Abstract
PSA may be elevated in non-malignant conditions such as prostatitis and leads to unnecessary prostate needle biopsy. Urine prostatic exosomal protein (PSEP) has been proved to be a promising biomarker of prostatic inflammation. The aim of this study is to determine the relationships between PSEP and the diagnosis of prostate cancer (PCa), and their association with histologic prostatic inflammation. Prostate needle biopsies from 674 patients were evaluated for the presence of histological inflammation and PCa. The urine PSEP levels were measured using an enzyme-linked immunosorbent assay kit. 286 cases were diagnosed as PCa and prostatic inflammation was observed in 33.7% of the biopsies. The presence of histological inflammation was significantly associated with a lower PCa risk (P < 0.001). The urine PSEP levels was significantly lower in PCa patients compared to the controls (P = 0.003). When subanalyzed by PSA levels, the difference was more evident in cases with PSA 4-10 ng/ml (P = 0.039). The urine PSEP levels was correlated with histological inflammation on prostate needle biopsy (P = 0.018, r = 0.12). Urine PSEP examination may be helpful to eliminate false positive PSA levels due to prostatic inflammation and reduce unnecessary prostate needle biopsy in cases with PSA grey zone.
Keywords: Prostate cancer, prostatic exosomal protein, inflammation, prostatitis
Introduction
Prostate cancer (PCa) is usually suspected on the basis of elevated prostatic specific antigen (PSA) levels. Men with a positive PSA test result may undergo a transrectal ultrasound-guided core needle biopsy of the prostate to diagnose PCa. PSA is organ but not cancer-specific, therefore, it may be elevated in benign prostatic hypertrophy, prostatitis and other non-malignant conditions [1]. Of biopsies performed, 60.6%-75.8% cases did not result in a PCa diagnosis [2,3].
Considering that inflammatory infiltrate is a common histological finding on prostate needle biopsy, varying from 68% to 82% [4], it is an important factor contributing to increased levels of PSA in men without PCa. Unfortunately, most prostatic inflammation are asymptomatic and can be detected only upon histological examination of a prostate specimen [5]. For urologists, a predisposing marker to eliminate false positive PSA levels due to prostatic inflammation would considerably avoid unnecessary prostate needle biopsy.
Prostasomes are extracellular vesicles produced by prostatic epithelial cells and excreted with urine [6]. Over 400 distinct protein compositions of prostasomes have been identified. Among these proteins, prostatic exosomal protein (PSEP) has been proved to be a promising marker of prostatic inflammation [7].
To date no studies have looked for associations between PSEP and risk of PCa. Herein, we evaluated the relationships between PSEP and the diagnosis of PCa, and their association with histologic prostatic inflammation.
Materials and methods
Study population
Prospective and observational study was carried out in 674 consecutive prostate biopsy done from January 2015 until December 2016 due to elevation of serum PSA (> 4.0 ng/ml) and/or suspicious DRE digital rectal examination (DRE) and/or positive imaging findings. Informed consent for both procedures and study participation was obtained. The study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center.
Transrectal Ultrasound Guided Prostate Needle Biopsy Prostate needle biopsy was performed as an out patient procedure under local anesthesia. An end-fire ultrasound transducer (Falcon 2101, B-K Medical, Inc.) and a 16-gauge automated biopsy needle (Bard, Inc.) were used. A minimum of 12 cores were obtained, and two to eight additional cores were taken as determined by age and prostate volume. Tissue specimens were fixed in 10% buffered formalin, processed and subjected to H&E staining according to routine protocol.
Clinical and demographic data were abstracted from patients’ medical records. Diagnoses of prostatic inflammation were based upon histology by experienced pathologists. The presence of prostatic inflammation was defined as presence of inflammatory cell infiltration in the glands and stroma of the prostate, using the Histopathological Classification System of Prostatic Inflammation [8].
PSEP measurement
In order to minimize the bacterial interference, midstream urine samples were collected without massage and stored at -80°C until PSEP measurement. PSEP levels were measured with PSEP diagnostic kits (enzyme-linked immunosorbent assay) (Onco Biomedical Technology, Suzhou, China) according to the manufacturer’s manuals [7]. The absorbance values were read in the dual-wavelength mode on a BIO-RAD 680 microplate reader. A linear standard curve was generated by plotting the graph using the standard concentrations on the y-axis and the corresponding absorbance on the x-axis. The sample concentration was determined according to the standard curve, and the value was multiplied by the dilution factor if there was one.
Statistical analysis
Quantitative variables were expressed as medians semi-interquartile range. Qualitative variables were expressed as rates. Univariate analysis included x2 test to analyze the association between qualitative variables. Mann-Whitney U test was performed to compare quantitative variables. SPSS program V.20 was used to perform statistical analysis.
Results
A total of 674 participating patients were analyzed. PCa was identified in biopsies from 286 cases. There were significant age and PSA levels difference between the cancer and non-cancer group (P < 0.001, Table 1). In general, histological inflammation was a frequent finding, observed in the biopsy specimens from 227 (33.7%) patients. A significant but inverse association was found between presence of inflammation and presence of PCa (P < 0.001, Table 1). PCa cases had lower levels of PSEP compared with normal subjects (P = 0.003, Table 1).
Table 1.
Characteristics of prostate needle biopsy cases
| Negative n = 388 | Positive n = 286 | P value | ||
|---|---|---|---|---|
| Age | 65 (33-91) | 69 (44-90) | < 0.001 | |
| PSA, ng/mla | ≤ 4 | 17 (4.5%) | 3 (1.1%) | < 0.001 |
| 4-10 | 203 (54.0%) | 45 (16.5%) | ||
| > 10 | 156 (41.5%) | 225 (82.4) | ||
| Inflammation | Yes | 180 (46.4%) | 47 (16.4%) | < 0.001 |
| No | 208 (53.6%) | 239 (83.6%) | ||
| PSEP, ng/ml | 1.01 (-4.99-18.8) | 0.61 (-7.28-18.5) | 0.003 |
25 cases’ PSA data were unavailable.
In PSA levels stratified analysis, the presence of prostatic inflammation were more prominently in cases with PSA 4-10 ng/ml than in cases with the other two PSA levels (P = 0.002, Table 2). Accordingly, decreased levels of PSEP were still associated with an increased risk of PCa in cases with PSA 4-10 ng/ml (P = 0.039, Figure 1). This association was not observed in the other two PSA levels (P = 0.564 and 0.379, respectively). An ROC curve was developed to identify the cut-off value distinguishing men with PCa from controls in PSA 4-10 ng/ml group. Youden’s Index reached a maximum at a cutoff value of 0.35 ng/ml. The diagnostic sensitivity was 62.2%, and the specificity was 72.4%. The negative predictive value was 89.6% and positive predictive value was 34.6%. In multivariate logistic regression analysis, PSEP levels < 0.35 ng/ml was not significantly associated with PCa risk in PSA grey zone. Compared with men with PSEP levels < 0.35 ng/ml, men with PSEP levels > 0.35 ng/ml were associated with a decreased risk of PCa (OR: 0.57, 95% CI = 0.29-1.12, P = 0.102). When the cut off value was 5.52 ng/ml, using PSEP levels would screen out 90% of PCa patients with PSA 4-10 ng/ml.
Table 2.
PSA levels and Histological prostate inflammation
| PSA ≤ 4 ng/ml | PSA 4-10 ng/ml | PSA > 10 ng/ml | P value | ||
|---|---|---|---|---|---|
| Inflammation | No | 12 (60.0%) | 145 (58.5%) | 274 (71.9%) | 0.002 |
| Yes | 8 (40.0%) | 103 (41.5%) | 107 (28.1%) |
Figure 1.
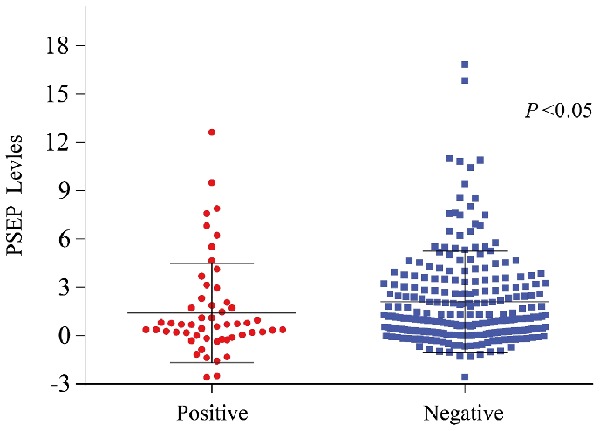
Urine PSEP levels in prostate needle biopsy cases with PSA 4-10 ng/ml.
The PSEP levels were positively correlated with prostatic inflammation (P = 0.018, r = 0.12). The PSEP levels were higher in urine isolated from prostatic inflammation patients [1.04 (-6.52-18.8) ng/ml] compared with normal subjects 0.76 (-7.28-18.5) ng/ml, P = 0.048]. The difference was more prominently in cases with PSA 4-10 ng/ml (Table 3).
Table 3.
Urine PSEP levels stratified by PSA levels
| Without Inflammation | With Inflammation | P value | ||
|---|---|---|---|---|
| PSA, ng/ml | ≤ 4 | 1.46 (-4.99-8.29) | -0.028 (-0.44-2.57) | 0.280 |
| 4-10 | 0.98 (-3.06-16.8) | 1.27 (-3.50-18.8) | 0.027 | |
| > 10 | 0.72 (-7.28-18.5) | 0.94 (-6.52-13.8) | 0.061 |
Discussion
Inflammatory infiltrates are frequently found in routine biopsies performed, although the prevalence is variable, perhaps because the inflammation is not frequently reported by pathologists [8]. In this study we report a high prevalence of prostatic inflammation on prostate needle biopsy. Multiple previous studies have evaluated the relationship between inflammation and the subsequent diagnosis of PCa and have reached differing conclusions. Iczkowski et al. reported that atrophy with inflammation showed some preferential spatial association to PCa [9]. Benedetti et al. and Gurel et al. found that prostate inflammation was associated with a higher incidence of PCa while some studies presented that inflammation in benign prostate is inversely associated with future PCa risk. Meta-analyses by Dennis [10] and Jiang [11] et al. showed a positive association between clinical prostatitis and PCa. These studies comprised pooled analyses of cohort or case-control studies and the presence or absence of prostatitis was determined by chart review or personal interview. While these studies provide an important clinical link between clinical prostatitis and PCa, they relied on patient definitions and recall of prior prostatitis, which may be vulnerable to recall bias. Moreover, clinical prostatitis is not always accompanied by histological signs of inflammation and vice versa. Thus, these studies are not well suited to determine the association of histological inflammation and PCa. Similar with our results, a meta-analysis of 25 studies reported that histological inflammation on prostate needle biopsy was associated with a lower PCs risk [12].
Prior studies have shown that asymptomatic histological inflammation and latent infection are common findings in men, particularly those with benign prostatic hyperplasia and concomitant urological conditions [13]. Given that subclinical inflammation may be present in many men, in our study we used histology to better determine the association between histological inflammation and the subsequent development of PCa. The high prevalence of inflammation in pathological samples of prostate tissue from surgery or biopsy suggested a possible link between inflammation and PCa.
While the molecular pathogenesis of PCa has been characterized by genes and proteins involved in pro-inflammatory pathways, previous study has shown that inflammation has a protective role on PCa incidence [12]. The ability of premalignant cells to evade and down-regulate the host immune defenses determines the survival and neoplastic potential of premalignant cells. The inflammatory response can prevent this carcinogenesis by recognizing and eliminating tumor specific antigens, a process known as immunosurveillance. Therefore, in the PCa milieu a balance exists between immune system up-regulation and downregulation. Inflammation is a hallmark of immune system up-regulation. Thus, it is plausible that it favors host defense mechanisms with a lower risk of PCa in the current study. Our results implicate inflammation and immunomodulation as candidate targets for pharmacological intervention to prevent and potentially treat PCa.
Proteomic profiling of prostasomes isolated from urine identified hundreds of proteins, many of which can serve as biomarkers for prostate diseases was recently validated for prostate cancer and prostatitis [6]. One advantage of collecting prostasomes from urine, as compared with blood, is that such isolates are more enriched in prostasomes relative to other constituents [6]. Cells and most apoptotic bodies are considerably larger than prostasomes and can thus be easily separated from prostasomes by differential centrifugation. In a recent study, PSEP displayed sensitivities above 60% at 94.2% specificity at a detection threshold with positive values for prostatitis patient samples and negative values for control samples [7]. Compared with expressed prostatic secretions, urine PSEP measurement was more convenient and less painful. In the current study, the PSEP levels were positively correlated with prostatic inflammation as expected. It is noteworthy that The PSEP levels were significantly lower in urine isolated from PCa patients with PSA 4-10 ng/ml compared with normal subjects. Histological inflammation was more frequently found (41.5%) in cases within PSA grey zone, which may account for the difference we observed. As a potential biomarker for prostatic inflammation, urine PSEP may be helpful to eliminate false positive PSA levels due to prostatic inflammation and reduce unnecessary prostate needle biopsy in cases within PSA grey zone.
There are several potential implications of our study. Since we found that histological prostate inflammation is associated with lower PCa risk, followup guidelines for these patients might be adjusted for the reduced PCa risk. As such, our results encourage pathologists to systematically evaluate and report prostate inflammation on biopsy as it may have implications for a repeat prostate biopsy strategy. Furthermore, the use of urine PSEP examination to predict prostatic histological inflammation is appealing, which could potentially allow for more tailored prostate needle biopsy decision.
This study had the limitation of not being designed to explore the mechanisms linking inflammation to lower prostate carcinogenesis. With these implications, studies evaluating the biology of inflammation and how it relates to carcinogenesis are needed since they may identify areas for PCa prevention therapies. As a non-invasive method, PSEP will be helpful to discriminate prostatic inflammation from PCa based on the relatively high negative predictive value. While the relatively small sample size of men in PSA grey zone in the current study might limit the power to reveal an optimal PSEP cut-off which should be confirmed in a larger-scale study.
In conclusion, histological inflammation was associated with lower PCa risk. Urine PSEP levels were correlated with histological inflammation on prostate needle biopsy. The urine PSEP levels were significantly decreased in PCa patients with PSA 4-10 ng/ml.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant No. 81702537).
Disclosure of conflict of interest
None.
Abbreviations
- PCa
prostate cancer
- PSA
prostate-specific antigen
- PSEP
prostatic exosomal protein
References
- 1.Roobol MJ, Steyerberg EW, Kranse R, Wolters T, van den Bergh RC, Bangma CH, Schröder FH. A risk-based strategy improves prostate-specific antigen-driven detection of prostate cancer. Eur Urol. 2010;57:79–85. doi: 10.1016/j.eururo.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, Kwiatkowski M, Lujan M, Määttänen L, Lilja H, Denis LJ, Recker F, Paez A, Bangma CH, Carlsson S, Puliti D, Villers A, Rebillard X, Hakama M, Stenman UH, Kujala P, Taari K, Aus G, Huber A, van der Kwast TH, van Schaik RH, de Koning HJ, Moss SM, Auvinen A ERSPC Investigators. Screening and prostate cancer mortality: results of the european randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinsky PF, Parnes HL, Andriole G. Mortality and complications after prostate biopsy in the prostate, lung, colorectal and ovarian cancer screening (PLCO) trial. BJU Int. 2014;113:254–9. doi: 10.1111/bju.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurel B, Lucia MS, Thompson IM Jr, Goodman PJ, Tangen CM, Kristal AR, Parnes HL, Hoque A, Lippman SM, Sutcliffe S, Peskoe SB, Drake CG, Nelson WG, De Marzo AM, Platz EA. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014;23:847–56. doi: 10.1158/1055-9965.EPI-13-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickel JC, True LD, Krieger JN, Berger RE, Boag AH, Young ID. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 2001;87:797–805. doi: 10.1046/j.1464-410x.2001.02193.x. [DOI] [PubMed] [Google Scholar]
- 6.Zijlstra C, Stoorvogel W. Prostasomes as a source of diagnostic biomarkers for prostate cancer. J Clin Invest. 2016;126:1144–51. doi: 10.1172/JCI81128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Jiang T, Liu F, Shao X, Xu Y, Sheng W, Sun W. Clinical evaluation of urine prostatic exosomal protein in the diagnosis of chronic prostatitis. Urol Int. 2018;100:112–118. doi: 10.1159/000479188. [DOI] [PubMed] [Google Scholar]
- 8.Benedetti I, Bettin A, Reyes N. Inflammation and focal atrophy in prostate needle biopsy cores and association to prostaticadenocarcinoma. Ann Diagn Pathol. 2016;24:55–61. doi: 10.1016/j.anndiagpath.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Iczkowski KA, Torkko KC, Wilson RS, Lucia MS, Bostwick DG. Prostatic atrophy: its spatial proximity to carcinoma and intraepithelial neoplasia based on annotation of digital slides. Hum Pathol. 2014;45:54–8. doi: 10.1016/j.humpath.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 11.Jiang J, Li J, Yunxia Z, Zhu H, Liu J, Pumill C. The role of prostatitis in prostate cancer: meta-analysis. PLoS One. 2013;8:e85179. doi: 10.1371/journal.pone.0085179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasavada SR, Dobbs RW, Kajdacsy-Balla AA, Abern MR, Moreira DM. Inflammation on prostate needle biopsy is associated with lower prostate cancer risk: a meta-analysis. J Urol. 2018;199:1174–1181. doi: 10.1016/j.juro.2017.11.120. [DOI] [PubMed] [Google Scholar]
- 13.Terakawa T, Kanomata N, Kumano M, Takenaka A, Fujisawao M. Inverse association between histologic inflammation in needle biopsy specimens and prostate cancer in men with serum PSA of 10-50 ng/mL. Urology. 2008;72:1194. doi: 10.1016/j.urology.2008.07.028. [DOI] [PubMed] [Google Scholar]


