Abstract
The identification of the epidermal growth factor mutation (EGFR) is a positive prognostic factor for survival and therapeutic response to tyrosine kinase inhibitors (TKIs) in patients with non-small cell lung cancer (NSCLC). TKIs are considered first line treatment in Patients with stages IIIB and IV NSCLC. We investigated the survival and prognostic factors in NSCLC patients with the mutation of the EGFR in routine clinical practice. We conducted a retrospective cohort observational study of 72 patients with non-small cell lung cancer (NSCLC) with EGFR gene mutations that received treatment with erlotinib from January 2009 to December 2015. Kaplan-Meier curves were presented. The association between independent variables and survival was analyzed using the Long-Rank test in bivariate analysis and for multivariate analysis, Cox proportional hazards method was used to calculate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). We included data from 72 patients, which were followed for a total of 1144 patient-months. The majority of patients were female (61.11%), non-smokers (62.50%), and with histological type corresponding to adenocarcinoma (76.38%). The most frequent EGFR gene mutation was the deletion of exon 19 (65.27%). The majority of patients presented with comorbidities (77.78%), most commonly hypertension. Almost all patients had stage IV NSCLC. Out of the 72 cases, 65 (90.28%) died. The median survival was 9.3 months (95% CI, 7.01-16.93). When comparing the survival curves when using the Log Rank Test, histological type (P = 0.01), place of mutation (P = 0.06), hemoglobin (P = 0.01) and age (P = 0.01) were significant associated to overall survival (OS). In multivariate analysis, only age (HR, 1.02; 95% CI, 1-1.04, P = 0.009) and hemoglobin (HR, 0.70; 95% CI, 0.55-0.89, P = 0.003) remained significant. In conclusion, the median OS of NSCLC patients with positive EGFR gene mutation treated with TKI was 9.3 months. Bivariate and multivariate analysis showed that younger age and a higher hemoglobin level were the most important factors associated with survival.
Keywords: Epidermal growth factor receptor mutation, non-small cell lung cancer, tyrosine kinase inhibitors, erlotinib
Introduction
Lung cancer is the deadliest neoplastic disease in Latin America [1] with non-small cell lung cancer (NSCLC) accounting for 85% to 90% of all lung cancers [2]. Every year, sixty thousand related deaths are reported in Latin American countries. In Peru the reported incidences of lung cancer for men and women are 13 and 9 cases respectively per 100000 inhabitants [1]. Some of these patients have mutations of the epidermal growth factor receptor (EGFR), which is a positive prognostic factor for survival and the therapeutic response to tyrosine kinase inhibitors (TKIs) [3-5]. In patients in advanced stages (IIIB and IV), TKIs have shown to be an effective treatment. Therefore, these drugs are considered as first line therapy in their treatment [6-8]. The TKIs with evidence of effectiveness in patients with NSCLC and mutation of the EGFR gene include erlotinib, gefitinib, and afatinib.
Several studies have found an improved survival in NSCLC patients with mutation of the EGFR treated with TKIs [7,9,10]. However, there is variability in the response according to the type of population studied. In Peru, the clinical response to this treatment has not been evaluated, so it is important to carry out studies that analyze the response to TKIs in the Peruvian population. In addition to assessing the survival of patients in treatment with TKIs it is important to identify the factors associated with the response to this treatment. The objective of the present study is to determine the overall survival and its associated factors in NSCLC patients in advanced stages with mutation of the EFGR gene treated with TKI at a reference center in Peru during the period 2009-2015.
Methods
A retrospective cohort observational study of patients with NSCLC in advanced stages (IIIB and IV) with EGFR gene mutation attended at the Hospital Nacional de Policía Luis N. Sáenz from Peru between 2009 and 2015. The clinical information of each patient was obtained from the data registered in medical records.
Data source & patients
Eligible patients were those with histological diagnosis of NSCLC in advanced stages IIIB and IV with mutation of EGFR treated with TKI. The identification of mutations as deletion of exon 19, point mutation of codon 858, insertion of exon 20 and mutation of exon 21 of the EGFR gene were carried out by bidirectional fluorescent cyclic sequencing amplified by real-time polymerase chain reaction (PCR) with the COBAS s 201 of Roche at a certified clinical laboratory.
A census evaluation was carried out including all the patients who met the inclusion and exclusion criteria. 72 medical records were obtained as shown in Figure 3. Based on a significance level of 95%, a power of 83% was calculated to detect a Hazard Ratio of 2.1 [12]. All patients with stage IIIB or IV NSCLC treated with TKI who had an EGFR gene mutation diagnosed between January 2009 and December 2015 and who had at least one medical follow-up visit after the start of the tyrosine kinase inhibitor were included. We excluded patients whose medical records were not found, those with small cell lung cancer diagnosis, patients who were negative for the EGFR gene mutation, and those without survival data or with no registered data of their last control.
Figure 3.
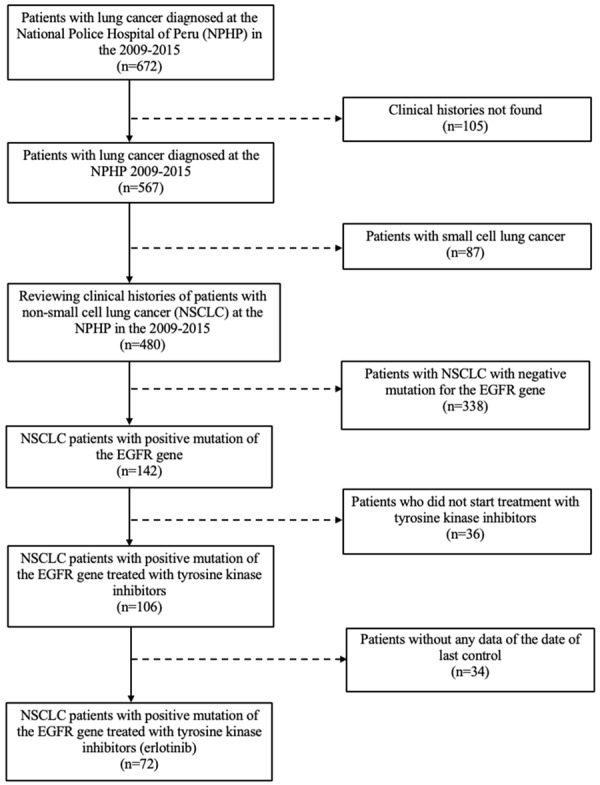
Flow chart of population cohort selection.
Statistical analyses
The outcome was the survival time starting from the diagnosis. The date of death was obtained from the review of medical records and the consultation with the National Registry of Identification and Civil Status records obtained by the Oncology service of the Hospital. The patients were followed up until December 31, 2016, after which the data was censored. The following data obtained from the review of medical records were included as independent variables: date of diagnosis of EGFR gene mutation, gender, age (in years) at the time of diagnosis of EGFR gene mutation, histological subtype (adenocarcinoma, squamous cell carcinoma, large cell carcinoma and others), clinical staging at the initial diagnosis, unilateral or bilateral pulmonary involvment, smoking history, previous treatment with chemotherapy, leukocyte count, hemoglobin, albumin, and response to treatment (no response, stable disease, partial response and total response). The type of EGFR mutation was determined through the polymerase chain reaction test in real time (quantitatively). The deletion of exon 19, the point mutation of codon 858, the mutation of exon 21 and the insertion of exon 20 were evaluated.
The categorical variables were presented as frequencies and percentages. The numerical variables as mean and standard deviation for those of normal distribution. For numerical variables with distributions other than normal, the median and interquartile range were used. The Kaplan Meier curve for OS was presented. In addition, the annual mortality of the patients was calculated. For the bivariate analysis, the association between independent variables and survival was analyzed using the Log-rank test. For the multivariate analysis, the Cox proportional hazards method was used to calculate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) using time-to-event outcomes. An initial multivariate model was elaborated, in which all those variables with a value of P < 0.15 in the bivariate analysis were included. Subsequently, those variables with higher values of p (Backward elimination) were eliminated, until a final model was obtained in which all the variables presented a value of P < 0.05. This methodology has been used in previous studies for the identification of clinical prediction models from a set of candidate variables [13,14]. A p value less than 0.05 was considered as statistically significant. All calculations were performed using the statistical package Stata V13.0 (Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Ethical aspects
This study was approved by the Ethics Committees of Universidad Peruana de Ciencias Aplicadas and the Hospital de la Policía Nacional del Perú. The study did not involve any intervention on human beings, as it was based on the retrospective review of clinical records.
Results
A total of 72 patients were included, followed for a total of 1144 patient-months. Most patients were females (61.11%). The average age at diagnosis was 58.8 years (SD: 18.66) and 25% were smokers. The majority of patients had comorbidities (77.78%), being the most frequent hypertension. Regarding the clinical stage of the diagnosis, almost all patients were in tage IV of NSCLC with adenocarcinoma being the most common histological subtype (76.38%). The most frequent mutation of the EGFR gene was the deletion of exon 19 (65.27%). Finally, the majority of patients did not show a response to treatment with TKIs. Table 1 shows other relevant population characteristics.
Table 1.
Clinical characteristics and prognostic factors of NSCLC patients with EGFR mutation treated with erlotinib
| Characteristics | Total (n = 72) | Vivos (n = 7) | Deceased (n = 65) | p Value† |
|---|---|---|---|---|
| Gender | 0.855 | |||
| Female | 44 (61.11%) | 4 (57.14%) | 40 (61.54%) | |
| Male | 28 (38.89%) | 3 (42.86%) | 25 (38.46%) | |
| Smoking Status | 0.245 | |||
| Non smoker | 45 (62.50%) | 4 (57.14%) | 41 (63.08%) | |
| Current smoker | 18 (25.01%) | 2 (28.57%) | 16 (24.62%) | |
| Former smoker | 9 (12.50%) | 1 (14.29%) | 8 (12.31%) | |
| Previous Treatment with Chemotherapy | 13 (18.06%) | 2 (28.57%) | 11 (16.92%) | 0.475 |
| Comorbidity | 56 (77.78%) | 6 (85.71%) | 50 (76.92%) | 0.543 |
| Clinical Stage | 0.468 | |||
| Stage IIIB | 16 (22.22%) | 1 (14.29%) | 15 (23.08%) | |
| Stage IV | 56 (77.78%) | 6 (85.71%) | 50 (76.92%) | |
| Baseline Nsclc Histology | 0.016* | |||
| Adenocarcinoma | 55 (76.38%) | 5 (71.43%) | 50 (76.92%) | |
| Squamous cell carcinomas | 9 (12.50%) | 1 (14.29%) | 8 (12.31%) | |
| Large cell carcinoma | 7 (9.72%) | 1 (14.29%) | 6 (9.23%) | |
| Other | 1 (1.39%) | 0 | 1 (1.54%) | |
| Egfr Mutations | 0.064* | |||
| Deletion of exon 19 | 47 (65.27%) | 6 (85.71%) | 41 (63.08%) | |
| Point mutation of codon 858 | 20 (27.78%) | 1 (14.29%) | 19 (29.23%) | |
| Exón 21 mutation | 4 (5.56%) | 0 | 4 (6.15%) | |
| Exon 20 insertion | 1 (1.39%) | 0 | 1 (1.54%) | |
| Lung Involvement | 0.823 | |||
| Unilateral | 46 (63.89%) | 3 (42.86%) | 43 (66.15%) | |
| Bilateral | 26 (36.11%) | 4 (57.14%) | 22 (33.85%) | |
| Initial Response to Treatment | ||||
| No response | 28 (38.89%) | 1 (14.29%) | 27 (41.54%) | |
| Stable disease | 24 (33.33%) | 0 | 24 (36.92) | |
| Partial response | 16 (22.22%) | 4 (57.14%) | 12 (18.46%) | |
| Total response | 4 (5.56%) | 2 (28.57%) | 2 (3.08%) | |
| Age in years | 65.52 (41.63-75.05) | 45.08 (34.84-71.84) | 65.62 (42.91-75.06) | 0.012* |
| Weight in Kg (RIC) | 68 (59-74.5) | 73 (58-80) | 66 (59-73) | |
| Height in metres | 1.62 (1.58-1.7) | 1.7 (1.57-1.73) | 1.62 (1.58-1.7) | |
| BMI | 24.9 (23.48-27.25) | 26.08 (22.74-27.68) | 24.8 (23.52-26.71) | 0.881 |
| Albumin in g/dl | 3 (2.4-3.45) | 3.1 (2.3-3.7) | 3 (2.4-3.4) | 0.502 |
| Lymphocytes in cells/uL | 3219.5 (2120-4675) | 4650 (2600-7038) | 3160 (2110-4560) | 0.466 |
| Hemoglobin in g/dl | 12 (11.05-13.1) | 13.6 (9.59-14) | 12 (11.1-13) | 0.001* |
The numerical variables are presented as median and interquartile range. The categorical variables are expressed as means and percentages. IMC, Body mass index; EGFR, epidermal growth factor receptor;
Significance level;
Long-Rank test.
Survival and prognostic factors
Sixtyfive (90.28%) out of the 72 patients died. The median survival was 9.3 months (95% CI, 7.01-16.93). The Overall survival Kaplan-Meier curve is shown in Figure 1.
Figure 1.

Kaplan-Meier curve showing overall survival in NSCLC patients with EGFR mutations.
When comparing the survival curves using the Long Rank test, histological type (P = 0.02), place of mutation (P = 0.06), hemoglobin level (P = 0.01) and age (P = 0.01) were significantly associated with survival. Figure 2 shows that patients with histology of squamous cell carcinoma, large cell carcinoma or other histological type had higher survival compared to adenocarcinomas. Likewise, hemoglobin greater than 12 g/dl and age under 65 years old had an association with a longer survival. Carriers of the point mutation of codon 858 had higher survival compared to carriers of the exon 19 deletion, mutation of exon 21 or insertion of exon 20.
Figure 2.
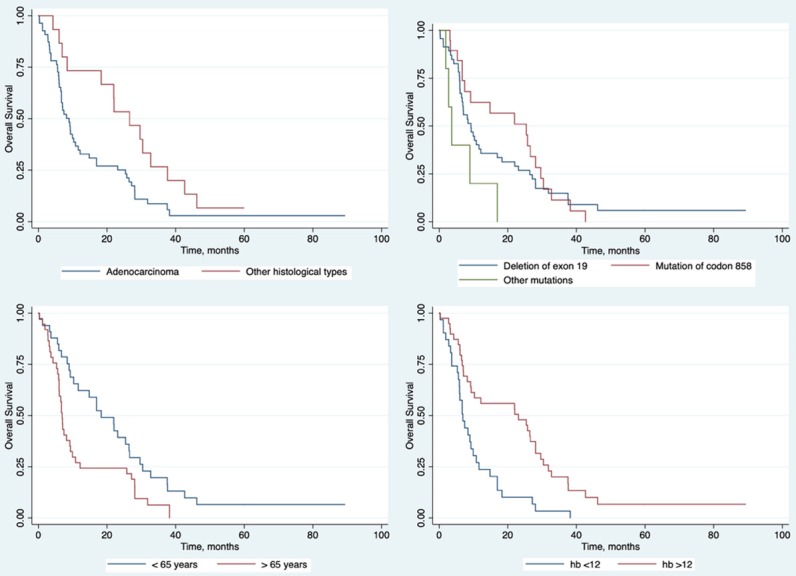
Kaplan-Meier curves showing the overall survival in NSCLC patients with EGFR mutations for those variables with significant association according to log rank-test.
In the multivariate analysis using the Cox regression only age and hemoglobin remained significant (Table 2) with Hazard Ratios of 1.02 (95% CI, 1.01-1.04) and 0.70 (95% CI, 0.55-0.89), respectively.
Table 2.
Multivariate analysis of prognostic factors of NSCLC patients with EGFR mutation treated with erlotinib
| Characteristics | Initial Model | Final Model | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Baseline Nsclc Histology (non adenocarcinoma) | 0.94 (0.42-2.10) | 0.882 | ||
| Egfr Mutations (compared to deletion of exon 19) | ||||
| Point mutation of codon 858 | 0.90 (0.51-1.59) | 0.712 | ||
| Other types of mutations | 1.73 (0.64-4.64) | 0.277 | ||
| Age in years | 1.02 (1.00-1.04) | 0.048 | 1.02 (1.01-1.04) | 0.009 |
| Hemoglobin in g/dl | 0.72 (0.56-0.92) | 0.008 | 0.70 (0.55-0.89) | 0.003 |
HR, harzard ratio; CI, confidence interval.
Discussion
Our study found a high mortality among patients with NSCLC and EGFR gene mutation treated with TKI in comparison to other latinamerican countries [15]. Notwithstanding, the median survival was 9.3 months, more than expected with conventional treatment according to the literature. In addition, there is a discrete difference in the median survival compared to other countries, such as United States (10.4 months [16]), Portugal (12 months [17]) and Colombia (9.8 months [15]). Several studies show that the prognosis of patients with NSCLC is poor with conventional treatment, reporting a median survival of 6 months [16]. However, it was observed that in patients treated with TKI (erlotinib) this can increase to 10.4 months [18]. Also, the median of progression-free survival (PFS) was of 4.8 months in the group that used TKI as a first-line treatment [19]; compared to 2.9 months in patients treated with chemotherapy [18]. In addition, those studies have shown that quality of life in patients who used erlotinib was better than in those receiving chemotherapy [8]. Finally, the side effect profile of TKI is clearly better than conventional chemotherpay [16,20,21]. We observed that patient age (< 65 years), hemoglobin (> 12 g/dl), histological type (no adenocarcinoma) and type of mutation (point mutation of codon 858) were significantly associated with OS. Other studies have found other related factors depending on study setting according to the setting reviewed literature including gender, clinical stage, functional status, history of smoking or second-line treatment with chemotherapy [22]. As was previously reported in other populations [23-25], mutations of the EGFR gene were more frequent in women, in patients who had never smoked, in those with histological adenocarcinoma subtype and the most EGFR gene mutation type found was the deletion of exon 19.
An additional finding during the sample selection was found that 29.6% of the patients with NSCLC were carriers of the EGFR mutation while in developed countries such as United States the patients with positive mutation only reach 18.5% [26]. This higher percentage of positivity to this mutation, if confirmed by further studies, would be specially relevant for peruvian oncologysts.
Among the limitations of our study were those of the retrospective nature of the study, the possible limitations in data collection due to incomplete clinical histories or the possibility of selection bias due missing data. In addition, the study was limited to a single health facility, which limited the generalization of its findings.
Finally, our study is the first Peruvian series that analyzes outcomes related to TKI therapy in Peru. Our results provide national evidence of the factors that are associated with survival in patients during treatment with TKI as first-line drugs for NSCLC patients with positive mutation of the EGFR gene.
Conclusion
This study provided evidence on overall survival and their prognostic factors during treatment with TKI from patients with NSCLC and positive mutation of the EGFR gene in Peru. Our data suggest that treatment with TKI could be an effective strategy for the Peruvian population with NSCLC. Younger age and higher hemoglobin levels were the most important factors associated with a longer survival both in bivariate and multivariate analysis. Further studies are needed to confirm the high frequency of EGFR mutations in Peruvian population and to compare responses to TKI therapy in specific subpopulations.
Disclosure of conflict of interest
None.
References
- 1.EIU Roche Lung cancer in Latin America: Time to stop looking away Español. pdf [Internet]. [cited 2019 Jan 6] Available from: http://www.vi-da.cl/wp-content/uploads/2018/09/EIU_Roche-Lung-cancer-in-Latin-America_Time-to-stop-looking-away_Espa%C3%B1ol.pdf.
- 2.D’Addario G, Früh M, Reck M, Baumann P, Klepetko W, Felip E ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–9. doi: 10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- 3.Jorge SE, Kobayashi SS, Costa DB. Epidermal growth factor receptor (EGFR) mutations in lung cancer: preclinical and clinical data. Braz J Med Biol Res. 2014;47:929–39. doi: 10.1590/1414-431X20144099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero-Ventosa EY, Mucientes-Molina A, Pedrido-Reino E, Lago-Rivero N, Constenla-Caramés L, Arias-Santos I. Efectividad y toxicidad de erlotinib en la farmacoterapia del cáncer de pulmón no microcítico. Farm Hosp. 2012;36:68–76. doi: 10.1016/j.farma.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Monteiro R, Antunes A, Gonçalves I, Lima C, Barroso A, Neves S, et al. Smoking habits and erlotinib response in non-small-cell lung cancer (NSCLC) treatment. European Respiratory Journal. 2011;38(Suppl 55):p2817. [Google Scholar]
- 6.Leon LF, Golsorkhi A, Liu S, Drozdowskyj A, Rosell R. Survival analyses of first-line erlotinib versus chemotherapy in the eurtac study population controlling for the use of post-study therapy. Ann Oncol. 2014;25(Suppl 4):iv447–iv448. [Google Scholar]
- 7.Inoue A, Yoshida K, Morita S, Imamura F, Seto T, Okamoto I, Nakagawa K, Yamamoto N, Muto S, Fukuoka M. Characteristics and overall survival of EGFR mutation-positive non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: a retrospective analysis for 1660 Japanese patients. Jpn J Clin Oncol. 2016;46:462–7. doi: 10.1093/jjco/hyw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, Jain P, Green JA. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2016:CD010383. doi: 10.1002/14651858.CD010383.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Saijo Y, Hagiwara K, Morita S, Nukiwa T North-East Japan Study Group. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–9. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 10.Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010;2:48–51. [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez X, Valdez N, Paredes R. Inhibidores de tirosin quinasa para el tratamiento de cáncer pulmonar de células no pequeñas: una necesidad desatendida. Rev Peru Med Exp Salud Publica. 2017;34:349–50. doi: 10.17843/rpmesp.2017.342.2690. [DOI] [PubMed] [Google Scholar]
- 12.Arrieta O, Cardona AF, Bramuglia GF, Gallo A, Campos-Parra AD, Serrano S, Castro M, Avilés A, Amorin E, Kirchuk R, Cuello M, Borbolla J, Riemersma O, Becerra H, Rosell R CLICaP. Genotyping non-small cell lung cancer (NSCLC) in Latin America. J Thorac Oncol. 2011;6:1955–9. doi: 10.1097/JTO.0b013e31822f655f. [DOI] [PubMed] [Google Scholar]
- 13.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, Ince WL, Jänne PA, Januario T, Johnson DH, Klein P, Miller VA, Ostland MA, Ramies DA, Sebisanovic D, Stinson JA, Zhang YR, Seshagiri S, Hillan KJ. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J. Clin. Oncol. 2005;23:5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 14.Marin-Marín D, Soto A. Comparación de sistemas de puntaje pronóstico en la predicción de mortalidad y complicaciones en sepsis. Rev Peru Med Exp Salud Pública. 2016;33:51–7. [PubMed] [Google Scholar]
- 15.Miguel OJ, Felipe CA, Ludovic R, Felipe C, Hernán C, Alberto VC, et al. Supervivencia en pacientes con adenocarcinoma de pulmón metastásico y registro de las primeras mutaciones en el receptor para el factor de crecimiento epidérmico documentado en Colombia Estudio del ONCOLGroup. Acta Med Colomb. 2009;34:55–65. [Google Scholar]
- 16.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 17.Aguiar F, Fernandes G, Queiroga H, Machado JC, Cirnes L, Souto Moura C, Hespanhol V. Overall survival analysis and characterization of an EGFR mutated non-small cell lung cancer (NSCLC) population. Arch Bronconeumol. 2018;54:10–7. doi: 10.1016/j.arbres.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Lee SM, Khan I, Upadhyay S, Lewanski C, Falk S, Skailes G, Marshall E, Woll PJ, Hatton M, Lal R, Jones R, Toy E, Chao D, Middleton G, Bulley S, Ngai Y, Rudd R, Hackshaw A, Boshoff C. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2012;13:1161–70. doi: 10.1016/S1470-2045(12)70412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 20.Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, Tan EH, Ho JC, Chu da T, Zaatar A, Osorio Sanchez JA, Vu VV, Au JS, Inoue A, Lee SM, Gebski V, Yang JC. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595–605. doi: 10.1093/jnci/djt072. [DOI] [PubMed] [Google Scholar]
- 21.Brugger W, Triller N, Blasinska-Morawiec M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R, Ward C, Mayne K, Trunzer K, Cappuzzo F. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011;29:4113–20. doi: 10.1200/JCO.2010.31.8162. [DOI] [PubMed] [Google Scholar]
- 22.Landi L, Cappuzzo F. Experience with erlotinib in the treatment of non-small cell lung cancer. Ther Adv Respir Dis. 2015;9:146–63. doi: 10.1177/1753465815588053. [DOI] [PubMed] [Google Scholar]
- 23.Basiri R, Hossein Jafarian A, Karimi M, Mohammadzadeh Lari S, Mortaza Haghgoo S. Expression of epidermal growth factor receptor and the association with demographic and prognostic factors in patients with non-small cell lung cancer. Journal of Cardio-Thoracic Medicine. 2015;3:297–302. [Google Scholar]
- 24.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sánchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 25.Soto A, Solari L, Agapito J, Acuna-Villaorduna C, Lambert ML, Gotuzzo E, Gotuzzo E, Van der Stuyft P. Development of a clinical scoring system for the diagnosis of smear-negative pulmonary tuberculosis. Braz J Infect Dis. 2008;12:128–32. doi: 10.1590/s1413-86702008000200006. [DOI] [PubMed] [Google Scholar]
- 26.Aguilar KM, Winfree KB, Muehlenbein CE, Zhu YE, Wilson T, Wetmore S, Nadler ES. Treatment patterns by egfr mutation status in non-small cell lung cancer patients in the USA: a retrospective database analysis. Adv Ther. 2018;35:1905–19. doi: 10.1007/s12325-018-0811-0. [DOI] [PubMed] [Google Scholar]


