Sharon R. (2007) Polyunsaturated fatty acids induce α-synuclein-related pathogenic changes in neuronal cells. Am. J. Pathol. 171, 2000–2011 10.2353/ajpath.2007.070373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. De Franceschi G., Frare E., Bubacco L., Mammi S., Fontana A., and de Laureto P. P. (2009) Molecular insights into the interaction between α-synuclein and docosahexaenoic acid. J. Mol. Biol. 394, 94–107 10.1016/j.jmb.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 100. Sharon R., Bar-Joseph I., Mirick G. E., Serhan C. N., and Selkoe D. J. (2003) Altered fatty acid composition of dopaminergic neurons expressing α-synuclein and human brains with α-synucleinopathies. J. Biol. Chem. 278, 49874–49881 10.1074/jbc.M309127200 [DOI] [PubMed] [Google Scholar]
- 101. Tsang A. H. K., and Chung K. K. K. (2009) Oxidative and nitrosative stress in Parkinson's disease. Biochim. Biophys. Acta 1792, 643–650 10.1016/j.bbadis.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 102. Dalfó E., Portero-Otín M., Ayala V., Martínez A., Pamplona R., and Ferrer I. (2005) Evidence of oxidative stress in the neocortex in incidental Lewy body disease. J. Neuropathol. Exp. Neurol. 64, 816–830 10.1097/01.jnen.0000179050.54522.5a [DOI] [PubMed] [Google Scholar]
- 103. Morel P., Tallineau C., Pontcharraud R., Piriou A., and Huguet F. (1998) Effects of 4-hydroxynonenal, a lipid peroxidation product, on dopamine transport and Na+/K+ ATPase in rat striatal synaptosomes. Neurochem. Int. 33, 531–540 10.1016/S0197-0186(98)00062-X [DOI] [PubMed] [Google Scholar]
- 104. De Franceschi G., Fecchio C., Sharon R., Schapira A. H. V., Proukakis C., Bellotti V., de Laureto P. P. (2017) α-Synuclein structural features inhibit harmful polyunsaturated fatty acid oxidation, suggesting roles in neuroprotection. J. Biol. Chem. 292, 6927–6937 10.1074/jbc.M116.765149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Aoki R., and Li Y. R. (2011) α-Synuclein promotes neuroprotection through NF-κB-mediated transcriptional regulation of protein kinase Cδ. Sci. Signal. 4, jc6 10.1126/scisignal.2002425 [DOI] [PubMed] [Google Scholar]
- 106. Jo E., Fuller N., Rand R. P., St George-Hyslop P., and Fraser P. E. (2002) Defective membrane interactions of familial Parkinson's disease mutant A30P α-synuclein. J. Mol. Biol. 315, 799–807 10.1006/jmbi.2001.5269 [DOI] [PubMed] [Google Scholar]
- 107. Bussell R. Jr., and Eliezer D. (2004) Effects of Parkinson's disease-linked mutations on the structure of lipid-associated α-synuclein. Biochemistry 43, 4810–4818 10.1021/bi036135+ [DOI] [PubMed] [Google Scholar]
- 108. van Maarschalkerweerd A., Vetri V., Langkilde A. E., Foderà V., and Vestergaard B. (2014) Protein/lipid coaggregates are formed during α-synuclein-induced disruption of lipid bilayers. Biomacromolecules 15, 3643–3654 10.1021/bm500937p [DOI] [PubMed] [Google Scholar]
- 109. van Maarschalkerweerd A., Vetri V., and Vestergaard B. (2015) Cholesterol facilitates interactions between α-synuclein oligomers and charge-neutral membranes. FEBS Lett. 589, 2661–2667 10.1016/j.febslet.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 110. van Rooijen B. D., Claessens M. M. A. E., and Subramaniam V. (2009) Lipid bilayer disruption by oligomeric α-synuclein depends on bilayer charge and accessibility of the hydrophobic core. Biochim. Biophys. Acta 1788, 1271–1278 10.1016/j.bbamem.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 111. Giehm L., Svergun D. I., Otzen D. E., and Vestergaard B. (2011) Low-resolution structure of a vesicle disrupting α-synuclein oligomer that accumulates during fibrillation. Proc. Natl. Acad. Sci. U.S.A. 108, 3246–3251 10.1073/pnas.1013225108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rhoades E., Ramlall T. F., Webb W. W., and Eliezer D. (2006) Quantification of α-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys. J. 90, 4692–4700 10.1529/biophysj.105.079251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jo E., McLaurin J., Yip C. M., St George-Hyslop P., and Fraser P. E. (2000) α-Synuclein membrane interactions and lipid specificity. J. Biol. Chem. 275, 34328–34334 10.1074/jbc.M004345200 [DOI] [PubMed] [Google Scholar]
- 114. Zigoneanu I. G., Yang Y. J., Krois A. S., Haque E., and Pielak G. J. (2012) Interaction of α-synuclein with vesicles that mimic mitochondrial membranes. Biochim. Biophys. Acta 1818, 512–519 10.1016/j.bbamem.2011.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nakamura K., Nemani V. M., Azarbal F., Skibinski G., Levy J. M., Egami K., Munishkina L., Zhang J., Gardner B., Wakabayashi J., Sesaki H., Cheng Y., Finkbeiner S., Nussbaum R. L., Masliah E., and Edwards R. H. (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein α-synuclein. J. Biol. Chem. 286, 20710–20726 10.1074/jbc.M110.213538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Grey M., Dunning C. J., Gaspar R., Grey C., Brundin P., Sparr E., and Linse S. (2015) Acceleration of α-synuclein aggregation by exosomes. J. Biol. Chem. 290, 2969–2982 10.1074/jbc.M114.585703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fantini J., Carlus D., and Yahi N. (2011) The fusogenic tilted peptide (67–78) of α-synuclein is a cholesterol binding domain. Biochim. Biophys. Acta 1808, 2343–2351 10.1016/j.bbamem.2011.06.017 [DOI] [PubMed] [Google Scholar]
- 118. Martinez Z., Zhu M., Han S. and Fink A. L. (2007) GM1 specifically interacts with α-synuclein and inhibits fibrillation. Biochemistry 46, 1868–1877 10.1021/bi061749a [DOI] [PubMed] [Google Scholar]
- 119. Di Pasquale E., Fantini J., Chahinian H., Maresca M., Taïeb N., and Yahi N. (2010) Altered ion channel formation by the Parkinson's-disease-linked E46K mutant of α-synuclein is corrected by GM3 but not by GM1 gangliosides. J. Mol. Biol. 397, 202–218 10.1016/j.jmb.2010.01.046 [DOI] [PubMed] [Google Scholar]
- 120. Park J. Y., Kim K. S., Lee S. B., Ryu J. S., Chung K. C., Choo Y. K., Jou I., Kim J., and Park S. M. (2009) On the mechanism of internalization of α-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J. Neurochem. 110, 400–411 10.1111/j.1471-4159.2009.06150.x [DOI] [PubMed] [Google Scholar]
- 121. Fantini J., and Yahi N. (2011) Molecular basis for the glycosphingolipid-binding specificity of α-synuclein: key role of tyrosine 39 in membrane insertion. J. Mol. Biol. 408, 654–669 10.1016/j.jmb.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 122. Bartels T., Kim N. C., Luth E. S., Selkoe D. J. (2014) N-α-Acetylation of α-synuclein increases its helical folding propensity, GM1 binding specificity and resistance to aggregation. PLoS One 9, e103727 10.1371/journal.pone.0103727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Volles M. J., and Lansbury P. T. Jr. (2002) Vesicle permeabilization by protofibrillar α-synuclein is sensitive to Parkinson's disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 41, 4595–4602 10.1021/bi0121353 [DOI] [PubMed] [Google Scholar]
- 124. Lashuel H. A., Hartley D., Petre B. M., Walz T., and Lansbury P. T. Jr. (2002) Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418, 291 10.1038/418291a [DOI] [PubMed] [Google Scholar]
- 125. Middleton E. R., and Rhoades E. (2010) Effects of curvature and composition on α-synuclein binding to lipid vesicles. Biophys. J. 99, 2279–2288 10.1016/j.bpj.2010.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pranke I. M., Morello V., Bigay J., Gibson K., Verbavatz J. M., Antonny B., and Jackson C. L. (2011) α-Synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding. J. Cell Biol. 194, 89–103 10.1083/jcb.201011118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kjaer L., Giehm L., Heimburg T., and Otzen D. (2009) The influence of vesicle size and composition on α-synuclein structure and stability. Biophys. J. 96, 2857–2870 10.1016/j.bpj.2008.12.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jensen M. B., Bhatia V. K., Jao C. C., Rasmussen J. E., Pedersen S. L., Jensen K. J., Langen R., and Stamou D. (2011) Membrane curvature sensing by amphipathic helices: a single liposome study using α-synuclein and annexin B12. J. Biol. Chem. 286, 42603–42614 10.1074/jbc.M111.271130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cui H., Lyman E., and Voth G. A. (2011) Mechanism of membrane curvature sensing by amphipathic helix containing proteins. Biophys. J. 100, 1271–1279 10.1016/j.bpj.2011.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nuscher B., Kamp F., Mehnert T., Odoy S., Haass C., Kahle P. J., and Beyer K. (2004) α-Synuclein has a high affinity for packing defects in a bilayer membrane: a thermodynamics study. J. Biol. Chem. 279, 21966–21975 10.1074/jbc.M401076200 [DOI] [PubMed] [Google Scholar]
- 131. Irizarry M. C., Kim T. W., McNamara M., Tanzi R. E., George J. M., Clayton D. F., and Hyman B. T. (1996) Characterization of the precursor protein of the non-Aβ component of senile plaques (NACP) in the human central nervous system. J. Neuropathol. Exp. Neurol. 55, 889–895 10.1097/00005072-199608000-00004 [DOI] [PubMed] [Google Scholar]
- 132. Lokappa S. B., and Ulmer T. S. (2011) α-Synuclein populates both elongated and broken helix states on small unilamellar vesicles. J. Biol. Chem. 286, 21450–21457 10.1074/jbc.M111.224055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ulmer T. S., Bax A., Cole N. B., and Nussbaum R. L. (2005) Structure and dynamics of micelle-bound human α-synuclein. J. Biol. Chem. 280, 9595–9603 10.1074/jbc.M411805200 [DOI] [PubMed] [Google Scholar]
- 134. Ulmer T. S., and Bax A. (2005) Comparison of structure and dynamics of micelle-bound human α-synuclein and Parkinson disease variants. J. Biol. Chem. 280, 43179–43187 10.1074/jbc.M507624200 [DOI] [PubMed] [Google Scholar]
- 135. Cheng C.-Y., Varkey J., Ambroso M. R., Langen R., and Han S. (2013) Hydration dynamics as an intrinsic ruler for refining protein structure at lipid membrane interfaces. Proc. Natl. Acad. Sci. U.S.A. 110, 16838–16843 10.1073/pnas.1307678110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bodner C. R., Dobson C. M., and Bax A. (2009) Multiple tight phospholipid-binding modes of α-synuclein revealed by solution NMR spectroscopy. J. Mol. Biol. 390, 775–790 10.1016/j.jmb.2009.05.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bodner C. R., Maltsev A. S., Dobson C. M., and Bax A. (2010) Differential phospholipid binding of α-synuclein variants implicated in Parkinson's disease revealed by solution NMR spectroscopy. Biochemistry 49, 862–871 10.1021/bi901723p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wang G. F., Li C., and Pielak G. J. (2010) 19F NMR studies of α-synuclein-membrane interactions. Protein Sci. 19, 1686–1691 10.1002/pro.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Fusco G., De Simone A., Arosio P., Vendruscolo M., Veglia G., Dobson C. M. (2016) Structural ensembles of membrane-bound α-synuclein reveal the molecular determinants of synaptic vesicle affinity. Sci. Rep. 6, 27125 10.1038/srep27125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Fusco G., De Simone A., Gopinath T., Vostrikov V., Vendruscolo M., Dobson C. M., and Veglia G. (2014) Direct observation of the three regions in α-synuclein that determine its membrane-bound behaviour. Nat. Commun. 5, 3827 10.1038/ncomms4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sarafian T. A., Ryan C. M., Souda P., Masliah E., Kar U. K., Vinters H. V., Mathern G. W., Faull K. F., Whitelegge J. P., and Watson J. B. (2013) Impairment of mitochondria in adult mouse brain overexpressing predominantly full-length, N-terminally acetylated human α-synuclein. PLoS One 8, e63557 10.1371/journal.pone.0063557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bender A., Desplats P., Spencer B., Rockenstein E., Adame A., Elstner M., Laub C., Mueller S., Koob A. O., Mante M., Pham E., Klopstock T., and Masliah E. (2013) TOM40 mediates mitochondrial dysfunction induced by α-synuclein accumulation in Parkinson's disease. PLoS One 8, e62277 10.1371/journal.pone.0062277 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 143. Martin L. J., Pan Y., Price A. C., Sterling W., Copeland N. G., Jenkins N. A., Price D. L., and Lee M. K. (2006) Parkinson's disease α-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 26, 41–50 10.1523/JNEUROSCI.4308-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Li L., Nadanaciva S., Berger Z., Shen W., Paumier K., Schwartz J., Mou K., Loos P., Milici A. J., Dunlop J., and Hirst W. D. (2013) Human A53T α-synuclein causes reversible deficits in mitochondrial function and dynamics in primary mouse cortical neurons. PLoS One 8, e85815 10.1371/journal.pone.0085815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhu Y., Duan C., Lü L., Gao H., Zhao C., Yu S., Uëda K., Chan P., and Yang H. (2011) α-Synuclein overexpression impairs mitochondrial function by associating with adenylate translocator. Int. J. Biochem. Cell Biol. 43, 732–741 10.1016/j.biocel.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 146. Chinta S. J., Mallajosyula J. K., Rane A., and Andersen J. K. (2010) Mitochondrial α-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci. Lett. 486, 235–239 10.1016/j.neulet.2010.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Devi L., Raghavendran V., Prabhu B. M., Avadhani N. G., and Anandatheerthavarada H. K. (2008) Mitochondrial import and accumulation of α-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 283, 9089–9100 10.1074/jbc.M710012200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Reeve A. K., Ludtmann M. H. R., Angelova P. R., Simcox E. M., Horrocks M. H., Klenerman D., Gandhi S., Turnbull D. M., and Abramov A. Y. (2015) Aggregated α-synuclein and complex I deficiency: exploration of their relationship in differentiated neurons. Cell Death Dis. 6, e1820 10.1038/cddis.2015.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Parihar M. S., Parihar A., Fujita M., Hashimoto M., and Ghafourifar P. (2008) Mitochondrial association of α-synuclein causes oxidative stress. Cell. Mol. Life Sci. 65, 1272–1284 10.1007/s00018-008-7589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Schapira A. H. V., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. (1989) Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1, 1269 [DOI] [PubMed] [Google Scholar]
- 151. Parker W. D. Jr., Boyson S. J., and Parks J. K. (1989) Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann. Neurol. 26, 719–723 10.1002/ana.410260606 [DOI] [PubMed] [Google Scholar]
- 152. Janetzky B., Hauck S., Youdim M. B. H., Riederer P., Jellinger K., Pantucek F., Zöchling R., Boissl K. W., and Reichmann H. (1994) Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson's disease. Neurosci. Lett. 169, 126–128 10.1016/0304-3940(94)90372-7 [DOI] [PubMed] [Google Scholar]
- 153. Nakamura K., Nemani V. M., Wallender E. K., Kaehlcke K., Ott M., and Edwards R. H. (2008) Optical reporters for the conformation of α-synuclein reveal a specific interaction with mitochondria. J. Neurosci. 28, 12305–12317 10.1523/JNEUROSCI.3088-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cole N. B., Dieuliis D., Leo P., Mitchell D. C., and Nussbaum R. L. (2008) Mitochondrial translocation of α-synuclein is promoted by intracellular acidification. Exp. Cell Res. 314, 2076–2089 10.1016/j.yexcr.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Shavali S., Brown-Borg H. M., Ebadi M., and Porter J. (2008) Mitochondrial localization of α-synuclein protein in α-synuclein overexpressing cells. Neurosci. Lett. 439, 125–128 10.1016/j.neulet.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Dixon C., Mathias N., Zweig R. M., Davis D. A., and Gross D. S. (2005) α-Synuclein targets the plasma membrane via the secretory pathway and induces toxicity in yeast. Genetics 170, 47–59 10.1534/genetics.104.035493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Furukawa K., Matsuzaki-Kobayashi M., Hasegawa T., Kikuchi A., Sugeno N., Itoyama Y., Wang Y., Yao P. J., Bushlin I., and Takeda A. (2006) Plasma membrane ion permeability induced by mutant α-synuclein contributes to the degeneration of neural cells. J. Neurochem. 97, 1071–1077 10.1111/j.1471-4159.2006.03803.x [DOI] [PubMed] [Google Scholar]
- 158. Danzer K. M., Haasen D., Karow A. R., Moussaud S., Habeck M., Giese A., Kretzschmar H., Hengerer B., and Kostka M. (2007) Different species of α-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 27, 9220–9232 10.1523/JNEUROSCI.2617-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Varkey J., Isas J. M., Mizuno N., Jensen M. B., Bhatia V. K., Jao C. C., Petrlova J., Voss J. C., Stamou D. G., Steven A. C., and Langen R. (2010) Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins. J. Biol. Chem. 285, 32486–32493 10.1074/jbc.M110.139576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shi Z., Sachs J. N., Rhoades E., and Baumgart T. (2015) Biophysics of α-synuclein induced membrane remodelling. Phys. Chem. Chem. Phys. 17, 15561–15568 10.1039/C4CP05883F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Pfefferkorn C. M., Heinrich F., Sodt A. J., Maltsev A. S., Pastor R. W., and Lee J. C. (2012) Depth of α-synuclein in a bilayer determined by fluorescence, neutron reflectometry, and computation. Biophys. J. 102, 613–621 10.1016/j.bpj.2011.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ouberai M. M., Wang J., Swann M. J., Galvagnion C., Guilliams T., Dobson C. M., and Welland M. E. (2013) α-Synuclein senses lipid packing defects and induces lateral expansion of lipids leading to membrane remodeling. J. Biol. Chem. 288, 20883–20895 10.1074/jbc.M113.478297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Devaux P. F., and Morris R. (2004) Transmembrane asymmetry and lateral domains in biological membranes. Traffic 5, 241–246 10.1111/j.1600-0854.2004.0170.x [DOI] [PubMed] [Google Scholar]
- 164. Darios F., Ruipérez V., López I., Villanueva J., Gutierrez L. M., and Davletov B. (2010) α-Synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 11, 528–533 10.1038/embor.2010.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lou X., Kim J., Hawk B. J., and Shin Y. K. (2017) α-Synuclein may cross-bridge v-SNARE and acidic phospholipids to facilitate SNARE-dependent vesicle docking. Biochem. J. 474, 2039–2049 10.1042/BCJ20170200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Fusco G., Chen S. W., Williamson P. T. F., Cascella R., Perni M., Jarvis J. A., Cecchi C., Vendruscolo M., Chiti F., Cremades N., Ying L., Dobson C. M., and De Simone A. (2017) Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers. Science 358, 1440–1443 10.1126/science.aan6160 [DOI] [PubMed] [Google Scholar]
- 167. Conway K. A., Rochet J. C., Bieganski R. M., and Lansbury P. T. Jr. (2001) Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science 294, 1346–1349 10.1126/science.1063522 [DOI] [PubMed] [Google Scholar]
- 168. Cappai R., Leck S.-L., Tew D. J., Williamson N. A., Smith D. P., Galatis D., Sharples R. A., Curtain C. C., Ali F. E., Cherny R. A., Culvenor J. G., Bottomley S. P., Masters C. L., Barnham K. J., and Hill A. F. (2005) Dopamine promotes α-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 19, 1377–1379 10.1096/fj.04-3437fje [DOI] [PubMed] [Google Scholar]
- 169. Lee H. J., Baek S. M., Ho D. H., Suk J. E., Cho E. D., and Lee S. J. (2011) Dopamine promotes formation and secretion of non-fibrillar α-synuclein oligomers. Exp. Mol. Med. 43, 216–222 10.3858/emm.2011.43.4.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bereczki E., Francis P. T., Howlett D., Pereira J. B., Höglund K., Bogstedt A., Cedazo-Minguez A., Baek J. H., Hortobágyi T., Attems J., Ballard C., and Aarsland D. (2016) Synaptic proteins predict cognitive decline in Alzheimer's disease and Lewy body dementia. Alzheimers Dement. 12, 1149–1158 10.1016/j.jalz.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 171. Vallortigara J., Whitfield D., Quelch W., Alghamdi A., Howlett D., Hortobágyi T., Johnson M., Attems J., O'Brien J. T., Thomas A., Ballard C. G., Aarsland D., and Francis P. T. (2016) Decreased levels of VAMP2 and monomeric α-synuclein correlate with duration of dementia. J. Alzheimers Dis. 50, 101–110 10.3233/JAD-150707 [DOI] [PubMed] [Google Scholar]
- 172. Bussell R. Jr., and Eliezer D. (2003) A structural and functional role for 11-mer repeats in α-synuclein and other exchangeable lipid binding proteins. J. Mol. Biol. 329, 763–778 10.1016/S0022-2836(03)00520-5 [DOI] [PubMed] [Google Scholar]
- 173. Llinás R., Sugimori M., and Silver R. B. (1992) Microdomains of high calcium concentration in a presynaptic terminal. Science 256, 677–679 10.1126/science.1350109 [DOI] [PubMed] [Google Scholar]
- 174. Schneggenburger R., and Neher E. (2000) Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature 406, 889–893 10.1038/35022702 [DOI] [PubMed] [Google Scholar]
- 175. Gross R. W. (2017) The evolution of lipidomics through space and time. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 731–739 10.1016/j.bbalip.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lohman D. C., Aydin D., Von Bank H. C., Smith R. W., Linke V., Weisenhorn E., Weisenhorn E., McDevitt M. T., Hutchins P., Wilkerson E. M., Wancewicz B., Russell J., Stefely M. S., Beebe E. T., Jochem A., Coon J. J., et al. (2019) An isoprene lipid-binding protein promotes eukaryotic coenzyme Q biosynthesis. Mol. Cell 73, 763–774.e10 10.1016/j.molcel.2018.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Saborio G. P., Permanne B., and Soto C. (2001) Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 10.1038/35081095 [DOI] [PubMed] [Google Scholar]
- 178. Herva M. E., Zibaee S., Fraser G., Barker R. A., Goedert M., Spillantini M. G. (2014) Anti-amyloid compounds inhibit α-synuclein aggregation induced by protein misfolding cyclic amplification (PMCA). J. Biol. Chem. 289, 11897–11905 10.1074/jbc.M113.542340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ma M. R., Hu Z. W., Zhao Y. F., Chen Y. X., and Li Y. M. (2016) Phosphorylation induces distinct α-synuclein strain formation. Sci. Rep. 6, 37130 10.1038/srep37130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Karampetsou M., Ardah M. T., Semitekolou M., Polissidis A., Samiotaki M., Kalomoiri M., Majbour N., Xanthou G., El-Agnaf O. M. A., and Vekrellis K. (2017) Phosphorylated exogenous α-synuclein fibrils exacerbate pathology and induce neuronal dysfunction in mice. Sci. Rep. 7, 16533 10.1038/s41598-017-15813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Samuel F., Flavin W. P., Iqbal S., Pacelli C., Sri Renganathan S. D., Trudeau L. E., Campbell E. M., Fraser P. E., and Tandon A. (2016) Effects of serine 129 phosphorylation on α-synuclein aggregation, membrane association, and internalization. J. Biol. Chem. 291, 4374–4385 10.1074/jbc.M115.705095 [DOI] [PMC free article] [PubMed] [Google Scholar]