Abstract
Slingshot phosphatase 3 (SSH3) is a member of the SSH phosphatase family that regulates actin filament dynamics. However, its role in cancer metastasis is relatively unclear compared to that of SSH1. Here, we showed that SSH3 was upregulated in colorectal cancer (CRC). Of note, SSH3 was upregulated in the tumor thrombus and lymph node metastasis compared with that in paired primary CRC tissues. High SSH3 expression was associated with the aggressive phenotype of CRC and may be an independent prognostic factor for the poor survival of patients with CRC. SSH3 significantly enhanced the invasion and metastasis of CRC cells in vitro and in vivo. Moreover, SSH3 regulated the remodeling of actin, which is involved in the cytoskeleton signaling pathway, through its interaction with LIMK1/Rac1 and subsequently promoted CRC cell invasion and metastasis. Our data elucidate an important role for SSH3 in the progression of CRC, and SSH3 may be considered a potential therapeutic target for CRC.
Keywords: SSH3, colorectal cancer, invasion, metastasis, LIMK1, Rac1
Introduction
Colorectal cancer (CRC) is one of the most common digestive malignancies, with high mobility and mortality [1]. It has been demonstrated that invasion and metastasis are the main biological features of malignant tumors, and metastasis is one of the major causes of CRC-related death [2]. However, the mechanisms underlying CRC metastasis are unclear. Therefore, it is important to explore the molecular signatures for the metastatic behavior of CRC cells.
In eukaryotes, the cytoskeleton consists of F-actin, microtubules and intermediate filaments. It is a system of intracellular filaments crucial for cell shape, division, and function in all three domains of life [3]. Previous studies have revealed that the cytoskeleton can change in shape and movement behavior in response to various stimulations anytime and anywhere in tumor cells [4-7]. Abnormal regulation of the cytoskeleton plays an essential role in the metastasis of tumors [8-10].
Slingshot phosphatase 3 (SSH3) is located on chromosome 11q13.2, includes 14 exons, and encodes the SSH3 protein. It is a member of the Slingshot phosphatase family that dephosphorylates and reactivates an inactive ADF/Cofilin [11]. Three members (SSH1, SSH2 and SSH3) comprise the Slingshot phosphatase family. It has been demonstrated that SSH1 is up-regulated in various types of tumors [12,13], and the upregulation of SSH1 could promote the metastasis of breast cancer [14]. However, the number of investigations of SSH3 is relatively less than that of SSH1. Although it has been revealed that SSH3 is upregulated in circulating cancer cells (CCCs) compared to primary cancer cells (PCCs) in an orthotopic model of breast cancer [15], there is currently less evidence suggesting the function and mechanism of SSH3 in invasion and metastasis.
In this research, we sought to investigate the expression of SSH3 in CRC tissues and cell lines, elucidate its function and explore the molecular mechanisms by which SSH3 promotes invasion and metastasis by regulating the cytoskeleton signaling pathway in CRC. To some extent, our investigation may help increase the understanding of the mechanisms of invasion and metastasis in CRC, which may assist in the development of new therapeutic strategies for CRC.
Materials and methods
Tissue specimens
All CRC tissues and matched adjacent normal mucosa samples were collected from Nanfang Hospital, Southern Medical University with the informed consent of patients. None of the patients had received radiotherapy or chemotherapy before surgery. The clinical research was performed according to the written approval obtained from the Southern Medical University Institutional Board (Guangzhou, China). In total, 83 CRC tissues and matched adjacent normal mucosa samples were collected between 2012 and 2015. For each tissue, one portion was snap-frozen in liquid nitrogen and stored at -80°C for quantitative real-time PCR (qRT-PCR) and Western blot analyses, and another protion was fixed in 10% formalin within 1 h after surgical removal, embedded in paraffin, and sectioned consecutively at 3 μm thickness using a rotary microtome for immunohistochemical assays. Notably, 35 of the primary CRC tissues were diagnosed with tumor thrombus in the vessels and paired lymph node metastasis. The medical records of 83 patients were reviewed for the acquisition of the following clinicopathological information: age, gender, differentiation, and TNM stage.
Cell cultures
Human CRC cell lines LS174T, HT29, SW620, SW480, DLD-1, RKO, LoVo, Caco2, and HCT116 were obtained from The Global Bioresource Center (ATCC, USA). The cells were cultured in RPMI1640 medium (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) at 37°C in a humidified atmosphere with 5% CO2.
Real-time quantitative PCR, Western blot, and immunohistochemistry
Real-time quantitative PCR (qRT-PCR), Western blot (WB) and Immunohistochemistry (IHC) were conducted according to previously described methods [16]. Further details are provided in the Supplementary Data Files (Table S1).
Vector construction and retroviral infection
The SSH3 construct was generated by subcloning PCR amplified full-length human SSH3 cDNA into pReceiver-Lv105. For the deletion of SSH3, the shRNA sequence (CCTGCTGGTAGTTTCTACA) was cloned into GV112. Retroviral production and infection were performed as previously described [17]. Stable cell lines expressing SSH3 or shSSH3 were selected for 10 days with 0.5 mg/mL puromycin.
Three-dimensional morphogenesis assay
Twenty-four-well plates were coated with Growth Factor Reduced Matrigel (BD Biosciences). Cells (1 × 104 per well) were suspended in growth medium containing 2% Matrigel and added to the top of the solidified Matrigel. The medium was replaced every 3 to 4 days. The three-dimensional morphological structure was observed by microscopy for 2-3 weeks. The filopodia formed by each cell sphere were counted according to the previous study, and the number of filopodia formed by each cell sphere reflects its invasive ability [18].
Transwell and wound healing assays
The transwell and wound healing assays were conducted as previously described [19]. Further details are provided in the Supplementary Data Files (Supplementary Materials and Methods).
Lung colonization assay
Female athymic BALB/c nude mice (4-6 weeks of age, 18-20 g) were obtained from the Central Laboratory of Animal Science at Southern Medical University and housed in laminar flow cabinets under specific pathogen-free conditions. All experimental procedures involving animals were performed in accordance with animal protocols approved by the Animal Care and Use Committee of Southern Medical University. A total of 2 × 106 cells were injected into the tail veins of nude mice (n = 6 per group); eight weeks later, the mice were sacrificed, and their lungs were removed and formalin fixed for histological analysis [20,21]. The metastatic tissue was analyzed using hematoxylin and eosin (H&E) staining. The number of metastatic lung nodules in individual mice was counted under a microscope.
Orthotopic mouse metastatic model
A surgical orthotopic implantation mouse model of CRC was established as previously described [17]. A total of 2 × 106 cells were subcutaneously injected into the right dorsal flanks of female BALB/c athymic nude mice. From the seventh day after injection, the size of the tumor was measured as described previously. For orthotopic metastasic assays, nude mice were anesthetized, and their caeca were exteriorized by laparotomy. The subcutaneous tumors were cut into small masses and embedded into the mesentery at the tail end of the caecum. The gut was repositioned to the abdominal cavity and subsequently closed with surgical sutures. Six weeks later, the mice were sacrificed, and all organs were resected for biopsy.
Immunofluorescence analysis
Cells were seeded onto coverslips at a density of 5 × 104 per well for 48 h and then probed with primary antibodies against SSH3 (Proteintech Group, Chicago, MA, USA) and LIMK1 (Abcam, USA) overnight at 4°C. The coverslips were then incubated with rhodamine-conjugated or fluorescein isothiocyanate (FITC)-conjugated goat antibodies against rabbit or mouse IgG (ZSGB-bio, Beijing, China). F-actin was stained with rhodamine-phalloidin. After counterstaining with DAPI (Sigma, St. Louis, MO, USA), images were taken using an Olympus FV1000 confocal laser-scanning microscope (Olympus America Inc, NY, USA). The experiment was performed with three replicates.
Co-immunoprecipitation (Co-IP)
Proteins were extracted from HT29 cells with lysis buffer. SSH3 (Proteintech Group, Chicago, MA, USA), LIMK1 (Abcam, USA) or Rac1 (Abcam, USA) antibodies were added to cell lysates. Subsequently, approximately 30 μl of agarose-protein G beads were added. After incubation for two hours, the beads were washed three times in PBS, and the proteins were eluted in Laemmli buffer. The interacting proteins were analyzed by Western blot.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 for Windows. The differences between groups were examined using one-way ANOVA or a two-tailed Student’s t-test. The relationships between SSH3 expression and clinicopathological characteristics were determined using the Mann-Whitney U-test. The survival curve was plotted using the Kaplan Meier method and compared using the log-rank test. Data are presented as the mean ± SD of at least 3 independent experiments. P < 0.05 was considered statistically significant.
Results
The upregulation of SSH3 was correlated with advanced progression and a poorer prognosis of CRC
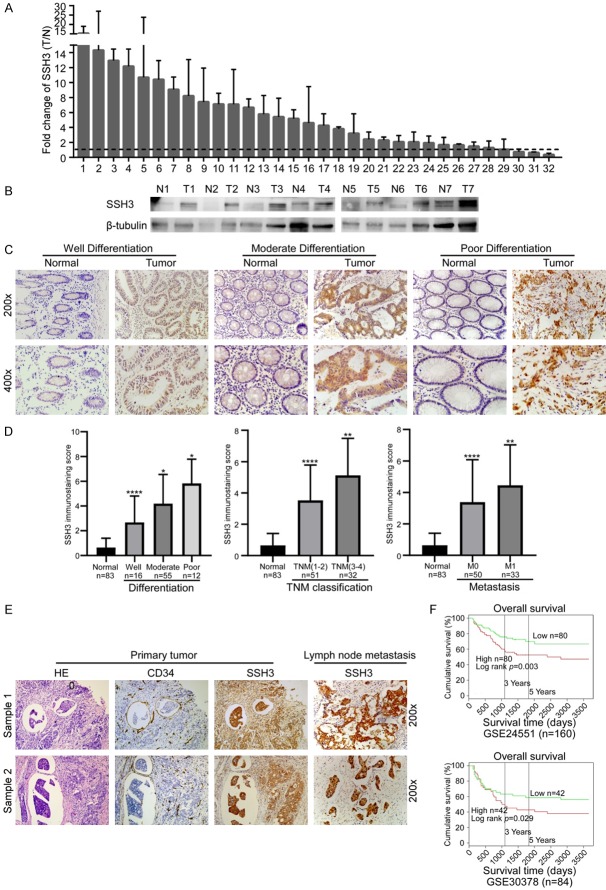
The public database Oncomine (https://www.oncomine.org) and GEO datasets (GSE24551 and GSE41258) were utilized to examine the expression of SSH3 in CRC tissues. SSH3 was upregulated in CRC, especially in liver metastasis compared to the primary tumor (Supplementary Data File: Figure S1A, S1B). Consistent with this result, the expression of SSH3 was higher in 29 of 32 CRC tissues compared to their adjacent normal mucosa tissues using qRT-PCR (Figure 1A). An increase in SSH3 expression was also observed by Western blot analysis in the same samples (Figure 1B). The expression levels of SSH3 were also examined in 83 CRC tissues using IHC. 35 of these samples were diagnosed with an endovascular tumor thrombus and lymph node metastasis. SSH3 was upregulated in the endovascular tumor thrombus and lymph node metastasis compared with the primary CRC tissues, in which SSH3 was overexpressed compared to their adjacent normal mucosa tissues (Figure 1C, 1E). Correlation analysis showed that increased SSH3 expression was significantly correlated with poorer tumor differentiation, advanced TNM stage, and metastasis (Figure 1C, 1D; Tables 1, 2). Kaplan Meier survival analyses published in PROGgene (http://www.compbio.iupui.edu/proggene) revealed that higher expression levels of SSH3 were significantly correlated with poor patient survival (Figure 1F). These results suggest that SSH3 may be a prognostic biomarker for CRC.
Figure 1.
Expression of SSH3 in CRC and its correlation with CRC prognosis. A. qRT-PCR analysis of SSH3 expression in 32 paired CRC tissues; SSH3 was quantified relative to the matched adjacent no tumor tissues. Error bars represent means ± SD calculated from three parallel experiments. B. Expression analyses of SSH3 protein in 7 surgical CRC tissues and the paired normal intestine epithelial samples using Western blot. β-tubulin was used as a loading control. C. Immunostaining of SSH3 protein in CRC tissue samples and normal colorectal tissues. D. Statistical analyses of the average SSH3 immunostaining score between normal intestinal tissues and CRC specimens with different degrees of differentiation, TNM classification and Metastasis (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). E. Immunostaining of SSH3 protein in CRC tissue samples diagnosed with tumor thrombus and the paired lymph node metastasis. F. Kaplan-Meier survival analyses published in PROG gene revealed that a higher expression of SSH3 was significantly correlated with poorer survival of patients.
Table 1.
The relationship between the expression of SSH3 and clinicopathological parameters
| Clinicopathological variables | SSH3 expression | Z | p value | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Age | ||||
| ≤ mean (58) | 18 | 20 | -0.538 | 0.590 |
| > mean (58) | 24 | 21 | ||
| Gender | ||||
| Male | 25 | 21 | -0.756 | 0.449 |
| Female | 17 | 20 | ||
| Differentiation | ||||
| Well | 6 | 10 | -2.609 | 0.009 |
| Moderate | 25 | 30 | ||
| Poor | 11 | 1 | ||
| TNM classification | ||||
| I-II | 21 | 30 | -2.155 | 0.031 |
| III-IV | 21 | 11 | ||
| Metastasis | ||||
| No | 20 | 30 | -2.364 | 0.018 |
| Yes | 22 | 11 | ||
Table 2.
Spearman correlation analysis between the expression of SSH3 and Clinicopathological Features
| Variables | SSH3 expression | |
|---|---|---|
|
| ||
| Spearman correlation | p value | |
| Age | -0.075 | 0.500 |
| Gender | -0.032 | 0.772 |
| Differentiation | 0.220 | 0.045 |
| TNM | 0.222 | 0.044 |
| Metastasis | 0.219 | 0.047 |
Upregulation of SSH3 promotes the invasion and metastasis of CRC cells, and exogenous SSH3 knockdown inhibits the invasion and metastasis of CRC cells
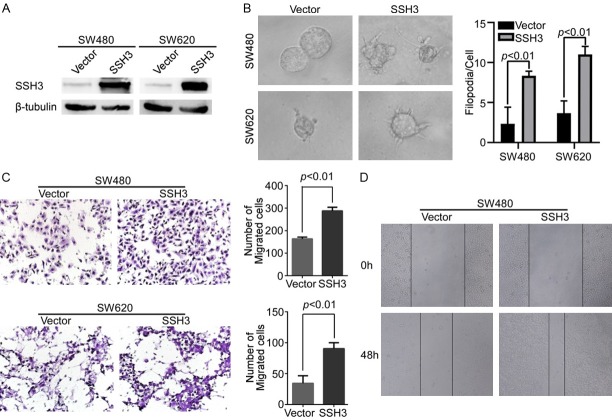
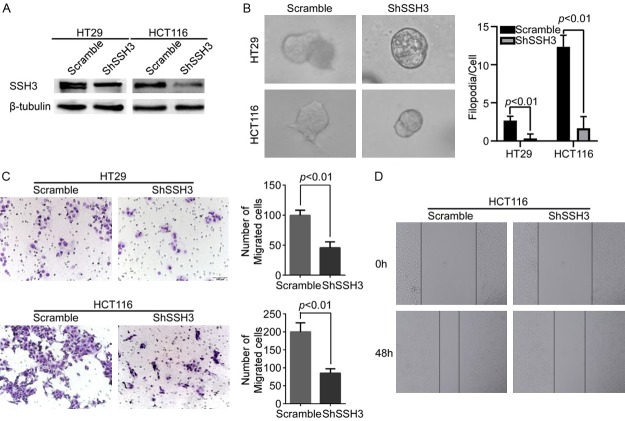
The expression of SSH3 was examined in different CRC cells (Supplementary Data File: Figure S2). SSH3 was stably expressed in SW480 and SW620 cells, generating subcell lines SW480-SSH3 and SW620-SSH3, respectively, and their controls were infected with lentiviruses containing empty vectors (Figure 2A). Correspondingly, SSH3 was permanently knocked down in HT29 and HCT116 cells by the shSSH3 lentiviruses, producing the HT29-shSSH3 and HCT116-shSSH3 subcell lines, respectively, and scramble was used as a control (Figure 3A). The three-dimensional morphogenesis analysis showed that forced SSH3 expression led to a marked increase in the number of protrusions in SW480 and SW620 cells (Figure 2B). Accordingly, the upregulation of SSH3 promoted cell migration compared with that in control cells, as measured by transwell migration and wound healing assays (Figure 2C, 2D). However, reducing SSH3 in CRC cell lines yielded the opposite effects (Figure 3B-D).
Figure 2.
Up-regulation of SSH3 promoted the invasion and metastasis of CRC cell in vitro. A. Overexpression of SSH3 in SW480 and SW620 cells analyzed through Western blot. β-tubulin was used as a loading control. B. The overexpression of SSH3 lead to a marked increase in the number of protrusions in SW480 and SW620 cells in three-dimensional morphogenesis assays. The bar chart represents the filopodia/cell. Error bars represent the means ± SD calculated from three parallel experiments. C. Overexpression of SSH3 increased cell migration as determined by transwell assays. The bar chart represents the migration cell numbers. Error bars represent the means ± SD of 5 different fields. D. Wound healing assay used to determine the migration ability of cells with up-regulated SSH3.
Figure 3.
Down-regulation of SSH3 inhibited the invasion and metastasis of CRC cell in vitro. A. Silencing of SSH3 in shRNA-transduced stable HT29 and HCT116 cells. β-tubulin was used as a loading control. B. Reduction of endogenous SSH3 lead to a marked decrease in the number of protrusions in three-dimensional morphogenesis assays. The bar chart represents the filopodia/cell. Error bars represent the means ± SD calculated from three parallel experiments. C. Down-regulation of SSH3 decreased cell migration as determined by transwell assays. The bar chart represents the migration cell numbers. Error bars represent the means ± SD of 5 different fields. D. Wound healing assay used to determine the migration ability of cells with down-regulated SSH3.
Moreover, we injected SW480-SSH3 or HT-29-shSSH3 cells and their control cells into nude mice via the tail vein to observe the effect of SSH3 on the potential of homing capacity. SSH3 significantly increased the lung homing capacity of SW480 cells compared with the effect of the vector (Figure 4A, 4B). However, fewer tumor nodules were formed in the lungs of the SSH3 knockdown group (Figure 4C, 4D). In addition, tiny tumor masses of subcutaneous tumors were transplanted into the mouse cecal subserosa. The number of hepatic metastatic lesions in the mice of the SW480-SSH3 group was obviously increased compared with that of the control group (Figure 4E, 4F). In particular, the expression of SSH3 was higher in the tumor thrombus than in the primary tumor in the mouse mucosa (Supplementary Data File: Figure S3), which was consistent with the expression of SSH3 in human CRC tissues.
Figure 4.
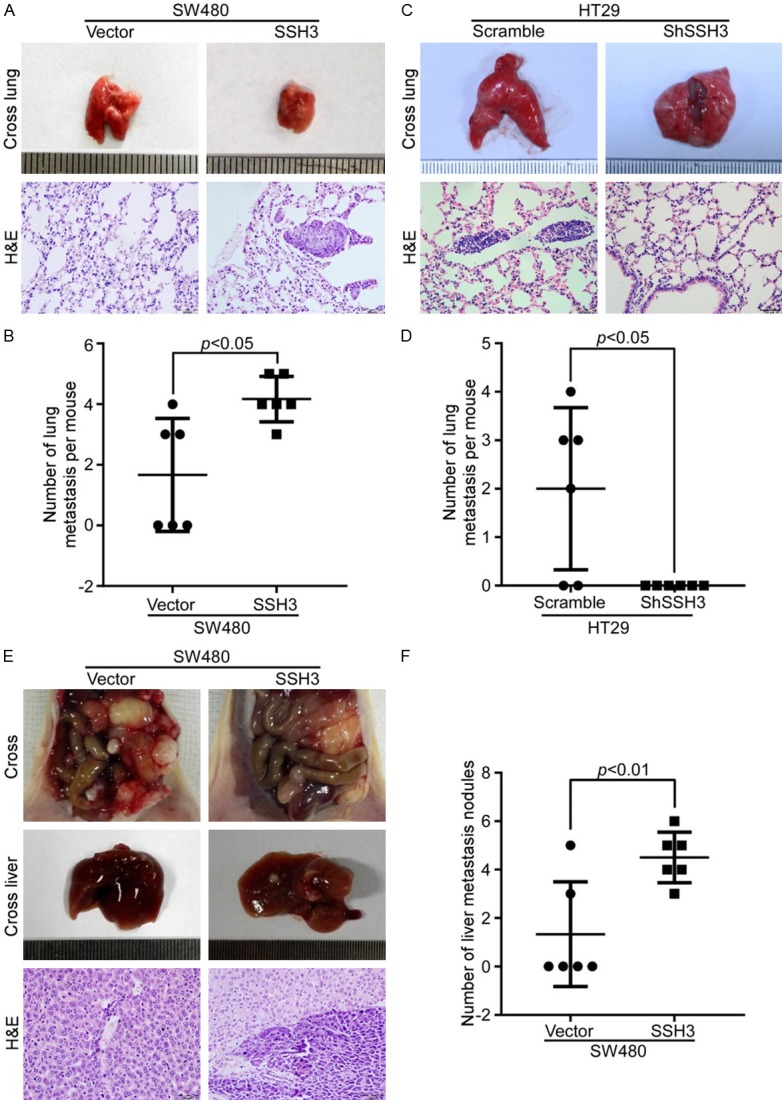
SSH3 promotes the metastatic potential of CRC cells in vivo. A and C. The representative gross images of the lungs from different experimental groups are shown. Sections of the liver were stained with H&E. B and D. Box-scatter plots show the number of metastatic nodules in the lungs as observed in each group. E. The representative gross images of the intestines and livers from different experimental groups are shown. Sections of the liver were stained with H&E. F. Box-scatter plot shows the number of metastatic nodules in the liver as observed in each group.
High SSH3 expression promotes the invasion and metastasis of CRC cells by regulating the cytoskeleton
Using GSEA (Gene Set Enrichment Analysis), we found an enrichment of cytoskeleton signaling pathway gene signatures (GSE13067 and GSE17538; Figure 5A) with high SSH3 expression.
Figure 5.
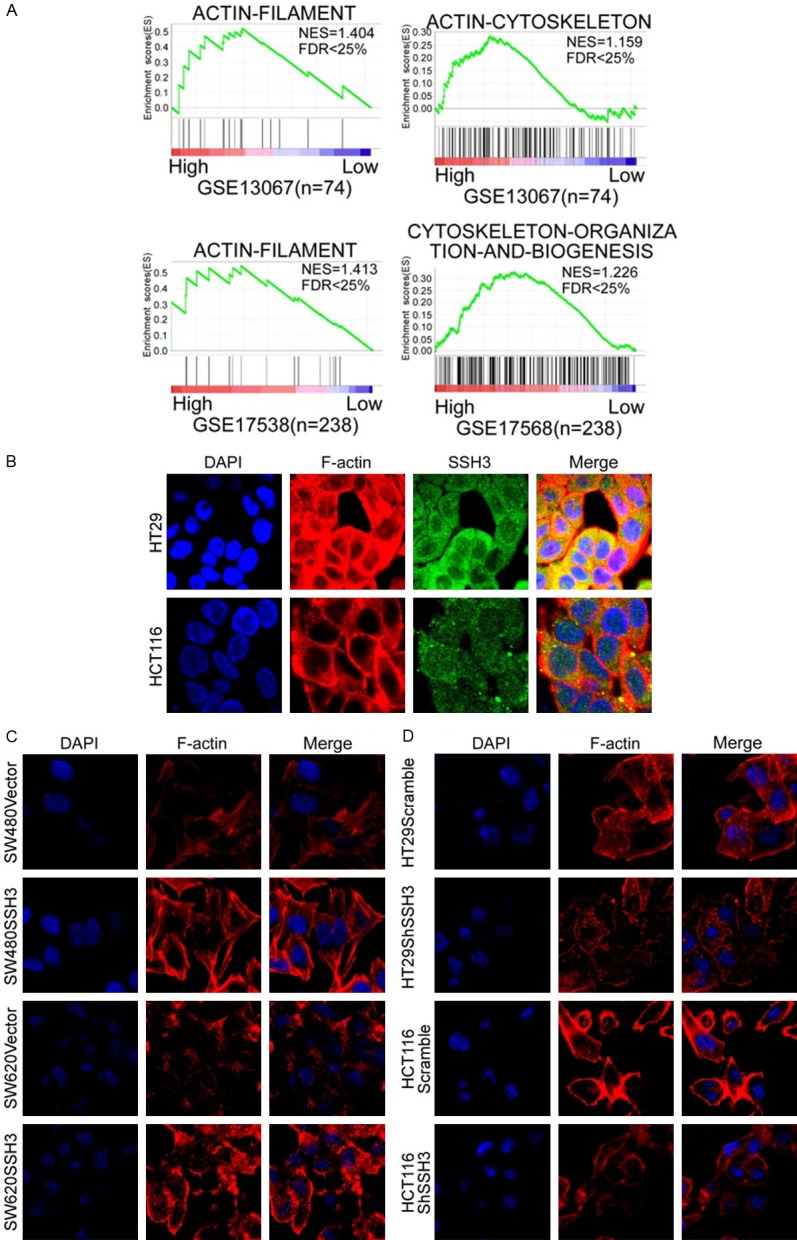
High expression of SSH3 promotes the invasion and metastasis of CRC cells by regulating the cytoskeleton. A. Analyses of SSH3-regulated gene signatures via GSEA. B. Co-localization of SSH3 and F-actin in CRC cells. C and D. Rearrangements of cytoskeleton in CRC cells when SSH3 was up- or downregulated.
Next, we explored the colocalization of SSH3 and F-actin in CRC cells. The results showed that the colocalization of SSH3 and F-actin existed in the cytoplasm of HT29 and HCT116 cells (Figure 5B). We also observed changes in the cytoskeleton in CRC cells when SSH3 was up- or downregulated. The actin cytoskeletons were reorganized when SSH3 was upregulated (Figure 5C), and the cytoskeletons disappeared when SSH3 was downregulated (Figure 5D).
SSH3 is involved in the cytoskeleton signaling pathway by interacting with LIMK1 and Rac1
To explore the molecular mechanisms underlying SSH3 in cell cytoskeleton regulation, we analyzed the potential proteins interacting with SSH3 using the public database PrePPI (http://bhapp.c2b2.columbia.edu/PrePPI) and found that LIMK1 is a candidate protein that may interact with SSH3 (Supplementary Data File: Figure S4A).
Pull-down assays were used to identify the SSH3 interacting proteins. The two most obvious pull-down bands identified by mass spectrometry (MS) were LIMK1 and Rac1 when (Supplementary Data File: Figure S4B, S4C). The immunofluorescent colocalization of SSH3 with LIMK1 was observed in CRC cells (Figure 6A).
Figure 6.
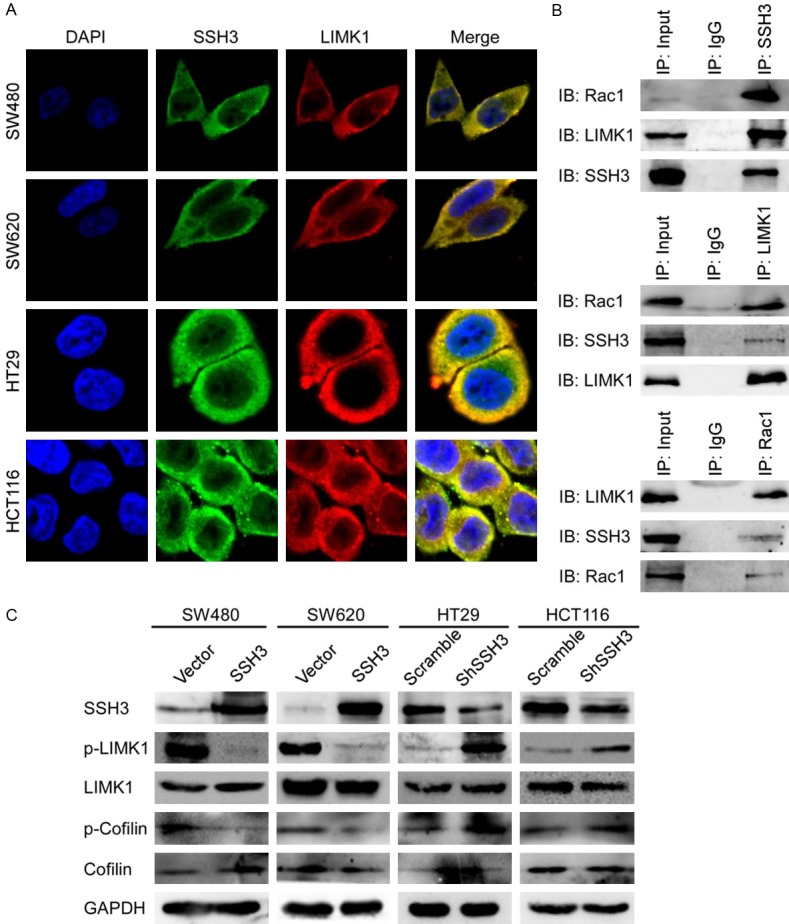
SSH3 is involved in the cytoskeleton signaling pathway by interacting with LIMK1 and Rac1. A. Colocalization of SSH3 and LIMK1 in CRC cells. B. Extracts of HT29 cells were subjected to Co-IP using SSH3 antibody or control IgG, and Western blot was performed with LIMK1 and Rac1 antibodies. Reciprocal Co-IPs were performed using LIMK1, Rac1 and IgG antibodies, followed by Western blot with SSH3, Rac1 or LIMK1 antibodies. C. Expression of LIMK1, p-LIMK1, Cofilin and p-Cofilin when SSH3 was up-regulated or down-regulated using Western blot. GAPDH was using as a loading control.
We performed Co-Immunoprecipitation (Co-IP) experiments and determined that SSH3, LIMK1 and Rac1 interact with each other (Figure 6B). The upregulation of SSH3 evidently inhibited the phosphorylation levels of LIMK1 and Cofilin (p-LIMK1 and p-Cofilin) (Figure 6C). Conversely, the downregulation of SSH3 increased LIMK1 and Cofilin phosphorylation (Figure 6C). These results suggest that SSH3 may promote the invasion and metastasis of CRC cells by interacting with LIMK1 and Rac1, which modulate the activity of Cofilin and the rearrangement of the cytoskeleton.
Discussion
Cellular movement in cancer cells is a fundamental biological behavior that contributes to invasion and metastasis. Actin protein and its regulatory protein, as basic engines for cellular mobility, play a critical role in the migration and metastasis of cancer cells [22]. Dynamic regulation of the actin strand is essential for cell migration and invasion [23]. The SSH family appears to play a role in actin dynamics by reactivating ADF/Cofilin proteins in vivo. It is well established that SSH1 contributes to actin remodeling by dephosphorylating and activating the actin-severing protein Cofilin [24]. However, there is no obvious evidence showing that SSH3 modulates actin dynamics. In the current work, we found that SSH3 was upregulated in the endovascular tumor thrombus and lymph node metastasis compared with the primary CRC tissues, in which SSH3 was overexpressed comparing to their paired normal mucosa tissues. Increased SSH3 expression was significantly associated with the aggressive cellular characteristics of CRC (e.g., poor differentiation and advanced TNM stage) and a poor outcome of patients, suggesting that SSH3 might be a tumor promoter and a prognostic marker of CRC progression. Analyses of Oncomine and the GEO datasets (GSE24551 and GSE41258) showed that SSH3 was upregulated in CRC, especially in liver metastasis compared to that in the primary tumor. Moreover, gene expression microarray analyses revealed that the expression of SSH3 was higher in the circulating cancer cells (CCCs) compared with the primary cancer cells (PCCs) in mice with 4T1 orthotopic tumors [15]. All of the analyses suggested that the overexpression of SSH3 might facilitate cancer progression and metastasis. Here, we demonstrated that the overexpression of SSH3 significantly enhanced the invasion and metastasis of CRC cells in vitro and in vivo. Moreover, the abnormal expression of SSH3 changed the cytoskeleton in CRC cells.
However, the relationships and molecular mechanisms through which SSH3 interacts with actin dynamics remain unknown. Bioinformatic and mass spectrometry (MS) analyses suggested that the candidate proteins interacting with SSH3 may be LIMK1 and Rac1.
LIMK1 (LIM motif-containing protein kinase-1) is a kinase that contains 2 N-terminal LIM motifs and a C-terminal protein kinase domain [25,26], phosphorylating the Ser3 of Cofilin directly, causing the inactivation of Cofilin and regulating actin polymerization [26-28]. Studies have demonstrated that the spatial and temporal regulation of Cofilin activity by LIM kinase and Slingshot is critical for directional cell migration [29]. Our present study also revealed that the colocalization of SSH3 and LIMK1 existed in CRC cells, and LIMK1 and Cofilin can be dephosphorylaed by SSH3. Therefore, it is perhaps not surprising that SSH3 may play central roles in invasion and metastasis by modulating actin dynamics.
Rac1 is a member of the Rho GTPase family which includes small GTP-binding proteins [30,31]. Rac1 is identified as a crucial regulator of actin cytoskeleton dynamics. Activated Rac1 can facilitate actin polymerization at the cell periphery to stimulate the formation of pseudopods [32]. Rac1 has been suggested to activate p21-activated kinases (PAKs), and the activation of PAKs is a regular manner for increasing cell motility. Active PAKs greatly enhance the phosphorylation and activation of LIMK1, and activated LIMK1 further exerts its effects on the architecture of the actin cytoskeleton by phosphorylating and regulating the activity of Cofilin proteins, resulting in the growth of actin filaments [33]. Our results showed that SSH3, LIMK1 and Rac1 can interact with each other. The interactions between SSH3, LIMK1 and Rac1 suggest that some functional coregulation might be involved.
We speculate that the functional coregulation of SSH3, LIMK1 and Rac1 may cause the rapid turnover of the cytoskeleton, and ultimately promote the invasion and metastasis of CRC (Figure 7). However, the underlying mechanism still needs further study.
Figure 7.
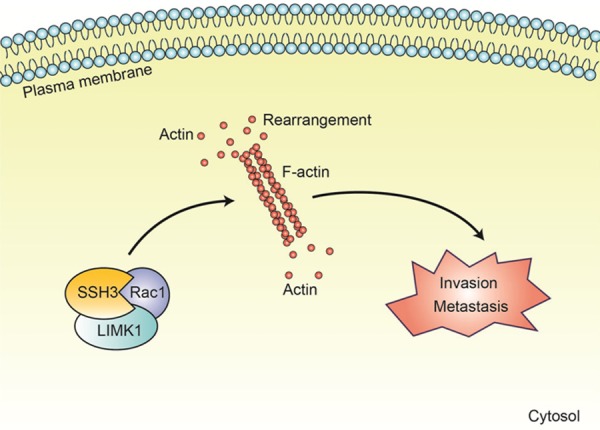
A model of SSH3 involvement in CRC. SSH3 may promote the invasion and metastasis of CRC by interacting with LIMK1 and Rac1 which can modulate the activity of Cofilin and the rearrangements of cytoskeleton.
Combining the results of our present study with other results from the literature led us to propose a working model in which SSH3 promotes colorectal cancer cell invasion and metastasis by affecting signaling cascades involving LIMK1/Rac1.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81672429, No. 81874074 and No. 81502479).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 3.Wickstead B, Gull K. The evolution of the cytoskeleton. J Cell Biol. 2011;194:513–525. doi: 10.1083/jcb.201102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Chan CK, Pan Y, Nyberg K, Marra MA, Lim EL, Jones SJ, Maar D, Gibb EA, Gunaratne PH, Robertson AG, Rowat AC. Tumour-suppressor microRNAs regulate ovarian cancer cell physical properties and invasive behaviour. Open Biol. 2016;6 doi: 10.1098/rsob.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinicola S, Fabrizi G, Masiello MG, Proietti S, Palombo A, Minini M, Harrath AH, Alwasel SH, Ricci G, Catizone A, Cucina A, Bizzarri M. Inositol induces mesenchymal-epithelial reversion in breast cancer cells through cytoskeleton rearrangement. Exp Cell Res. 2016;345:37–50. doi: 10.1016/j.yexcr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhan H, Ma J, Ruan F, Bedaiwy MA, Peng B, Wu R, Lin J. Elevated phosphatase of regenerating liver 3 (PRL-3) promotes cytoskeleton reorganization, cell migration and invasion in endometrial stromal cells from endometrioma. Hum Reprod. 2016;31:723–733. doi: 10.1093/humrep/dew015. [DOI] [PubMed] [Google Scholar]
- 8.Huang D, Cao L, Zheng S. CAPZA1 modulates EMT by regulating actin cytoskeleton remodelling in hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36:13. doi: 10.1186/s13046-016-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Wang Z, Huang D, Wu C, Li H, Zhang X, Meng B, Li Z, Zhu T, Yang S, Sun W. LMO2 promotes tumor cell invasion and metastasis in basal-type breast cancer by altering actin cytoskeleton remodeling. Oncotarget. 2017;8:9513–9524. doi: 10.18632/oncotarget.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazazian K, Go C, Wu H, Brashavitskaya O, Xu R, Dennis JW, Gingras AC, Swallow CJ. Plk4 promotes cancer invasion and metastasis through Arp2/3 complex regulation of the actin cytoskeleton. Cancer Res. 2017;77:434–447. doi: 10.1158/0008-5472.CAN-16-2060. [DOI] [PubMed] [Google Scholar]
- 11.Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- 12.Maimaiti Y, Maimaitiming M, Li Y, Aibibula S, Ainiwaer A, Aili A, Sun Z, Abudureyimu K. SSH1 expression is associated with gastric cancer progression and predicts a poor prognosis. BMC Gastroenterol. 2018;18:12. doi: 10.1186/s12876-018-0739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggelou H, Chadla P, Nikou S, Karteri S, Maroulis I, Kalofonos HP, Papadaki H, Bravou V. LIMK/cofilin pathway and Slingshot are implicated in human colorectal cancer progression and chemoresistance. Virchows Arch. 2018;472:727–737. doi: 10.1007/s00428-018-2298-0. [DOI] [PubMed] [Google Scholar]
- 14.Wang LH, Xiang J, Yan M, Zhang Y, Zhao Y, Yue CF, Xu J, Zheng FM, Chen JN, Kang Z, Chen TS, Xing D, Liu Q. The mitotic kinase Aurora-A induces mammary cell migration and breast cancer metastasis by activating the Cofilin-F-actin pathway. Cancer Res. 2010;70:9118–9128. doi: 10.1158/0008-5472.CAN-10-1246. [DOI] [PubMed] [Google Scholar]
- 15.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, Asara JM, Kalluri R. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. 1001–1015. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu YH, Chen Q, Lu YX, Zhang JM, Lin C, Zhang F, Zhang WJ, Li XM, Zhang W, Li XN. Hypermethylation of NDN promotes cell proliferation by activating the Wnt signaling pathway in colorectal cancer. Oncotarget. 2017;8:46191–46203. doi: 10.18632/oncotarget.17580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu YX, Yuan L, Xue XL, Zhou M, Liu Y, Zhang C, Li JP, Zheng L, Hong M, Li XN. Regulation of colorectal carcinoma stemness, growth, and metastasis by an miR-200c-Sox2-negative feedback loop mechanism. Clin Cancer Res. 2014;20:2631–2642. doi: 10.1158/1078-0432.CCR-13-2348. [DOI] [PubMed] [Google Scholar]
- 18.Ye YP, Jiao HL, Wang SY, Xiao ZY, Zhang D, Qiu JF, Zhang LJ, Zhao YL, Li TT, Li L, Liao WT, Ding YQ. Hypermethylation of DMTN promotes the metastasis of colorectal cancer cells by regulating the actin cytoskeleton through Rac1 signaling activation. J Exp Clin Cancer Res. 2018;37:299. doi: 10.1186/s13046-018-0958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan L, Zhou C, Lu Y, Hong M, Zhang Z, Zhang Z, Chang Y, Zhang C, Li X. IFN-gamma-mediated IRF1/miR-29b feedback loop suppresses colorectal cancer cell growth and metastasis by repressing IGF1. Cancer Lett. 2015;359:136–147. doi: 10.1016/j.canlet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Zhou C, Chang Y, Zhang Z, Hu Y, Zhang F, Lu Y, Zheng L, Zhang W, Li X, Li X. Long non-coding RNA CASC11 interacts with hnRNP-K and activates the WNT/beta-catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Lett. 2016;376:62–73. doi: 10.1016/j.canlet.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Shen Z, Zhou R, Liu C, Wang Y, Zhan W, Shao Z, Liu J, Zhang F, Xu L, Zhou X, Qi L, Bo F, Ding Y, Zhao L. MicroRNA-105 is involved in TNF-alpha-related tumor microenvironment enhanced colorectal cancer progression. Cell Death Dis. 2017;8:3213. doi: 10.1038/s41419-017-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajakyla EK, Vartiainen MK. Rho, nuclear actin, and actin-binding proteins in the regulation of transcription and gene expression. Small GTPases. 2014;5:e27539. doi: 10.4161/sgtp.27539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinbacher T, Ebnet K. The regulation of junctional actin dynamics by cell adhesion receptors. Histochem Cell Biol. 2018;150:341–350. doi: 10.1007/s00418-018-1691-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Kuramitsu Y, Kitagawa T, Baron B, Yoshino S, Maehara S, Maehara Y, Oka M, Nakamura K. Cofilin-phosphatase slingshot-1L (SSH1L) is over-expressed in pancreatic cancer (PC) and contributes to tumor cell migration. Cancer Lett. 2015;360:171–176. doi: 10.1016/j.canlet.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno K, Okano I, Ohashi K, Nunoue K, Kuma K, Miyata T, Nakamura T. Identification of a human cDNA encoding a novel protein kinase with two repeats of the LIM/double zinc finger motif. Oncogene. 1994;9:1605–1612. [PubMed] [Google Scholar]
- 26.Yang N, Higuchi O, Mizuno K. Cytoplasmic localization of LIM-kinase 1 is directed by a short sequence within the PDZ domain. Exp Cell Res. 1998;241:242–252. doi: 10.1006/excr.1998.4053. [DOI] [PubMed] [Google Scholar]
- 27.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 28.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 29.Nishita M, Tomizawa C, Yamamoto M, Horita Y, Ohashi K, Mizuno K. Spatial and temporal regulation of cofilin activity by LIM kinase and Slingshot is critical for directional cell migration. J Cell Biol. 2005;171:349–359. doi: 10.1083/jcb.200504029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobes C, Hall A. Regulation and function of the Rho subfamily of small GTPases. Curr Opin Genet Dev. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 31.Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 32.Fort L, Batista JM, Thomason PA, Spence HJ, Whitelaw JA, Tweedy L, Greaves J, Martin KJ, Anderson KI, Brown P, Lilla S, Neilson MP, Tafelmeyer P, Zanivan S, Ismail S, Bryant DM, Tomkinson NCO, Chamberlain LH, Mastick GS, Insall RH, Machesky LM. Fam49/CYRI interacts with Rac1 and locally suppresses protrusions. Nat Cell Biol. 2018;20:1159–1171. doi: 10.1038/s41556-018-0198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong H, Qi D, Guan X, Jiang G, Liao Z, Zhang X, Chen P, Li N, Wu M. c-Abl tyrosine kinase regulates neutrophil crawling behavior under fluid shear stress via Rac/PAK/LIMK/cofilin signaling axis. J Cell Biochem. 2018;119:2806–2817. doi: 10.1002/jcb.26453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.