Abstract
Many cancer management approaches including immunotherapies can not achieve desirable therapeutic efficacies if targeting tumors alone or could not effectively reach tumor cells. The concept of tumor microenvironment and its induced gene reprogramming have largely extended our current understandings on the determinants of tumor initiation/progression and fostered our hope in establishing first-line therapies targeting cancer microenvironment or adjuvant therapies enhancing the efficacies of existing oncotherapeutic modalities such as immunotherapies for efficient cancer management. This review identifies key indexes of tumor microenvironment, i.e., hypoxia, acidosis, hypo-nutrition and inflammation, which collectively determine the feature and the fate of adjacent tumor cells, and proposes cold atmospheric plasma, the fourth state of matter that is largely composed of various reactive oxygen and nitrogen species, as a promising tool for tumor microenvironment editing. We propose that cold atmospheric plasma represents an emerging onco-therapeutic strategy alone or complementing existing treatment approaches given its multi-modal nature through tumor microenvironment modulation.
Keywords: Tumor microenvironment, cold atmospheric plasma, reactive oxygen and nitrogen species, microenvironment editing
Introduction
Tumor microenvironment (TME) refers to the environment where a tumor originates. It dynamically alters during carcinogenesis and constantly interchanges signals and biomasses with tumors. Tumors can edit TME by, e.g., promoting tumor angiogenesis, creating metabolic symbiosis with stromal cells, and inducing peripheral immune tolerance, while immune cells in the microenvironment can affect the proliferation and evolution of cancerous cells [1]. The critical roles of TME and its interplay with tumors during cancer initiation and progression such as vascularization and immunosuppression [2,3] have been increasingly recognized. Understanding the unique features of TME not only helps us create desired efficacies from conventional anticancer therapies (e.g., immunotherapies [4]), but also leads us to the identification of novel onco-therapeutic targets and treatment possibilities. We are thus motivated to comprehensively review key indexes for TME measurement, based on which we propose cold atmospheric plasma (CAP), incompletely ionized plasma and the fourth state of matter besides solid, liquid and gas, as an emerging first line or adjuvant approach for cancer control given its efficacies in TME editing.
Factors influencing tumor microenvironment
Hypoxia
Hypoxia, being one of the most characterized properties of TME, arises in tumors through excess tumor mass resulting from uncontrolled tumor cell proliferation and insufficient oxygen supply. Hypoxia, in turn, up-regulates the production of angiogenic factors and triggers the vascularization of the tumor mass, resulting in tumor angiogenesis [5]. Unlike normal vascularization, vessels formed from tumor angiogenesis have chaotic architecture that often lead to vascular leakiness and non-laminar blood flow [6]. Therefore, the functionalities of these abnormally generated vessels are not guaranteed, and typically subjected to alterations in the direction and velocity of the flow that likely to lead to blood clotting and local tissue oedema [7,8]. Adaptation to hypoxia is primarily mediated through transcription factor hypoxia-induced factors (HIFs). There are two forms of HIFs, HIF1α and HIF2α, each being composed of a β subunit and an oxygen-labile α subunits that distinguishes the two HIFs. The α subunit is rapidly degraded through PHD-mediated hydroxylation following pVHL-dependent ubiquitylation under normoxic conditions [9-11]. HIFs regulate the expression of target genes playing critical roles in, e.g., tumor angiogenesis and metabolic adaptations through recognizing hypoxia-responsive elements located in either proximal or distal to their promoters [12-16].
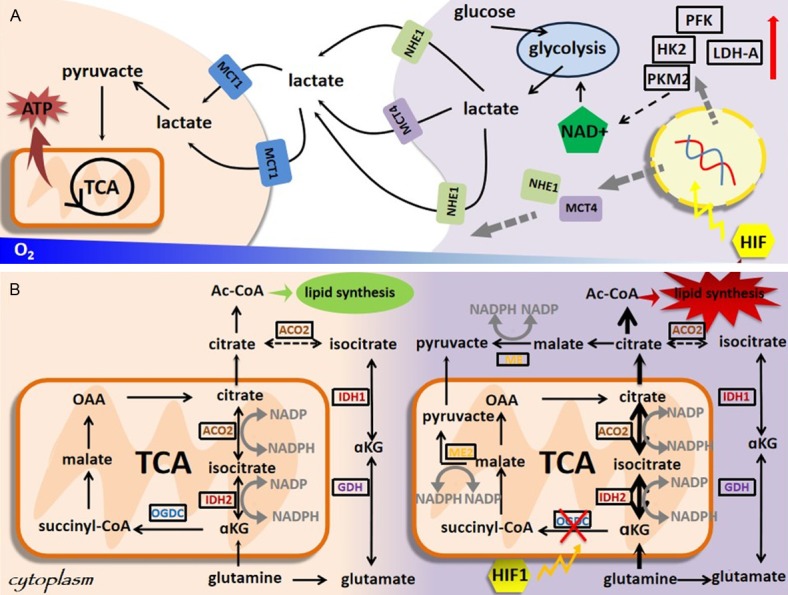
Hypoxia can rewire the glucose metabolic fate of cancer cells (Figure 1A). Under hypoxic conditions, glycolytic rate is enhanced by up-regulating and/or activating a series of enzymes boosting glycolysis including, e.g., lactate dehydrogenase A (LDH-A), pyruvate kinase M2 (PKM2), hexokinase II (HK2), and phosphofructokinase (PFK) [17,18]; several transporters extruding acids and/or lactates such as monocarboxylate transporter isoform 4 (MCT4) and the Na+/H+ exchanger NHE1 are up-regulated by HIF-1α [19,20], leading to the secretion of various acid equivalents including lactates by tumor cells; these lactates produced by hypoxic tumor cells are taken up by normoxic cancer cells via MCT1 and spared for tumor energy and biomass supply [21], resulting in metabolic coupling between cancer cells located in differentially oxygenated regions, and between cancer and normal cells.
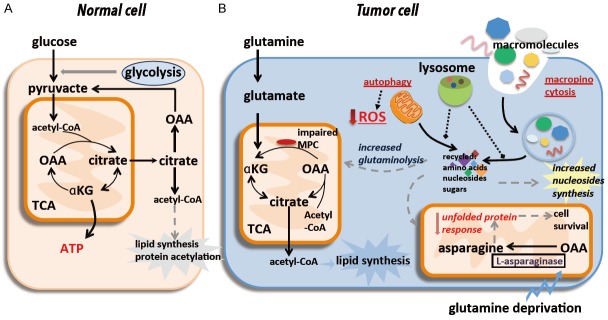
Figure 1.
Hypoxia is a characterized feature of tumor microenvironment. Hypoxia can (A) rewire the glucose metabolic fate of cancer cells, and (B) alter glutamine flux. In (A), under hypoxic conditions, glycolytic rate is enhanced by up-regulating and/or activating a series of glycolysis-stimulating enzymes such as PFK, HK2, PKM2 and LDH-A, several acid and/or lactate-extruding transporters such as MCT4 and NHE1 are up-regulated by hypoxia through HIF-1α signaling, leading to the secretion of lots of lactates and other acid equivalents by tumor cells. Metabolic coupling occurs between cancer cells in hypoxic and well-oxygenated tumor regions, and between cancer and stromal cells, i.e., lactates produced by hypoxic tumor cells are taken up via MCT1 in normoxic cancer cells, followed by conversion to pyruvate, sparing the limited supply of glucose for the hypoxic tumor regions. In (B), despite decreased mitochondrial respiration and increased activity of reductive carboxylation, hypoxic cells can maintain and in some cases even up-regulate oxidative glutamine metabolism, accounting for the majority of ATP synthesis through oxidative phosphorylation under hypoxic conditions.
Hypoxia can alter glutamine flux (Figure 1B). Being essential to the anabolism of most cells [22], glutamine is oxidized through both the tricarboxylic acid cycle (TCA) and anabolic building blocks to support cell proliferation under normoxic conditions. Once exposed to hypoxia, glutamine flux is decreased due to reduced pyruvate oxidation and mitochondrial respiration, glutamine oxidation dropped which can be mimicked in cells with dysfunctional mitochondria [23-26], reductive carboxylation of glutamine-derived alpha-ketoglutarate (αKG) occurs in response to increases in the αKG/citrate ratio to produce sufficient citrate for lipid synthesis [27-31]. It is worth mentioning that hypoxic cells can maintain or even up-regulate glutamine oxidation in some cases to make cells generate ATP mostly via oxidative phosphorylation under hypoxic conditions, regardless of reduced mitochondrial respiration and enhanced reductive carboxylation [32-34].
Acidosis
Tumor acidosis has been gaining increased recognition as another major TME index. Tumor acidosis mainly results from lactic acid excretion and CO2 hydration. H+ ions may alter the functionalities of various proteins by influencing the ionization of some of their amino acid residues [35], underlying a delicate balance between the intracellular pH (pHi) and the extracellular pH (pHe) that represents the metabolic features of a given cohort of cancer cells.
Acidosis alters the glucose metabolism of cancer cells. A lactate gradient is generated to adapt to TME acidosis, where the most hypoxic tumor areas has the lowest pH. While lactates are released through MCT4 from cancer cells as the end-products of glycolysis, other cancer cells can capture lactates via MCT1 and convert them into pyruvates [21,36-40], resulting in metabolic symbiosis [23,41-49]. The fact that H+ ions need to be co-transported with lactates for inward flux boosts the requirement on neutralizing intracellular H+ for sustainable lactate shuttle, where carbonic anhydrase helps solving this problem by neutralizing H+ ions and hydrating CO2 (Figure 2A). Using lactates secreted from cancer cells to support the TCA cycle and fatty acid synthesis of other cancer cells is one important survival strategy for cancer cells under low glucose availability. First, local acidification plays promotive roles on tumor invasion [50], partly through increasing extracellular levels of VEGF-A [51,52] and proteases [53,54]. Second, lactates can be used as energy substrate by adjacent stromal cells to support growth or produce pyruvates that are extruded to TME and taken up by cancer cells [55,56].
Figure 2.
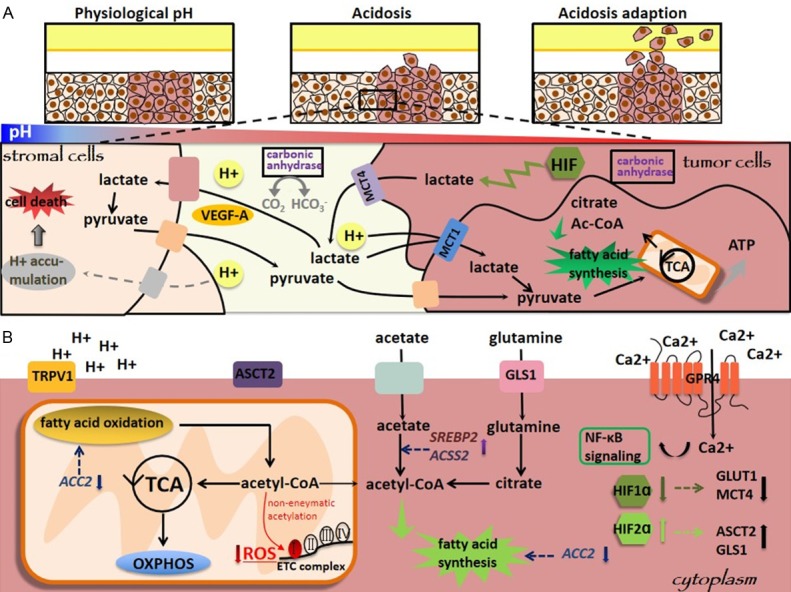
Tumor acidosis is a major tumor microenvironment index. Acidosis alters (A) the glucose and (B) lipid metabolism of cancer cells. In (A), acidosis adaption occurs, i.e., generating a lactate gradient, with the highest concentration being in the most hypoxic tumor areas, following metabolic symbiosis, i.e., while lactates are released through MCT4 from cancer cells as the end-products of glycolysis, other cancer cells can capture lactates via MCT1 and convert them into pyruvates. In (B), both fatty acid synthesis and fatty oxidation are enhanced under acidosis, where fatty acid synthesis is mediated via the production of acetyl-CoA from citrates and acetates.
Lipid metabolism is also metabolically reprogrammed under acidosis (Figure 2B). Acidosis can rewire the fates of citrates and/or acetates toward acetyl-CoA production and ultimately fatty acid synthesis, accompanied with fatty acid oxidation [41,42]. It is worth noting that though these two fatty acids synthesis supporting pathways are also stimulated in response to hypoxic stress [25,26,43,44], fatty acid oxidation is only concomitantly activated under acidosis. In many cancers such as colon, oropharyngeal and cervical tumors, fatty acid synthesis and oxidation are simultaneously promoted under acidosis by down-regulating ACC2 [45]. Fatty acid oxidation derived acetyl-CoA can fuel the TCA cycle and lead to a sharp increase in the non-enzymatic acetylation of many proteins, including electron transport chain (ETC) complex members [45]. However, restraining the activity of acetylated ETC complex I could limit the production of reactive oxygen species (ROS) that enhances the fitness of cancer cells, and reduced activities of acetylated ETC complex I has been measured in various types of cancer cells adapted to acidosis [45].
Hypoxia is advantageous for tumor cells to survive under acidosis exposure [47]. Alteration in the key pHi regulating proteins under hypoxia provides tumor cells with survival advantages over normal cells [48]. The hypoxia-induced transcription factor HIF2α plays critical roles in the regulation of metabolic adaptation to acidosis [41,46,57,58], and the activity of the bona fide HIF1α is generally down-regulated under such conditions [41,59,60]. Acidosis leads to increased activities of NAD-dependent protein deacetylase sirtuin-1 (SIRT1) and -6 (SIRT6) which differentially affect the activities of both HIFs [52,61,62]. While deacetylation of lysine residues in the amino-terminal transactivation domain of HIF2α is associated with its increased regulatory activities [62], similar deacetylation in HIF1α represses its transcriptional functionalities [63]. In cancer cells of the cervix, colon and pharynx, Up-regulated HIF2α and down-regulated HIF1α could drive the switch from glucose to glutamine metabolism on acidosis exposure via elevating the expression of the glutamine transporters ASCT2 and GLS1 (regulated by HIF2α) [41] and reducing the expression of the glucose transporters GLUT1 and MCT4 (modulated by HIF1α).
Various types of pH sensors function in sensing extracellular acidosis and transducing it into intracellular reprogramming [64]. G protein-coupled receptors (GPCRs) such as GPR4, GPR65 and GPR68 could be activated if the histidine residues of their extracellular domains were protonated [65], and transduce signals via activating various pathways such as phospholipase C and adenylyl cyclase through the use of different G proteins [66] (Figure 2B). Non-GPCRs such as transient receptor potential cation channel subfamily V member 1 (TRPV1) and acid-sensing ion channel 1 (ASIC1) can also sense extracellular acidosis [66]. Calcium influx, under acidosis exposure, directly or indirectly opens these channels that activates NF-κB signaling in, e.g., breast [67] and prostate [68] cancers. Alterations in pH can also be detected through the protonation of various signalling proteins [35,69]. For instance, the R337H substitution in the tetramerization domain of TP53, a famous tumor suppressor, under increased pHi can result in DNA binding inhibition [70]. Unveiling various types of pH sensors and altered signaling pathways in response to their activation can significantly advance our understandings towards tumor acidosis and avail in any oncotherapeutic design targeting tumor acidosis and the associated metabolic reprogramming.
Hypo-nutrition
As nutrient supply cannot typically support the growth of tumor cells due to the rapid expansion of tumors and relatively poor angiogenesis, cells residing in the inner part of tumors typically suffer from hypo-nutrition. Warburg effect, the observation that cancer cells favor anaerobic glycolysis rather than the more energy-efficient aerobic glycolysis suggests that tumor cells require enhanced nutrient supply to support their increased metabolism and biosynthesis than normal cells. To survive under hypo-nutrition, cancer cells show extreme metabolic flexibilities. It was recently reported both in vitro and in vivo that, glucose deprivation imposes a selective pressure for KRAS mutations in colon cancer cells [71]. Similarly, cancer cells are capable of rewiring their metabolism towards the use of alternative nutrients to compensate for the loss of, e.g., glucose [32,72-75].
Glutamine can complement the synthesis of acetyl-CoA as an alternative of glucose [76], which is crucial for cell survival on lack of glucose (Figure 3). For instance, converting glutamine to lactate can produce sufficient NADPH that is needed for the synthesis of fatty acids [39]. Lymphoma cells with Myc over-expression can reroute glutamine carbon to produce acetyl-CoA once deprived of glucose [32], and such metabolic reprogramming can also be produced by silencing the mitochondrial pyruvate carrier (MPC) [77,78]. Therefore, metabolic vulnerability as such is required and glutamine oxidation is indispensable for tumor cells to survive if MPC was impaired [78].
Figure 3.
Hypo-nutrition is characterized in tumor microenvironment. Tumors (A) use glutamine to fuel alternative forms of metabolism and (B) activate autophagic degradation of macromolecules under hypo-nutrition. In (A), glucose deprivation in cancer cells stimulates a pathway whereby glutamine carbon is re-routed to acetyl-CoA. In (B), autophagy functions in degrading damaged organelles and their macromolecular components to provide recycled small molecule nutrients to feed intermediary metabolism under hypo-nutrition, and functions to eliminate defective mitochondria, thereby reducing ROS accumulation and improving cellular fitness.
Maintaining an asparagine pool provides survival advantages to tumor cells when deprived of glutamine. For example, citrate synthase maintains the functionalities of the TCA cycle by condensing glutamine-derived OAA with acetyl-CoA under normal conditions [76], which was found to be lost once deprived of glutamine [79]; further, shunting OAA towards asparagine rather than citrate was shown to be favorable for cell survival [79]. On the other hand, asparagine synthetase expression is positively associated with poor prognosis in cancers such as glioma and neuroblastoma [79], providing an in vivo evidence of our notion that an asparagine pool favors cancer cell survival.
Macropinocytosis adds further metabolic flexibilities to cancer cells under glutamine deprivation. Macropinocytosis enables cells to scavenge fluid and macromolecules, where extracellular proteins were important cargoes captured and internalized in macropinosomes, and these proteins provide starved cells with materials to generate pools of glutamine and other amino acids required for survival [80]. It was reported that glutamine deprivation could stimulate macropinocytosis in cancer cells expressing Ras [80]. Therefore, macropinocytosis provides another mode of metabolic flexibility for cancer cells to overcome hypo-nutrition.
Cancer cells can recycle molecule nutrients by degrading macromolecules through autophagy under hypo-nutrition [81-84] (Figure 3). During autophagy, damaged organelles are degraded and recycled to feed intermediary metabolism [83,85,86]. Autophagy can also reduce cellular redox level by eliminating defective mitochondria, leading to improved cellular fitness. Autophagy was shown to be required in Kras-driven pancreatic tumors [87] and BRafV600E lung tumors [88] for maximal growth, and both autophagy and normal mitochondrial function were needed in forming aggressive Kras-driven carcinomas [89]. Therefore, autophagy provides tumor survival advantages through extracellular nutrient supply and intracellular nutrient recycle.
Inflammation
Inflammation is a defensive response against foreign invasion or in response to physical and chemical hazards [90,91]. A clear evidence between inflammation and tumorigenesis was established in the last decade [92], and tumor-associated inflammation was implicated as a enabling cancer hallmark in 2011 [93].
Tumor-associated inflammation is characterized by the presence of a large amount of leukocytes in tumor tissues and high expression of inflammatory mediators in TME (Figure 4). Macrophages in the TME are composed of tumor-associated macrophages (TAMs) and monocytes recruited from blood vessels, where TAMs have two phenotypes, i.e., M1 and M2 macrophages which are pro-inflammatory and anti-inflammatory, respectively [94]. M2 macrophages are polarized by factors derived from tumors to sustain tumor proliferation and enable immunosuppression [95-97]. Cancer-associated fibroblasts (CAFs) are originated from normal fibroblasts with acquired characteristics similar to myofibroblasts. Both TAMs and CAFs are abundant in a TME and play crucial roles in tumor initiation, progression, evasion, and chemotherapeutic resistance [98].
Figure 4.
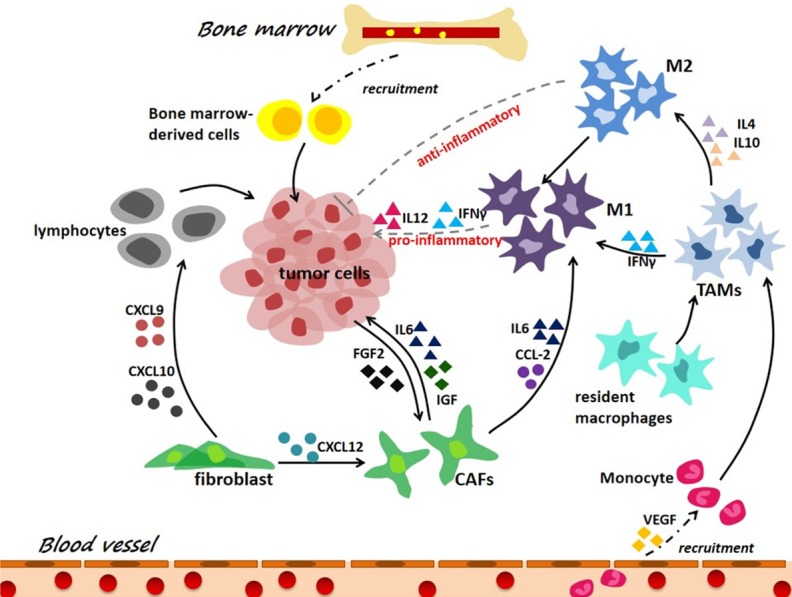
Tumor associated inflammation is an essential mark of tumor microenvironment. Tumor-associated inflammation is characterized by a high number of leukocytes in tumor tissues, and high expression of inflammatory mediators in TME, where tumor-associated macrophages (TAMs) have two phenotypes, i.e., M1 macrophages (pro-inflammatory) and M2 macrophages (anti-inflammatory). During tumor-associated inflammation, innate immune cells such as TAMs and activated resident cells such as CAFs produce a variety of chemokines and cytokines in response to the danger signals originated from tumor cells.
Tumor-associated inflammation is a chronic process that fosters tumor progression [99] (Figure 4). During tumorigenesis, cancer cells, TAMs, CAFs or other innate immune or activated resident cells produce a variety of chemokines and cytokines such as interleukins and interferons in response to signals originated from tumor cells. Cytokines are major players in chronic inflammation, and are indispensible throughout the whole process of cancer initiation and progression mediated by inflammation [100]. For example, cytokines can activate and constitute the so-called cytokine storm (a type of systemic inflammatory response which can be caused by infection or adverse effect of some immunotherapies) by recruiting massive amounts of additional bone marrow-derived innate immune cells [101]; and this prolonged reaction favors immunosuppression via accumulating myeloid suppressive cells and inhibiting effector immune cells, and promotes tumor cell proliferation as well as angiogenesis [102]. With our increased knowledge on signalings involved in tumor inflammation and its cross-talk with TME, we will be able to create more effective immunotherapies with little side effects by concomitantly modulating TME [103,104].
Cold atmospheric plasma targets tumor microenvironment indexes
CAP is a near room temperature ionized gas comprised of various reactive species, such as charged particles, neutral gas molecules, UV radiation, localized electric, reactive species and so on [105-107]. Dominant radical sources are reactive oxygen and nitrogen species (RONS) formed from oxygen and nitrogen molecules [105]. These complicated substances lead to numerous interactions between CAP and cells or tissues [107,108], and triggers complex chemical kinetics. RONS have been implicated in tumor microenvironment modulation [109]. CAP has been proposed as a promising oncotherapeutic approach [106,110,111], which largely relies on its ability in tumor microenvironment modulation apart from its other functionalities controlling cancer progression (Figure 5).
Figure 5.
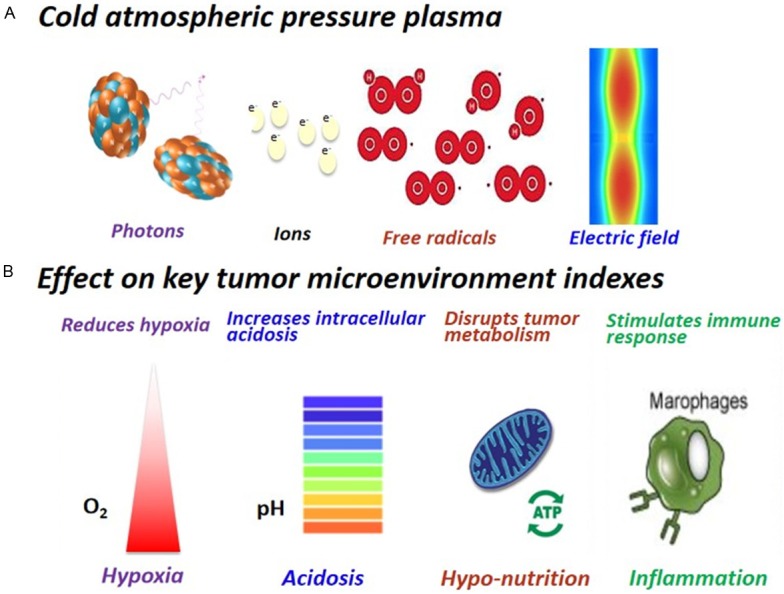
Cold atmospheric plasma composition and its effects on modulating key tumor microenvironment indexes. A. CAP is a cocktail therapy with multi-modality nature. It is composed of, e.g., free radicals, photons, ions and elective fields. B. CAP reduces hypoxia, increases intracellular acidosis, disrupts tumor metabolism and stimulates immune response.
Cold atmospheric plasma reduces hypoxia
Hypoxia is a major cause of cancer cell resistance to some treatment modalities such as radiotherapy. CAP can reduce hypoxia, where a rapid fourfold increase in tissue oxygen partial pressure (pO2) was observed in mouse skin upon plasma treatment [112].
As oxygen is the terminal electron acceptor, electrons leak out from the mitochondrial electron transport chain under hypoxia [9,10], which creates a redox stress in tumor mitochondria, CAP can create temporal openings of the cell membrane, usually over a microsecond time scale, to allow for the transportation of RONS into cells [113,114]. Once entering cells, RONS increases cellular redox level that leads to selective death of cancer cells as the baseline redox level of malignant cells is higher than that of normal cells and cells undergo apoptosis when cellular redox level exceeds a certain threshold [115-118]. Redox change is quite often accompanied with signalings tilting apoptosis. CAP can induce ATM expression that phosphorylates p53 [119] and p73 [120], where phosphorylated p53 and p73 can induce mitochondria mediated apoptosis through activating the expression of pro-apoptotic factors such as Bax, PUMA, and NOXA [120,121].
Cold atmospheric plasma increases intracellular acidosis
Reactive species generated by CAP at the gas-water interface triggers intricate reactions within the bio-system that often lead to acidification of the culture medium and ultimately increased intracellular acidosis. For example, with the increased dose of CAP, the pH of the cell culture medium might drop from 8.5 to 5.5 [122]. An alkaline pHi was shown necessary for mechanisms involved in driving or facilitating cellular transformation and proliferation [123-125], as many intracellular metabolic enzymes, such as phosphofructokinase (the rate-limiting step of glycolysis), have alkaline pH optima [123]. As elevated pHi is associated with both cell transformation and cell proliferation [126], increased intracellular acidosis, though does not necessarily lead to cell apoptosis [122,127,128], may arrest cell growth.
Cold atmospheric plasma disrupts tumor cell metabolism
Cancer cells adopt aerobic glycolysis to support their excess needs on biomass supply required for rapid growth [129]. Through whole metabolism profiling, CAP was found to suppress beta-alanine metabolism in myeloma cells [130]. Beta-alanine is crucial for acetyl-CoA synthesis that plays critical roles in TCA cycle and biomass production such as the synthesis of fatty acids, cholesterols and acetylcholines [112,131]. Therefore, CAP can disrupt cellular metabolism favorable for tumor cell growth through, e.g., suppressing biomass production.
Cold atmospheric plasma stimulates immune response
Immunogenic cell death (ICD) can induce an effective antitumor immune response through activation of dendritic cells and consequent activation of specific T cell response [132]. ICD is accompanied by changes in the influx of many signal molecules on the surface of cell membrane, and the synthesis and release of immune-effector factors. These substances are called damage-associated molecular patterns (DAMPs), including early cell death calreticulin (CRT), release of heat-shock proteins, late cell death ATP and HMGB1 [133]. CRT is a main cause of ICD, which is capable of emitting ‘eat me’ signaling to mediate the engulfment of tumor cells by macrophages and dendritic cells [134] and recruiting immune cells to the tumor immune site to promote inflammation. ATP and HMGB1 are important signaling molecules of DAMPs, where extracellular ATP promotes secretion of key pro-inflammatory cytokines, activates macrophages that play an important role in anti-tumor effects. CAP can significantly enhance the secretion of CRT, ATP and HMGB1 in tumor tissues, inducing ICD [135,136].
Macrophages are crucial mediators of cellular inflammation [135]. M1 macrophages are pro-inflammatory, including secrete pro-inflammatory cytokines and cytotoxic substances, and M2 macrophages are immunosuppressive and release anti-inflammatory cytokines [134]. Tumor cells can polarize macrophages into M2 to support tumor growth, and CAP can modulate this switch in reverse [136]. It was demonstrated that the expression of CD206 (marker of M2) decreased remarkably while iNOs (Marker of M1) significantly increased on CAP treatment [135,136]. CAP has also been shown to upregulate the influx of INF-γ in cell culture supernatants derived from splenocytes [133], which play a key role in activating M1 macrophages. Altogether, M1 macrophages were enhanced to induce antitumor responses including secretion of cytokines such as TNFα, IL-1 and IL-6, kill tumor cells, and provoke inflammation in the tumor microenvironment upon CAP treatment. Other immune cells including monocytes and neutrophils have also been detected increased in CAP activated medium that lead to enhanced immune response against tumor cells [129].
Endoplasmic reticulum (ER) stress is a protective stress response of eukaryotic cells that reduces abnormal aggregation of intracellular proteins by activating unfolded protein response (UPR) [137]. UPR is coupled with inflammatory signaling pathways through various mechanisms including RONS, NFkB, and release of calcium ions in ER. ATF4 and STC2 are typical markers of ER stress, and CAP was shown capable of increasing ATF4 and STC2 generation that triggers inflammatory signaling [111].
Conclusion
Given the multi-modality nature, CAP has demonstrated its unique properties in TME modulation including, e.g., reducing hypoxia, increasing intracellular acidosis, disrupting tumor cell metabolism and stimulating immune response. Importantly, CAP can selectively target cancer cells [130,138-140] with its safety being systematically validated by several studies [110,140-143]. Thus, CAP represents a promising onco-therapeutic approach, alone or in combination with existing treatment modalities to achieve improved efficacies with little side effects.
Acknowledgements
This study was funded by the Natural Science Foundation of Jiangsu Province (Grant No. BK20161130), the Six Talent Peaks Project in Jiangsu Province (Grant No. SWYY-128), Major Project of Science and Technology in Henan Province (Grant No. 161100311400), National Science and Technology Major project (Grant No. 2018ZX10302205-004-002), Research Funds for the Medical School of Jiangnan University ESI special cultivation project (Grant No. 1286010241170320), The Major Province of Science and Technology in Henan Province (Grant No. 2018ZX10302-205-004), The Technology Development Funding of Wuxi (Grant No. WX18IVJN017). These funding sources have no role in the writing of the manuscript or the decision to submit it for publication. The Translational Research Institute receives support from the Australian Federal Government.
Disclosure of conflict of interest
None.
References
- 1.Korneev KV, Atretkhany KN, Drutskaya MS, Grivennikov SI, Kuprash DV, Nedospasov SA. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine. 2017;89:127–135. doi: 10.1016/j.cyto.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 3.Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–48. doi: 10.1016/j.copbio.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Hugues S. Warming up the tumor microenvironment in order to enhance immunogenicity. Oncoimmunology. 2019;8:e1510710. doi: 10.1080/2162402X.2018.1510710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldmann E. The growth of malignant disease in man and the lower animals, with special reference to the vascular system. Proc R Soc Med. 1908;1:1–13. doi: 10.1177/003591570800101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 8.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 10.Berra E, Roux D, Richard DE, Pouyssegur J. Hypoxia-inducible factor-1 alpha (HIF-1 alpha) escapes O-2-driven proteasomal degradation irrespective of its subcellular localization: nucleus or cytoplasm. Embo Reports. 2001;2:615–620. doi: 10.1093/embo-reports/kve130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor-1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular O-2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 13.Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:E207–E217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 15.Shah T, Krishnamachary B, Wildes F, Mironchik Y, Kakkad SM, Jacob D, Artemov D, Bhujwalla ZM. HIF isoforms have divergent effects on invasion, metastasis, metabolism and formation of lipid droplets. Oncotarget. 2015;6:28104–28119. doi: 10.18632/oncotarget.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Icard P, Lincet H. A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim Biophys Acta. 2012;1826:423–433. doi: 10.1016/j.bbcan.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L941–L949. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 20.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1 alpha-dependent mechanism. Am J Pathol. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 21.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang YF, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–U171. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filipp FV, Scott DA, Ronai ZA, Osterman AL, Smith JW. Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res. 2012;25:375–383. doi: 10.1111/j.1755-148X.2012.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang JJ, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–U166. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker SJ, Metallo CM. Metabolic consequences of oncogenic IDH mutations. Pharmacol Ther. 2015;152:54–62. doi: 10.1016/j.pharmthera.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fendt SM, Bell EL, Keibler MA, Olenchock BA, Mayers JR, Wasylenko TM, Vokes NI, Guarente L, Vander Heiden MG, Stephanopoulos G. Reductive glutamine metabolism is a function of the alpha-ketoglutarate to citrate ratio in cells. Nat Commun. 2013;4:2236. doi: 10.1038/ncomms3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gameiro PA, Yang J, Metelo AM, Perez-Carro R, Baker R, Wang Z, Arreola A, Rathmell WK, Olumi A, Lopez-Larrubia P, Stephanopoulos G, Iliopoulos O. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab. 2013;17:372–385. doi: 10.1016/j.cmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberta L, Chitra S, Suzanne J, Rock CO. Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J Biol Chem. 2012;287:14615–20. doi: 10.1074/jbc.C112.353946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smolková K, Dvořák A, Zelenka J, Vítek L, Ježek P. Reductive carboxylation and 2-hydroxyglutarate formation by wild-type IDH2 in breast carcinoma cells. Int J Biochem Cell Biol. 2015;65:125–133. doi: 10.1016/j.biocel.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang HX, Zimmerman LJ, Liebler DC, Slebos RJ, Lorkiewicz PK, Higashi RM, Fan TW, Dang CV. Glucose-independent glutamine metabolism via tca cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassian AR, Parker SJ, Davidson SM, Divakaruni AS, Green CR, Zhang X, Slocum KL, Pu M, Lin F, Vickers C, Joud-Caldwell C, Chung F, Yin H, Handly ED, Straub C, Growney JD, Vander Heiden MG, Murphy AN, Pagliarini R, Metallo CM. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res. 2014;74:3317–3331. doi: 10.1158/0008-5472.CAN-14-0772-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, Rabinowitz JD. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol. 2013;9:712. doi: 10.1038/msb.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava J, Barber DL, Jacobson MP. Intracellular pH sensors: design principles and functional significance. Physiology (Bethesda) 2007;22:30–39. doi: 10.1152/physiol.00035.2006. [DOI] [PubMed] [Google Scholar]
- 36.Boidot R, Vegran F, Meulle A, Le Breton A, Dessy C, Sonveaux P, Lizard-Nacol S, Feron O. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 2012;72:939–948. doi: 10.1158/0008-5472.CAN-11-2474. [DOI] [PubMed] [Google Scholar]
- 37.Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappa B/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 38.Allen E, Mieville P, Warren CM, Saghafinia S, Li L, Peng MW, Hanahan D. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. 2016;15:1144–1160. doi: 10.1016/j.celrep.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimenez-Valerio G, Martinez-Lozano M, Bassani N, Vidal A, Ochoa-de-Olza M, Suarez C, Garcia-del-Muro X, Carles J, Vinals F, Graupera M, Indraccolo S, Casanovas O. Resistance to antiangiogenic therapies by metabolic symbiosis in renal cell carcinoma PDX models and patients. Cell Rep. 2016;15:1134–1143. doi: 10.1016/j.celrep.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pisarsky L, Bill R, Fagiani E, Dimeloe S, Goosen RW, Hagmann J, Hess C, Christofori G. Targeting metabolic symbiosis to overcome resistance to anti-angiogenic therapy. Cell Rep. 2016;15:1161–1174. doi: 10.1016/j.celrep.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corbet C, Draoui N, Polet F, Pinto A, Drozak X, Riant O, Feron O. The SIRT1/HIF2 alpha axis drives reductive glutamine metabolism under chronic acidosis and alters tumor response to therapy. Cancer Res. 2014;74:5507–5519. doi: 10.1158/0008-5472.CAN-14-0705. [DOI] [PubMed] [Google Scholar]
- 42.Kondo A, Yamamoto S, Nakaki R, Shimamura T, Hamakubo T, Sakai J, Kodama T, Yoshida T, Aburatani H, Osawa T. Extracellular acidic pH activates the sterol regulatory element-binding protein 2 to promote tumor progression. Cell Rep. 2017;18:2228–2242. doi: 10.1016/j.celrep.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Sun RC, Denko NC. Hypoxic regulation of glutamine metabolism through HIF1 and SIA-|H2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 2014;19:285–292. doi: 10.1016/j.cmet.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, Goodwin LM, Smethurst E, Mason S, Blyth K, McGarry L, James D, Shanks E, Kalna G, Saunders RE, Jiang M, Howell M, Lassailly F, Thin MZ, Spencer-Dene B, Stamp G, van den Broek NJ, Mackay G, Bulusu V, Kamphorst JJ, Tardito S, Strachan D, Harris AL, Aboagye EO, Critchlow SE, Wakelam MJ, Schulze A, Gottlieb E. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27:57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbet C, Pinto A, Martherus R, Santiago de Jesus JP, Polet F, Feron O. Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metab. 2016;24:311–323. doi: 10.1016/j.cmet.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Filatova A, Seidel S, Bogurcu N, Graf S, Garvalov BK, Acker T. Acidosis acts through HSP90 in a PHD/VHL-independent manner to promote HIF function and stem cell maintenance in glioma. Cancer Res. 2016;76:5845–5856. doi: 10.1158/0008-5472.CAN-15-2630. [DOI] [PubMed] [Google Scholar]
- 47.Parks SK, Mazure NM, Counillon L, Pouyssegur J. Hypoxia promotes tumor cell survival in acidic conditions by preserving ATP levels. J Cell Physiol. 2013;228:1854–1862. doi: 10.1002/jcp.24346. [DOI] [PubMed] [Google Scholar]
- 48.Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–623. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- 49.Pawel S, Vaughan-Jones RD, Harris AL, Alzbeta H. The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130099. doi: 10.1098/rstb.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 51.Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- 52.Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, Xie K. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20:3751–3756. doi: 10.1038/sj.onc.1204500. [DOI] [PubMed] [Google Scholar]
- 53.Baumann F, Leukel P, Doerfelt A, Beier CP, Dettmer K, Oefner PJ, Kastenberger M, Kreutz M, Nickl-Jockschat T, Bogdahn U, Bosserhoff AK, Hau P. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. 2009;11:368–380. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994;54:6517–6525. [PubMed] [Google Scholar]
- 55.Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92:329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 56.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 57.Mekhail K, Gunaratnam L, Bonicalzi ME, Lee S. HIF activation by pH-dependent nucleolar sequestration of VHL. Nat Cell Biol. 2004;6:642–647. doi: 10.1038/ncb1144. [DOI] [PubMed] [Google Scholar]
- 58.Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan A, Rich JN. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011;18:829–840. doi: 10.1038/cdd.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang X, Lucas JE, Chen JL, LaMonte G, Wu J, Wang MC, Koumenis C, Chi JT. Functional interaction between responses to lactic acidosis and hypoxia regulates genomic transcriptional outputs. Cancer Res. 2012;72:491–502. doi: 10.1158/0008-5472.CAN-11-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen JL, Lucas JE, Schroeder T, Mori S, Wu J, Nevins J, Dewhirst M, West M, Chi JT. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 2008;4:e1000293. doi: 10.1371/journal.pgen.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Molecular Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 62.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 63.Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 64.Glitsch M. Protons and Ca2+: ionic allies in tumor progression? Physiology (Bethesda) 2011;26:252–265. doi: 10.1152/physiol.00005.2011. [DOI] [PubMed] [Google Scholar]
- 65.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 66.Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol. 2013;4:370. doi: 10.3389/fphys.2013.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta SC, Singh R, Pochampally R, Watabe K, Mo YY. Acidosis promotes invasiveness of breast cancer cells through ROS-AKT-NF-kappaB pathway. Oncotarget. 2014;5:12070–12082. doi: 10.18632/oncotarget.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen B, Liu J, Ho TT, Ding X, Mo YY. ERK-mediated NF-kappaB activation through ASIC1 in response to acidosis. Oncogenesis. 2016;5:e279. doi: 10.1038/oncsis.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 70.DiGiammarino EL, Lee AS, Cadwell C, Zhang W, Bothner B, Ribeiro RC, Zambetti G, Kriwacki RW. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat Struct Biol. 2002;9:12–16. doi: 10.1038/nsb730. [DOI] [PubMed] [Google Scholar]
- 71.Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou S, Diaz LA Jr, Velculescu VE, Lengauer C, Kinzler KW, Vogelstein B, Papadopoulos N. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or akt signaling. Cancer Res. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci U S A. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H, Zhou F, Green MR, Chen L, Monti S, Marto JA, Shipp MA, Danial NN. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vacanti NM, Divakaruni AS, Green CR, Parker SJ, Henry RR, Ciaraldi TP, Murphy AN, Metallo CM. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol Cell. 2014;56:425–435. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, Rutter J, Merritt ME, DeBerardinis RJ. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56:414–424. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng EH, Judkins AR, Pawel B, Baggs J, Cherry S, Rabinowitz JD, Thompson CB. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell. 2014;56:205–218. doi: 10.1016/j.molcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldsmith J, Levine B, Debnath J. Autophagy and cancer metabolism. Methods Enzymol. 2014;542:25–57. doi: 10.1016/B978-0-12-416618-9.00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 85.Deberardinis RJ, Lum JJ, Thompson CB. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem. 2006;281:37372–37380. doi: 10.1074/jbc.M608372200. [DOI] [PubMed] [Google Scholar]
- 86.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 87.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, McMahon M, White E. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Dis. 2013;3:1272–1285. doi: 10.1158/2159-8290.CD-13-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo JY, Karsli-Uzunbas G, Mathew R, Aisner SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, Snyder E, Santanam U, Dipaola RS, Jacks T, Rabinowitz JD, White E. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 2013;27:1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin C, Zhang J. Inflammasomes in inflammation-induced cancer. Front Immunol. 2017;8:271. doi: 10.3389/fimmu.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colotta F, Allavena PA. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 92.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 93.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 94.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 96.Kang FB, Wang L, Li D, Zhang YG, Sun DX. Hepatocellular carcinomas promote tumor-associated macrophage M2-polarization via increased B7-H3 expression. Oncol Rep. 2015;33:274. doi: 10.3892/or.2014.3587. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y, Ye YC, Chen Y, Zhao JL, Gao CC, Han H, Liu WC, Qin HY. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9:793. doi: 10.1038/s41419-018-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 99.Pesic M, Greten FR. Inflammation and cancer: tissue regeneration gone awry. Curr Opin Cell Biol. 2016;43:55–61. doi: 10.1016/j.ceb.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 100.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 102.Becht E, Giraldo NA, Germain C, de Reynies A, Laurent-Puig P, Zucman-Rossi J, Dieu-Nosjean MC, Sautes-Fridman C, Fridman WH. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv Immunol. 2016;130:95–190. doi: 10.1016/bs.ai.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Ohman T, Teirila L, Lahesmaa-Korpinen AM, Cypryk W, Veckman V, Saijo S, Wolff H, Hautaniemi S, Nyman TA, Matikainen S. Dectin-1 pathway activates robust autophagy-dependent unconventional protein secretion in human macrophages. J Immunol. 2014;192:5952–5962. doi: 10.4049/jimmunol.1303213. [DOI] [PubMed] [Google Scholar]
- 104.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Judée F, Fongia C, Ducommun B, Yousfi M, Lobjois V, Merbahi N. Short and long time effects of low temperature plasma activated media on 3D multicellular tumor spheroids. Sci Rep. 2016;6:21421. doi: 10.1038/srep21421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hirst AM, Frame FM, Arya M, Maitland NJ, O’Connell D. Low temperature plasmas as emerging cancer therapeutics: the state of play and thoughts for the future. Tumour Biol. 2016;37:7021–7031. doi: 10.1007/s13277-016-4911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Musarat I, Evans MDM, Kostya Ken O. Atmospheric pressure gas plasma-induced colorectal cancer cell death is mediated by Nox2-ASK1 apoptosis pathways and oxidative stress is mitigated by Srx-Nrf2 anti-oxidant system. Biochimica Et Biophysica Acta. 2014;1843:2827–2837. doi: 10.1016/j.bbamcr.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 108.Mirpour S, Piroozmand S, Soleimani N, Faharani NJ, Ghomi H, Eskandari HF, Sharifi AM, Mirpour S, Eftekhari M, Nikkhah M. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci Rep. 2016;6:29048. doi: 10.1038/srep29048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hornsveld M, Dansen TB. The hallmarks of cancer from a redox perspective. Antioxid Redox Signal. 2016;25:300–325. doi: 10.1089/ars.2015.6580. [DOI] [PubMed] [Google Scholar]
- 110.Han D, Jin HC, Lee RH, Bang W, Park K, Kim MS, Shim JH, Chae JI, Moon SY. Antitumorigenic effect of atmospheric-pressure dielectric barrier discharge on human colorectal cancer cells via regulation of Sp1 transcription factor. Sci Rep. 2017;7:43081. doi: 10.1038/srep43081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan D, Sherman JH, Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget. 2017;8:15977–15995. doi: 10.18632/oncotarget.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Collet G, Robert E, Lenoir A, Vandamme M, Darny T, Dozias S, Kieda C, Pouvesle JM. Plasma jet-induced tissue oxygenation: potentialities for new therapeutic strategies. Plasma Sources Science & Technology. 2014;23:184–195. [Google Scholar]
- 113.Chang JW, Kang SU, Shin YS, Kim KI, Seo SJ, Yang SS, Lee JS, Moon E, Baek SJ, Lee K, Kim CH. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: involvement of DNA-damage-triggering sub-G(1) arrest via the ATM/p53 pathway. Arch Biochem Biophys. 2014;545:133–140. doi: 10.1016/j.abb.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 114.Sameer K, Kelly CM, Ekaterina C, Behzad T, Oleg A, Alexander F, Gary F, Jane AC. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011;6:e16270. doi: 10.1371/journal.pone.0016270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaushik NK, Kaushik N, Park D, Choi EH. Altered antioxidant system stimulates dielectric barrier discharge plasma-induced cell death for solid tumor cell treatment. PLoS One. 2014;9:e103349. doi: 10.1371/journal.pone.0103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Musarat I, Shailesh K, Hilal V, Jun HZ, Rider AE, Evans MD, Murphy AB, Kostya O. Atmospheric gas plasma-induced ROS production activates TNF-ASK1 pathway for the induction of melanoma cancer cell apoptosis. Mol Biol Cell. 2014;25:1523–1531. doi: 10.1091/mbc.E13-10-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keidar M. Plasma for cancer treatment. Post Communist Economies. 2015;24 [Google Scholar]
- 118.Yan D, Talbot A, Nourmohammadi N, Sherman JH, Cheng X, Keidar M. Toward understanding the selective anticancer capacity of cold atmospheric plasma--a model based on aquaporins (Review) Biointerphases. 2015;10:040801. doi: 10.1116/1.4938020. [DOI] [PubMed] [Google Scholar]
- 119.Kastan M. DNA damage responses: mechanisms and roles in human disease: 2007 g.h.a. Clowes memorial award lecture. Mol Cancer Res. 2008;6:517–524. doi: 10.1158/1541-7786.MCR-08-0020. [DOI] [PubMed] [Google Scholar]
- 120.Ahn HJ, Kim KI, Kim G, Moon E, Yang SS, Lee JS. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One. 2011;6:e28154. doi: 10.1371/journal.pone.0028154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ishaq M, Bazaka K, Ostrikov K. Intracellular effects of atmospheric-pressure plasmas on melanoma cancer cells. Physics of Plasmas. 2015;22:996–1002. [Google Scholar]
- 122.Rouven LK, Eric F, Christine H, Claus-Dieter H, Lars-Ivo P, Sander B. A myeloid and lymphoid infiltrate in murine pancreatic tumors exposed to plasma-treated medium. Clinical Plasma Medicine. 2018 [Google Scholar]
- 123.McLean LA, Roscoe J, Jorgensen NK, Gorin FA, Cala PM. Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am J Physiol Cell Physiol. 2000;278:C676–88. doi: 10.1152/ajpcell.2000.278.4.C676. [DOI] [PubMed] [Google Scholar]
- 124.Rich IN, Worthington-White D, Garden OA, Musk P. Apoptosis of leukemic cells accompanies reduction in intracellular pH after targeted inhibition of the Na(+)/H(+) exchanger. Blood. 2000;95:1427–1434. [PubMed] [Google Scholar]
- 125.Shrode LD, Tapper H, Grinstein S. Role of intracellular ph in proliferation, transformation, and apoptosis. J Bioenerg Biomembr. 1997;29:393–399. doi: 10.1023/a:1022407116339. [DOI] [PubMed] [Google Scholar]
- 126.Humez S, Monet M, Coppenolle F, Delcourt P, Prevarskaya N. The role of intracellular pH in cell growth arrest induced by ATP. Am J Physiol Cell Physiol. 2004;287:C1733–C1746. doi: 10.1152/ajpcell.00578.2003. [DOI] [PubMed] [Google Scholar]
- 127.Kong MG, Keidar M, Ostrikov K. Plasmas meet nanoparticles-where synergies can advance the frontier of medicine. Journal of Physics D Applied Physics. 2011;4417:174018–174014. [Google Scholar]
- 128.Liedtke KR, Hackbarth C, Partecke LI, Bekeschus S. Reduction of metastasis with plasma-treated liquid in mice: the role of myleoid cells in the tumor microenvironment. Clinical Plasma Medicine. 2018;9:45–46. [Google Scholar]
- 129.Xu D, Cui Q, Xu Y, Wang B, Tian M, Li Q, Liu Z, Liu DX, Chen H, Kong G. Systemic study on the safety of immuno-deficient nude mice treated by atmospheric plasma-activated water. Plasma Science & Technology. 2018;20:044003. [Google Scholar]
- 130.Xu D, Xu Y, Ning N, Cui Q, Liu Z, Wang X, Liu D, Chen H, Kong MG. Alteration of metabolite profiling by cold atmospheric plasma treatment in human myeloma cells. Cancer Cell Int. 2018;18:42. doi: 10.1186/s12935-018-0541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fernie AR, Carrari F, Sweetlove LJ. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol. 2004;7:254–261. doi: 10.1016/j.pbi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 132.Spisek R, Dhodapkar MV. Towards a better way to die with chemotherapy: role of heat shock protein exposure on dying tumor cells. Cell Cycle. 2007;6:1962–1965. doi: 10.4161/cc.6.16.4601. [DOI] [PubMed] [Google Scholar]
- 133.Reverendo M, Mendes A, Argüello RJ, Gatti E, Pierre P. At the crossway of ER-stress and pro-inflammatory responses. FEBS J. 2019;286:297–310. doi: 10.1111/febs.14391. [DOI] [PubMed] [Google Scholar]
- 134.Graves DB. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Journal of Physics D Applied Physics. 2012;45:263001. [Google Scholar]
- 135.Bekeschus S, Clemen R, Metelmann HR. Potentiating anti-tumor immunity with physical plasma. Clinical Plasma Medicine. 2018 [Google Scholar]
- 136.Lin A, Truong B, Pappas A, Kirifides L, Oubarri A, Chen S, Lin S, Dobrynin D, Fridman G, Fridman A. Uniform nanosecond pulsed dielectric barrier discharge plasma enhances anti-tumor effects by induction of immunogenic cell death in tumors and stimulation of macrophages. Plasma Processes & Polymers. 2016;12:1392–1399. [Google Scholar]
- 137.Xu D, Xu Y, Cui Q, Liu D, Liu Z, Wang X, Yang Y, Feng M, Liang R, Chen H. Cold atmospheric plasma as a potential tool for multiple myeloma treatment. Oncotarget. 2018;9:18002–18017. doi: 10.18632/oncotarget.24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mian W, Benjamin H, Xiaoqian C, Wei Z, Michael K, Lijie Grace Z. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS One. 2013;8:e73741. doi: 10.1371/journal.pone.0073741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Volotskova O, Hawley TS, Stepp MA, Keidar M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci Rep. 2012;2:636. doi: 10.1038/srep00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rafael GP, Takenori O, Mamoru U, Gary S, Valle BL, Francesca P, Rajani R, David S, Michael K, Barry T. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int J Mol Med. 2014;34:941–946. doi: 10.3892/ijmm.2014.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Isbary G, Heinlin J, Shimizu T, Zimmermann JL, Morfill G, Schmidt HU, Monetti R, Steffes B, Bunk W, Li Y, Klaempfl T, Karrer S, Landthaler M, Stolz W. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol. 2012;167:404–10. doi: 10.1111/j.1365-2133.2012.10923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stephanie A, Eva W, Yang-Fang L, Tetsuji S, Thomas HM, Morfill GE, Sigrid K, Zimmermann JL, Anja-Katrin B. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp Dermatol. 2013;22:284–289. doi: 10.1111/exd.12127. [DOI] [PubMed] [Google Scholar]
- 143.Georg I, Gregor M, Julia Z, Tetsuji S, Wilhelm S. Cold atmospheric plasma: a successful treatment of lesions in Hailey-Hailey disease. Arch Dermatol. 2011;147:388. doi: 10.1001/archdermatol.2011.57. [DOI] [PubMed] [Google Scholar]