Abstract
Peripheral neurotoxicity is a common adverse reaction in cancer patients undergoing chemotherapy. The neuropathologic changes were partly associated with mitochondrial dysfunction and autophagy. Tanshinone IIA, a compound extracted from the medicinal herb Salvia miltiorrhiza, has been shown to exhibit neuroprotective effects. The present study investigated the effects of tanshinone IIA on chemotherapy-induced neurotoxicity and to study the underlying mechanism. Neuroma cell line N2a and rats were treated with oxaliplatin and/or tanshinone IIA. The effects on neurotoxicity were evaluated using cell viability assay, flow cytometry detection of apoptosis, measurement of intracellular reactive oxygen species (ROS) and mitochondrial membrane potential (Ψm), autophagy detection, nerve function assessment, and behavior assessment. The results showed that tanshinone IIA prevented oxaliplatin-induced inhibition of cell viability and reduced apoptosis. Tanshinone IIA also prevented excessive oxidative stress, as demonstrated by decreased ROS levels and reduced Ψm loss. Lastly, treatment with tanshinone IIA promoted autophagy through the PI3K/Akt/mTOR signaling pathway. The in vivo experiment showed that tanshinone IIA ameliorated oxaliplatin-induced allodynia and sciatic nerve dysfunction. An increase in serum nerve growth factor level was observed. In conclusion, the results of the study suggested a protective role of tanshinone IIA in neurotoxicity induced by oxaliplatin via mitochondrial protection and autophagy promotion.
Keywords: Autophagy, chemotherapy, neurotoxicity, tanshinone IIA
Introduction
Chemotherapy-induced peripheral neuropathy is a common adverse effect of several chemotherapeutics such as oxaliplatin [1]; approximately 90% of patients who received oxaliplatin developed peripheral neurotoxicity [2-4]. The main clinical manifestations of peripheral neurotoxicity include numbness of limbs and loss of peripheral sensation accompanied by painful spasms [5]. The neuropathologic changes were associated with the accumulation of platinum product in the dorsal root ganglion, but the underlying mechanism remains to be elucidated [6]. The symptoms of peripheral neurotoxicity could impact on patient’s physical, psychological, and social life, and treatment may be discontinued in some circumstances [7]. A treatment strategy that alleviates the neurotoxicity of oxaliplatin is of great clinical importance.
Tanshinone IIA (molecular formula: C19H18O3; molecular weight: 294.34) is a compound extracted from the plant Salvia miltiorrhiz-a well-known herbal medicine used in China. Studies showed that tanshinone IIA exhibits various physiological effects such anti-cancer activities, antioxidant effects, and anti-inflammatory activities [8-11]. Tanshinone IIA also has a neuroprotective effect. In a classical Alzheimer’s disease-like mouse model, tanshinone IIA improved the learning and memory ability of mice. Further analysis showed a reduction in reactive oxygen species (ROS) in the hippocampus and cortex. Tanshinone IIA significantly induced mitochondrial protection through upregulation of antioxidant enzymes [12]. Tanshinone IIA also appeared to promote apoptosis through beclin-1-dependent autophagy in osteosarcoma and oral squamous cell carcinoma [10,13]. Indeed, accumulating evidence has suggested a crosstalk between autophagy and mitochondrial function [14,15]. To date, little is known on the role of tanshinone IIA on mitochondrial function and autophagy in the context of oxaliplatin-mediated neuropathy. Clinically, patients with malignant tumor benefited from chemotherapy with oxaliplatin and tanshinone IIA. Reduced incidences of oxaliplatin-induced peripheral neuropathy were observed in the tanshinone IIA group (27.8%) compared to the control (55.6%) [16]. In the present study, we investigated the mechanisms underlying the neuroprotective role of tanshinone IIA in oxaliplatin-induced neurotoxicity.
Materials and methods
In vitro assays
Cell culture and reagents
Mouse brain neuroma cell line N2a was purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in minimum essential medium (MEM), containing 10% fetal bovine serum and 1% streptomycin/penicillin, under 5% CO2 environment at 37°C. Oxaliplatin and tanshinone IIA were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell counting kit 8 (CCK8) cell viability test kit, JC1 (3,3’-diethyloxacarbocyanine iodide), and DCFDA (2’7’-dichlorofluorescin diacetate) assay kits were obtained from Beyotime (China). Tissue protein extraction reagents and immunofluorescence detection reagents were purchased from Boster (Wuhan, China).
CCK8 cell viability assays
N2a cells were seeded in a 96-well plate at a density of 1 × 105 cells per well. Different concentrations of oxaliplatin (0, 0.1, 0.2, 0.5, and 1.2 μM) and tanshinone IIA (0, 1, 2, 5, 10, and 20 μM) were used to stimulate N2a cells. The effect of 20 μM oxaliplatin and 5 μM tanshinone on cell viability was also evaluated. After 24 h of stimulation, CCK8 solution (10 μL) was added to each well containing 100 μL medium. The plate was incubated at 37°C for 90 min protected from light, and the optical density value was measured at a wavelength of 450 nm using a microplate reader. The cell viability in each group was determined relative to the control group.
Immunofluorescence detection of autophagy
N2a cells were seeded in a 24-well plate at a density of 1 × 105 cells per well and stimulated with appropriate concentrations of oxaliplatin and/or tanshinone IIA. After 24 h, the medium was replenished with 250 μL of Autophagy Green working solution in accordance to the manual of Cell Meter Autophagy Fluorescence Imaging Kit (AAT Bioquest, Sunnyvale, CA, USA). The cells were then incubated under 5% CO2 at 37°C for 30 min in the dark. After wash, the expression of autophagy was detected under a fluorescence microscope and the differences between groups were analyzed.
JC1 mitochondrial membrane potential
N2a cells were seeded in a 24-well plate at a density of 1 × 105 per well with appropriate concentrations of oxaliplatin and/or tanshinone IIA. After 24 h, the JC1 working solution was prepared according to the manufacturer’s instruction, 250 μL of culture medium and 250 μL JC1 working solution were added to each well, and the cells were incubated under 5% CO2 at 37°C for 20 min in the dark. The cells were then washed twice with staining buffer, and 250 μL fresh medium was added. The cells were observed under a fluorescence microscope, and the results were analyzed using a flow cytometer.
DCFDA cellular reactive oxygen species (ROS) detection
N2a cells were seeded in a 24-well plate at 1 × 105 cells per well with appropriate concentrations of drugs. After drug treatment, the medium was replaced with 500 μL DCFDA working solution, followed by 20 min incubation at 37°C. After wash, cells were observed under a fluorescence microscope, and a flow cytometer was used to measure ROS production.
Annexin V for apoptosis
N2a cells were seeded in a 6-well plate at a density of 1 × 105 cells per well. After drug treatment for 24 h, the cells were harvested and washed with phosphate-buffered saline. Then, the cells were labeled with annexin V (incubated at 37°C for 30 min) and analyzed by a flow cytometer.
In vivo assays
All animal treatments and experiments were approved by the Institutional Animal Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Grouping and modeling
Forty male Sprague-Dawley (SD) rats, weighing 200-250 g, were randomly divided into four groups. All the rats had access to food and water ad libitum. The ambient temperature was kept at 23 ± 1°C, and a 12:12-h dark and light cycle was maintained. After acclimatization for 1 week, rats in the control group received an intraperitoneal injection of 5% glucose, while rats in the oxaliplatin group received an oxaliplatin injection 20 mg/(kg × day) (diluted in 5% glucose) for 7 days, and rats in the tanshinone group received an injection with tanshinone IIA 25 mg/(kg × day) (diluted in 5% glucose). Rats in the tanshinone IIA plus oxaliplatin group first received a tanshinone IIA injection for 3 days, and then both drugs were injected for additional 7 days.
Mechanical threshold detection
The mechanical threshold was determined before drug injection and 1, 3, and 7 days after drug injection. During examination, the rats were first allowed to adapt to the environment for 15 min. Then, the von Frey fibers were used to stimulate the middle part of the hind paws until the fibers were bent slightly for 6-8 s. A sudden withdrawal during the stimulation period with a paw licking sign was defined as a positive response, and the paw withdrawal caused by other physical activities was excluded. The test was conducted three times at a 5-min interval, and the average pressure recorded by the von Frey fibers was defined as the mechanical threshold.
Electrophysiological measurements
The rats were anesthetized with 2% pentobarbital (40 mg/kg), and the neurophysiologic activities of the sciatic nerve were measured using a multichannel biological signal recording system. The amplitude, latency, and conduction velocity of the sciatic nerve action potential were measured before drug injection and 6 h, and 1, 3 and 7 days after drug injection.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was used to detect the plasma level of nerve growth factor (NGF) in rats. The rats were first anesthetized with 2% pentobarbital (40 mg/kg). Blood sample was collected from the rat tail before drug injection or 6, 24, and 72 h after drug injection. ELISA was performed to detect the level of NGF in the serum samples. The measurement of absorbance was performed with a microplate reader at 450 nm wavelength.
Western blot analysis
Proteins from cells and tissues were extracted using RIPA (Radio Immunoprecipitation Assay) lysis buffer. Western blot analysis was performed as previously described [17]. Total protein (20 μg/lane) was loaded and separated using sodium dodecyl sulfate-polyacrylamide gels. The primary antibodies against the following proteins were used: autophagy-related protein LC3A/B (#12741, 1:1000, CST, MA, USA), beclin1 (#3738, 1:1000, CST, MA, USA), PI3 Kinase (#4255, 1:1000, CST, MA, USA), Akt (#4685, 1:1000, CST, MA, USA), and mTOR (#2972, 1:1000, CST, MA, USA). In addition, the rats were given a deep anesthesia after drug injection for 7 days, and the protein in the dorsal horn of the spinal cord was extracted for detection of NGF expression level.
Statistical analysis
Data were presented as mean ± standard deviation (SD), and one-way analysis of variance was used to compare mean differences among groups. A P-value < 0.05 was considered as statistically significant. SPSS 20.0 (IBM, Armonk, NY, USA) was used for statistical analysis.
Results
Tanshinone IIA prevents oxaliplatin-induced apoptosis and alleviates inhibition on cell growth
The cell viability assays showed that oxaliplatin inhibited the viability of N2a cells in a concentration-dependent manner (Figure 1A). Less than 50% of growth ratio of cells (relative to control) was observed at the highest concentration of 2 μM oxaliplatin. On the other hand, low concentrations of tanshinone IIA (1-5 μM) had a slightly positive effect on cell viability (not statistically significant), whereas high concentration of tanshinone IIA (10 μM) significantly inhibited cell growth (P < 0.05) (Figure 1B). The combination of oxaliplatin and tanshinone IIA reduced the inhibitory effect on cell viability when compared to oxaliplatin alone (Figure 1C).
Figure 1.
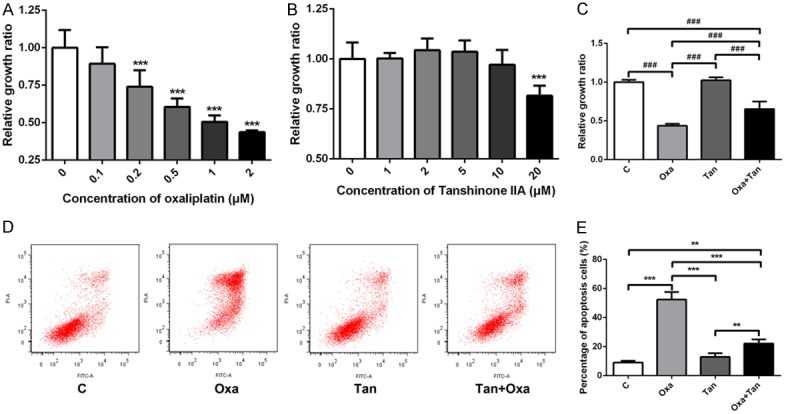
Effects of oxaliplatin and tanshinone IIA on N2a cell viability and apoptosis. Relative growth ratio after stimulation with (A) different concentrations of oxaliplatin, (B) different concentrations of tanshinone IIA, and (C) co-treatment with oxaliplatin and tanshinone IIA. ***P < 0.001 compared with 0 μM group, ###P < 0.001 compared with each other (D, E). The percentage of apoptotic cells was detected by flow cytometric analysis of annexin V stained cells. **P < 0.01, ***P < 0.001, compared with each other.
Annexin V was used to discriminate the living and apoptotic cells by flow cytometry. The results showed that oxaliplatin promoted N2a cell apoptosis in a concentration-dependent manner. Tanshinone IIA and oxaliplatin co-treated cells exhibited a lower percentage of apoptosis compared with the oxaliplatin alone group (Figure 1D and 1E).
Tanshinone IIA prevents oxaliplatin-induced oxidative stress and loss of mitochondrial membrane
The intracellular ROS level was detected by DCFDA assays to investigate the effect of oxaliplatin on oxidative stress. As shown in Figure 2A (upper panel), oxaliplatin significantly increased the intracellular ROS levels compared with the control group. However, tanshinone IIA alone had no obvious effect on ROS, but co-treatment with oxaliplatin and transhinone IIA reduced the ROS levels (Figure 2B).
Figure 2.
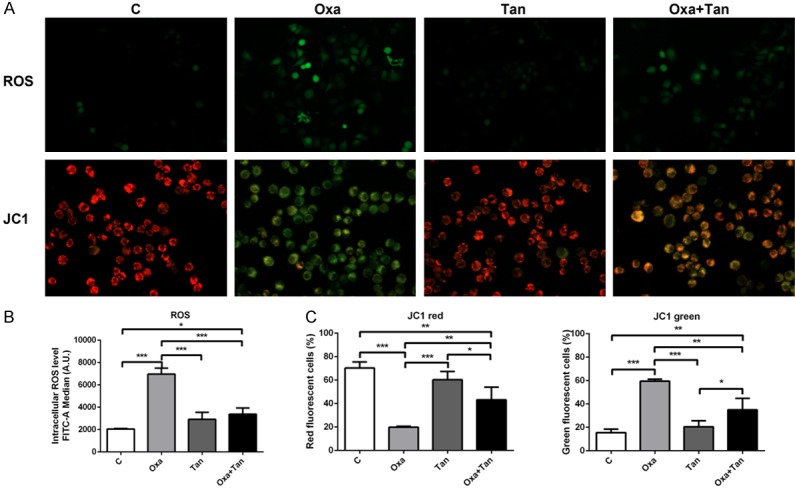
Effects of oxaliplatin and tanshinone IIA on reactive oxygen species (ROS) and mitochondrial membrane potential (Ψm) in N2a cells. A. Immunofluorescence staining shows the effects of oxaliplatin and tanshinone IIA on ROS (upper panel) and JC1 (lower panel) in cells. B. Respective graphs represent the intensity of ROS staining and the percentage of JC1 red and green cells. C. The percentage of red to orange stained cells (590 ± 17.5 nm) due to high mitochondrial membrane potential, and the percentage of green stained cells (530 ± 15 nm) due to low mitochondrial membrane potential. *P < 0.05, **P < 0.01, ***P < 0.001.
Changes in mitochondrial membrane potential (Ψm) can reflect the effect of extracellular environment on cells, and a decreased Ψm is an early indication of apoptosis. The JC1 assay was used to detect changes in Ψm (Figure 2A lower panel). The oxaliplatin group had an increase in green fluorescence intensity compared with the control, suggesting a decrease in Ψm. On the contrary, the tanshinone IIA group showed a bright red fluorescence signal, with only a low level of green signal. This was similar to the control group. The results of the combination treatment indicated that tanshinone alleviated the reduction in Ψm induced by oxaliplatin (Figure 2C).
Tanshinone IIA reduces oxaliplatin-induced neurotoxicity in rats
Allodynia is one of the neurotoxicity manifestations induced by oxaliplatin [18]. A rat model with oxaliplatin-induced neurotoxicity was successfully established. After 72 h of oxaliplatin treatment, a marked reduction in mechanical threshold was observed. The most significant drop in threshold was observed in the first 24 h, and the threshold reached a nadir at 72 h. This low level of threshold was remained until day 7 after treatment. However, rats received treatment with tanshinone IIA exhibited higher mechanical thresholds than oxaliplatin-treated rats at all the time points examined, and the differences became more apparent after 24 h. After 7 days of treatment, the threshold of the tanshinone group was comparable to the control group. Overall, the results suggested that tanshinone IIA alleviated allodynia induced by oxaliplatin (Figure 3A).
Figure 3.
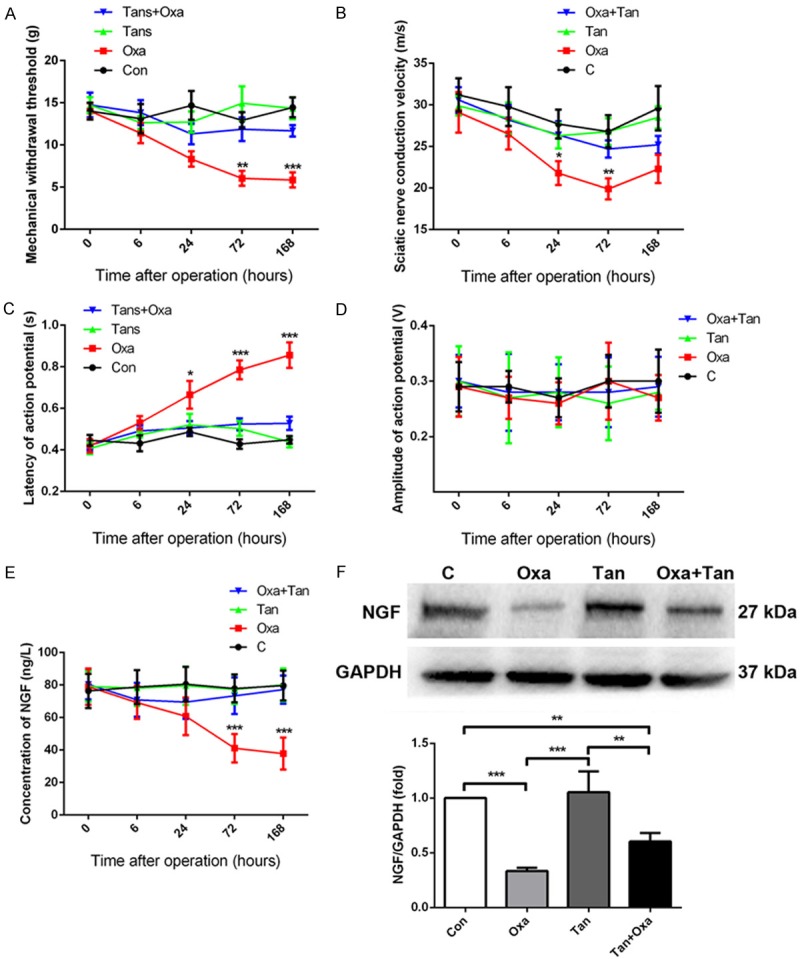
Effects of oxaliplatin and tanshinone IIA on the behavior and sciatic nerve function in rats (A-D), and the serum level of nerve growth factor (NGF) was measured (E, F). (A) Mechanical withdrawal threshold changes. (B) Effects on sciatic nerve conduction velocity. (C) Latency changes of action potential. (D) Action potential amplitude changes. *P < 0.05, **P < 0.01, ***P < 0.001, Oxa vs. Oxa + Tan. (E) The expression of NGF in serum after treatment with oxaliplatin and/or tanshinone IIA. (F) Representative Western blotting images of NGF expression in the dorsal horn of the spinal cord, and the graphical representation of the relative expression compared with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) loading control. *P < 0.05, **P < 0.01, ***P < 0.001.
Action potential is an important index to evaluate neurological function. It includes the evaluation of nerve conduction velocity, latency, and potential amplitude. We found that the conduction velocity of the sciatic nerve was significantly reduced at 24 h after oxaliplatin injection and reached the lowest velocity at 72 h. No significant effect was observed in the conduction velocity in the tanshinone IIA group. Meanwhile, oxaliplatin injection significantly prolonged the latency at 24 h, which peaked at day 7, whereas tanshinone IIA inhibited the prolongation of latency induced by oxaliplatin (Figure 3C). Both oxaliplatin and tanshinone IIA had no significant effect on the action potential amplitude of the sciatic nerve (Figure 3D).
Tanshinone IIA upregulated NGF level
The level of NGF expression was reduced significantly at 3 and 7 days after injection of oxaliplatin when compared to controls. Meanwhile, the serum level of NGF increased progressively after treatment with tanshinone IIA, and the level was comparable to the control group at day 7 (Figure 3E). In addition, Western blotting assays showed that NGF expression decreased in the dorsal horn of the spinal cord in the oxaliplatin group, but increased in the combination treatment group (Figure 3F).
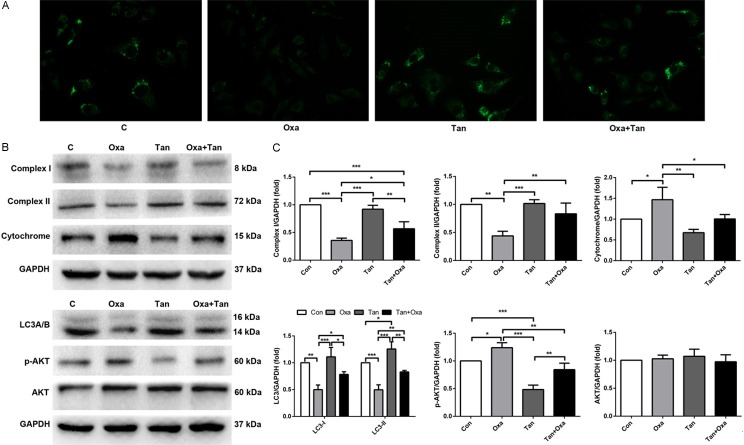
Tanshinone IIA promotes autophagy by regulatingPI3K/Akt signaling
As shown in Figure 4, oxaliplatin significantly inhibited the expression of cellular autophagy in N2a cells compared to control (LC3A/B, beclin 1). The level of autophagy was slightly upregulated in the tanshinone IIA alone group, and the addition of tanshinone IIA with oxaliplatin alleviated the inhibition on autophagy (Figure 4A). The Western blotting assays showed that a decrease in activity of mitochondrial enzyme complexes I and II was observed upon oxaliplatin treatment I. The expression level of autophagy-related protein LC3A/B and its upstream PI3K/AKT/mTOR signaling pathway were also detected. The results confirmed that oxaliplatin inhibited autophagy by promoting the phosphorylation of the PI3K/AKT/mTOR signaling pathway, and tanshinone IIA reversed the inhibitory effect of oxaliplatin on autophagy. It is likely that tanshinone IIA blocked the reduction of autophagy induced by oxaliplatin through regulating the upstream PI3K/Akt/mTOR signaling pathway, thereby blocking the neurotoxicity induced by oxaliplatin.
Figure 4.
Effects of oxaliplatin and tanshinone IIA on autophagy expression. A. The expression of autophagosome in different groups. B. Western blotting images shows the expression of mitochondrial function-related proteins (complex I, complex II, and cytochrome), autophagy-related LC3A/B, and related signaling proteins p-PI3K/PI3K, p-Akt/Akt, and p-mTOR/mTOR. C. Relative protein expression compared with GAPDH. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The present study investigated the mechanisms underlying the protective role of tanshinone IIA in the context of oxaliplatin-induced neurotoxicity. Mitochondrial dysfunction has been proposed as one of the important mechanisms underlying peripheral neuropathy [19]. Our findings showed that oxaliplatin induced ROS in N2a cells, reduced cell viability, and increased cell apoptosis. In addition, a loss in Ψm was observed, indicating a decrease in activities of mitochondrial enzyme complexes I/II and an impairment of ATP production.
A recent study demonstrated that exposure to high concentrations of oxaliplatin increased the mitochondrial ROS levels in neuronal cultures, further supporting the association of mitochondrial oxidative stress and oxaliplatin-induced peripheral neuropathy [20]. Tanshinone IIA has strong antioxidant effects [21]. Studies have shown that tanshinone IIA prevented oxidative stress-triggered atherogenesis as well as cardiac injury [22]. In the H9c2 rat myoblast cell line, pretreatment with tanshinone IIA protected cells from doxorubicin-induced injury and oxidative stress [23], which is consistent with our findings.
Autophagy, a major catabolic process for degrading proteins and organelles [24], appeared to have a dual role in nerve injury. On one hand, autophagy eliminates unwanted macromolecules and recycles components, allowing cells to adapt to the changing environment [25]. On the other hand, nerve injury may up-regulate autophagy, resulting in Schwann cell myelin breakdown [26]. In the present study, we found that LC3A/B was down-regulated upon oxaliplatin treatment, indicating suppressed autophagy, which was partly attributed to the inhibition of the PI3K/AKT/mTOR pathway. The addition of tanshinone IIA released the inhibitory effect of oxaliplatin on autophagy. It was reported that tanshinone IIA could activate PI3K/Akt/mTOR signaling, resulting in an improvement of myocardial ischemia reperfusion injury in rats [9]. The data suggested an important role of the signaling pathway in the pharmacological actions of tanshinone IIA. The fact that allodynia induced by oxaliplatin was attenuated by tanshinone IIA, a promoter of autophagy, indicated a beneficial role of autophagy in this type of neuropathy.
The protective role of tanshinone IIA on nerve tissues was further supported in animal studies. In a rat model of Alzheimer’s disease, tanshinone IIA markedly improved the symptoms caused by learning and memory disruption [27,28]. Our in vivo study showed that tanshinone IIA alleviated neurotoxicity caused by oxaliplatin, as demonstrated by the shorter latency of action potential and promotion of sciatic nerve conduction velocity. In addition, tanshinone IIA upregulated serum NGF levels. NGF plays a protective role in various neurological diseases, such as brain injury and stroke [19,29]. It has been reported that rats that received intrathecal infusion of NGF had reduced lidocaine-induced neurotoxicity in the spinal cord, likely through inhibition of neuronal apoptosis [30]. Another study showed that cisplatin impaired peripheral sensory function in adult rodents, and exogenous NGF administration restored the structural and functional changes induced by cisplatin, indicating a direct role of NGF in cisplatin-induced peripheral neuropathies [31]. The role remains to be determined of tanshinone IIA-induced NGF in the pathogenesis of neuropathy evoked by oxaliplatin treatment.
To the best of our knowledge, this is the first study to describe various neuroprotective effects of tanshinone IIA on oxaliplatin-induced neurotoxicity, including promotion of NGF release, prevention of mitochondrial dysfunction and neuronal apoptosis, as well as enhancement of autophagy. The findings provide a basis for future clinical studies investigating the role of tanshinone IIA in oxaliplatin-induced neuropathy.
Acknowledgements
The study was supported by the National Natural Science Foundation (81672652).
Disclosure of conflict of interest
None.
References
- 1.Coriat R, Alexandre J, Nicco C, Quinquis L, Benoit E, Chereau C, Lemarechal H, Mir O, Borderie D, Treluyer JM, Weill B, Coste J, Goldwasser F, Batteux F. Treatment of oxaliplatin-induced peripheral neuropathy by intravenous mangafodipir. J Clin Invest. 2014;124:262–72. doi: 10.1172/JCI68730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40:872–82. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Weickhardt A, Wells K, Messersmith W. Oxaliplatin-induced neuropathy in colorectal cancer. J Oncol. 2011;2011:201593. doi: 10.1155/2011/201593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broomand A, Jerremalm E, Yachnin J, Ehrsson H, Elinder F. Oxaliplatin neurotoxicity--no general ion channel surface-charge effect. J Negat Results Biomed. 2009;8:2. doi: 10.1186/1477-5751-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek KK, Lee J, Park SH, Park JO, Park YS, Lim HY, Kang WK, Cho YB, Yun SH, Kim HC, Lee WY, Chun HK. Oxaliplatin-induced chronic peripheral neurotoxicity: a prospective analysis in patients with colorectal cancer. Cancer Res Treat. 2010;42:185–90. doi: 10.4143/crt.2010.42.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamieson SM, Liu J, Connor B, McKeage MJ. Oxaliplatin causes selective atrophy of a subpopulation of dorsal root ganglion neurons without inducing cell loss. Cancer Chemother Pharmacol. 2005;56:391–9. doi: 10.1007/s00280-004-0953-4. [DOI] [PubMed] [Google Scholar]
- 7.Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother. 2005;39:128–35. doi: 10.1345/aph.1E319. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Han X, Zhang H, Wu J, Li B. The interplay between autophagy and apoptosis induced by tanshinone IIA in prostate cancer cells. Tumour Biol. 2016;37:7667–74. doi: 10.1007/s13277-015-4602-9. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Shen L, Wang Z, Jiang HP, Liu LX. Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2016;84:106–114. doi: 10.1016/j.biopha.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Ma K, Zhang C, Huang MY, Guo YX, Hu GQ. Crosstalk between Beclin-1-dependent autophagy and caspasedependent apoptosis induced by tanshinone IIA in human osteosarcoma MG-63 cells. Oncol Rep. 2016;36:1807–18. doi: 10.3892/or.2016.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang J, Wang W. Potential anticancer activity of tanshinone IIA against human breast cancer. Int J Cancer. 2005;116:799–807. doi: 10.1002/ijc.20880. [DOI] [PubMed] [Google Scholar]
- 12.Xu QQ, Xu YJ, Yang C, Tang Y, Li L, Cai HB, Hou BN, Chen HF, Wang Q, Shi XG, Zhang SJ. Sodium tanshinone IIA sulfonate attenuates scopolamine-induced cognitive dysfunctions via improving cholinergic system. Biomed Res Int. 2016;2016:9852536. doi: 10.1155/2016/9852536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Y, Li C, Wang Q, Zeng X, Ji P. Tanshinone IIA induces cell death via Beclin-1-dependent autophagy in oral squamous cell carcinoma SCC-9 cell line. Cancer Med. 2018;7:397–407. doi: 10.1002/cam4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Abraham N, Gao G, Yang Q. Dysregulation of autophagy and mitochondrial function in Parkinson’s disease. Transl Neurodegener. 2016;5:19. doi: 10.1186/s40035-016-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–40. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K, Cheng WT, Hu ZW, Wang S. Effect of tanshinone IIA in preventing and treating oxaliplatin induced peripheral neuropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36:559–63. [PubMed] [Google Scholar]
- 17.Xiang W, Jiang T, Guo F, Xu T, Gong C, Cheng P, Zhao L, Cheng W, Xu K. Evaluating the role of PTH in promotion of chondrosarcoma cell proliferation and invasion by inhibiting primary cilia expression. Int J Mol Sci. 2014;15:19816–31. doi: 10.3390/ijms151119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Areti A, Komirishetty P, Akuthota M, Malik RA, Kumar A. Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy. J Pineal Res. 2017;62 doi: 10.1111/jpi.12393. [DOI] [PubMed] [Google Scholar]
- 19.Bennett GJ, Doyle T, Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol. 2014;10:326–336. doi: 10.1038/nrneurol.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley MR, Jiang Y, Guo C, Reed A, Meng H, Vasko MR. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS One. 2014;9:e106485. doi: 10.1371/journal.pone.0106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao YF, Wang SF, Li X, Zhang YL, Qiao YJ. The anticancer mechanism investigation of Tanshinone IIA by pharmacological clustering in protein network. BMC Syst Biol. 2018;12:90. doi: 10.1186/s12918-018-0606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220:3–10. doi: 10.1016/j.atherosclerosis.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z, Yan M, Chen L, Fang P, Li Z, Wan Z, Cao S, Hou Z, Wei S, Li W, Zhang B. Nrf2-dependent antioxidant response mediated the protective effect of tanshinone IIA on doxorubicin-induced cardiotoxicity. Exp Ther Med. 2018;16:3333–3344. doi: 10.3892/etm.2018.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferraro E, Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Arch Biochem Biophys. 2007;462:210–9. doi: 10.1016/j.abb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–9. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martinez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, Jessen KR. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015;210:153–68. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang P, Li C, Xiang Z, Jiao B. Tanshinone IIA reduces the risk of Alzheimer’s disease by inhibiting iNOS, MMP2 and NFkappaBp65 transcription and translation in the temporal lobes of rat models of Alzheimer’s disease. Mol Med Rep. 2014;10:689–94. doi: 10.3892/mmr.2014.2254. [DOI] [PubMed] [Google Scholar]
- 28.Maione F, Piccolo M, De Vita S, Chini MG, Cristiano C, De Caro C, Lippiello P, Miniaci MC, Santamaria R, Irace C, De Feo V, Calignano A, Mascolo N, Bifulco G. Down regulation of pro-inflammatory pathways by tanshinone IIA and cryptotanshinone in a non-genetic mouse model of Alzheimer’s disease. Pharmacol Res. 2018;129:482–490. doi: 10.1016/j.phrs.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Areti A, Yerra VG, Komirishetty P, Kumar A. Potential therapeutic benefits of maintaining mitochondrial health in peripheral neuropathies. Curr Neuropharmacol. 2016;14:593–609. doi: 10.2174/1570159X14666151126215358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao G, Ding X, Guo Y, Chen W. Intrathecal lidocaine neurotoxicity: combination with bupivacaine and ropivacaine and effect of nerve growth factor. Life Sci. 2014;112:10–21. doi: 10.1016/j.lfs.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Aloe L, Manni L, Properzi F, De Santis S, Fiore M. Evidence that nerve growth factor promotes the recovery of peripheral neuropathy induced in mice by cisplatin: behavioral, structural and biochemical analysis. Auton Neurosci. 2000;86:84–93. doi: 10.1016/S1566-0702(00)00247-2. [DOI] [PubMed] [Google Scholar]