Abstract
In order to avoid the occurrence of “abnormal liver biochemical tests” caused by diet in Phase I clinical trial, the effect of a low-calorie and low-carbohydrate diet on biochemical indices of liver function of healthy volunteers in a Phase I unit was investigated. A single-centered, non-randomized and parallel-controlled study was designed with 46 healthy subjects consuming two types of diet (24 in the experimental group and 22 in the control group). The diets comprised a balanced normal calorie diet for the control group and a low-calorie and low-carbohydrate diet for the experimental group. All subjects were required to reside on site for 15 consecutive days. Liver biochemical indices such as alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) were detected on Day-1 (baseline), Day 1, Day 2, Day 3, Day 5, Day 7, Day 9, Day 12, Day 15 and Day 22 (follow-up). There was no significant difference in age, ethnicity, occupation and other demographic data between two groups with balanced baseline. There was no significant difference in ALP between two groups and ALP remained unchanged as a function of time. However, ALT level of the experimental group remained constant as a function of time while ALT level of the control group increased with time and the level was significantly higher than the experimental group after Day 2. The difference between two groups increased with time but decreased on Day 22 as follow-up. After controlling the baseline of AST, AST exhibited a similar trend as ALT. After Day 9, 5%-18% of the patients in the control group showed hepatocyte injury, while no hepatic injury was found in the experimental group. In summary, a low-calorie, low-carbohydrate diet can effectively avoid the occurrence of liver biochemical abnormalities caused by diet in Phase I clinical trials.
Keywords: Diet, Phase I clinical trial, healthy subjects, liver biochemical indices
Introduction
Adverse drug reactions (ADRs) are important indicators for drug safety evaluation. The U.S. Food and Drug Administration (FDA) clearly stipulates in the regulation of drug registration and management of ADRs that new drugs must provide complete reports of relevant evaluation indicators including safety and effectiveness before the drugs going to market [1]. “Abnormal liver biochemical tests” and “drug-induced liver injury” belong to the most common and serious ADRs [2,3]. They are also the most common reasons for FDA to take regulatory action on approved drugs. However, pre-clinical tests usually fails to provide a reliable risk assessment of the hepatotoxicity of new drugs, and the pre-clinical studies conducted among highly selected populations might not efficiently monitor the possibility of liver injury during a short period either [4-6].
Drug-induced liver injury (DILI) refers to the liver injury induced by various prescription or non-prescription drugs, biological agents, traditional Chinese medicine (TCM), natural drugs (NM), healthy products (HP), dietary supplements (DS), and their metabolites and accessories [2,7-9]. In developed countries, the incidence of DILI is estimated between 1/100,000 to 20/100,000 [2,10]. The annual incidence of DILI reported by France in 2002 was approximately 13.9/100,000, and around 19.1/100,000 as reported by Iceland in 2013 [2,11].
The risk factors resulting in liver biochemical abnormalities and DILI include not only drug factors, but also host and environmental factors [12]. Although Drug factors have been widely investigated, there were little studies on identification of the causes of liver biochemical abnormalities in healthy volunteers in Phase I unit. Phase I clinical trial provides a test for preliminary clinical pharmacology and human safety evaluation. The purpose of a Phase I clinical trial is to observe the tolerance and pharmacokinetics of new drugs to determine the metabolism and pharmacological effects of drugs in human body, as well as the ADRs which are related to an increase in drug dosage, Phase I clinical trial provides basis for formulating drug delivery plan [13]. The subjects of phase I clinical trials are mainly healthy volunteers, they are normally required to resident in our clinical research center for several days and accept our food and exercise guidance and supervision. As the liver is not only an organ for drug and toxic metabolism, but also a factory for the synthesis, decomposition and metabolism of sugar, protein and fat. Previous studies [14,15] have demonstrated that dietary control is conducive to the reduction of liver enzymes in patients with fatty liver, but the effects of dietary control on liver function indices of healthy volunteers in Phase I clinical trials have never been reported. The exclusion of non-drug-related liver biochemical abnormalities and DILI is very important to formulate the treatment plan for healthy volunteers with abnormal liver biochemical indices, and to improve the accuracy on judgement of ADRs and drug safety. Therefore, this study was designed and the research was conducted to investigate the effect of diet on biochemical indices of blood and liver in healthy subjects in a Phase I clinical trial.
Objectives and methods
Research objectives
The current study has been approved by biomedical ethics committee of Peking University. A convenient entry method was applied to select healthy volunteers from the drug clinical trial center at Peking University Third Hospital. This study involved 46 healthy subjects aged 18-45 years old with 24 subjects in the experimental group and 22 subjects in the control group. Subjects are all male Chinese citizens and their Body Mass Indexes (BMIs) are between 18.0-24.0 kg/m2. The exclusion criteria including: First, Refusal to be included in the trial; Second, existence of cardiovascular, endocrine, neurological, digestive, hematological, immune, respiratory and urogenital abnormalities or related medical history; Third, researchers believe that any other medical history may confuse the results obtained from current study or the subjects may face extra risks after participation in the trial; Fourth, subjects participated in another clinical study within three months before the current trial.
Methods
A single-centered, non-randomized and parallel-controlled method was applied in this study. Subjects in both groups resided in the drug clinical trial center at Peking University Third Hospital for 15 consecutive days. During this period, all diets were provided by the center. The specific types of food and total calories were designed and determined by researchers and dietitians. All subjects were accompanied by researchers for one hour of outdoor walking on each day during their stay in the center.
Diets
The total calorie of diets for subjects in the experimental group was 2,300 Kcal per day. The dietary structure was designed according to “the American Diabetes Association (ADA) 2017 Nutrition Guidelines” [16] and “the Chinese Medical Nutrition Guidelines for Diabetes 2013” [17]. The principles for diets during resident in the center including: First, low-calorie and low-carbohydrate diet with total calorie not more than 2,300 Kcal/day; Second, quantitative staple diet with whole grains accounting for one third of staple diet intake; Third, light diet with a sodium intake not more than 2,300 mg/day; Fourth, regular quantitative diets with controlled dining speed, diet time was monitored by on-site researchers, time for each diet was beyond 30 minutes.
There is no restriction on the total calories of diets provided for subjects in the control group every day. The dietary structure was formulated in accordance with “the Dietary Guidelines for Chinese Residents 2016” [18]. The principles for providing diets including: diversified foods, mainly cereals; vegetables and fruits; fish, eggs and meat; less salt and oil, less sodium intake not more than 6,000 mg/day, less sugar intake not more than 50 g/day.
Laboratory analyses
Body mass of subjects were measured at a fixed time with empty stomach and bladder. Blood samples for routine biochemical analyses were collected on Day-1 (baseline), Day 1, Day 2, Day 3, Day 5, Day 7, Day 9, Day 12, Day 15 and Day 22 (follow-up) before the start of each diet in the morning. Alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), direct bilirubin (D_Bil), total bilirubin (T_Bil), albumin (ALB), total protein (TP) and γ-Glutamyltransferase (γ_GT) were measured on serum from fasting morning blood samples.
Statistical analysis
SPSS 22.0 (IBM, NewYork, USA) was used for statistical analysis. Categorical variables were expressed by frequencies and percentages. Chi-square test or Fisher test were used for comparison between groups. Normal test was used for measuring variables, the yielded data which in agreement with normal distribution were described by mean ± standard deviation. Independent sample t test was used for comparison between groups. Covariance analysis was applied to control the impact of baseline on subsequent measurements when baseline was not balanced. P<0.05 indicated that there are no significant differences between the data.
Results
After screening, 24 subjects were selected for the experimental group and 22 subjects were selected for the control group. The general information of the subjects in two groups is listed in Table 1. There was no significant difference in age, sex, nationality, occupation and body weight between two groups. The baseline data of two groups were comparable and balanced.
Table 1.
General information of the subjects in the experimental group and the control group
| Experimental group (n=24) | Control group (n=22) | t/χ2 | P | |
|---|---|---|---|---|
| Age | 30.0±6.6 | 30.5±6.9 | -0.271 | 0.788 |
| Nationality | ||||
| Han | 24 (100%) | 21 (95.45%) | 0.002 | 0.965 |
| Other | 0 (0%) | 1 (4.55%) | ||
| Occupation | ||||
| Employed | 12 (50%) | 8 (36.36%) | 0.402 | 0.526 |
| Unemployed | 12 (50%) | 14 (63.64%) | ||
| Weight | ||||
| D-1 (baseline) | 63.5±7.4 | 63.0±6.6 | 0.217 | 0.830 |
| D3 | 62.8±7.5 | 63.1±6.4 | -0.142 | 0.888 |
| D4 | 62.1±7.5 | 63.1±6.4 | -0.501 | 0.619 |
| D14 | 62.1±7.6 | 63.1±6.4 | -0.572 | 0.571 |
The ALP levels of two groups were compared. As shown in Table 2, from Day 2 to Day 22 (follow-up), ALP levels of the experimental group were all higher compared with the control group, but there was no significant difference between the groups. ALP levels of all 46 subjects in two groups were lower than 125 U/l during the trial period, no abnormal cases were reported. The trends of mean of ALP level as a function of time for two groups are shown in Figure 1.
Table 2.
Comparison of ALP level at different time points between the experimental group and the control group
| Time | Experimental group (U/l) | Control group (U/l) | t | P |
|---|---|---|---|---|
| D-1 (baseline) | 59.0±13.1 | 58.0±13.6 | 0.536 | 0.595 |
| D1 | 58.7±13.1 | 58.8±13.5 | 0.019 | 0.985 |
| D2 | 62.3±13.6 | 57.5±12.9 | 1.204 | 0.235 |
| D3 | 63.8±14.0 | 56.6±12.1 | 1.830 | 0.074 |
| D5 | 62.7±13.6 | 59.0±13.4 | 0.919 | 0.363 |
| D7 | 61.2±13.3 | 56.6±11.6 | 1.230 | 0.225 |
| D9 | 60.5±13.9 | 59.4±12.4 | 0.272 | 0.787 |
| D12 | 63.2±13.9 | 58.4±12.1 | 1.235 | 0.223 |
| D15 | 63.9±14.8 | 59.6±12.8 | 1.045 | 0.302 |
| D22 (follow-up) | 63.9±15.7 | 58.4±11.2 | 1.336 | 0.189 |
Figure 1.
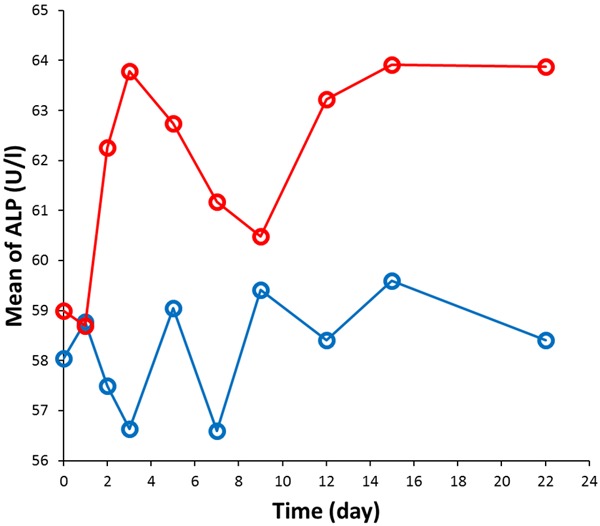
Mean of ALP level as a function of time for the control group (blue data points) and experimental group (red data points).
Table 3 shows that ALT levels of the control group were higher compared with the experimental group at all time points. The difference between two groups was statistically significant from the first day (Day 1) to the last follow-up (Day 22). From Day 5, ALT levels of the control group were two-fold greater than those of the experimental group. From Day 9, ALT levels of the control group was three-fold larger than those of the experimental group. Figure 2 indicates that ALT level of the experimental group did not exhibit significant change with time, while ALT level of the control group showed continuous increase with time and decreased on the last day as follow-up.
Table 3.
Comparison of ALT level at different time points between the experimental group and the control group
| Time | Experimental group (U/l) | Control group (U/l) | t | P |
|---|---|---|---|---|
| D-1 (baseline) | 17.9±8.8 | 24.2±14.9 | -1.471 | 0.149 |
| D1 | 17.4±10.2 | 25.1±13.1 | -2.198 | 0.034 |
| D2 | 19.0±9.8 | 27.5±14.6 | -2.271 | 0.029 |
| D3 | 20.4±9.0 | 32.0±18.4 | -2.667 | 0.012 |
| D5 | 19.5±7.3 | 39.6±21.1 | -4.219 | 0.000 |
| D7 | 19.5±7.6 | 46.6±30.8 | -4.020 | 0.001 |
| D9 | 20.4±7.6 | 62.8±49.8 | -3.946 | 0.001 |
| D12 | 20.7±7.4 | 71.2±65.8 | -3.579 | 0.002 |
| D15 | 22.8±11.2 | 82.0±87.5 | -3.145 | 0.005 |
| D22 (follow-up) | 18.6±10.3 | 42.9±38.4 | -2.869 | 0.008 |
Figure 2.
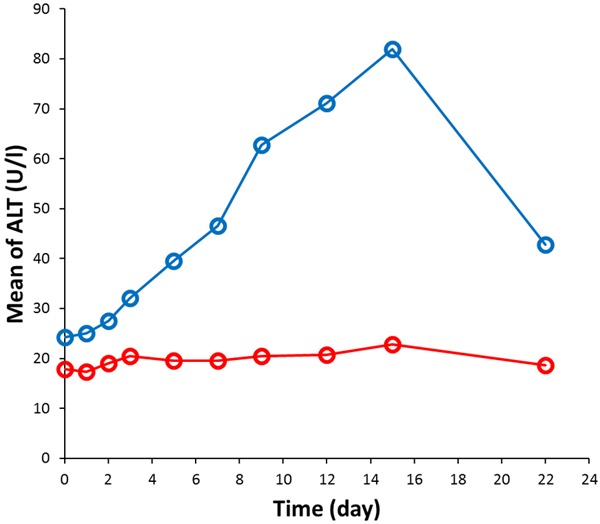
Mean of ALT level as a function of time for the control group (blue data points) and experimental group (red data points).
We consider the ALT levels greater than 50 U/l as abnormal cases, the percentage of abnormal cases was compared for two groups (see Table 4). There are almost no abnormal cases in the experimental group, while the percentage of abnormal cases in the control group increases from 5% to 50%.
Table 4.
Comparison of abnormal ALT level at different time points between the experimental group and the control group
| Time | Experimental group (n=24) | Control group (n=22) | χ2 | P |
|---|---|---|---|---|
| D-1 (baseline) | 0 (0%) | 1 (5%) | - | 0.478 |
| D1 | 0 (0%) | 1 (5%) | - | 0.489 |
| D2 | 0 (0%) | 2 (9%) | - | 0.233 |
| D3 | 0 (0%) | 4 (18%) | - | 0.049 |
| D5 | 0 (0%) | 7 (32%) | - | 0.004 |
| D7 | 0 (0%) | 10 (46%) | - | <0.001 |
| D9 | 0 (0%) | 11 (50%) | - | <0.001 |
| D12 | 0 (0%) | 11 (50%) | - | <0.001 |
| D15 | 1 (4%) | 11 (50%) | - | 0.001 |
| D22 (follow-up) | 1 (4%) | 6 (27%) | - | 0.047 |
The comparison results of AST between two groups are similar to those obtained for ALT. As the baseline level of AST (23.9±7.2 U/l) in the control group was higher compared with the experimental group (17.5±3.6 U/l) with statistically significant difference (t=-3.733, P=0.001). Covariance analysis was applied to compare AST. After adjusting the baseline, the AST levels of two groups were compared and listed in Table 5. There was significant difference between two groups from the baseline. From Day 9, AST levels of the control group were two-fold higher than those of the experimental group.
Table 5.
Comparison of AST level at different time points between the experimental group and the control group
| Time | Experimental group (U/l) | Control group (U/l) | F | P |
|---|---|---|---|---|
| D1 | 18.8±0.8 | 20.5±0.8 | 1.805 | 0.186 |
| D2 | 21.0±0.9 | 21.5±1.0 | 0.112 | 0.739 |
| D3 | 21.6±1.6 | 24.5±1.7 | 1.338 | 0.254 |
| D5 | 20.7±2.1 | 28.0±2.1 | 5.237 | 0.027 |
| D7 | 18.8±2.5 | 30.9±2.6 | 10.042 | 0.003 |
| D9 | 18.3±3.9 | 40.5±4.0 | 13.721 | 0.001 |
| D12 | 17.1±4.3 | 40.2±4.4 | 12.579 | 0.001 |
| D15 | 18.2±4.9 | 42.5±5.0 | 10.546 | 0.002 |
| D22 (follow-up) | 19.1±1.6 | 23.2±1.7 | 2.665 | 0.110 |
According to the Council for International Organizations of Medical Sciences (CIOMS) standard [8,19,20], liver injury in this study was classified and the results were listed in Table 6. From Day 9, subjects in the control group showed some cases of hepatocyte damage, while subjects in the experimental group did not exhibit any relevant cases for live injury.
Table 6.
Comparison of classified liver injury at different time points between the experimental group and the control group
| Time | Experimental group | Control group | χ2 | P |
|---|---|---|---|---|
| D-1 (baseline) | ||||
| Abnormal case | 0 (0%) | 1 (5%) | - | 0.478 |
| D1 | ||||
| Abnormal case | 1 (4%) | 1 (5%) | - | 1.000 |
| D2 | ||||
| Abnormal case | 1 (4%) | 2 (9%) | - | 0.600 |
| D3 | ||||
| Abnormal case | 1 (4%) | 4 (18%) | - | 0.178 |
| D5 | ||||
| Abnormal case | 1 (4%) | 7 (32%) | - | 0.020 |
| D7 | ||||
| Abnormal case | 1 (4%) | 10 (46%) | - | 0.001 |
| D9 | ||||
| Abnormal case | 1 (4%) | 10 (46%) | - | 0.001 |
| Hepatocyte injury type | 0 (0%) | 1 (5%) | ||
| D12 | ||||
| Abnormal case | 1 (4%) | 8 (36%) | - | 0.001 |
| Hepatocyte injury type | 0 (0%) | 3 (14%) | ||
| D15 | ||||
| Abnormal case | 2 (8%) | 7 (32%) | - | 0.004 |
| Hepatocyte injury type | 0 (0%) | 4 (18%) | ||
| D22 (follow-up) | ||||
| Abnormal case | 2 (8%) | 6 (27%) | - | 0.128 |
Discussion
In a Phase I clinical trial, subjects are usually required to reside in a research center for several continuous days or weeks, their diets during resident period will be provided on site. Basically the diets for Phase I clinical trial should meet some general requirements such as safety and hygiene, as well as special dietary requirements for specific clinical trials. This study was designed primarily to investigate the effect of a low-calorie and low-carbohydrate diet on biochemical indices of liver function of healthy volunteers in a Phase I unit. The results indicate that a low-calorie and low-carbohydrate diet is able to ensure that the blood and liver biochemical indicators of all subjects in the experimental group remain within the normal range for a relatively long period during their stay in the research center. ALT, ALP, AST, γ_GT and T_Bil in serum are important indices for liver function. Currently their changes are considered main laboratory indicators for diagnosing liver injury and DILI, an increase in serum ALT is more significant than AST in diagnosing DILI [21]. In this study, ALT, AST and other indicators in the control group were significantly higher compared with the experimental group. The highest levels of ALT and AST were five- and three-fold greater than the upper limits of their normal ranges, respectively.
The biochemical indices of liver function of subjects in the control group were significantly higher compared with the experimental group, suggesting that uncontrolled dietary calorie during a Phase I clinical trial will significantly improve the level of liver enzymes, leading to the detection of liver biochemical abnormalities in serum. Previous work on effects of diet on liver function mostly selected patients with non-alcoholic fatty liver as subjects [22,23]. It is difficult to extrapolate these results to healthy subjects to understand the relationship between liver function tests and diet in a Phase I unit. Such dietary effects might be neglected to result in abnormal liver function reported in a Phase I clinical trial and eventually cause interruption of new drug R&D or drug withdrawal from the market. The results of current study suggest that it is necessary to control the dietary structure of subjects in any Phase I clinical trials for a long time in order to avoid any adverse events of liver dysfunction caused by diet, which affect the results of a Phase I clinical trial and the direction of future drug R&D.
Based on the results obtained from previous diet-related studies with only controlled calories, the carbohydrate intake from diet in current study was also restricted. The results are consistent with recent studies which have demonstrated that low carbohydrate is more helpful to improve blood lipid and liver function indices [24]. Early studies on dietary control focused on low-fat diet [25-27]. However, recent research on dietary control has changed to carbohydrate restriction. Many studies have shown that low-carbohydrate diet is more conducive to improve insulin resistance [24,28]. In this study, dietary structure of the experimental group was designed and formulated based on the Dietary Guidelines for Chinese Residents 2016 [18], and combined with the ADA 2017 Nutrition Guidelines [16] and the Chinese Medical Nutrition Guidelines for Diabetes 2013 [17]. The blood and liver biochemical indicators of all subjects in the experimental group exhibited no abnormal increase, suggesting that a low-carbohydrate and low-calorie diet for subjects in Phase I clinical trials is more effective to prevent abnormal liver biochemical tests from uncontrolled diet.
The biochemical levels of liver function of subjects in the control group were significantly higher compared with the experimental group. The highest ALT level was 297 U/l. According to the diagnostic criteria of DILI established and further revised by CIOMS [8,19,20], the case with ALT≥3×ULN and R≥5 is defined as the hepatocyte injury type; the case with ALP≥2×ULN and R≤2 is defined as the cholestasis type; and the case with ALT≥3×ULN, ALP≥2×ULN, and 2<R<5 is considered as the mixed type. R is expressed by Equation 1.
R = (ALT measured value/ALT × ULN)/(ALP measured value/ALP × ULN) (1)
If ALT and ALP do not meet these criteria, it is called “abnormal liver biochemical tests”. From Day 9, the subjects in the control group showed “abnormal liver biochemical tests” cases and then developed to DILI cases on Day 15 (discharge) and returned to “abnormal liver biochemical tests” on Day 22 (follow-up). All the subjects who have been diagnosed to DILI belonged to “hepatocyte injury type”. Currently, acute DILI is classified to grade 1 (slight hepatic injury) according to the international classification on the severity of DILI [29]. Moreover, all these subjects did not show any clinical symptoms and imaging evidences, suggesting that diet-induced liver damage can cause not only abnormal liver biochemical tests, but also DILI diagnostic criteria as time goes on. In this study, the DILI subjects with abnormal biochemical indices of blood and liver in the control group showed a decreased tendency from Day 15 (discharge) to Day 22 (follow-up), and they all reduced to the normal range (<3 ULN), which is in agreement with the disease progression of acute liver injury. It is suggested that the abnormal biochemical indices of blood and liver caused by diet do not require drug treatment, they can return to normal range after the subjects back to their daily lives.
Figure 2 shows that ALT level of subjects in the experimental group increased continuously from Day 1 until the day of discharge on Day 15, the level exceeded the upper limit of the normal range on Day 8 and gradually reduced from Day 15 to Day 22 (follow-up). The results indicate that the abnormal biochemical indices of blood and liver caused by diet have been found since the subject resided in the research center. It is suggested that the abnormal biochemical indices of blood and liver caused by uncontrolled diet should be firstly excluded in future Phase I clinical trials with time longer than 8 days, thus non-drug liver injury can be excluded to avoid any misjudgments of drug-related adverse events. At the same time, it is suggested that low-carbohydrate and low-calorie diets should be always formulated in all Phase I clinical trials more than 8 consecutive days, regular and quantitative diets should be also used to control the dining speed [17]. Furthermore, for the Phase I clinical trial less than 8 days, low-carbohydrate and low-calorie diet should be also provided for subjects to ensure that the biochemical indicators of liver function will not fluctuate in a large range.
Due to some limitations of current study such as small-scale subjects and short period of the follow-up, large-scale studies should be used in the future to confirm the results obtained from this study. Also, the follow-up time should be extended to further track the “return-to-normal” process for subjects with abnormal blood and liver biochemical tests caused by diet. Furthermore, studies have demonstrated that the number of exercise days per week is negatively correlated with an increase in level of liver enzymes [32]. Martins et al [33] showed that sedentary behavior was positively correlated with increased ALT, while moderate to intense exercise was negatively correlated with ALT [33]. In this study, the subjects had less activity in the center compared with their daily lives, the effect of reduced exercise on blood and liver biochemical indicators cannot be completely ignored. Therefore further studies are required to investigate the effects of combined diet and exercise on blood and liver function indices in Phase I clinical trials.
Conclusions
This study used healthy volunteer as the subjects and investigated the effect of diet on liver function indices of the subjects in a Phase I clinical trial. Based on the low calorie and low fat dietary structure, a low calorie and low carbohydrate diet was designed. The results showed that a designed dietary structure can effectively reduce non-drug-related liver injury caused by dietary factors, and avoid dietary interference factors in drug test. Compared with the normal seven-day study, in this study the time was extended to 15 days, and a follow-up period was added. It has been found that a Phase I clinical trial without restricted dietary calorie and food structure will lead to liver and blood biochemical abnormalities and even acute liver injury of the healthy subjects, the probability of liver blood biochemical abnormalities as adverse events will increase. Because the symptoms are easy to confuse with DILI, it is difficult for the researchers to establish appropriate treatment strategies, evaluate the relationship between adverse events and drugs and estimate process of the Phase I process of clinical trial. The further R&D work and future marketing of the drugs will be significantly delayed. Therefore, a strict dietary plan in Phase I clinical trials should be established and executed, including total calories, daily dietary structure, dining time, etc. It is also necessary to monitor trends in blood and liver function, the reasons for liver biochemical abnormalities should be carefully investigated to distinguish drug-induced liver injury and non-drug-related injury to correctly determine the correlation between blood biochemical abnormalities and drug.
Disclosure of conflict of interest
None.
References
- 1.Food and drug administration. IND safety reporting. 2018 21 CRF§312.32. FDA. [Google Scholar]
- 2.Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Miguel A, Azevedo LF, Araújo M, Pereira AC. Frequency of adverse drug reactions in hospitalized patients: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2012;21:1139–1154. doi: 10.1002/pds.3309. [DOI] [PubMed] [Google Scholar]
- 4.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM U.S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 5.Lee WM, Senior JR. Recognizing drug-induced liver injury: current problems, possible solutions. Toxciol Pathol. 2005;33:155–164. doi: 10.1080/01926230590522356. [DOI] [PubMed] [Google Scholar]
- 6.Watkins PB, Seeff LB. Drug induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43:618–631. doi: 10.1002/hep.21095. [DOI] [PubMed] [Google Scholar]
- 7.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, Rochon J DILIN Study Group. Drug-Induced LiverInjury Network (DILIN) prospective study: rationale, design and Conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ Practice Parameters Committee of the American College of Gastroenterology. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950–966. doi: 10.1038/ajg.2014.131. quiz 967. [DOI] [PubMed] [Google Scholar]
- 9.Devarbhavi H. An update on drug-induced liver injury. J Clin Exp Hepatol. 2012;2:247–259. doi: 10.1016/j.jceh.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis. 2002;22:145–155. doi: 10.1055/s-2002-30105. [DOI] [PubMed] [Google Scholar]
- 11.Bjornsson ES. Epidemiology and risk factors for idiosyncraticdrug-induced liver injury. Semin Liver Dis. 2014;34:115–122. doi: 10.1055/s-0034-1375953. [DOI] [PubMed] [Google Scholar]
- 12.Drug-induced Liver Disease Study Group; Chinese Society of Hepatology; Chinese Medical Association. Guidelines for the management of drug-induced liver injury. J Clin Hepatol. 2015;31:1752–1769. [Google Scholar]
- 13.Food and drug administration. 2014 Phases of an investigation: phase 1.21 CFR § 312.21(a).FDA. [Google Scholar]
- 14.Nobili V, Alisi A, Massimilino R. Pediatric fatty liver disease: prevention and therapeutic value of lifestyle intervention. World J Gastroenterolgy. 2009;15:6017–22. doi: 10.3748/wjg.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caporaso N, Morisco F, Camera S, Graziani G, Donnarumma L, Ritieni A. Dietary approach in the prevention and treatment of NAFLD. Front Biosci. 2012;17:2259–2268. doi: 10.2741/4049. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes-2017. Diabetes Care. 2017;40(Suppl 1):S1–135. [Google Scholar]
- 17.Chinese Diabetes Society; Chinese Medical Doctor Association Nutritionist Specialized Committee. China medical nutrition therapy guideline for diabetes 2013. Chinese Journal of Diabetes Mellitus. 2015;7:73–88. (in Chinese) [Google Scholar]
- 18.The Chinese Nutrition Society. The Food Guide Pagoda for Chinese Residents. 2016. Available from om http://dg.cnsoc.org/ [A/[Accessed on June 20, 2016] (in Chinese)
- 19.Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34:134–144. doi: 10.1055/s-0034-1375955. [DOI] [PubMed] [Google Scholar]
- 20.Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Cáliz I, González-Jimenez A, Ulzurrun E, Gonzalez AF, Fernandez MC, Romero-Gómez M, Jimenez-Perez M, Bruguera M, Prieto M, Bessone F, Hernandez N, Arrese M, Andrade RJ Spanish DILI Registry; SLatinDILI Network; Safer and Faster Evidence-based Translation Consortium. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014;147:109–118. doi: 10.1053/j.gastro.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Gerrn RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367–1384. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- 22.Sogabe M, Okahisa T, Nakagawa T, Fukuno H, Nakasono M, Tomonari T, Tanaka T, Tanaka H, Taniguchi T, Muguruma N, Takayama T. Influence of light alcohol consumption on lifestyle-related diseases: a predictor of fatty liver with liver enzyme elevation in Japanese females with metabolic syndrome. BMC Gastroenterology. 2016;16:17. doi: 10.1186/s12876-016-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kani AH, Alavian SM, Esmaillzadeh A, Adibi P, Azadbakht L. Effects of a novel therapeutic diet on liver enzymes and coagulating factors in patients with non-alcoholic fatty liver disease: a parallel randomized trial. Nutrition. 2014;30:814–821. doi: 10.1016/j.nut.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Nazarenko LI, Petrova IuN , Raĭkhel’son KL, Baranovskiĭ AIu Nutrition mistakes in patients with nonalcoholic fatty liver disease and the ways of correction. Eksp Klin Gastroenterol. 2012:19–24. [PubMed] [Google Scholar]
- 25.Liu T, Yang LL, Zhang L, Song HY, Li DF, Ji G. Comparative study on the effects of different therapeutic methods in preventing and treating nonalcoholic fatty liver in rats. Zhong Xi Yi Jie He Xue Bao. 2012;10:1120–1126. doi: 10.3736/jcim20121008. [DOI] [PubMed] [Google Scholar]
- 26.Morita M, Ishida N, Uchiyama K, Yamaguchi K, Itoh Y, Shichiri M, Yoshida Y, Hagihara Y, Naito Y, Yoshikawa T, Niki E. Fatty liver induced by free radicals and lipid peroxidation. Free Radic Res. 2012;46:758–765. doi: 10.3109/10715762.2012.677840. [DOI] [PubMed] [Google Scholar]
- 27.van der Meer RW, Hammer S, Lamb HJ, Frölich M, Diamant M, Rijzewijk LJ, de Roos A, Romijn JA, Smit JW. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab. 2008;93:2702–2708. doi: 10.1210/jc.2007-2524. [DOI] [PubMed] [Google Scholar]
- 28.Agius L. High-carbohydrate diets induce hepatic insulin resistance to protectthe liver from substrate overload. Biochem Pharmacol. 2013;85:306–312. doi: 10.1016/j.bcp.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Watkins PB, Seligman PJ, Pears JS, Avigan MI, Senior JR. Using controlled clinical trials to learn more about acute drug- induced liver injury. Hepatology. 2008;48:1680–1689. doi: 10.1002/hep.22633. [DOI] [PubMed] [Google Scholar]
- 30.Carvalhana S, Machado MV, Cortez-Pinto H. Improving dietary patterns in patients with nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15:468–473. doi: 10.1097/MCO.0b013e3283566614. [DOI] [PubMed] [Google Scholar]
- 31.Scaglioni F, Marino M, Ciccia S, Procaccini A, Busacchi M, Loria P, Lonardo A, Malavolti M, Battistini NC, Pellegrini M, Carubbi F, Bellentani S. Short-term multidisciplinary non-pharmacological intervention is effective in reducing liver fat content assessed non-invasively in patients with nonalcoholic fatty liver disease (NAFLD) Clin Res Hepatol Gastroenterol. 2013;37:353–358. doi: 10.1016/j.clinre.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Lee EY, Choi HY, Cho H, Kim BH, Ki M. Health behavior associated with liverenzymes among obese Korean adolescents, 2009-2014. PLoS One. 2018;13:e0190535. doi: 10.1371/journal.pone.0190535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martins C, Aires L, Júnior IF, Silva G, Silva A, Lemos L, Mota J. Physical activity is related to fatty liver marker in obese youth, independently of central obesity or cardiorespiratory fitness. J Sports Sci Med. 2015;14:103–109. [PMC free article] [PubMed] [Google Scholar]


