Abstract
Triple negative breast cancers (TNBC) without BRCA1/2 gene mutation or BRCAness are nowadays the breast malignancies most difficult to treat. Improvement of their treatment, for all phases of the disease, is an important unmet medical need. We analyzed the effect of homoharringtonine (HHT), a natural protein synthesis inhibitor approved for treatment of chronic myeloid leukemia, on four cell lines representing aggressive, BRCA1/2 non-mutated, TNBC genomic categories. We show that HHT inhibits in vitro growth of all cell lines for more than 80%, after 48-72 h exposure to 20-100 ng/mL, the concentrations achievable in human plasma after subcutaneous administration of the drug. HHT, at 100 ng/mL, strongly reduced levels of a major TNBC survival factor, anti-apoptotic protein Mcl-1, after only 2 h of exposure, in all cell lines except MDA-MB-231. Other anti-apoptotic proteins, Bcl-2, survivin and XIAP, were also strongly downregulated. Moreover, in vivo growth of the least sensitive cell line to HHT in vitro, MDA-MB-231, was inhibited for 36.5% in mice, by 1 mg/kg of the drug, given subcutaneously, bi-daily, over 7 days. These results demonstrate marked antineoplastic activity of homoharringtonine in TNBC, making further development of the drug in this disease highly warranted.
Keywords: Homoharringtonine, breast cancer, protein synthesis, translation inhibitor, Mcl-1
Introduction
Triple-negative breast cancer (TNBC) is today the breast cancer subtype most difficult to treat, due to exquisite heterogeneity at the clinical, phenotypic, genotypic and microenvironmental level [1]. Some TNBC subtypes are orphan diseases [2], whereas, in certain regions of the world, TNBC is a frequent, devastating cancer of young women, affecting reproductive and economic power of entire countries [3]. Current standard treatment for most TNBC patients is neoadjuvant chemotherapy (NACT), followed by breast surgery [4]. NACT leaves around two thirds of patients with residual disease, carrying a risk for metastatic recurrences [5]. The armamentarium for systemic treatment of metastatic TNBC being very limited [6], patients in this phase of the disease have the worst prognosis among all breast cancer patients, with overall survival often shorter than 12 months [7].
Therefore, improvement of TNBC treatment, for all disease phases, is still a big unmet medical need. However, after the initial success of an anticancer agent or regimen, TNBC, as many other cancers, frequently develops resistance. Efficacious, rapid and affordable ways to prevent or/and combat that resistance need to be found. In such a situation, repositioning into TNBC treatment the drugs which have shown capacity to suppress other cancers, even after several prior treatments, is an approach that should not be neglected.
Homoharringtonine (HHT), also named omacetaxine mepesuccinate (international non-proprietary name), is approved by the US FDA for treatment of patients with chronic myeloid leukemia (CML) resistant or intolerant to two or more tyrosine kinase inhibitors (TKI) [8]. HHT has been used in China for more than 50 years in treatment of pts with myeloid leukemias. Current regimens, which combine HHT with cytarabine and daunorubicin or aclarubicin, induce up to 85% of complete response in acute myeloid leukemia (AML), after only two cycles [9]. So, HHT is a potent anticancer agent, efficacious in several aggressive blood malignancies, even in cases of resistance to specific, targeted therapy like TKI. HHT has recently been confirmed to efficaciously kill also the lung cancer cells with primary or TKI-induced resistance [10,11].
HHT fixes to the ribosomes, disabling elongation of the nascent peptide chain and further protein synthesis [12]. The antileukemic actions of HHT are considered to be exerted principally by this mechanism, which occurs downstream all other ways of RNA translation inhibition [13]. Inhibition of protein synthesis by HHT has been shown to strongly reduce abundance of oncodrivers (like Bcr-abl protein in CML) or other proteins crucial for cancer cell survival [14].
Increased protein synthesis is present in cancers, both as a part of increased global anabolic activity and as local adaptative event [15-17]. In particular, resistance of cancer cell to death is enabled by increased abundance of anti-apoptotic proteins [18,19]. One of them, Mcl-1, is a crucial determinant of breast cancer survival [20,21]. Most TNBC have increased level of Mcl-1 protein, either due to the amplification of MCL1 gene [22], or to hyperactivation of the PI3K-mTOR axis, found in up to 70% of cases [23]. An efficacious reduction of Mcl-1 levels highly facilitates induction of cancer cell death by other anti-neoplastic agents [24]. This has encouraged development of several Mcl-1 inhibitors [25-27], however none of them is approved yet for clinical use.
As several studies have shown rapid and marked reduction of Mcl-1 protein level by HHT in blood malignancies [28-30], we evaluated efficacity of this agent on the models of aggressive TNBC. Here we are showing that HHT induces the same effect on TNBC in vitro as the one reported in blood cancers (leukemias, myeloma). In addition, HHT alone inhibits TNBC growth in mice after a short and easy to administer treatment (7 days, subcutaneous). These results support development of HHT in the treatment of TNBC patients, thus enlarging the area of exploitation of protein synthesis inhibition as an efficacious anticancer approach.
Material and methods
Cell lines and culture conditions
We used MDA-MB-231 (ATCC® HTB-26™), MDA-MB-468 (ATCC® HTB-132™), MDA-MB-157 (ATCC® HTB-24™) and CAL-51, chosen to represent the BRCA1/2 non-mutated TNBC transcriptomic subtypes [31]. The MDA lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). CAL-51 was kindly provided by Dr Gerard Milano, from the Centre Antoine Lacassagne, Nice, France, where the line has been established [32]. The cell line stock vials were conserved at the Biological Resources Facility of Centre Jean Perrin (biobank ID BB-0033-00075, Clermont-Ferrand, France).
The MDA cell lines were cultured as previously described [33]. CAL-51 was maintained in DMEM, supplemented with 10% heat-inactivated fetal bovine serum and 20 mg/mL gentamicin (both media from Invitrogen Life Technologies, Carlsbad, CA, USA), in a humidified atmosphere with 5% CO2.
HHT solution preparation
Highly purified HHT of natural origin was supplied by LeukePharma Inc. (Houston, TX, USA) in the form of water-soluble, crystalline tartaric acid salt. HHT-hydrogen (2R,3R)-(+)-tartrate (named below “HHT tartrate”; empirical formula C33H45NO15; extended formula C29H40NO9, C4H5O6; molecular weight 695.7 g/mol) was prepared from natural HHT by fractional crystallization which yields HHT of the same purity level as the semi-synthetic form (US Patent WO 2015101628 (2018) and related US patents).
Initial stock solution of HHT tartrate was prepared at 10 mM in sterile deionized water, aliquoted and kept at -80°C. For each experiment, a fresh HHT tartrate solution was prepared by dissolving the HHT tartrate stock in sterile phosphate-buffered saline.
Cell viability assay
Cell viability was assessed using the sulforhodamine B (SRB) assay (Sigma-Aldrich, St Louis, MO, USA), as previously described [33]. Cells were seeded in hexaplicates, in 96-well plates at 5 × 103 (MDA cell lines) or 1 × 103 cells/well (CAL-51).
Cell cycle analysis
Cells were seeded into 6-well plates at 5 × 104 cells per well (each cell line), left overnight to adhere, and then treated or not by 20-50 ng/mL HHT for 48 h. The assay was further preformed as previously described [33].
Apoptosis assay
Cell preparation was performed as in the cell cycle assays. The cells were treated by 20-100 ng/mL of HHT for 6, 24, 48 or 72 hours. Apoptosis measurement was performed by flow-cytometry, as previously described [33].
Western blotting
Cells were plated in 10 cm Petri dishes at 5 × 105 cells per dish, left overnight to adhere and treated, the following day, with 100 ng/mL of HHT over 2-48 h. Further processing, ending with the signal revelation was done as previously described [33]. The following primary antibodies were used: anti-Mcl-1 (clone D35A5), anti-Bcl-2 (50E3), anti-XIAP (D2Z8W), anti-survivin (71G4B7), anti-caspase 3 (8G10), anti-PARP (rabbit polyclonal, cat. number 9542), anti-Myc (D84C12), anti-Akt (C67E7), anti-phospho-Akt (D9E), anti-p44/p42 MAPK (rabbit polyclonal, cat. number 9102), anti-phospho-p44/p42 MAPK (D13134E), anti-S6 ribosomal protein (5G10), anti-phospho-S6 ribosomal protein (D57.2.2E), anti-β-actin (D6A8), all from Cell Signaling Technology (Danvers, MA, USA). All antibodies were used at final concentration 1:1000. The chemiluminescent signal detection was performed using a ChemiDoc XRS + gel imager (Bio-Rad).
siRNA experiments
MDA-MB-468 and CAL-51 cells were seeded in 6-well plates, at 2 × 105 cells and 2.5 mL of DMEM per well. After 24 h, the cells were transfected by MCL1 siRNA and mock siRNA using Lipofectamine® (Invitrogen, ThermoFisher Scientific, Waltham, MA, USA). Briefly, the solution of MCL1 siRNA or mock siRNA were prepared by dissolving 3 µl of the respective reagents (ON-TARGETplus Human MCL1 siRNA and ON-TARGETplus Non-targeting siRNA, both from Dharmacon, Lafayette, CO, USA) in 150 µl of medium (Gibco®Opti-MEM™, Life Technologies, Carlsbad, CA, USA). Lipofectamine® RNAiMAX medium (9 µl) was also diluted in Opti-MEM™ (150 µl). Finally, the solution of each siRNA was added to the Lipofectamine solution, mixed well, and 250 µl of the mixture was added to the cells. The cells were then treated by 100 ng/mL HHT for 24 h and 48 h, lysed, proteins extracted and western blots performed.
Animal studies
The experiments were conducted according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication n°85-23, revised 1996) and approved by the Ethic Committee of Clermont-Ferrand, France. All mouse studies were conducted in the animal facility of the INSERM U1240. Six weeks old Swiss nu/nu female mice were purchased from Charles River Laboratories France and housed in standard conditions (n = 5 per cage on ventilated racks, at 21-24°C, 60% humidity, 12 h light/12 h dark cycle) with ad libitum access to irradiated food and water.
For establishment of xenografts, groups of 10 mice were injected subcutaneously, in the right flank area, with 1 × 107 MDA-MB-231 or 5 × 106 MDA-MB-468 cells suspended in 150 μl of culture medium/Matrigel® (BD Biosciences, San Jose, CA, USA) in a 4:1 ratio.
For assessment of HHT toxicity, groups of 6 mice were injected with sterile saline water (0.9% NaCl) or 1, 2, 4 or 8 mg/kg of the drug, subcutaneously, bi-daily, over 7 days. The animals were left to rest 2 more days and sacrificed at day 10. The solution for injection were freshly prepared by serial dilutions of the stock HHT-tartrate in sterile saline water and filtered through sterile filters having 0.25 µM pore size (Merck/Millipore, Burlington, MA, USA). The number of live animals was determined at days 4, 8 and 10, after treatment initiation.
For evaluation of HHT efficacy in vivo, mice were randomized into the control or the treated group when xenografts reached a volume of 200 ± 20 mm3. The MDA-MB-231 xenografts were treated by 1 mg/kg of HHT, using the same protocol as for determination of HHT toxicity. The MDA-MB-468 xenografts were treated by 0.5 mg/kg, as 1 mg/kg was too toxic for the animals (all mice died after only a few days, in a preliminary experiment; data not shown). Tumor size was non-invasively assessed using a digital caliper on days 3, 6, 8 and 10 after treatment initiation. Tumor volume was calculated using the following formula: V = (length × width2)/2.
The drug was evaluated in a total of 21 mice (2 independent experiments on MDA-MB-231 tumors, with 8 or 9 mice per experiment, as well as in one experiment on MDA-MB-468 tumors, with 4 mice). Xenografting of CAL-51 cells was not successful, after several attempts.
Statistical analysis
The IC50 of HHT after 24, 48 or 72 h were determined by the Chou-Talalay method [34], using Compusyn software (freely downloadable from www.combosyn.com). Mean ± standard deviation was used for presentation of all values. Variations of the viable or apoptotic cell fraction according to cell type, exposure time or drug concentration were studied using two-way ANOVA. Comparison of mean values between two time-points was tested by Kruskal-Wallis H-test because distributions were not Gaussian or heteroscedastic. Tests were two-sided and the standard level P ≤ 0.05 was considered statistically significant.
Results
HHT inhibits growth of TNBC cell lines, in concentrations achievable in patients’ plasma
As shown on Figure 1A, inhibitory effect of HHT on TNBC cell growth in vitro was observed already after 24 h. At that time, IC50 were 15.7 ng/mL, 19.9 ng/mL, 23.1 ng/mL and 80.5 ng/mL, for MDA-MB-157, MDA-MB-468, CAL-51 and MDA-MB-231, respectively. IC50 for the first three cell lines are below the lowest concentration of HHT (31.3 ng/mL), found 5 days after the last injection, in plasma of AML patients treated subcutaneously, bi-daily, by 5 mg/m2 for 9 days [35]. IC50 for MDA-MB-231 is comparable to the maximal plasmatic concentration of HHT found in patients, at the same time point, after the same regimen.
Figure 1.
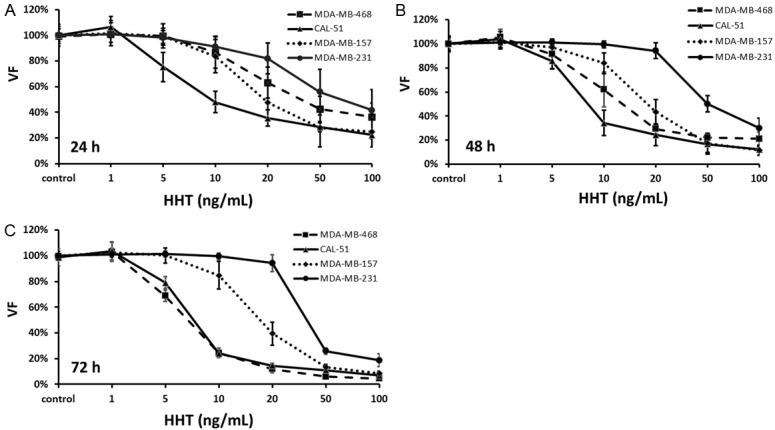
Cell viability after exposure to homoharringtonine in vitro. HHT, homoharringtonine; VF, viable fraction. As HHT-tartrate is soluble in water, the control samples were omitted the drug and the volume was replaced with the culture media. The concentrations are given as the HHT equivalent in HHT tartrate. The values represent a mean ± SD of three independent experiments.
HHT effect on cell line growth was dose- but not time-dependent in MDA-MB-157, MDA-MB-468 and CAL-51 (no significant change of IC50 after 48 h or 72 h, P > 0.05, Figure 1B and 1C). However, growth inhibition on MDA-MB-231 cells was both dose- and time-dependent: concentrations below 50 ng/mL could not significantly reduce cell growth, even after 72 h. However, when MDA-MB-231 was exposed to higher concentrations of HHT, the viable fraction (VF) was significantly lower after 72 h, compared to 24 h (at 50 ng/mL, VF: 25.9 ± 2.4% vs 58.1 ± 14.5%, 72 h vs 24 h resp., P < 0.01; at 100 ng/mL, VF: 18.7 ± 4.8% vs 41.7 ± 13.3%, 72 h vs 24 h, P < 0.01, Figure 1A and 1C). Thus, the viable fraction of MDA-MB-231 cells, after 72 h of exposure to high concentrations of HHT, was similar to the viable fractions in other cell lines (Figure 1C).
Taken together, these results indicate that HHT, in vitro, strongly inhibits (more than 80%) growth of TNBC cells with different molecular characteristics (3 transcriptomic subtypes: mesenchymal (CAL-51), mesenchymal stem-like (MDA-MB-157 and MDA-MB-231, basal-like 1 (MDA-MB-468) [31]).
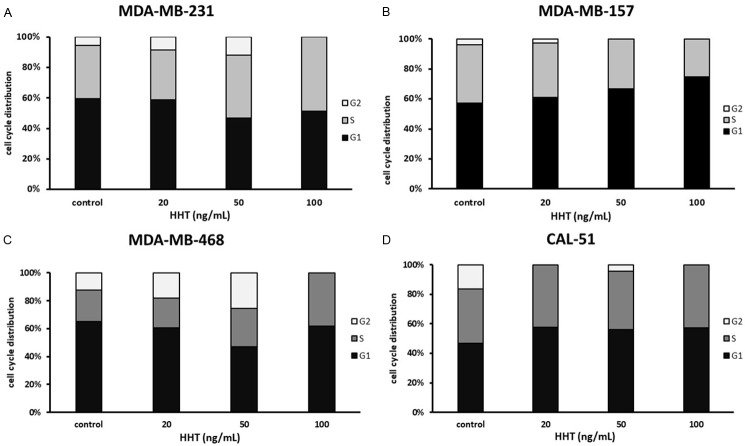
To get a better insight into cell growth-inhibitory action of HHT, we first analyzed changes in the size of cell populations engaged in different phases of the cell cycle, after 48 h-exposure to 20-100 ng/mL of the drug. As shown on Figure 2B and 2D, HHT, already at 20 ng/mL, induced accumulation in the G1 (MDA-MB-157) or/and in the S- and G1-phase (CAL-51). Under such conditions, MDA-MB-468 and MDA-MB-231 cells were first arrested in the G2/M phase; a stronger arrest occurred at 50 ng/mL (Figure 2A and 2C). The maximal concentration of HHT, 100 ng/mL, arrested both MDA-MB-468 and MDA-MB-231 cells in the S- and G1-phase of the cell cycle (Figure 2A and 2C).
Figure 2.
Distribution of cells in the cell cycle phases after exposure to homoharringtonine for 48 h. HHT, homoharringtonine. As HHT-tartrate is soluble in water, the control samples were omitted the drug and the volume was replaced with the culture media. The concentrations are given as the HHT equivalent in HHT tartrate. The figure represents the most representative of three independent experiments.
HHT induces apoptosis of TNBC cells, in cell-, dose- and time-dependent manner
We next evaluated the capacity of HHT to induce apoptosis in TNBC cell lines, as well as the kinetics of that phenomenon, knowing that in blood malignancies HHT can induce cell death as early as after 3-4 hours of exposure [28-30].
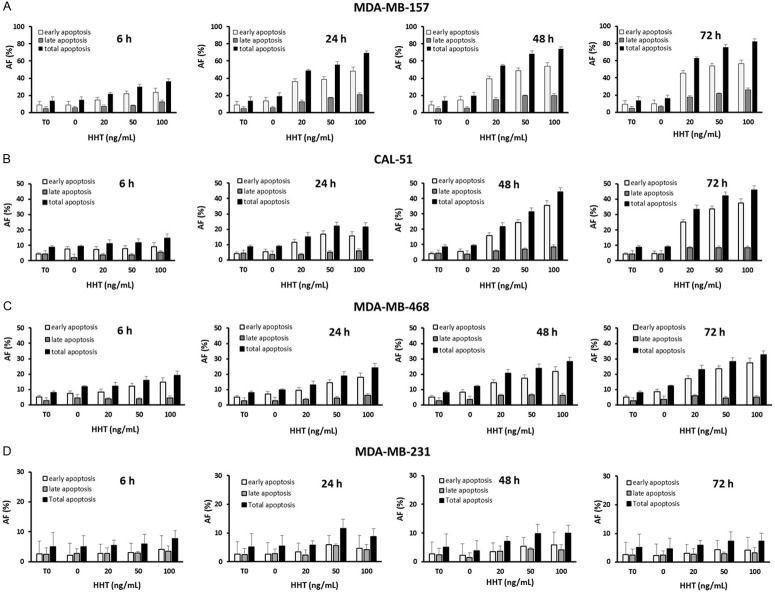
As shown on Figure 3, the four analyzed cell lines showed different levels of apoptosis after exposure to 20-100 ng/mL of the drug, over a time course from 6 to 72 h. MDA-MB-231 cells were most resistant to apoptosis induction: the total apoptotic fraction (AF) did not exceed 11.6 ± 3.6% at any concentration and at any time point (Table 1). In other three cell lines (MDA-MB-157, MDA-MB-468 and CAL-51) the AF was cell type-dependent (P = 0.0016): MDA-MB-157 cells were markedly more sensitive to the apoptosis induction than MDA-MB-468 and CAL-51 cells, as the mean value of AF obtained by all drug concentrations, at all time-points, was twice higher in MDA-MB-157 cells (44.9%) than the corresponding mean value obtained either in MDA-MB-468 (18.4%) or in CAL-51 cells (21.2%) (P = 6.5 × 10-5 for MDA-MB-157 vs MDA-MB-468 and P = 1.4 × 10-3 for MDA-MB-157 vs CAL-51).
Figure 3.
Apoptotic fractions in the cell lines exposed to homoharringtonine. HHT, homoharringtonine; AF, apoptotic fraction; T0, beginning of the experiment (before adding the drug). As HHT tartrate is soluble in water, the control samples were omitted the drug and the volume was replaced with the culture media. The concentrations are given as the HHT equivalent in HHT tartrate. The values represent a mean ± SD of three independent experiments.
Table 1.
Apoptotic fractions in TNBC cell lines exposed to homoharringtonine
| HHT (ng/ml) | AF | T0 | T6 | T24 | ||||||
|
|
|
|||||||||
| 0 | 0 | 20 | 50 | 100 | 0 | 20 | 50 | 100 | ||
|
| ||||||||||
| MDA-MB-157 | ||||||||||
| Early | 9.1 ± 4.2 | 8.9 ± 4.0 | 14.7 ± 3.0 | 21.8 ± 3.1 | 23.7 ± 4.5 | 13.6 ± 2.1 | 36.2 ± 3.3 | 38.6 ± 2.8 | 48.2 ± 3.5 | |
| Late | 4.7 ± 2.2 | 5.9 ± 1.6 | 7.2 ± 1.9 | 8.2 ± 0.6 | 12.6 ± 1.8 | 5.5 ± 1.3 | 12.7 ± 1.5 | 17.3 ± 3.3 | 21.1 ± 1.6 | |
| Total | 13.7 ± 4.7 | 14.8 ± 3.7 | 21.9 ± 1.6 | 30.0 ± 3.2 | 36.3 ± 2.7 | 19.1 ± 3.3 | 48.9 ± 4.8 | 55.9 ± 0.5 | 69.3 ± 4.1 | |
| MDA-MB-468 | ||||||||||
| Early | 5.2 ± 1.7 | 7.4 ± 1.2 | 8.3 ± 1.4 | 12.1 ± 4.1 | 14.7 ± 4.5 | 7.2 ± 0.8 | 9.5 ± 1.3 | 14.4 ± 4.9 | 18.1 ± 5.5 | |
| Late | 2.7 ± 1.2 | 4.4 ± 1.1 | 3.8 ± 0.9 | 3.8 ± 0.5 | 4.6 ± 0.9 | 2.7 ± 0.9 | 3.5 ± 1.7 | 4.5 ± 0.7 | 6.2 ± 0.6 | |
| Total | 7.9 ± 2.8 | 11.8 ± 1.7 | 12.1 ± 2.3 | 15.9 ± 4.2 | 19.2 ± 3.8 | 9.9 ± 1.5 | 13.0 ± 2.8 | 19.0 ± 5.4 | 24.3 ± 5.7 | |
| MDA-MB-231 | ||||||||||
| Early | 2.7 ± 0.5 | 2.2 ± 0.5 | 2.8 ± 1.2 | 3.0 ± 0.7 | 4.2 ± 1.7 | 2.6 ± 0.7 | 3.4 ± 1.0 | 6.0 ± 1.6 | 4.6 ± 1.3 | |
| Late | 2.4 ± 1.0 | 2.8 ± 1.0 | 2.7 ± 0.5 | 2.9 ± 0.8 | 3.5 ± 1.1 | 2.8 ± 0.8 | 2.3 ± 0.2 | 5.6 ± 2.3 | 4.2 ± 1.8 | |
| Total | 5.1 ± 1.4 | 5.0 ± 1.4 | 5.5 ± 1.7 | 6.0 ± 0.9 | 7.7 ± 2.1 | 5.5 ± 1.2 | 5.7 ± 1.0 | 11.6 ± 3.6 | 8.8 ± 1.8 | |
| CAL-51 | ||||||||||
| Early | 4.2 ± 1.0 | 7.4 ± 1.6 | 7.2 ± 1.8 | 7.8 ± 2.0 | 9.1 ± 2.8 | 5.3 ± 1.5 | 11.6 ± 2.8 | 16.9 ± 0.7 | 15.5 ± 1.3 | |
| Late | 4.4 ± 2.0 | 1.9 ± 2.2 | 3.8 ± 0.9 | 3.9 ± 0.9 | 5.4 ± 1.2 | 3.7 ± 1.2 | 3.6 ± 0.1 | 5.1 ± 0.6 | 6.1 ± 1.0 | |
| Total | 8.6 ± 1.1 | 9.3 ± 0.7 | 11.0 ± 2.6 | 11.7 ± 2.6 | 14.5 ± 2.8 | 9.0 ± 1.2 | 15.2 ± 2.8 | 22.0 ± 0.2 | 21.6 ± 1.1 | |
|
| ||||||||||
| HHT (ng/ml) | AF | T48 | T72 | |||||||
|
|
|
|||||||||
| 0 | 20 | 50 | 100 | 0 | 20 | 50 | 100 | |||
|
| ||||||||||
| MDA-MB-157 | ||||||||||
| Early | 14.6 ± 3.8 | 39.4 ± 2.7 | 48.8 ± 2.8 | 53.9 ± 4.2 | 9.9 ± 2.7 | 45.5 ± 3.9 | 53.7 ± 4.6 | 56.3 ± 3.0 | ||
| Late | 5.1 ± 1.3 | 15.2 ± 1.9 | 19.7 ± 0.6 | 20.0 ± 4.6 | 6.4 ± 2.5 | 17.5 ± 2.8 | 22.0 ± 4.6 | 26.1 ± 1.2 | ||
| Total | 19.7 ± 3.7 | 54.6 ± 3.9 | 68.5 ± 2.8 | 73.9 ± 4.6 | 16.3 ± 4.7 | 62.9 ± 1.1 | 75.7 ± 4.0 | 82.4 ± 2.5 | ||
| MDA-MB-468 | ||||||||||
| Early | 8.3 ± 1.2 | 14.5 ± 3.3 | 17.6 ± 4.1 | 21.9 ± 5.8 | 8.7 ± 1.2 | 17.1 ± 4.1 | 23.6 ± 5.5 | 27.5 ± 4.5 | ||
| Late | 3.8 ± 1.3 | 6.3 ± 0.2 | 6.4 ± 0.9 | 6.3 ± 1.5 | 3.6 ± 0.5 | 6.0 ± 0.2 | 4.6 ± 1.8 | 5.0 ± 1.3 | ||
| Total | 12.1 ± 1.7 | 20.7 ± 3.5 | 24.0 ± 3.2 | 28.3 ± 6.0 | 12.3 ± 0.8 | 23.1 ± 4.1 | 28.2 ± 6.1 | 32.5 ± 5.7 | ||
| MDA-MB-231 | ||||||||||
| Early | 2.2 ± 0.9 | 3.5 ± 1.7 | 5.4 ± 1.4 | 5.8 ± 1.3 | 2.3 ± 1.0 | 3.2 ± 2.1 | 4.4 ± 2.2 | 4.1 ± 1.3 | ||
| Late | 1.5 ± 0.4 | 3.7 ± 2.2 | 4.4 ± 1.0 | 4.2 ± 1.8 | 2.4 ± 1.2 | 2.8 ± 1.0 | 2.9 ± 0.7 | 3.2 ± 0.2 | ||
| Total | 3.7 ± 0.5 | 7.2 ± 3.1 | 9.8 ± 1.5 | 10.0 ± 0.5 | 4.7 ± 1.7 | 6.0 ± 2.8 | 7.3 ± 2.9 | 7.4 ± 1.4 | ||
| CAL-51 | ||||||||||
| Early | 5.5 ± 0.8 | 16.0 ± 2.8 | 24.4 ± 2.7 | 35.6 ± 4.2 | 4.7 ± 1.4 | 25.0 ± 2.9 | 33.6 ± 4.4 | 37.6 ± 4.0 | ||
| Late | 3.9 ± 0.3 | 5.8 ± 0.5 | 6.9 ± 0.4 | 8.6 ± 1.1 | 4.3 ± 1.7 | 8.4 ± 0.9 | 8.5 ± 1.4 | 8.4 ± 1.2 | ||
| Total | 9.4 ± 0.7 | 21.8 ± 2.6 | 31.3 ± 3.1 | 44.2 ± 3.5 | 9.1 ± 2.5 | 33.5 ± 2.1 | 42.1 ± 2.9 | 46.0 ± 3.4 | ||
AF, apoptotic fraction; HHT, homoharringtonine; T0, start of the experiment; T6, after 6 h of exposure; T24, after 24 h of exposure; T48, after 48 h of exposure; T72, after 72 h of exposure. All values are given in percentages (%) and represent mean ± SD of three independent experiments.
The apoptotic effect of HHT was dose- and time-dependent in cells other than MDA-MB-231 (Figure 3). The total AF in MDA-MB-157 cells, after the lowest drug concentration tested, 20 ng/mL, was 21.9 ± 1.6% already after 6 h of exposure, and reached the maximum, 82.4 ± 2.5%, after 72 h of exposure to 100 ng/mL. In CAL-51 and MDA-MB-468 cells the total AF was 11.0 ± 2.6% and 12.1 ± 2.3%, respectively, after the lowest concentration and the shortest exposure, and reached 46.0 ± 3.4% and 32.5 ± 5.7% after maximal concentration and exposure time (Table 1).
The cell lines other than MDA-MB-231 also differed in kinetics of apoptosis induction. Indeed, apoptosis occurred most rapidly in MDA-MB-157 cells, in which AF more than doubled from 6 h to 24 h of exposure to any concentration (Figure 3A; Table 1). When incubated with 20-50 ng/mL of HHT, CAL-51 cells showed a steady rise in AF over 72 h, however, after exposure to 100 ng/mL, the AF reached its maximum already after 48 h and did not exceed that level during next 24 h (Figure 3B; Table 1). Similar kinetics of apoptosis induction was observed in MDA-MB-468 cells (Figure 3C; Table 1).
The difference in AF size between MDA-MB-157 and CAL-51 cells, after exposure to 100 ng/mL of HHT during 48 h (73.9 ± 4.6% vs 44.2 ± 3.5%, respectively; Table 1) corresponded to the difference in size of the G0/G1 fraction in those cells (74.6% vs 57.4%, respectively/data not shown, Figure 2B and 2D). The MD-MB-468 cells were more arrested in the G0/G1 phase (61.9%, data not shown) than apoptotic (AF = 28.3 ± 6.0%, Table 1). In MDA-MB-231 cells, despite a similar cell cycle arrest like in CAL-51 cells after 100 ng/mL for 48 h (Figure 2A), apoptotic fraction was much smaller (Figure 3D). On that basis, we conclude that, in MDA-MB-157, CAL-51 and MDA-MB-468 cells, apoptosis likely takes place in the G1/G0-phase of the cell cycle. However, HHT effect on MDA-MB-231 cells is mostly cytostatic (arrest in the S-phase) and not cytotoxic.
HHT rapidly reduces abundance of several anti-apoptotic proteins, in particular of Mcl-1, which precedes apoptosis onset
As previous reports demonstrated that HHT most impacts the short-lived cellular proteins [28-30], we investigated how exposure to this drug affects abundance of several anti-apoptotic proteins, known to have a short half-life [36].
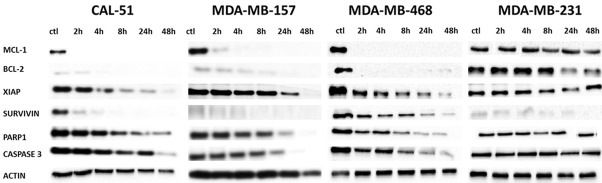
As shown on Figure 4, HHT provoked an extremely rapid and strong reduction of Mcl-1 levels (almost to total loss, after only 2 h of exposure) in all cell lines except in MDA-MB-231. Similar reduction of Bcl-2 was observed in MDA-MB-468 cells. In addition, survivin level was strongly and rapidly reduced in CAL-51 and MDA-MB-468 cells. XIAP abundance diminished as well, in all cells except MDA-MB-231, however the reduction was visible after a longer exposure to HHT.
Figure 4.
Levels of anti-apoptotic proteins in cell lines exposed to homoharringtonine. ctl, control, beginning of the experiment (before adding the drug). All cells were incubated with 100 ng/ml of HHT. The figure represents the most representative of three independent experiments.
Levels of caspase 3, the main apoptosis effector protein, started to lower several hours after the moment when Mcl-1 was almost undetectable. PARP1 change had similar kinetics (Figure 4).
We next compared Mcl-1 abundance reduction obtained by exposure to HHT or to MCL1 small inhibiting RNA (siRNA). As shown on Figure 5, in both CAL-51 and MDA-MB-468 cell line, the exposure to either siRNA or to 100 ng/mL of HHT resulted in a strong reduction of Mcl-1 levels, already after 2 h. Addition of HHT, after 24 h incubation with MCL1 siRNA, further lowered Mcl-1 levels, in both cell lines, and already after 2 h of incubation (Figure 5). In CAL-51 cells, level of Mcl-1 was reduced practically to zero, either by 100 ng/mL of HHT alone, by siRNA or by both agents together, after 24 h of exposure (Figure 5). Interestingly, under identical conditions, in MDA-MB-468 cells there is still a residual quantity of Mcl-1 protein after 24 h of treatment with MCL1 siRNA only (Figure 5).
Figure 5.
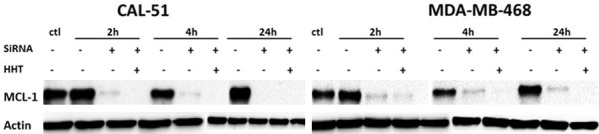
Mcl-1 protein level after exposure of cell lines to MCL1 siRNA, to homoharringtonine or to both components. ctl, control, beginning of the experiment (before adding the drug); HHT, homoharringtonine. All cells were incubated with 100 ng/ml of HHT. The figure represents the most representative of two independent experiments.
These results demonstrate that exposure to HHT in vitro reduces Mcl-1 protein level more effectively than one of the most powerful ways to achieve that effect, inhibition by siRNA of the gene which encodes for the protein (MCL1).
HHT rapidly and strongly reduces Myc levels, even in MDA-MB-231 cells
After observation that HHT reduced abundance of major anti-apoptotic proteins in all cells but MDA-MB-231, we wanted to see whether it will reduce abundance of a short-lived protein not from the anti-apoptotic class. For example, Myc protein has a very short half-life [37] and an important role in TNBC resistance to therapy [22,38].
As shown on Figure 6, in each MDA-MB-231 and CAL-51 cells HHT strongly reduced Myc levels already after 2 h. At that time, in MDA-MB-231 cells, Myc levels were reduced almost to zero and did not increase after (Figure 6).
Figure 6.
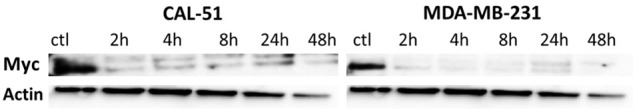
Myc protein level after exposure of cell lines to homoharringtonine. ctl, control, beginning of the experiment (before adding the drug). All cells were incubated with 100 ng/ml of HHT. The figure represents the most representative of two independent experiments.
HHT inhibits growth of MDA-MB-231 and MDA-MB-468 in vivo
As shown on Figure 7, the Maximal Tolerated Dose (MTD) of HHT, in a 7-day regimen, was 2 mg/kg (two subcutaneous injections, each with 1 mg/kg) per day, for the mice carrying MDA-MB-231 xenografts.
Figure 7.
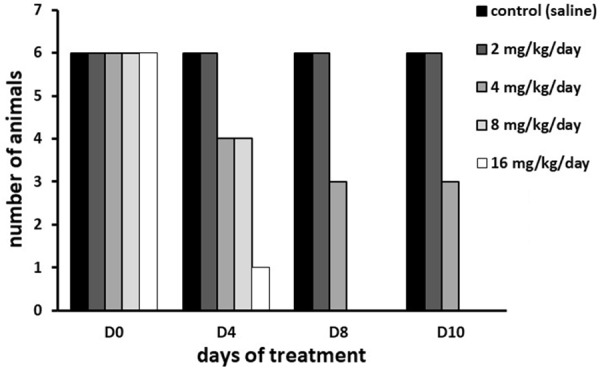
Determination of Maximal Tolerated Dose of homoharringtonine in mice after subcutaneous bi-daily administration over 7 days. D0, beginning of the experiment; D4, D8, D10, days 4, 8 and 10 after the treatment start.
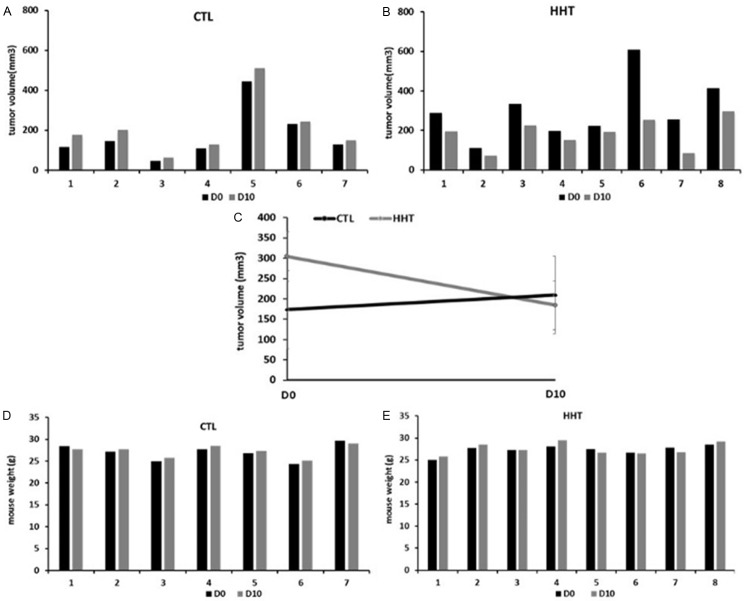
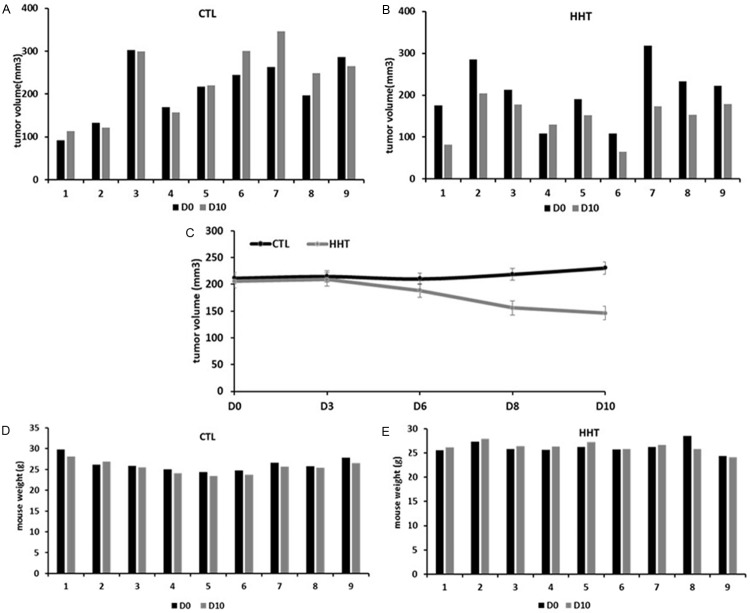
HHT had a growth-suppressive effect on MDA-MB-231 xenografts, in two experiments. In the first (Figure 8), the mean volume of the tumors in the treated animals was, at the end of observation (day 10), for 12% smaller than in the untreated animals (184.4 mm3 vs 209.5 mm3, treated vs untreated, respectively, Figure 8E). Moreover, the mean tumor volume before the initiation of the treatment by was almost twice bigger in the group that will be treated by HHT than in the group planned for treatment with saline only (304.5 mm3 vs 173.3 mm3, respectively). In the second experiment (Figure 9), with less difference in tumor volume before initiation of the treatment, the mean tumor volume, at day 10, was reduced for 36.5% in the treated animals, compared to the untreated group (146.2 ± 15.5 mm3 vs 230.4 ± 27.7 mm3, respectively, Figure 9E).
Figure 8.
Effect of bi-daily subcutaneous administration of 1 mg/kg homoharringtonine over 7 days on MDA-MB-231 xenografts in mice (experiment 1). A and B: Tumor volumes, absolute values. C: Tumor volume before and after treatment, mean ± SD. D and E: Mouse weight, absolute values. CTL, control, mice treated with saline water only; HHT, homoharringtonine; D0, beginning of the experiment; D10, day 10 after the treatment start.
Figure 9.
Effect of bi-daily subcutaneous administration of 1 mg/kg homoharringtonine over 7 days on MDA-MB-231 xenografts in mice (experiment 2). A and B: Tumor volumes, absolute values. C: Tumor volume before and after treatment, mean ± SD. D and E: Mouse weight, absolute values. CTL, control, mice treated with saline water only; HHT, homoharringtonine; D0, beginning of the experiment; D3, D6, D8, D10, day 3, 6, 8 and 10 after the treatment start.
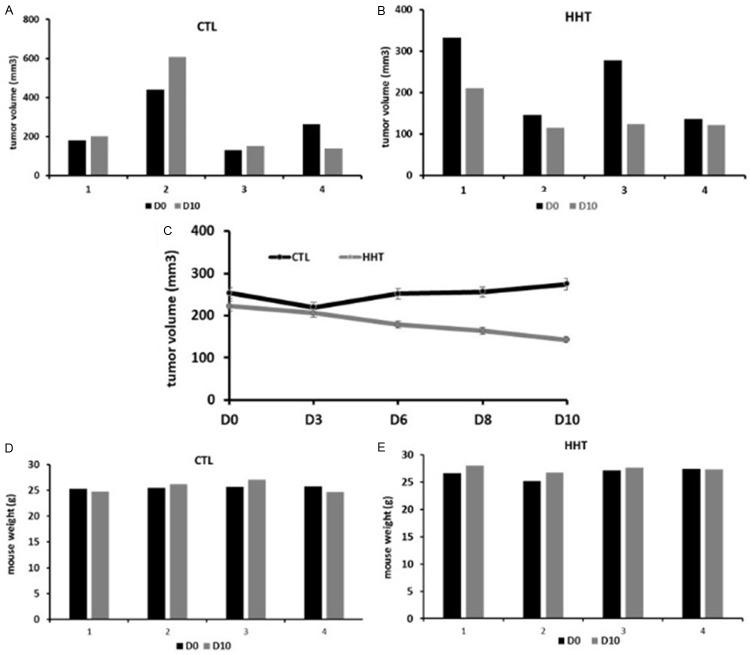
In the experiment on MDA-MB-468 xenografts (Figure 10), the mean tumor volume, at day 10, was for almost half-smaller in the treated mice than in the untreated ones (142.8 ± 22.5 mm3 vs 273.8 ± 110.5 mm3, respectively, Figure 10E).
Figure 10.
Effect of bi-daily subcutaneous administration of 0.5 mg/kg homoharringtonine over 7 days on MDA-MB-468 xenografts in mice. A and B: Tumor volumes, absolute values. C: Tumor volume before and after treatment, mean ± SD. D and E: Mouse weight, absolute values. CTL, control, mice treated with saline water only; HHT, homoharringtonine; D0, beginning of the experiment; D3, D6, D8, D10, day 3, 6, 8 and 10 after the treatment start.
The difference in animal weight after treatment, in comparison to the weight before treatment, was minimal (Figures 9 and 10, panels C and D on each).
Taken together, these results indicate that HHT inhibits in vivo growth of two different models of aggressive TNBC, without general toxicity for the animals, when administered in a 7-day regimen.
Discussion
Here we report that an approved anti-leukemic agent, HHT/omacetaxine [8], has a growth-reducing effect on one of the most aggressive solid cancers, TNBC. This is the first study of HHT alone using transcriptomically defined, BRCA1/2 non-mutated TNBC models. These models represent the TNBC subgroups nowadays most in need of new treatment approaches, as the currently available standard chemotherapy, as well as the most promising new agents, PARP or immune checkpoint inhibitors, all work best in TNBC with defects in DNA damage repair (BRCA1/2-mutated or with BRCAness) [4].
HHT has been principally evaluated in blood malignancies and not in solid tumors. A couple of early clinical trials, which tested HHT in the form of leaf extract, reported no effect of this molecule on breast cancer [39,40]. Very recently, a natural analog of HHT, isoharringtonine, was reported to inhibit growth and migration of two TNBC cell lines, one BRCA1-mutated, and another highly sensitive to a standard chemotherapeutic component, paclitaxel [41]. That indicates inhibitory action of the harringtonine class components on the TNBC already well responding to the classical chemotherapy. Our study demonstrates that HHT also suppresses growth of the TNBC strains quite resistant to the available chemotherapy drugs [31]. In addition, IC50 of HHT were at the low-medium nanomolar level (after 72 h of incubation, from 7 to 45 ng/mL /data not shown/, which is equivalent to 13 to 82.5 nM, molecular weight of HHT being 545.6 g/mol). Similar IC50 have been seen in myeloid leukemia cell lines [28], indicating that HHT is a potent anti-neoplastic agent.
We showed that HHT very rapidly induces significant growth inhibition and cell death in 3 out of 4 cell TNBC cell lines tested. This is in concordance with studies performed on AML and myeloma cell lines, in which HHT induced apoptosis of malignant cells as early as after 4-6 h of incubation [28,29,42,43]. In our study, HHT exerted the maximal apoptotic effect after a continuous exposure for 48-72 h, depending on the cell type. HHT is highly stable in cell cultures [44], so the experimental conditions we used are an example of continuous exposure to the drug. In patients, HHT gets progressively degraded; its mean steady-state half-life is around 7 h [45], which, in the bi-daily regimens, can assure exposure of cancer cells to sufficient concentration of the drug to permit its anti-cancer action.
Induction of apoptosis by HHT occurred after an arrest of TNBC cells in the cell cycle. In one of the TNBC cell lines we tested, MDA-MB-231, HHT dominantly induced an S-phase arrest, without much apoptosis. However, we observed a significant reduction in MDA-MB-231 tumor volume after 7 days of HHT treatment in vivo. This discrepancy between in vitro and in vivo data could be explained by experimental design: in our in vitro studies, the maximal exposure time to HHT was 48 h (cell cycle) or 72 h (apoptosis), whereas in vivo it was 7 days, plus additional 2 days, without the treatment, before the measure of tumor volume. That way the tumor cells were, in vivo, exposed to HHT for almost 5-fold longer time then in vitro. Even during the 2 days without the drug, in vivo, HHT which has already entered the cells and fixed to ribosomes, could continue inhibiting the protein synthesis and tumor growth. Indeed, there was no difference in the MDA-MB-231 tumor volume after 3 days of HHT injection, but it became visible after 6 days and was maximal after 10 days (Figure 7).
We could not test HHT on sufficient number of mice carrying xenografts of MDA-MB-468 and CAL-51 cell line, so we cannot make a strong conclusion about its efficiency in those models. Anyway, a remarkable growth-inhibitory effect of HHT was demonstrated in 4 mice carrying MDA-MB-468 xenografts. As we showed that HHT does reduce in vivo growth of the cell line the least sensitive in vitro (MDA-MB-231), it seems plausible to hypothesize that it would inhibit in vivo growth of the cell lines it most inhibited in vitro (CAL-51, MDA-MB-157, MDA-MB-468). However, that remains to be proven in future studies.
MDA-MB-468 and CAL-51 cell lines have in common hyperactivated PI3K-AKT-mTOR pathway, due to PTEN absence in the former and PIK3CA activating mutation in the latter [31]. These cell lines may have an increased RNA translation/increased protein synthesis so its inhibition may have a deleterious effect on their growth. We observed, on a luminal breast cancer cell line MCF-7, a growth-inhibitory effect of HHT similar to the one observed on MDA-MB-468 cells (in vitro data, not shown). MCF-7 also carries an activating mutation of PIK3CA, and the increased sensitivity of that cell line to HHT indicates that the drug could be efficacious in suppression of all breast cancers having the PI3K-AKT-mTOR pathway activated, at least if the activation is caused by a PI3K activating mutation or PTEN loss, irrespective of molecular subtype.
We hypothesize that the strong and rapid Mcl-1 protein level reduction, is the main event which induces apoptosis of TNBC after exposure to HHT. Our experiments showed that a significant reduction of Mcl-1 protein level, either by HHT or by sRNA, is rapidly followed by apoptosis induction. Mcl-1 protein has been demonstrated to have crucial importance for TNBC survival [21]. In addition, overexpression of Mcl-1 is associated with worse survival of pts with all breast cancer subtypes [20]. Finally, downregulation of Mcl-1 levels enables sensitization of breast cancer cells to the current chemotherapy [46]. However, all reported Mcl-1 inhibitors are far earlier in the clinical development than HHT, which has already been approved in one malignant disease [8].
HHT is a universal inhibitor of protein synthesis in eukaryotes, and, as many natural products, can be considered as a multi-target agent, although all its action targets are now known. It has been demonstrated that HHT inhibits stem cells of chronic myeloid leukemia and multiple myeloma [47,48]. In TNBC, HHT-induced downregulation of Myc protein may also have an important role in stem cell suppression [49]. We demonstrated that HHT rapidly reduces Myc abundance to very low levels even in a highly metastatic TNBC cell line, MDA-MB-231. This implies that HHT might be efficacious in suppression of TNBC metastatic progression. We showed, in two independent experiments, that HHT markedly suppresses in vivo growth of MDA-MB-231. In additional experiments, which need verification, we also observed that migration of this line was strongly inhibited by HHT (data not shown), indicating possible anti-metastatic properties of the drug.
In conclusion, our results show that HHT effectively suppresses an important mechanism of cancer cell resistance to death, the increased levels of Mcl-1 and other anti-apoptotic proteins. As it has been shown that Mcl-1 downregulation is of primordial importance for sensitization to many anti-cancer therapies, HHT is worth developing in TNBC, as a single agent in the metastatic phase of the disease and as a sensitizer of standard chemo- or targeted therapy. Development of another natural product (HHT) for breast cancer therapy should be encouraged by the eribulin case, recently approved as a single agent in metastatic breast cancer and one of the most efficacious treatments in this setting. In addition, new purification procedures (like the one exploited for production of HHT used in this study) which yield a highly purified HHT form at a several-fold lower cost compared to semi-synthesis used for production of the currently approved omacetaxine (personal communication, LeukePharma Inc.). This will render HHT and translation/protein synthesis inhibition more available for cancer treatment, and in particular, in the regions where TNBC and other cancers are devastating the population but numerous patients cannot afford even the standard chemotherapy.
Acknowledgements
We are sincerely grateful to Professor Marie-Paule Vasson, from the ECREIN Team at the University Clermont Auvergne in Clermont-Ferrand, France, for offering us to use her laboratory equipment in cell viability assays. We also express our gratitude to Ms Christelle Blavignac, from the Cell Imaging Center of the Medical School, University Clermont Auvergne in Clermond-Ferrand, France, for the assistance in flow-cytometry assays. Finally, a big thanks to Mr Nicolas Sonnier, senior laboratory technician at INSERM UMR1240, for help with siRNA assays.
Disclosure of conflict of interest
None.
References
- 1.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieci MV, Orvieto E, Dominici M, Conte P, Guarneri V. Rare breast cancer subtypes: histological, molecular, and clinical peculiarities. Oncologist. 2014;19:805–813. doi: 10.1634/theoncologist.2014-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakur KK, Bordoloi D, Kunnumakkara AB. Alarming burden of triple-negative breast cancer in India. Clin Breast Cancer. 2018;18:e393–e399. doi: 10.1016/j.clbc.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Ahn JH, Kim SB. How shall we treat early triple-negative breast cancer (TNBC): from the current standard to upcoming immunomolecular strategies. ESMO Open. 2018;3:e000357. doi: 10.1136/esmoopen-2018-000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 6.Khosravi-Shahi P, Cabezon-Gutierrez L, Custodio-Cabello S. Metastatic triple negative breast cancer: optimizing treatment options, new and emerging targeted therapies. Asia Pac J Clin Oncol. 2018;14:32–39. doi: 10.1111/ajco.12748. [DOI] [PubMed] [Google Scholar]
- 7.Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, Fralick M, Kumar R, Clemons M. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9:29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 8.Alvandi F, Kwitkowski VE, Ko CW, Rothmann MD, Ricci S, Saber H, Ghosh D, Brown J, Pfeiler E, Chikhale E, Grillo J, Bullock J, Kane R, Kaminskas E, Farrell AT, Pazdur R. U.S. food and drug administration approval summary: omacetaxine mepesuccinate as treatment for chronic myeloid leukemia. Oncologist. 2014;19:94–99. doi: 10.1634/theoncologist.2013-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu S, Wang J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J Hematol Oncol. 2014;7:2. doi: 10.1186/1756-8722-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao W, Liu Y, Zhang R, Zhang B, Wang T, Zhu X, Mei L, Chen H, Zhang H, Ming P, Huang L. Homoharringtonine induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal pathway in Gefitinib-resistant lung cancer cells. Sci Rep. 2015;5:8477. doi: 10.1038/srep08477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weng TY, Wu HF, Li CY, Hung YH, Chang YW, Chen YL, Hsu HP, Chen YH, Wang CY, Chang JY, Lai MD. Homoharringtonine induced immune alteration for an efficient anti-tumor response in mouse models of non-small cell lung adenocarcinoma expressing Kras mutation. Sci Rep. 2018;8:8216. doi: 10.1038/s41598-018-26454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tujebajeva RM, Graifer DM, Karpova GG, Ajtkhozhina NA. Alkaloid homoharringtonine inhibits polypeptide chain elongation on human ribosomes on the step of peptide bond formation. FEBS Lett. 1989;257:254–256. doi: 10.1016/0014-5793(89)81546-7. [DOI] [PubMed] [Google Scholar]
- 13.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015;14:261–278. doi: 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi V, Plunkett W, Cortes JE. Omacetaxine: a protein translation inhibitor for treatment of chronic myelogenous leukemia. Clin Cancer Res. 2014;20:1735–1740. doi: 10.1158/1078-0432.CCR-13-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaklavas C, Blume SW, Grizzle WE. Translational dysregulation in cancer: molecular insights and potential clinical applications in biomarker development. Front Oncol. 2017;7:158. doi: 10.3389/fonc.2017.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindqvist LM, Tandoc K, Topisirovic I, Furic L. Cross-talk between protein synthesis, energy metabolism and autophagy in cancer. Curr Opin Genet Dev. 2018;48:104–111. doi: 10.1016/j.gde.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao G, Mingle L, Van De Water L, Liu G. Control of cell migration through mRNA localization and local translation. Wiley Interdiscip Rev RNA. 2015;6:1–15. doi: 10.1002/wrna.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–1424. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto T, Coultas L, Metcalf D, van Delft MF, Glaser SP, Takiguchi M, Strasser A, Bouillet P, Adams JM, Huang DC. Enhanced stability of Mcl1, a prosurvival Bcl2 relative, blunts stress-induced apoptosis, causes male sterility, and promotes tumorigenesis. Proc Natl Acad Sci U S A. 2014;111:261–266. doi: 10.1073/pnas.1321259110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell KJ, Dhayade S, Ferrari N, Sims AH, Johnson E, Mason SM, Dickson A, Ryan KM, Kalna G, Edwards J, Tait SWG, Blyth K. MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis. 2018;9:19. doi: 10.1038/s41419-017-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin CM, Rossanese OW, Olejniczak ET, Fesik SW. Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Differ. 2015;22:2098–2106. doi: 10.1038/cdd.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balko JM, Giltnane JM, Wang K, Schwarz LJ, Young CD, Cook RS, Owens P, Sanders ME, Kuba MG, Sanchez V, Kurupi R, Moore PD, Pinto JA, Doimi FD, Gomez H, Horiuchi D, Goga A, Lehmann BD, Bauer JA, Pietenpol JA, Ross JS, Palmer GA, Yelensky R, Cronin M, Miller VA, Stephens PJ, Arteaga CL. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014;4:232–245. doi: 10.1158/2159-8290.CD-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millis SZ, Gatalica Z, Winkler J, Vranic S, Kimbrough J, Reddy S, O’Shaughnessy JA. Predictive biomarker profiling of > 6000 breast cancer patients shows heterogeneity in TNBC, with treatment implications. Clin Breast Cancer. 2015;15:473–481. e473. doi: 10.1016/j.clbc.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–147. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- 25.Doi K, Li R, Sung SS, Wu H, Liu Y, Manieri W, Krishnegowda G, Awwad A, Dewey A, Liu X, Amin S, Cheng C, Qin Y, Schonbrunn E, Daughdrill G, Loughran TP Jr, Sebti S, Wang HG. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J Biol Chem. 2012;287:10224–10235. doi: 10.1074/jbc.M111.334532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, Chanrion M, Kelly GL, Gong JN, Moujalled DM, Bruno A, Csekei M, Paczal A, Szabo ZB, Sipos S, Radics G, Proszenyak A, Balint B, Ondi L, Blasko G, Robertson A, Surgenor A, Dokurno P, Chen I, Matassova N, Smith J, Pedder C, Graham C, Studeny A, Lysiak-Auvity G, Girard AM, Grave F, Segal D, Riffkin CD, Pomilio G, Galbraith LC, Aubrey BJ, Brennan MS, Herold MJ, Chang C, Guasconi G, Cauquil N, Melchiore F, Guigal-Stephan N, Lockhart B, Colland F, Hickman JA, Roberts AW, Huang DC, Wei AH, Strasser A, Lessene G, Geneste O. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Fletcher S. Mcl-1 inhibitors: a patent review. Expert Opin Ther Pat. 2017;27:163–178. doi: 10.1080/13543776.2017.1249848. [DOI] [PubMed] [Google Scholar]
- 28.Tang R, Faussat AM, Majdak P, Marzac C, Dubrulle S, Marjanovic Z, Legrand O, Marie JP. Semisynthetic homoharringtonine induces apoptosis via inhibition of protein synthesis and triggers rapid myeloid cell leukemia-1 down-regulation in myeloid leukemia cells. Mol Cancer Ther. 2006;5:723–731. doi: 10.1158/1535-7163.MCT-05-0164. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda J, Kamitsuji Y, Kimura S, Ashihara E, Kawata E, Nakagawa Y, Takeuichi M, Murotani Y, Yokota A, Tanaka R, Andreeff M, Taniwaki M, Maekawa T. Anti-myeloma effect of homoharringtonine with concomitant targeting of the myeloma-promoting molecules, Mcl-1, XIAP, and beta-catenin. Int J Hematol. 2008;87:507–515. doi: 10.1007/s12185-008-0081-8. [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Guo L, Chen Y, Jiang Y, Wierda WG, Plunkett W. Homoharringtonine reduced Mcl-1 expression and induced apoptosis in chronic lymphocytic leukemia. Blood. 2011;117:156–164. doi: 10.1182/blood-2010-01-262808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioanni J, Le Francois D, Zanghellini E, Mazeau C, Ettore F, Lambert JC, Schneider M, Dutrillaux B. Establishment and characterisation of a new tumorigenic cell line with a normal karyotype derived from a human breast adenocarcinoma. Br J Cancer. 1990;62:8–13. doi: 10.1038/bjc.1990.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Guerrab A, Bamdad M, Bignon YJ, Penault-Llorca F, Aubel C. Anti-EGFR monoclonal antibodies enhance sensitivity to DNA-damaging agents in BRCA1-mutated and PTEN-wild-type triple-negative breast cancer cells. Mol Carcinog. 2017;56:1383–1394. doi: 10.1002/mc.22596. [DOI] [PubMed] [Google Scholar]
- 34.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 35.Levy V, Zohar S, Bardin C, Vekhoff A, Chaoui D, Rio B, Legrand O, Sentenac S, Rousselot P, Raffoux E, Chast F, Chevret S, Marie JP. A phase I dose-finding and pharmacokinetic study of subcutaneous semisynthetic homoharringtonine (ssHHT) in patients with advanced acute myeloid leukaemia. Br J Cancer. 2006;95:253–259. doi: 10.1038/sj.bjc.6603265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opferman JT. Attacking cancer’s Achilles heel: antagonism of anti-apoptotic BCL-2 family members. FEBS J. 2016;283:2661–2675. doi: 10.1111/febs.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hann SR, Thompson CB, Eisenman RN. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. Nature. 1985;314:366–369. doi: 10.1038/314366a0. [DOI] [PubMed] [Google Scholar]
- 38.Lee KM, Giltnane JM, Balko JM, Schwarz LJ, Guerrero-Zotano AL, Hutchinson KE, Nixon MJ, Estrada MV, Sanchez V, Sanders ME, Lee T, Gomez H, Lluch A, Perez-Fidalgo JA, Wolf MM, Andrejeva G, Rathmell JC, Fesik SW, Arteaga CL. MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab. 2017;26:633–647. e637. doi: 10.1016/j.cmet.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao TP, Ding GX, Gao HY, Shen ZZ, Li KY. A clinical trial of homoharringtonine in the treatment of advanced breast cancer. Tumori. 1986;72:395–398. doi: 10.1177/030089168607200409. [DOI] [PubMed] [Google Scholar]
- 40.Ajani JA, Dimery I, Chawla SP, Pinnamaneni K, Benjamin RS, Legha SS, Krakoff IH. Phase II studies of homoharringtonine in patients with advanced malignant melanoma; sarcoma; and head and neck, breast, and colorectal carcinomas. Cancer Treat Rep. 1986;70:375–379. [PubMed] [Google Scholar]
- 41.Chen W, Wang H, Cheng M, Ni L, Zou L, Yang Q, Cai X, Jiao B. Isoharringtonine inhibits breast cancer stem-like properties and STAT3 signaling. Biomed Pharmacother. 2018;103:435–442. doi: 10.1016/j.biopha.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Xia LJ, Jiang C, Han R. [Induction of apoptosis by harringtonine and homoharringtonine in HL-60 cells] . Yao Xue Xue Bao. 1994;29:667–672. [PubMed] [Google Scholar]
- 43.Yinjun L, Jie J, Weilai X, Xiangming T. Homoharringtonine mediates myeloid cell apoptosis via upregulation of pro-apoptotic bax and inducing caspase-3-mediated cleavage of poly(ADP-ribose) polymerase (PARP) Am J Hematol. 2004;76:199–204. doi: 10.1002/ajh.20100. [DOI] [PubMed] [Google Scholar]
- 44.Marenah L, Allan EK, Mountford JC, Holyoake TL, Jorgensen HG, Elliott MA. Investigation into omacetaxine solution stability for in vitro study. Biomed Chromatogr. 2012;26:545–547. doi: 10.1002/bmc.1686. [DOI] [PubMed] [Google Scholar]
- 45.Nemunaitis J, Mita A, Stephenson J, Mita MM, Sarantopoulos J, Padmanabhan-Iyer S, Nanda N, Gleich L, Benichou AC, Craig A. Pharmacokinetic study of omacetaxine mepesuccinate administered subcutaneously to patients with advanced solid and hematologic tumors. Cancer Chemother Pharmacol. 2013;71:35–41. doi: 10.1007/s00280-012-1963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merino D, Whittle JR, Vaillant F, Serrano A, Gong JN, Giner G, Maragno AL, Chanrion M, Schneider E, Pal B, Li X, Dewson G, Grasel J, Liu K, Lalaoui N, Segal D, Herold MJ, Huang DCS, Smyth GK, Geneste O, Lessene G, Visvader JE, Lindeman GJ. Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam7049. [DOI] [PubMed] [Google Scholar]
- 47.Allan EK, Holyoake TL, Craig AR, Jorgensen HG. Omacetaxine may have a role in chronic myeloid leukaemia eradication through downregulation of Mcl-1 and induction of apoptosis in stem/progenitor cells. Leukemia. 2011;25:985–994. doi: 10.1038/leu.2011.55. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Shi F, Fei X, Wu S, Wu D, Pan M, Luo S, Gu N, Dou J. PEGylated long-circulating liposomes deliver homoharringtonine to suppress multiple myeloma cancer stem cells. Exp Biol Med (Maywood) 2017;242:996–1004. doi: 10.1177/1535370216685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin S, Cheryan VT, Xu L, Rishi AK, Reddy KB. Myc mediates cancer stem-like cells and EMT changes in triple negative breast cancers cells. PLoS One. 2017;12:e0183578. doi: 10.1371/journal.pone.0183578. [DOI] [PMC free article] [PubMed] [Google Scholar]