Abstract
Renal ischemia-reperfusion injury (RIRI) is one of the main causes for acute kidney injury (AKI). Many previous attempts failed to adopt a suitable treatment regimen for AKI. Recently, combined melatonin (Mel) and mesenchymal stem cell (MSC)-derived exosomes (Exo) therapy gave a promising therapeutic option for acute liver ischemic injury, however this treatment approach has not been tested against RIRI yet. This study tested the hypothesis that administration of exosomes derived from MSCs preconditioned with Mel gave best protection against RIRI as compared to therapy by MSCs or exosomes derived from non-preconditioned MSCs. Female adult rats (n = 60) equally divided into control group, sham group, RIRI group (induced by bilateral renal arteries clamping), RIRI + MSCs group (1 × 106 bone marrow derived MSCs), RIRI + Exo group (250 μg Exo derived from no-preconditioned MSCs), and RIRI + Mel + Exo group (250 μg Exo derived from Mel preconditioned MSCs). MSCs or Exo was bilaterally injected once in each renal artery during reperfusion. The obtained results revealed notable improvement in RIRI following all treatment (MSCs, Exo, and Exo + Mel) with best improvement in Exo + Mel group as evidenced by: 1) decreased kidney injury histopathological score; 2) reduced blood levels of kidney damage markers [blood urea nitrogen (BUN) and creatinine]; 3) declined oxidative stress status (MDA level, HIF1α gene, and NOX2 protein); 4) increased anti-oxidant status (HO1 gene, and SOD, CAT, GPX activities); 5) declined apoptosis (caspase 3 activity and mRNA, and PARP1, Bax genes), 6) induced anti-apoptotic effect (Bcl2 gene); 7) inhibition of inflammation (decreased MPO activity and ICAM1, IL1B, NFkB genes and increased IL10 genes); 8) improved regeneration (bFGF, HGF and SOX9 proteins); and 9) enhanced angiogenesis (VEGF gene). These data indicate that treatment with exosomes derived from MSCs preconditioned with melatonin gave best protective effect against renal ischemia-reperfusion injury as compared to therapy by non-preconditioned MSCs or their exosomes.
Keywords: Melatonin, exosomes, renal ischemia, mesenchymal stem cells
Introduction
Renal ischemia-reperfusion injury (RIRI) is one of the main causes for acute kidney injury (AKI). The latter is a devastating renal dysfunction syndrome that has the potential to accelerate the occurrence of chronic kidney disease (CKD) and further lead to renal failure and high morbidity and mortality. The end phase of CKD characterized by irreversible loss of kidney function obligates dialysis or kidney transplantation [1]. It was reported that release of free radicals, mitochondrial dysfunction, induction of apoptosis and inflammation are among the main causes of RIRI [2,3]. Hence, therapeutic agents targeting oxidative stress, apoptosis, and inflammation would be beneficial in clinical approach of RIRI. Many previous attempts failed to adopt a suitable treatment regimen for RIRI [3]. However, cell-based therapies using mesenchymal stem cells (MSCs) give a promising hope in that regard and could be used as effective treatment for AKI due to MSCs inherent capacity to produce cell phenotype of host organs and to improve tissue repair and regeneration [4,5]. Thus, more MSCs within the damaged tissue would meaningfully advance the positive effects of MSCs therapy. However, a major hindrance of this method is the higher early death rates of injected MSCs due to hostile microenvironment [6,7]. Oxidative stress, hypoxia-induced apoptosis, and in-site inflammation are different explanations that were highlighted in the early death of injected MSCs [8,9].
Previous studies have hypothesized three tactics to overcome these setbacks. The first approach is the co-administration of MSCs with another agent (adjuvant) that has anti-inflammatory and antioxidant characteristics. Ideally, melatonin (Mel), a hormone secreted mainly by the pineal gland, can improve MSCs viability and differentiation [10,11]. Mel acts as a powerful scavenger of free radicals with ability of oxidative stress and inflammation alleviation, also it stabilizes the cell membrane making the cells less susceptible to inflammatory, oxidative, and renal injury [12,13]. Furthermore, Mel is widely used as a human dietary supplement without reporting any side effects [14]. It has also the ability to reduce RIRI in rats [15]. Mel was used as an adjuvant for MSCs to foster their efficacy in the treatment of chronic diseases and cancer [16,17]. Additionally, a number of studies showed that MSCs preconditioned by Mel have beneficial therapeutic effect against ischemic injury in kidney [11], heart [10], brain [18], lung [19], and bowel [20]. This may be because MSCs have the melatonin receptors MT1 and MT2, [12] which showed higher expression in Mel-pretreated MSCs than in non-pretreated MSCs [16].
The second approach is injection of microvesicles secreted by MSCs instead of direct MSCs injection. Exosomes, 20-130 nm vesicles produced physiologically by all types of cells and present in all fluids of the body, were found to be crucial vehicles for the transfer of membrane-enclosed signaling molecules and gene products including protein, mRNA, and microRNA that are vital modulators for intercellular communications [7,21-23]. Recent publications from my laboratory have shown that exosomes derived from MSCs possess anti-apoptotic, immunomodulatory, anti-inflammatory, and pro-angiogenic properties [7,23]. Therefore, it was not surprising to find that exosomes derived from renal tubular cells inhibited most symptoms of RIRI/AKI [1,24-27]. However, the actual mechanism by which exosomes can mitigate RIRI/AKI have not elucidated yet. A previous study has reported the ability of MSCs-derived exosomes to protect tubular epithelial cells against death in an in vitro model of RIRI and this effect involved targeting of some molecules related to apoptosis and hypoxia by exosomal miRNAs [28], suggesting a potential role for exosomal miRNAs in relieving RIRI.
The third approach is a combined therapy of Mel and MSCs-derived exosomes. The combined therapy gave a promising therapeutic option for experimental acute liver ischemic injury [29], but has not been applied in RIRI/AKI yet. Besides, this previous study only investigated the effect of a combined therapy of Mel and MSCs-derived exosomes, but did not address the effect of exosomes derived from MSCs preconditioned with Mel. Therefore, this study was conducted to test the hypothesis that exosomes derived from MSCs preconditioned with Mel may offer superior protection against RIRI compared to therapy by MSCs or exosomes from non-preconditioned MSCs.
Materials and methods
Isolation and characterization of MSCs and their exosomes
MSCs were isolated from bone marrow (BM) of rat femur and tibia as previously described in two recent publications from my laboratory [7,23]. The isolated cells were further confirmed to be MSCs by flow cytometry (Attune, Applied Biosystem, USA) using specific stem cell markers, including two positive markers; anti-CD44, and -CD90, and a negative marker; anti-CD34 (a mark for hematopoietic cells), according to the manufacturer’s instruction (Becton, Dickinson). Exosomes were isolated from MSCs by ultracentrifugation at 100,000 g (Optima L-90K; Beckman Coulter) as previously described by us [7,23]. The isolated exosomes were verified by transmission electron microscopy (JEM2100, Joel Inc.) and western blotting detection for specific exosomal proteins [CD63 (1:500), CD81 (1:500); Santa Cruz].
Animals and experimental design
The experimental protocol was approved by the Animal Ethics Committee of King Abdulaziz University and was performed in accordance with the NIH guidelines on animal care. Adult female rats fed a standard diet ad libitum with free access to water were housed in plastic cages under a controlled temperature (25-27°C) with a 12 h light/dark cycle. Animals were acclimated for 7 days before surgery.
Adult female rats (n = 60) with matched weights (280-320 g) and age were randomized into six groups (n = 10/group and 5/each time point, 3 days and 4 weeks): control (Cnt), sham, renal ischemia-reperfusion injury (RIRI), RIRI + MSCs (MSCs), RIRI + Exo (Exo) and RIRI + Exo+ Mel (Exo + Mel) groups. Sham-operated animals were only subjected to laparotomy without renal artery clamping or any injection. In RIRI group, rats were anesthetized by intraperitoneal injection of ketamine (100 mg/kg body weight, bw) combined with xylazine (100 mg/Kg bw) and then a midline laparotomy was done followed by bilateral ischemia for 45 min by clamping renal vessels using atraumatic vascular microclamps. During the 30 min reperfusion, vehicle (DMEM, RIRI group) or either MSCs (1 × 106 cells in 30 µl of DMEM, MSCs group) or exosomes (250 μg exosomes into 30 µl of DMEM, Exo and Exo + Mel groups) were injected in right and left renal arteries in each animal [23,30]. In Exo + Mel group, MSCs were pretreated with 5 µM Mel (Sigma Aldrich, a Mel solution was prepared at a concentration of 1.15 μg/ml ethanol) for 24 h [11,31] and the media was collected to isolate exosomes.
Blood and tissue sampling
Blood samples were collected from the venous plexuses at the medial canthus of rat eye and serum was separated by centrifugation as previously described [22]. Following rats euthanization by cervical dislocation under light anesthesia by ether, kidneys were excised and rinsed by saline and then were divided into three portions. The first portion was prepared for ROS/antioxidant and myeloperoxidase (MPO) assays via homogenization in ice-cold PBS. The second portion was immediately frozen to be used for RNA extraction and subsequently in real time PCR or for protein extraction and western blot. The third portion was preserved in 10% neutral-buffered formalin solution for histological analysis. All blood and tissues samples were collected at two different time points, 3 day and 4 week post-ischemia.
Biochemical analysis
The levels of the two kidney damage markers creatinine and blood urea nitrogen (BUN) were determined in serum and plasma, respectively, using commercial available kits. The concentration of the lipid peroxidation biomarker malondialdehyde (MDA) and the activities of antioxidant enzymes (SOD, CAT, GPX) determined in kidney homogenates as previously described [32]. The activity of neutrophil infiltration indicator MPO was determined in renal tissues as previously described [3]. The cleaved caspase-3 activity assay was determined on kidney homogenate as previously described [33].
Histopathological examination
Right and left kidney specimens were dehydrated in ethanol, cleared in xylene, and impeded in paraffin, sectioned (4-5 µm), and stained with hematoxylin and eosin (H&E) as previously described [34]. The histopathology scoring was blindly performed depending on the grading of pathological changes including tubular necrosis, cast formation, brush border loss, and tubular dilatation in 10 randomly chosen, non-overlapping fields (400 ×) as follows: 0 (none), 1 (≤ 10%), 2 (11-25%), 3 (26-45%), 4 (46-75%), and 5 (≥ 76%) as previously described [35].
Real time PCR
Real time PCR was applied to determine the changes in the relative expression of the oxidative stress-, antioxidant-, apoptosis-, inflammation-, and angiogenesis-related genes in kidney tissues from all rat groups. Total RNA was extracted from kidney tissue using RNeasy Mini kit (Qiagen) and its concentration and purity were detected by a Nanodrop. Purified RNA samples (4 mg for each) were reverse transcribed into cDNA by Quantiscript reverse transcriptase as previously described [36]. Gene specific primers were designed by the Primer 3 web-based tool based on the published rat sequences (Table 1). The real-time PCR reaction was carried out in a tube containing cDNA, QuantiTect SYBR Green qPCR Master Mix and gene specific primers which was put in StepOnePlus real time PCR system (Applied Biosystem, USA). The conditions of reaction cycles and temperature of melting curves were set as previously described [37]. The quantities critical threshold (Ct) of target genes were normalized with quantities (Ct) of the internal control (β actin) as previously described [38].
Table 1.
Primers used for real-time PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Oxidative stress | ||
| HIF1α | GAAAGAGCCCGATGCCCT | TGATATGATCGTGTCCCCAGC |
| Antioxidant | ||
| HO1 | GGAAAGCAGTCATGGTCAGTCA | CCCTTCCTGTGTCTTCCTTTGT |
| Apoptosis | ||
| Bax | ACACCTGAGCTGACCTTG | AGCCCATGATGGTTCTGATC |
| Bcl2 | AGTACCTGAACCGGCATCTG | CATGCTGGGGCCATATAGTT |
| Caspase 3 | GGTATTGAGACAGACAGTGG | CATGGGATCTGTTTCTTTGC |
| PARP1 | ACGCACAATGCCTATGAC | CCAGCGGAACCTCTACAC |
| Inflammation | ||
| IL1β | CACCTCTCAAGCAGAGCACAG | GGGTTCCATGGTGAAGTCAAC |
| NFκβ | CCTAGCTTTCTCTGAACTGCAAA | GGGTCAGAGGCCAATAGAGA |
| IL10 | GTTGCCAAGCCTTGTCAGAAA | TTTCTGGGCCATGGTTCTCT |
| ICAM1 | AGATCATACGGGTTTGGGCTTC | TATGACTCGTGAAAGAAATCAGCTC |
| Angiogenesis | ||
| VEGF | GATCATGCGGATCAAACCTCACC | CCTCCGGACCCAAAGTGCTC |
| Housekeeping | ||
| β actin | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC |
Western blotting
Western blotting was performed to detect the changes in proteins expression of target proteins following different treatments. It was carried out following the manufacturers’ guidelines and as detailed previously by my laboratory team [23]. In brief, following determination of protein concentration by the Bradford method, equal amounts (50 µg) of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to a polyvinylidene difluoride (PVDF) membrane. This membrane was then incubated in blocking buffer (5% nonfat dry milk in PBS containing 0.05% Tween 20) for 12 h to block the nonspecific sites. The membranes were then incubated with primary antibodies against the following protein NOX2, bFGF, HGF, SOX9 (1:1000, Santa Cruz) for 1 h at room temperature. The used secondary antibody was horse radish peroxidase (HRP)-conjugated goat anti-rabbit antibodies (1:5000; Santa Cruz) for 1 h at room temperature. The washing procedure was repeated eight times within one hour. The specific protein bands were developed using tetramethylbenzidine (TMB, Sigma). The density of target protein bands was normalized by β actin and all bands were analyzed by Image J software.
Statistical analysis
Data were presented as mean ± standard error of mean (SEM). The statistical analysis was performed using one way ANOVA using GraphPad Prism 8 (GraphPad Software, Inc., LaJolla, CA, USA) followed by Tukey’s Honestly Significant Difference (Tukey’s HSD) test. Significance was declared at P < 0.05.
Results
Identification and characterization of MSCs and their exosomes
Bone marrow (BM) derived MSCs were isolated and identified as previously showed by us [7,23]. Briefly, BM derived cells from rats were isolated and were cultured in vitro. Examination of these cells using an inverted microscope showed presence of adhesive and fusiform cells assumed to be MSCs (Figure 1A). The identity of these cells was further confirmed by flow cytometry which revealed presence of high percent of BM-MSCs as indicated by increased expression of the two MSCs positive markers CD90 and CD44 and only very few cells expressed CD34 (a mark for hematopoietic cells, Figure 1B).
Figure 1.
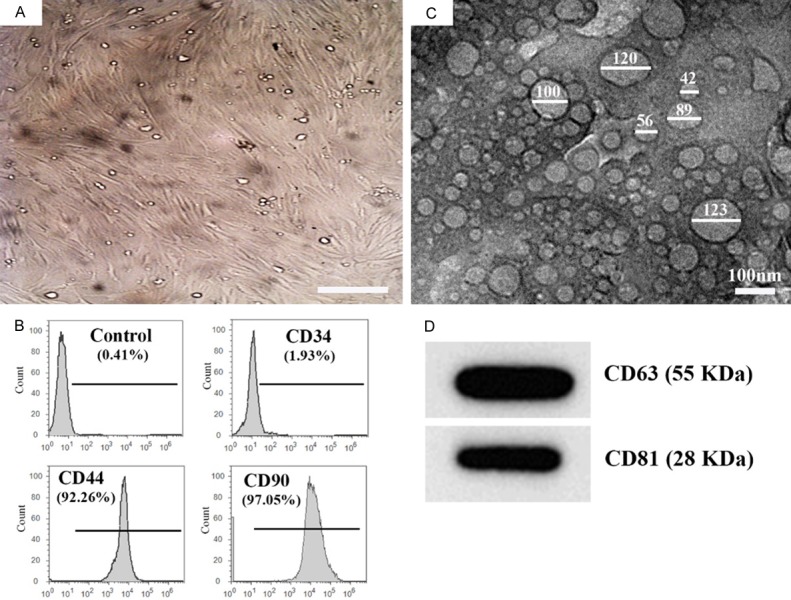
Characterization and identification of cultured MSCs and their exosomes. A. MSCs with their characteristic fusiform (fibroblast-like) shape were grown after passage 8, Scale bar = 50 μm. B. Characterization of MSCs by flow cytometer analysis showed that 92.26% and 97.05% of the bone marrow derived MSCs were positive to CD44 and CD90, respectively, while only 1.93% positive for CD34 which considered as negative results. C. Transmission electron microscopic examination shows small nanovesicles (20-130 nm) in the sample isolated from the MSCs culture media by ultracentrifugation. D. Western blot analysis of the exosomal protein shows presence of CD63 and CD81.
Exosomes were successfully isolated and characterized as previously described by my research team [7,23]. In brief, the identity of exosomes isolated by ultracentrifugation were confirmed using both transmission electron microscopy (TEM) and western blot (WB). TEM examination revealed the presence of cup-shaped or irregular-shaped nanovesicles (20 to 130 nm) in the samples isolated from BM-MSCs media (Figure 1C). These nanovesicles were confirmed to be exosomes through detection of the MSCs-exosomal specific markers CD63 and CD81 using WB (Figure 1D).
Exosomes from Mel pre-conditioned MSCs decrease kidney damage score
To assess the level of renal tubules injury, H&E staining was carried out. Kidneys of rats in the control and sham groups showed normal histological structure of renal tubules and glomeruli at the two time points, 3 day and 4 week (Figure 2A-D). However, the kidneys of rats in RIRI group showed tubular epithelial cell necrosis (green arrowheads), tubular dilation (yellow arrowheads), casts formation and desquamated tubular epithelia within the tubular lumen (blue arrowheads) (Figure 2E, 2F). These typical ischemic lesions were more prominent during the acute stage (3 days) than the chronic stage (4 weeks) of renal injury. However, mono-nuclear cells infiltration (assumed to be inflammatory cells) in the interstitium was only noticed at 4 weeks post-ischemia (yellow arrow, Figure 2F). These ischemic and pro-inflammatory lesions were alleviated following treatment with Exo (Exo group) or MSCs (MSCs group) with or without Mel preconditioning, with the best improvement in the Exo + Mel group at 4 weeks (Figure 2G-L). Figure 2M, 2N shows the pathological scoring of kidney at the two time points and reveals the presence of highest score in RIRI group, followed by MSCs group, then Exo group and finally Exo + Mel group. The latter showed a very low score close to that of the control and sham groups, especially at 4 weeks. These data suggest that Exo derived from Mel preconditioned MSCs treatment was superior to either treatment with MSCs or Exo from non-preconditioned MSCs.
Figure 2.
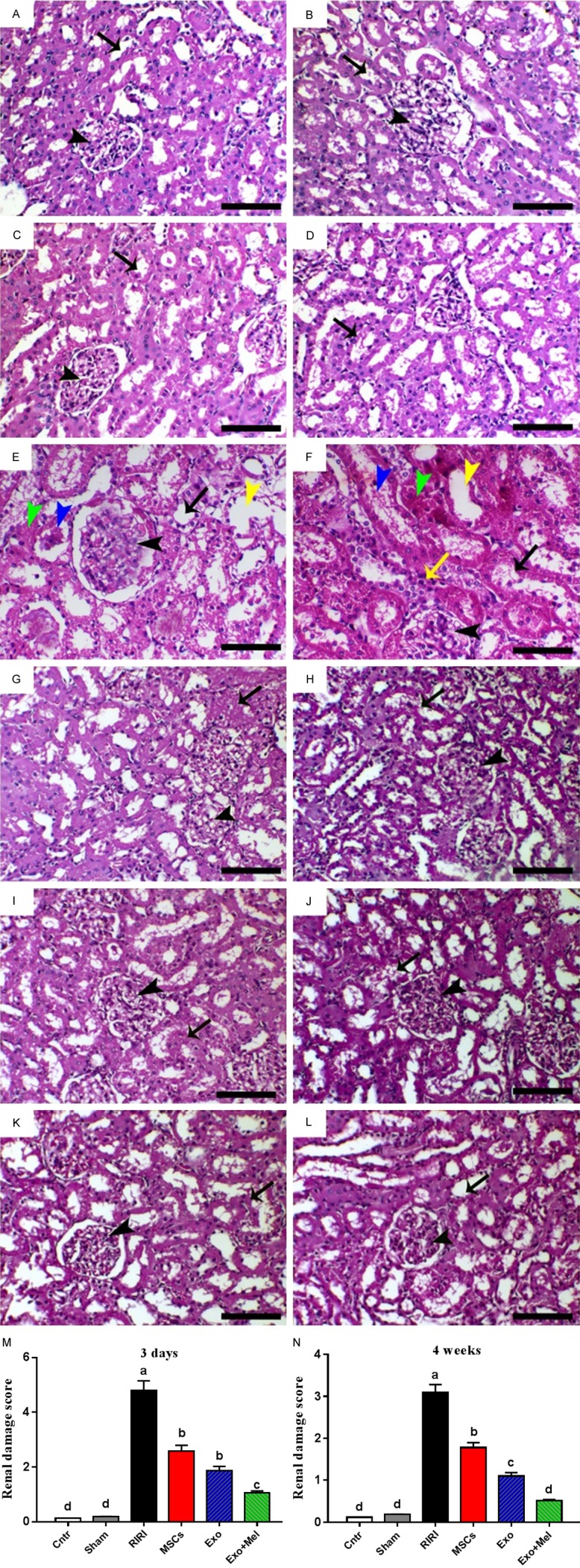
H&E staining and the analysis of renal damage score. (A, B) Control group; (C, D) Sham group; (E, F) RIRI group; (G, H) MSCs group; (I, J) Exo group; and (K, L) Mel + Exo group at 3 days (A, C, E, G, I, K) and 4 weeks (B, D, F, H, J, L) post-ischemia. In all images, black arrows and arrowheads refer to renal tubules and glomeruli, respectively. (M and N) Pathological scoring of kidney at 3 days (M) and 2 weeks (N). Values were means ± SEM (n = 10/group and 5/each time point). *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars = 50 µm in (E and F); 100 µm in all remaining images.
Exosomes from Mel pre-conditioned MSCs improve kidney function
The extent of renal function were evaluated by detection of the two renal dysfunction indices BUN in plasma and creatinine in serum. BUN and creatinine significantly increased in rats of RIRI group at the two time points (3 days and 4 weeks), with highest levels at 3 days, as compared to the control and sham groups (Table 2). Although the BUN and creatinine levels were dramatically declined in RIRI group at 4 weeks post-ischemia, they remained higher than other groups. These elevated levels were significantly reduced following treatment with Exo (Exo group) or MSCs (MSCs group) with or without Mel preconditioning, with the best improvement in the Exo + Mel group at 4 weeks (Table 2). Surprisingly, values returned to normal in Exo and Exo + Mel groups at 4 weeks post-ischemia. Thus, the disrupted renal function in RIRI group was relieved after Exo or MSCs treatment, with best effect for Exo derived from MSCs pretreated with Mel. This implies that treatment with Mel preconditioned MSCs-Exo offered an additional benefit for reno-protection against RIRI.
Table 2.
Effect of treatment with MSCs and Exo on kidney damages parameters in RIRI rats
| Animal groups | Plasma BUN (mg/dl) | Serum creatinine (mg/dl) | ||
|---|---|---|---|---|
|
|
|
|||
| 3 days | 4 weeks | 3 days | 4 weeks | |
| Control | 19.24±1.05e | 18.91±0.91c | 1.22±0.03c | 1.20±0.02b |
| Sham | 19.78±0.81e | 19.11±0.78c | 1.25±0.04c | 1.21±0.02b |
| RIRI | 59.05±1.30a | 33.12±1.02a | 2.51±0.09a | 1.60±0.04a |
| MSCs | 45.20±1.25b | 27.20±0.94b | 1.82±0.06b | 1.37±0.03b |
| Exo | 40.36±0.85c | 22.34±0.82b,c | 1.64±0.07b | 1.29±0.02b |
| Exo + Mel | 29.10±0.91d | 20.07±0.79c | 1.30±0.04c | 1.24±0.03b |
Data are presented as mean ± SEM. Mean values with different superscript letters (a-e) in the same column are significantly different at (P ≤ 0.05).
Exosomes from Mel pre-conditioned MSCs reduce oxidative stress and enhance antioxidant status
To check whether Exo can relive RIRI through reduction of oxidative stress and improvement of endogenous antioxidant status, we evaluated the content of the lipid peroxidation marker MDA, the activities of the antioxidant enzymes SOD, GPX and CAT, the mRNA levels of oxidative stress-related marker HIF1α and the antioxidant-related marker HO1, and protein expression of the oxidative stress marker NOX2 in rat kidney from all the groups and the results were presented in Figure 3. The RIRI group showed highest levels of MDA content, HIF1α mRNA, and NOX2 protein, and the lowest levels of the three antioxidant enzymes and HO1 mRNA as compared to the control and sham groups. Injection of MSCs (MSCs group) or Exo derived from MSCs with (Exo + Mel group) or without (Exo group) preconditioning by Mel resulted in a significant decrease in the levels of MDA, HIF1α mRNA, and NOX2 protein, and a significant increase in the levels of the three antioxidant enzymes and HO1 mRNA, with the best improvement in the Exo + Mel group, compared with the RIRI group. The changes in oxidative stress/antioxidant related parameters were more prominent at 3 days than at 4 weeks post-ischemia. However, in either time point the treatment failed to return the parameters to the normal values. These results once again indicate that administration of Mel preconditioned MSCs-Exo give better attenuated effect on RIRI and this effect could be mediated, at least in part, by induction of antioxidant activities and inhibition of oxidative stress in injured renal tissues.
Figure 3.
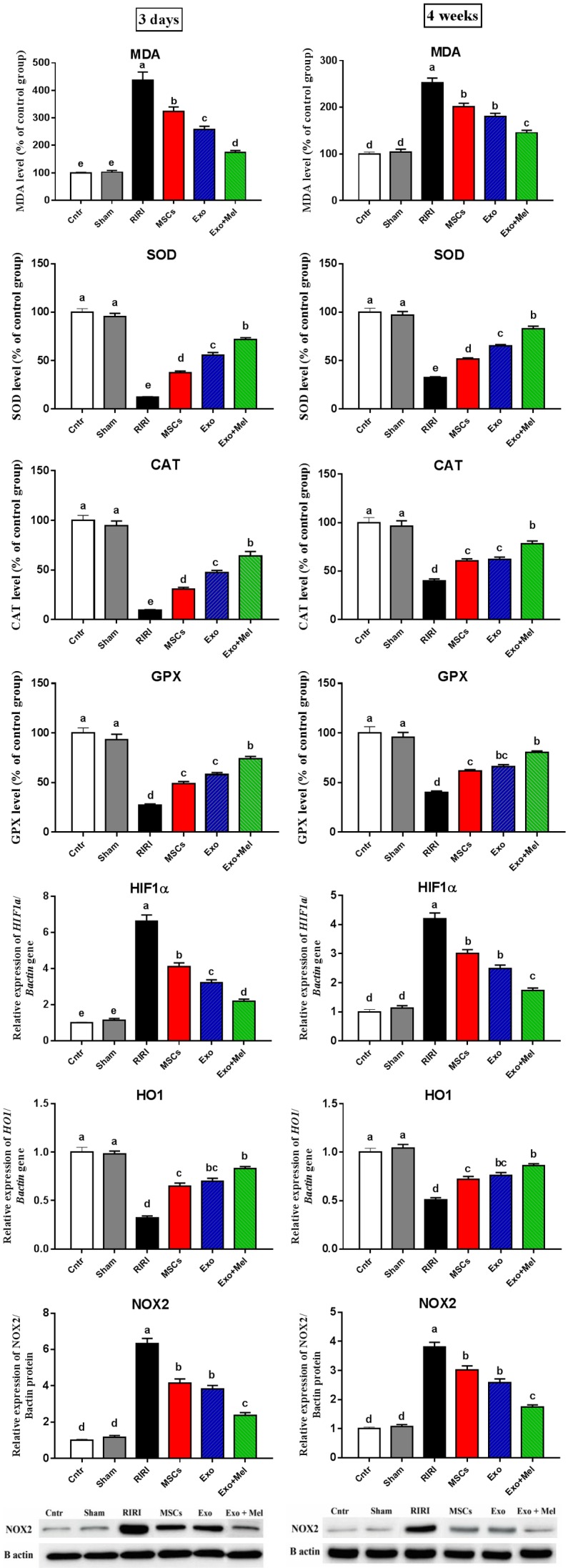
Effect of MSCs or exosomes treatment on oxidative stress (MDA, HIF1α gene, NOX2 protein) and antioxidant (SOD, CAT, GPX enzymes and HO1 gene) status of kidney in RIRI rats at 3 days and 4 weeks time points. Values are expressed as mean ± SEM. Columns carrying different letters are significantly different at P < 0.05.
Exosomes from Mel pre-conditioned MSCs decline apoptosis
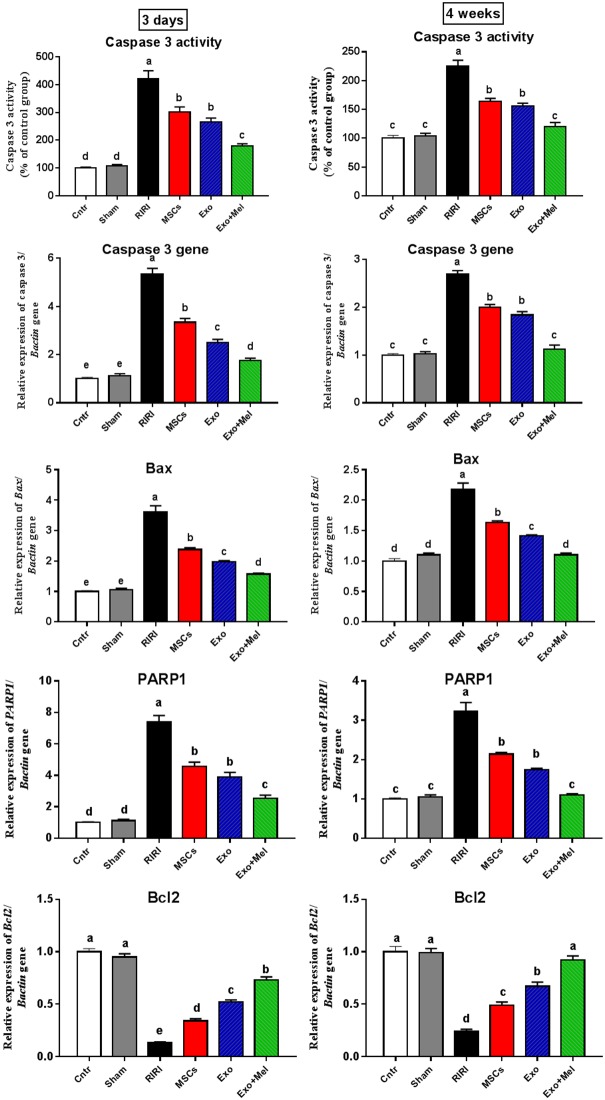
Another possible pathway by which Exo can exert their ameliorative effect on RIRI is the interference with apoptosis in renal cells. Herein, this possible apoptosis inhibitory effect was assessed by measuring the activity of the final marker of apoptosis, cleaved caspase-3. Expectedly, the RIRI group exhibited the highest cleaved caspase-3 activity relative to the control and sham groups (Figure 4), while administration of MSCs or their Exo significantly decreased this activity, with lowest activity observed in the Exo + Mel group.
Figure 4.
Effect of MSCs or exosomes treatment on apoptosis (caspase 3 activity and mRNA level, Bax, PARP1, and Bcl2 genes). Data presented as fold change (mean) ± SEM from the control group. Columns carrying different letters are significantly different at P < 0.05.
Results of caspase 3 activity assay was further confirmed at a molecular level. The obtained qPCR results showed a significant upregulation of the apoptotic markers Bax, PARP1, and caspase 3 and a significant downregulation of the anti-apoptotic marker Bcl2 in kidneys of RIRI rats relative to the control and sham rats (Figure 4). In contrast, treatments with MSCs or their Exo resulted in a significant reduction in mRNA levels of Bax, PARP1, and caspase 3 and a significant elevation in mRNA level of Bcl2, with best results in the Exo + Mel group, compared with the RIRI group (Figure 4). Again, the altered apoptotic and anti-apoptotic conditions were more remarkable at 3 days than at 4 weeks time point. Interestingly, Exo + Mel group exhibited values of apoptotic and anti-apoptotic parameters at 4 weeks close to those in the control and sham groups. Collectively, these results suggest that the ameliorative effect MSCs and their Exo on RIRI associated with inhibition of apoptosis in damaged kidney, with best results obtained when Exo was obtained from MSCs preconditioned with Mel.
Exosomes from Mel pre-conditioned MSCs inhibit inflammation
Figure 5 displays the change in MPO activity (as a pro-inflammatory marker and an indicator for neutrophil infiltration) and mRNA levels of inflammation markers ICAM1, IL1β, NFκB, and the anti-inflammatory marker IL10 in kidneys from all groups. At the two time points, the RIRI group showed the most significantly increased and highest MPO level and ICAM1, IL1β, NFκB mRNA levels and the lowest IL10 mRNA level. Administration of MSCs or their Exo with or without preconditioning significantly decreased MPO level and mRNA levels of these inflammation markers and increased the mRNA levels of the anti-inflammatory marker, with the best improvement in the Exo + Mel. This effect was more notable at 4 weeks than at 3 days time point but none of these improved parameter was returned to normal values. These findings suggest an important role for Exo, with best effect for those derived from Mel pre-conditioned MSCs, in mitigation of RIRI via at least in part reduction of the inflammation.
Figure 5.
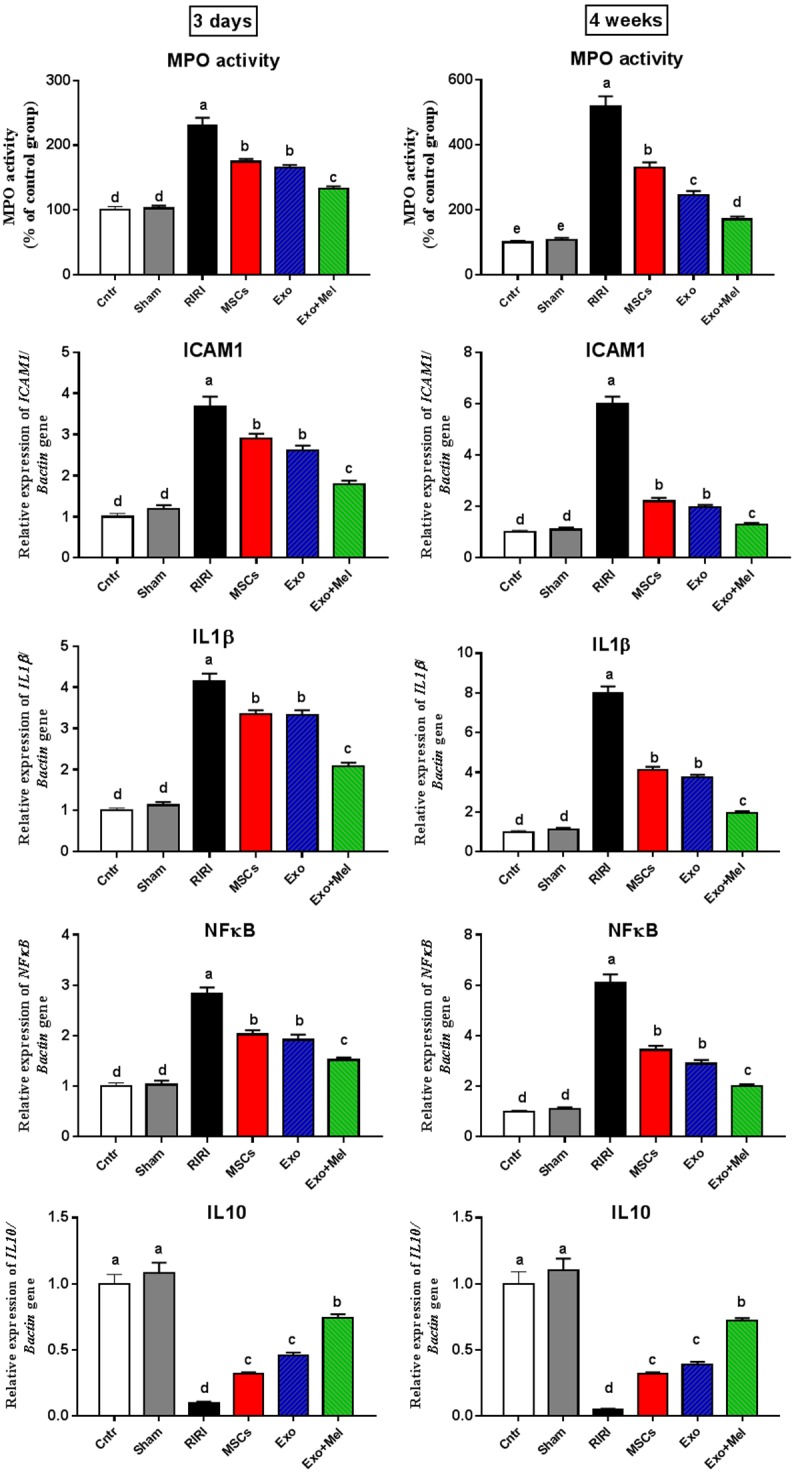
Effect of MSCs or exosomes treatment on inflammation (MPO, and mRNA levels of ICAM-1, IL1β, NFκB, and IL10). Data presented as fold change (mean) ± SEM from the control group. Columns carrying different letters are significantly different at P < 0.05.
Exosomes from Mel pretreated MSCs improve regeneration and angiogenesis
Previous studies revealed important roles for bFGF, HGF, and SOX9 in regeneration of renal tubules following RIRI [39]. The overexpression of these regeneration markers was also induced following treatment with Exo or MSCs [26]. Herein, we determined the change in the relative protein expression of these regeneration markers in RIRI kidney after treatment with Exo or MSCs using western blot. The expression of bFGF, HGF, and SOX9 proteins were very weak in control and sham kidneys and RIRI induced a slight significant increase of these proteins in kidneys, however, MSCs or Exo treatment significantly upregulated the expression of these proteins, with highest expression in Exo + Mel group, suggested that MSCs or Exo treatment activated tubular bFGF, HGF, SOX9 expression (Figure 6). In addition, we also investigated whether MSCs and their Exo can mitigate RIRI through modulation of angiogenesis. Indeed, administration of MSCs or Exo significantly increased the mRNA levels of the angiogenesis-related gene (VEGF), with best effect in Exo + Mel group, as compared to RIRI group which showed lower expression than that of the control and sham groups (Figure 6). Again the most prominent effect was noticed at 4 weeks post-ischemia. Thus, MSCs or Exo could exert its ameliorative effect against RIRI through induction of regeneration and angiogenesis in kidney, with better effect for Exo derived from MSCs preconditioned with Mel.
Figure 6.
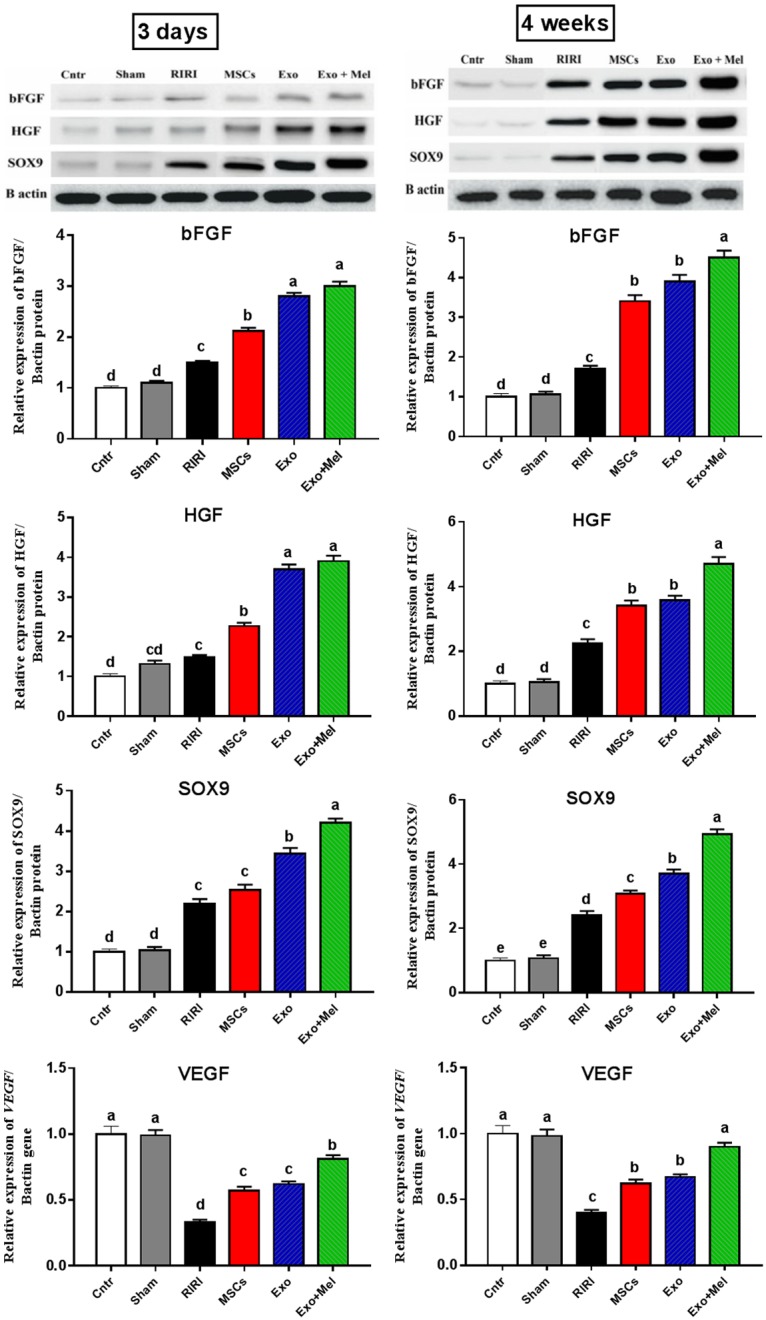
Effect of MSCs or exosomes treatment on regeneration (bFGF, HGF, and SOX9 proteins) and angiogenesis (VEGF gene). Data presented as fold change (mean) ± SEM from the control group. Columns carrying different letters are significantly different at P < 0.05.
Discussion
To the best of my knowledge, this may be the first study to report that administration of exosomes derived from MSCs preconditioned with melatonin (Exo + Mel group) give better ameliorative effect against renal ischemic reperfusion injury (RIRI), induced by bilateral clamping of renal arteries, than using MSCs or their exosomes without precondition. Our overall conclusion was based on the following evidences that favor the best effect of Exo + Mel group over that of other treated groups: 1) restored renal function as indicated by reduced blood levels of kidney damage markers BUN and creatinine; 2) declined oxidative stress as revealed by decreased MDA content and HIF1α mRNA level, and NOX2 protein expression; 3) increased anti-oxidants status as indicated by higher activities of SOD, CAT, GPX and upregulated HO1 gene expression; 4) decreased apoptosis as evidenced by decreased activity and gene expression of caspase 3, and declined mRNA levels of PARP1, Bax and increased mRNA level of anti-apoptotic marker Bcl2; 5) inhibited inflammation as showed by decreased MPO activity and mRNA levels of ICAM1, IL1β, NFκB and increased IL10 mRNA level; 6) increased tissue repair and regeneration as noticed by decreased kidney injury histopathological score; improved expression of regeneration-related proteins bFGF, HGF and SOX9; and 7) enhanced angiogenesis as judged by increased expression of angiogenesis-related VEGF gene.
Several previous studies have investigated the protective effects of autologous and heterologous MSCs-derived Exo on in vivo models of RIRI and found not only a potential ameliorative effect for these exosomes on RIRI-induced kidney damage but also a subsequent enhancement in tissue regeneration and renal function [1,24-27,40]. However, none of these studies have investigated the effect of Exo derived from MSCs preconditioned with Mel. To date, only one study reported a beneficial effect for combined therapy of Mel and MSCs-derived exosomes on acute liver ischemic injury [29]. Again, this previous study did not report the effect of Exo derived from MSCs preconditioned with Mel. This prompts us to conduct this study to compare between the effect of MSCs alone and Exo derived from either MEl preconditioned or non-preconditioned MSCs on RIRI. Indeed, we found a superior ameliorative effect for Mel preconditioned MSCs-derived Exo on RIRI. Importantly, the obtained data revealed that the disrupted renal function and structure in RIRI rats were significantly restored following MSCs treatment, significantly more restored with non-preconditioned MSCs-derived Exo treatment, and most significantly preserved with Mel preconditioned MSCs-derived Exo treatment.
It is well known that organ ischemic injury and the resulting necrosis have the potential to activate a prompt immune response which further induce inflammatory reaction, and release of free radicals, which ultimately lead to subsequent notable organ damage [41]. Exo+Mel reno-protective effect involved five possible mechanisms of action targeting oxidative stress, apoptosis, and inflammation and modulating regeneration and angiogenesis. The first possible mechanism of action by which Exo+Mel could attenuate RIRI is the inhibition of oxidative stress and induction of antioxidant status. In the present study, administration of Mel preconditioned MSCs-derived exosomes mitigated renal oxidative stress and damage by decreasing MDA content and HIF1α mRNA level, and NOX2 protein expression and increasing anti-oxidants status by inducing activities of SOD, CAT, GPX and upregulating HO1 gene expression in a way much better than MSCs or exosomes from non-preconditioned MSCs. Attenuation of renal oxidative stress by these preconditioned exosomes was compatible with improved renal function (as indicated by reduced serum creatinine and BUN) and enhanced renal structure (lowest histopathology score) after the ischemic insult. In agreement, previous studies showed similar reactive oxygen species (ROS) inhibitory effect for MSCs-derived exosomes on RIRI [42,43].
The second possible mechanism which may be targeted by Exo+Mel to relive RIRI is the apoptosis. We found that administration of Mel preconditioned MSCs-derived Exo significantly mitigated apoptosis by decreasing activity and gene expression of caspase 3, and mRNA levels of PARP1, Bax and by increasing gene expression of the anti-apoptotic marker, Bcl2 in kidneys of RIRI in a way much better than treatment with either MSCs or non-preconditioned-derived Exo. Similar anti-apoptotic effect was reported for MSCs-derived exosomes against RIRI [27,44], cisplatin-induced AKI [45,46], and glycerol-induced AKI [47] in rodents. Again, this anti-apoptotic effect was also associated with, and could explain, the enhanced tubular epithelial cells proliferation and improved renal function and structure.
The third possible mechanism which may be targeted by Exo+Mel to relive RIRI is the inflammation. Inflammatory cytokines play a pivotal role in RIRI and so previous studies evoked some tactics to inhibit inflammation through targeting macrophages and pro-inflammatory cytokines to exert reno-protection against RIRI and AKI [48]. In the present study, administration of MSCs-derived exosomes inhibited inflammation as indicated by decreased MPO level and ICAM1, IL1β, NFκB mRNA levels and increased IL10 mRNA level in kidney of RIRI rats. Consistent with our findings, several previous studies found severe depletion of macrophages, decreased expression of the pro-inflammatory cytokines IL 1β, IL6 and increased expression of the anti-inflammatory cytokines IL10 in RIRI kidney following administration of MSCs-derived exosomes, with best effect when MSCs and their exosomes co-administrated [30,44,49]. This ameliorative effect for exosomes is unlikely to be specific for RIRI only, as it also works against other models of AKI including those chemically induced. In rodents with gentamycin- or glycerol-induced AKI, administration of exosomes associated with downregulation of IL6 and TNFα, and upregulation of IL10 [50,51]. Thus, our results and those of the previous studies proved that MSC-Exo possessed a therapeutic potential on RIRI, with better effect for Mel preconditioned MSCs-derived Exo, possibly through amelioration of inflammation.
The fourth possible mechanism by which Exo+Mel may mitigate RIRI is the induction of tissue regeneration. It is well known that the transcription factor Sox9 plays a pivotal role in the development of kidney since its disruption can cause severe renal dysplasia [52]. Moreover, it was reported that the ameliorative effect of MSCs and Exo on AKI is mediated through upregulation of Sox9 expression in renal tubular cells [26]. In agreement, we also found a significant upregulation in SOX9 protein expression in kidney of RIRI group treated with MSCs or Exo with best effect for Exo + Mel group. We also found a significant higher expression of bFGF and HGF proteins in kidney of RIRI following treatment with Exo. Similarly, administration of MSCs-derived Exo also increased gene and protein expression of the bFGF and HGF in kidney of RIRI rats, thereby improving renal tissue regeneration and decreased tubular fibrosis [53,54].
The fifth possible mechanism of action by which Exo+Mel could exert reno-protection against RIRI is induction of angiogenesis. Treatment of RIRI rats with exosomes derived from Mel pre-conditioned MSCs showed a significantly higher expression of the angiogenesis-related marker VEGF than treatment with MSCs or exosomes from non-preconditioned MSCs. Consistent with our data, injection of exosomes derived from either umbilical cord MSCs or kidney resident MSCs in rats with RIRI increased renal capillary density by inducing the expression of several proangiogenic factors, such as VEGF and IGF1 [54,55], thereby leading to improved renal function and suggesting that these proangiogenic factors may be involved in exosomes-induced renal repair.
The most important question is why the best improvement effect was found when injecting Exo derived from MSCs preconditioned with Mel rather than when giving either MSCs or exosomes from non-preconditioned MSCs. Before answering this question, we have to know the importance of preconditioning treatment strategy. It is well known that Mel induces proliferation of MSCs through binding to MT1 and MT2 receptors [56,57]. Another important thing is that most of beneficial effect of MSCs is mediated by their exosomes which contain miRNA, mRNA and LncRNA. These exosomal nucleic acid cargoes are responsible for cell-to-cell communication through which RNA-based information transferred to recipient cells [58]. Interestingly, the ameliorative effect of Exo on RIRI was eliminated when exosomes were pre-treated with RNase, implying the importance of exosomal RNA cargo transfer in triggering renal repair [55]. Similarly, injection of exosomes lacked IL10 into RIRI rats restricted exosomal renal repair competence, indicating mediation of exosomal antioxidant effect via exosomal IL10 [49]. Moreover, Lindoso, et al. [28] reported that the ameliorative effect of MSCs-derived exosomes on RIRI and metabolic syndrome was associated with decreased expression of some miRNAs involved in apoptosis and hypoxia, implying that exosomes can mediate their ameliorative effect by post-transcriptional targeting for some genes in receipt cells through exosomal miRNAs. Moreover, it is well known that exosomes have different subsets of miRNAs dependent on the cell type from which they are originated [23]. Even within the same population of cells, the exosomal miRNAs may differ depending on the physiological status and heterogeneity of MSCs and method of exosomes isolation [59]. Hence, we would speculate that Mel preconditioning may induce MSCs to produce exosomes with higher expression of beneficial RNA cargoes that when transfer to the recipient cells (renal tissues of RIRI in that case), they induce renal repair through inhibition of certain molecules involved in oxidative stress, apoptosis, and inflammation. Thus, this preconditioning treatment strategy maximizes the therapeutic outputs of exosomes compared with the non-preconditioning strategy. However, we and most of other studies did not determine the specific downstream target molecules in kidney for these exosomal RNA cargoes. Therefore, further gain and loss of function experiments are required to specifically determine the downstream targets for exosomal cargoes and the downstream targets regulated by melatonin in MSCs and their exosomes.
The present study showed some limitations. First, although extensive work was performed in the current study, the actual underlying mechanisms of Exo reno-protective effect for RIRI remain unclear. However, the obtained data suggested presence of many factors, but not a single factor, modulating this reno-protective effect. Second, using only a single dose regimen treatment is doubtful as this dose may be insufficient to get a sustained effect, thereby limiting their efficacy for renal repair, although the present study supported by previous studies [24,35,51,54] showed that a single dose of MSCs-exosomes can relieve RIRI/AKI in rats. This may be attributed to targeting renal tissues macrophages by the majority of injected exosomes which further transfer them from kidney to circulation and other remote off target organs [60]. A previous study reported that injection of multiple doses of exosomes give better ameliorative effect on RIRI than single dose [46]. To date, no available published data investigated the in vivo effect of gradually increased doses of exosomes on RIRI/AKI or chronic renal diseases. Therefore, future investigation are required to unveil a standard treatment strategy for exosomes to adequately indicate number of doses injections and the interval between them.
Despite their competence to relive RIRI and induce renal cells regeneration, some challenges should be addressed before considering MSC-derived exosomes as effective therapeutic tool for AKI patients. Among these challenges are lacking of data to ascertain their safety and practical limitation for production of large number of exosomes from MSCs, for identifying the most appropriate cellular source for exosomes (exosomes heterogeneity may lead to different actions on their recipient cells), and for choosing the most suitable isolation method without altering their RNA and protein contents. Until these challenges could be resolved, exosomes based therapy for RIRI/AKI should be carefully done. Therefore, future preclinical and clinical trials are required to elucidate these uncertainties and overwhelm the challenges accompanied exosome therapy in RIRI/AKI patients.
Conclusion
The present study reported for the first time that administration of exosomes derived from MSCs preconditioned with melatonin gave better therapeutic effect on renal ischemia-reperfusion induced injury than treatment with MSCs or exosomes from non-preconditioned MSCs. This exosomal ameliorative effect mediated, at least in part, through inhibition oxidative stress, apoptosis and inflammation and induction of antioxidant status, regeneration and angiogenesis, thereby leading to improved renal repair and function. This preconditioned treatment strategy using exosomes could also be useful in therapy of RIRI/AKI patients. However, future clinical studies are required to assure whether this treatment strategy is safe and clinically relevant to patients.
Acknowledgements
This work was supported by the Deanship of Scientific of Research (DSR), King Abdulaziz University, Jeddah, under grant no. (D-183-662-1439). The author therefore, gratefully acknowledges the DSR technical and financial support. The author would like also to acknowledge Dr. Ayman Saleh and his lab team; for careful reading and efforts towards improving the manuscript. I also would like to thank Dr. Alaa Ghazy for helping in animal surgery.
Disclosure of conflict of interest
None.
References
- 1.Aghajani Nargesi A, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther. 2017;8:273. doi: 10.1186/s13287-017-0727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zager RA, Johnson AC, Lund S. Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury. Am J Physiol Renal Physiol. 2009;297:F961–70. doi: 10.1152/ajprenal.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams P, Lopez H, Britt D, Chan C, Ezrin A, Hottendorf R. Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods. 1997;37:1–7. doi: 10.1016/s1056-8719(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 4.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 5.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–35. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 6.Maurel A, Azarnoush K, Sabbah L, Vignier N, Le Lorc’h M, Mandet C, Bissery A, Garcin I, Carrion C, Fiszman M, Bruneval P, Hagege A, Carpentier A, Vilquin JT, Menasche P. Can cold or heat shock improve skeletal myoblast engraftment in infarcted myocardium? Transplantation. 2005;80:660–665. doi: 10.1097/01.tp.0000172178.35488.31. [DOI] [PubMed] [Google Scholar]
- 7.Alzahrani F, Saadeldin I, Alkarim S. Ameliorative effect of mesenchymal stem cells-derived exosomes on diethylnitrosamine-induced liver injury in albino rats. International Journal of Pharmacology. 2018;14:1128–1135. [Google Scholar]
- 8.Suzuki K, Murtuza B, Beauchamp JR, Smolenski RT, Varela-Carver A, Fukushima S, Coppen SR, Partridge TA, Yacoub MH. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 2004;18:1153–5. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- 9.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–50. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 10.Han D, Huang W, Li X, Gao L, Su T, Li X, Ma S, Liu T, Li C, Chen J, Gao E, Cao F. Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the sirt1 signaling pathway. J Pineal Res. 2016;60:178–192. doi: 10.1111/jpi.12299. [DOI] [PubMed] [Google Scholar]
- 11.Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, Daniel L, Bianchi P, Calise D, Bourin P, Parini A, Cussac D. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749–57. doi: 10.1634/stemcells.2007-1000. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57:131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 13.Sun CK, Lee FY, Kao YH, Chiang HJ, Sung PH, Tsai TH, Lin YC, Leu S, Wu YC, Lu HI, Chen YL, Chung SY, Su HL, Yip HK. Systemic combined melatonin-mitochondria treatment improves acute respiratory distress syndrome in the rat. J Pineal Res. 2015;58:137–150. doi: 10.1111/jpi.12199. [DOI] [PubMed] [Google Scholar]
- 14.Bandyopadhyay D, Chattopadhyay A. Reactive oxygen species-induced gastric ulceration: protection by melatonin. Curr Med Chem. 2006;13:1187–202. doi: 10.2174/092986706776360842. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadiasl N, Banaei S, Alihemmati A, Baradaran B, Azimian E. The anti-inflammatory effect of erythropoietin and melatonin on renal ischemia reperfusion injury in male rats. Adv Pharm Bull. 2014;4:49–54. doi: 10.5681/apb.2014.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortezaee K, Khanlarkhani N, Sabbaghziarani F, Nekoonam S, Majidpoor J, Hosseini A, Pasbakhsh P, Kashani IR, Zendedel A. Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by ccl4. Cell Tissue Res. 2017;369:303–312. doi: 10.1007/s00441-017-2604-1. [DOI] [PubMed] [Google Scholar]
- 17.Cho YA, Noh K, Jue SS, Lee SY, Kim EC. Melatonin promotes hepatic differentiation of human dental pulp stem cells: clinical implications for the prevention of liver fibrosis. J Pineal Res. 2015;58:127–135. doi: 10.1111/jpi.12198. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Cai B, Yuan F, He X, Lin X, Wang J, Wang Y, Yang GY. Melatonin pretreatment improves the survival and function of transplanted mesenchymal stem cells after focal cerebral ischemia. Cell Transplant. 2014;23:1279–1291. doi: 10.3727/096368913x667510. [DOI] [PubMed] [Google Scholar]
- 19.Yip HK, Chang YC, Wallace CG, Chang LT, Tsai TH, Chen YL, Chang HW, Leu S, Zhen YY, Tsai CY, Yeh KH, Sun CK, Yen CH. Melatonin treatment mproves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J Pineal Res. 2013;54:207–221. doi: 10.1111/jpi.12020. [DOI] [PubMed] [Google Scholar]
- 20.Chang CL, Sung PH, Sun CK, Chen CH, Chiang HJ, Huang TH, Chen YL, Zhen YY, Chai HT, Chung SY, Tong MS, Chang HW, Chen HH, Yip HK. Protective effect of melatonin-supported adipose-derived mesenchymal stem cells against small bowel ischemia-reperfusion injury in rat. J Pineal Res. 2015;59:206–220. doi: 10.1111/jpi.12251. [DOI] [PubMed] [Google Scholar]
- 21.Gamez-Valero A, Lozano-Ramos SI, Bancu I, Lauzurica-Valdemoros R, Borras FE. Urinary extracellular vesicles as source of biomarkers in kidney diseases. Front Immunol. 2015;6:6. doi: 10.3389/fimmu.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badawy AA, El-Magd MA, AlSadrah SA. Therapeutic effect of camel milk and its exosomes on mcf7 cells in vitro and in vivo. Integr Cancer Ther. 2018;17:1235–1246. doi: 10.1177/1534735418786000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alzahrani FA, El-Magd MA, Abdelfattah-Hassan A, Saleh AA, Saadeldin IM, El-Shetry ES, Badawy AA, Alkarim S. Potential effect of exosomes derived from cancer stem cells and mscs on progression of den-induced hcc in rats. Stem Cells Int. 2018;2018:8058979. doi: 10.1155/2018/8058979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez JM 2nd, Dominguez JH, Xie D, Kelly KJ. Human extracellular microvesicles from renal tubules reverse kidney ischemia-reperfusion injury in rats. PLoS One. 2018;13:e0202550. doi: 10.1371/journal.pone.0202550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez JH, Liu Y, Gao H, Dominguez JM 2nd, Xie D, Kelly KJ. Renal tubular cell-derived extracellular vesicles accelerate the recovery of established renal ischemia reperfusion injury. J Am Soc Nephrol. 2017;28:3533–3544. doi: 10.1681/ASN.2016121278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu F, Chong Lee Shin OLS, Pei G, Hu Z, Yang J, Zhu H, Wang M, Mou J, Sun J, Wang Y, Yang Q, Zhao Z, Xu H, Gao H, Yao W, Luo X, Liao W, Xu G, Zeng R, Yao Y. Adipose-derived mesenchymal stem cells employed exosomes to attenuate aki-ckd transition through tubular epithelial cell dependent sox9 activation. Oncotarget. 2017;8:70707–70726. doi: 10.18632/oncotarget.19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–83. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 28.Lindoso RS, Collino F, Bruno S, Araujo DS, Sant’Anna JF, Tetta C, Provero P, Quesenberry PJ, Vieyra A, Einicker-Lamas M, Camussi G. Extracellular vesicles released from mesenchymal stromal cells modulate mirna in renal tubular cells and inhibit atp depletion injury. Stem Cells Dev. 2014;23:1809–19. doi: 10.1089/scd.2013.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun CK, Chen CH, Chang CL, Chiang HJ, Sung PH, Chen KH, Chen YL, Chen SY, Kao GS, Chang HW, Lee MS, Yip HK. Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am J Transl Res. 2017;9:1543–1560. [PMC free article] [PubMed] [Google Scholar]
- 30.Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, Chai HT, Chang CL, Chen CH, Lee MS. Combination of adipose-derived mesenchymal stem cells (admsc) and admsc-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–85. doi: 10.1016/j.ijcard.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 31.Rafat A, Mohammadi Roushandeh A, Alizadeh A, Hashemi-Firouzi N, Golipoor Z. Comparison of the melatonin preconditioning efficacy between bone marrow and adipose-derived mesenchymal stem cells. Cell J. 2019;20:450–458. doi: 10.22074/cellj.2019.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdelhadya DH, El-Magd MA, Elbialy ZI, Saleh AA. Bromuconazole-induced hepatotoxicity is accompanied by upregulation of pxr/cyp3a1 and downregulation of car/cyp2b1 gene expression. Toxicol Mech Methods. 2017;27:544–550. doi: 10.1080/15376516.2017.1333555. [DOI] [PubMed] [Google Scholar]
- 33.El-Magd MA, Khalifa SF, A Alzahrani FA, Badawy AA, El-Shetry ES, Dawood LM, Alruwaili MM, Alrawaili HA, Risha EF, El-Taweel FM, Marei HE. Incensole acetate prevents beta-amyloid-induced neurotoxicity in human olfactory bulb neural stem cells. Biomed Pharmacother. 2018;105:813–823. doi: 10.1016/j.biopha.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 34.El-Magd MA, Abdo WS, El-Maddaway M, Nasr NM, Gaber RA, El-Shetry ES, Saleh AA, Alzahrani FAA, Abdelhady DH. High doses of s-methylcysteine cause hypoxia-induced cardiomyocyte apoptosis accompanied by engulfment of mitochondaria by nucleus. Biomed Pharmacother. 2017;94:589–597. doi: 10.1016/j.biopha.2017.07.100. [DOI] [PubMed] [Google Scholar]
- 35.Chang CL, Sung PH, Chen KH, Shao PL, Yang CC, Cheng BC, Lin KC, Chen CH, Chai HT, Chang HW, Yip HK, Chen HH. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am J Transl Res. 2018;10:1053–1070. [PMC free article] [PubMed] [Google Scholar]
- 36.El-Magd MA, Abbas HE, El-kattawy AM, Mokhbatly A. Novel polymorphisms of the igf1r gene and their association with average daily gain in egyptian buffalo (bubalus bubalis) Domest Anim Endocrinol. 2013;45:105–10. doi: 10.1016/j.domaniend.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Elkeiy MM, Khamis AA, El-Gamal MM, Abo Gazia MM, Zalat ZA, El-Magd MA. Chitosan nanoparticles from artemia salina inhibit progression of hepatocellular carcinoma in vitro and in vivo. Environ Sci Pollut Res Int. 2018 doi: 10.1007/s11356-018-3339-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.El-Magd MA, Khamis A, Nasr Eldeen SK, Ibrahim WM, Salama AF. Trehalose enhances the antitumor potential of methotrexate against mice bearing ehrlich ascites carcinoma. Biomed Pharmacother. 2017;92:870–878. doi: 10.1016/j.biopha.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Little MH, Kairath P. Does renal repair recapitulate kidney development? J Am Soc Nephrol. 2017;28:34–46. doi: 10.1681/ASN.2016070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Lin M, Li L, Li L, Qi G, Rong R, Xu M, Zhu T. Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats. Zhonghua Yi Xue Za Zhi. 2014;94:3298–303. [PubMed] [Google Scholar]
- 41.Chen HH, Lin KC, Wallace CG, Chen YT, Yang CC, Leu S, Chen YC, Sun CK, Tsai TH, Chen YL, Chung SY, Chang CL, Yip HK. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J Pineal Res. 2014;57:16–32. doi: 10.1111/jpi.12140. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Zou X, Huang Y, Wang F, Miao S, Liu G, Chen M, Zhu Y. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing nrf2/are activation in rats. Kidney Blood Press Res. 2016;41:119–28. doi: 10.1159/000443413. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G, Zou X, Miao S, Chen J, Du T, Zhong L, Ju G, Liu G, Zhu Y. The anti-oxidative role of micro-vesicles derived from human wharton-jelly mesenchymal stromal cells through nox2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS One. 2014;9:e92129. doi: 10.1371/journal.pone.0092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y. Microvesicles derived from human wharton’s jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing cx3cl1. Stem Cell Res Ther. 2014;5:40. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Soares Lindoso R, Neri F, Kholia S, Giunti S, Wen S, Quesenberry P, Camussi G. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A. 2017;23:1262–1273. doi: 10.1089/ten.tea.2017.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinuesa E, Hotter G, Jung M, Herrero-Fresneda I, Torras J, Sola A. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J Pathol. 2008;214:104–13. doi: 10.1002/path.2259. [DOI] [PubMed] [Google Scholar]
- 49.Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114–124. doi: 10.1016/j.kint.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ, Camussi G. Aki recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying micrornas. J Am Soc Nephrol. 2015;26:2349–60. doi: 10.1681/ASN.2014070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar S, Liu J, Pang P, Krautzberger AM, Reginensi A, Akiyama H, Schedl A, Humphreys BD, McMahon AP. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Rep. 2015;12:1325–38. doi: 10.1016/j.celrep.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 53.Ju GQ, Cheng J, Zhong L, Wu S, Zou XY, Zhang GY, Gu D, Miao S, Zhu YJ, Sun J, Du T. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS One. 2015;10:e0121534. doi: 10.1371/journal.pone.0121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi HY, Moon SJ, Ratliff BB, Ahn SH, Jung A, Lee M, Lee S, Lim BJ, Kim BS, Plotkin MD, Ha SK, Park HC. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS One. 2014;9:e87853. doi: 10.1371/journal.pone.0087853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G, Zhu Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res. 2016;8:4289–4299. [PMC free article] [PubMed] [Google Scholar]
- 56.Luchetti F, Canonico B, Bartolini D, Arcangeletti M, Ciffolilli S, Murdolo G, Piroddi M, Papa S, Reiter RJ, Galli F. Melatonin regulates mesenchymal stem cell differentiation: a review. J Pineal Res. 2014;56:382–397. doi: 10.1111/jpi.12133. [DOI] [PubMed] [Google Scholar]
- 57.Mortezaee K, Pasbakhsh P, Ragerdi Kashani I, Sabbaghziarani F, Omidi A, Zendedel A, Ghasemi S, Dehpour AR. Melatonin pretreatment enhances the homing of bone marrow-derived mesenchymal stem cells following transplantation in a rat model of liver fibrosis. Iran Biomed J. 2016;20:207–216. doi: 10.7508/ibj.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quesenberry PJ, Dooner MS, Aliotta JM. Stem cell plasticity revisited: the continuum marrow model and phenotypic changes mediated by microvesicles. Exp Hematol. 2010;38:581–92. doi: 10.1016/j.exphem.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, Quesenberry P, Camussi G. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev. 2017;13:226–243. doi: 10.1007/s12015-016-9713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, Matsumoto A, Charoenviriyakul C, Takakura Y. Macrophage-dependent clearance of systemically administered b16bl6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238. doi: 10.3402/jev.v4.26238. [DOI] [PMC free article] [PubMed] [Google Scholar]