Abstract
High annexin A7 expression is a potential indicator of lymphatic metastasis and poor prognosis in patients with gastric cancer (GC). The mechanism underlying the effects of annexin A7 on GC cells remains unclear. In patients with GC, primary adenocarcinoma tissues had higher annexin A7 expression than adjacent non-cancerous tissues (P < 0.05). Among three human GC cell lines with high, moderate, and low levels of differentiation, respectively, the cell line with the lowest level of differentiation displayed the highest level of annexin A7 expression. We transfected cells of the human GC cell line BGC823 with short interfering RNAs (siRNAs) targeting annexin A7 and investigated the effects on signaling pathways related to cancer progression by quantitative real-time PCR and western blot. The silencing of endogenous annexin A7 suppressed the proliferation, migration, and invasion abilities of the BGC823 cells. In the cells treated with annexin A7 siRNA, the expression of p16, p21, and p27 was significantly upregulated while that of proliferating cell nuclear antigen (PCNA), cyclin A, cyclin D1, cyclin E1, matrix metalloproteinase-2 (MMP-2), MMP-9, and intercellular cell-adhesion molecule-1 (ICAM-1) was significantly downregulated compared with that in control cells. Our results suggest that the downregulation of endogenous annexin A7 inhibits GC cell proliferation, migration, and invasion by impacting cell cycle regulators and the expression of MMP-1, MMP-2, and ICAM-1. Targeting annexin A7 may represent a valuable strategy for the diagnosis and clinical treatment of GC.
Keywords: Gastric cancer, annexin A7, proliferation, migration, invasion, matrix metalloproteinases
Introduction
Gastric cancer (GC) has the fourth highest incidence among types of malignancies worldwide and is the second-leading cause of cancer-related death [1,2]. Despite improvements in surgical techniques and anticancer drugs, the five-year survival rate among patients with GC is still unsatisfactory, although the incidence of GC has declined in recent years [3-5]. GC is the deadliest cancer in Asian countries, including China, Japan, and Korea. In China, an estimated 679,100 new cases of GC are diagnosed each year, and 498,000 Chinese patients died of GC in 2015 [6]. Surgical resection is the most common treatment strategy for GC. However, because early-stage GC does not always involve obvious symptoms, many cases are already in an advanced stage when diagnosis is confirmed [7]. Advances in genome sequencing and precision therapy have led to new cancer treatments, but neither targeted treatments nor neoadjuvant chemotherapy has substantially improved the outcomes for patients with advanced GC [8]. Therefore, the development of new diagnostic and prognostic biomarkers for GC is an urgent priority.
Annexins are Ca2+-binding and phospholipid-binding proteins that are associated with cell transformation, cancer progression, and metastasis [9]. Annexin A7, also named synexin, belongs to the group A annexin family and contains an extraordinarily long amino terminus. Annexin A7 plays an important role in membrane fusion during exocytosis, localizing predominantly in the cytoplasm and showing a high prevalence in the brain, heart, and skeletal muscle [10]. Up to now, the effects of annexin A7 on cellular processes have been studied mostly in the context of cell apoptosis and membrane fusion. Recently, annexin A7 was reported to potentially play a role in promoting gastrointestinal cancer [11]. In a previous study, we showed that high annexin A7 expression was associated with poor differentiation of cancer cells in patients with GC and might be a predictor of lymphatic metastasis [12]. On the basis of those results, we hypothesize that expression of annexin A7 increases the proliferation, migration, and invasion abilities of GC cells.
Our overall aim is to provide a new therapeutic strategy for GC. To discover the mechanism by which annexin A7 promotes tumorigenesis and metastasis, we investigated the effects of annexin A7 expression on the biological characteristics of GC cells and its link to matrix metalloproteinases (MMPs) and intercellular cell-adhesion molecule-1 (ICAM1).
Materials and methods
Patient-derived specimens
We collected carcinoma tissues samples (1.0 cm × 1.0 cm × 1.0 cm) by gastrectomy from a total of 159 patients that were diagnosed with primary adenocarcinoma GC in the Fourth Hospital of Hebei Medical University from October 2018 to January 2019. We excluded patients receiving neoadjuvant chemotherapy or radiotherapy from our analysis. The study was approved by the Ethical Committee of the Fourth Hospital of Hebei Medical University.
Immunohistochemistry
Tissue samples were fixed in formaldehyde (10%), embedded them paraffin, and serially sectioned into 10 separate 4 μm sections. After xylene dewaxing and rehydration using an ethanol gradient, we incubated the sections at room temperature in H2O2 (3%) for 15 min to remove any endogenous peroxidase activity. We then washed the sections with PBS and performed antigen retrieval using citrate buffer (0.01 M, pH 6.0), followed by heterogenetic antigen blocking with normal goat serum (5%) at room temperature for 40 min. We then added rabbit anti-human annexin 7 polyclonal antibody (Proteintech Group, Inc., 1:100 dilution), incubated the sections at 4°C overnight, and washed them with PBS. We then added biotin-conjugated, goat anti-rabbit IgG polyclonal antibody (Zhongshan Golden Bridge Inc.; 1:100 dilution), incubated the sections at 37°C for 30 min, and washed them again with PBS. Next, we added a horseradish peroxidase-conjugated streptavidin working solution, washed the sections with PBS, and applied DAB chromogen for color development. Finally, the sections were stained with hematoxylin, dehydrated, cleared, and mounted with neutral gum. PBS was substituted for the primary antibody as a negative control.
Two independent examiners determined the staining intensity and percentage of the total area with positive staining for each section. We scored the expression of annexin A7 by multiplying the intensity scores by the percentage of the area of the sections that was positively stained. Briefly, we categorized the intensity scores into four groups: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. Similarly, we categorized the percentages of positively stained cells into four groups: 0, < 5%; 1, 5-25%; 2, 25-50%; 3, 51-75%; 4, >75%. We then calculated a composite score by multiplying the intensity score and the percentage of staining. We grouped the composite scores into four grades: grade 1, score 0-1; grade 2, score 2-4; grade 3, score 5-8; grade 4, score 9-12.
Cell lines and cultures
Cells of the cancer cell lines MKN28, SGC7901, and BGC823 were purchased from Cell Resource Center of Life Sciences (Shanghai, China). All cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% FBS, 100 units/ml penicillin, and 10 mg/100 ml streptomycin at 37°C in an incubator containing 5% CO2. The cells were dissociated with 0.25% trypsin containing 0.02% EDTA and then passaged them every 2-3 days.
Quantitative RT-PCR
We isolated total RNA from the cell lines using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. We synthesized cDNA from 2 μg total RNA using Superscript II reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed using the SYBR Green RT-PCR Kit (Applied Biosystems, Foster City, CA, USA) in a total volume of 20 μl containing 2 μl reverse-transcription product, 5 pmol each primer, 10 μl 2 × SYBR Green mix (Applied Biosystems), and 0.4 μl 1,000 × diluted reference dye (Applied Biosystems). The primers [11] used to detect annexin A7 expression were:
5’-GTATCCACAGCCACCTTCACAGTC-3’ (forward) and 5’-TCCAAADAAACAGGAGAGAAAACAG-3’ (reverse). As an internal control, we detected the expression of GAPDH mRNA using the following primers: 5’-CGCTGAGTACGTCGTGGAGTC-3’ (forward) and 5’-GCTGATGATCTTGAGGCTGTTGTC-3’ (reverse).
The PCR was performed using an Applied Biosystems Prism model 7900HT Sequence Detection System with the following settings: initial denaturation at 95°C for 5 min to ensure complete denaturation of the DNA and activation of the Taq polymerase, followed by 45 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and elongation at 72°C for 30 s. Fluorescence was detected after each cycle. All reactions were performed in triplicate. We normalized the relative expression levels to those of the GAPDH internal control and calculated the annexin A7 expression levels using the 2-ΔΔCT method.
Western blot
Cellular protein was extracted from the cell lines using lysis buffer and protease inhibitors (Beyotime, China). We separated 60 μg of protein from each sample by 12% SDS-PAGE and then electroblotted the gels onto PVDF membranes (Roche, Basel Switzerland). The membranes were then blocked in Tris-buffered saline with 0.1% Tween-20 (TBS-T) containing 5% non-fat milk for 1 h at room temperature, followed by incubation at 4°C overnight with primary antibodies for annexin A7, proliferating cell nuclear antigen (PCNA), p16, p21, p27, cyclin D1, cyclin E1, cyclin A, tissue inhibitor of metalloproteinase-1 (TIMP-1), TIMP-2, ICAM-1, MMP-1, MMP-2, and MMP-9 (all antibodies were purchased from Santa Cruz, USA). After several washes with TBS-T, the blots were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. We used the GAPDH protein level as a control for equal protein loading.
Annexin A7 siRNA transfections
We designed small interfering RNAs (siRNAs) targeting annexin A7 according to the guidelines of the “Dharmacon siDESIGN Center” (www. dharmacon.com). The sequences of each siRNA pair were as follows: siRNA1, 5’-CCUCUAAGUCUGCUUGAUATT-3’ and 5’-UAUCAAGCAGACUUAGAGGGA-3’; siRNA2, 5’-GGCUAAUCGAGACUUGUUATT-3’ and 5’-UAACAAGUCUCGAUUAGCCAT-3’; siRNA3, 5’-GGAGCUUACGGAAAGCAAUTT-3’ and 5’-AUUGCUUUCCGUAAGCUCCAG-3’; non-targeting siRNA (NS siRNA), 5’-UUCUCCGAACGUGUCACGUTT-3’ and 5’-ACGUGACACGUUCGGAGAATT-3’. The siRNAs were dissolved in an RNase-free solution at a concentration of 20 μmol/l.
The cell lines were cultured in six-well plates for 24 h and washed with RPMI 1640 prior to transfection. We divided the cells into control, NS siRNA, and Annexin A7 siRNA groups. The control group was treated with Lipofectamine 2000 only. The cells were transfected with the siRNAs using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After transfection for 24 h, we evaluated the transfection efficiency.
MTT cell proliferation assay
BGC823 cells were incubated on 96-well plates at a density of 6 × 104 cells/ml. When the cells reached 70% confluence, they were transfected with Annexin A7 siRNA or NS siRNA. At the end of the experiment, 20 μl methyl thiazolyl tetrazolium (MTT, 5 mg/ml) was added to each well, and the cells were incubated for another 4 h. We then discarded the medium, added 150 μl dimethyl sulfoxide (DMSO) to each well, and applied shaking at room temperature for 15 min. The absorbance was then recorded at 490 nm on a microplate reader.
Wound healing assay
Cells were seeded on a six-well plate at a density of 1 × 106 cells/well and then transfected with Annexin A7 siRNA or NS siRNA under the aforementioned conditions. When the cultured cells reached 80-90% confluence, the medium was discarded, and the cells were washed with PBS. We then created a wound by making a scratch between the cells with a sterile pipette tip. We observed the wound healing by counting the number of cells that crossed the scratch in five arbitrary visual fields under microscopy. All wound healing assays were performed in triplicate.
Cell invasion assay
Transwell chambers (8 μm pore size, polycarbonate filters, 6.5 mm diameter; Corning Costar, Corning, NY, USA) were coated with 100 μl growth-factor-reduced Matrigel. BGC823 cells were suspended on a six-well plate at a density of 1 × 106 cells per ml or per well and transfected with Annexin A7 siRNA or NS siRNA under the aforementioned conditions. After 24 h, we dissociated the cells with 0.25% trypsin containing 0.02% EDTA and placed 200 μl of the dissociated cells (1 × 106 cells/ml) in the upper layer of the transwell chamber, which was then placed on a 24-well plate. RPMI 1640 medium containing 10% fetal calf serum was added to the lower chamber. The cells were then incubated for 24 h, and non-invading cells were then removed from the top of the transwell membrane by scraping.
The transwell membranes were fixed in methanol for 10 min and stained with crystal violet. The cells that had invaded the Matrigel on the underside of the membranes were then counted under a microscope. We counted five arbitrary visual fields for each membrane.
Statistical analysis
Results are expressed as means ± standard deviation. Student’s t test or one-way ANOVA were used to determine the significance of differences between groups. We used SPSS software version 22.0 for all statistical analyses, with P < 0.05 as the threshold for statistical significance.
Results
Expression of annexin A7 in patients with GC
Annexin A7 protein expression was graded in 159 GC tissues from patients with or without lymph node metastasis. Of the samples from the 136 patients with lymph node metastasis, 87 showed positive annexin A7 expression. The patients with lymph node metastasis displayed high-grade (grade 3-4) annexin A7 expression, whereas those without lymph node metastasis displayed low-grade (grade 1-2) annexin A7 expression. As shown in Figure 1, the level of annexin A7 expression in GC patients with lymph node metastasis was significantly higher than that in patients without lymph node involvement (P < 0.05).
Figure 1.
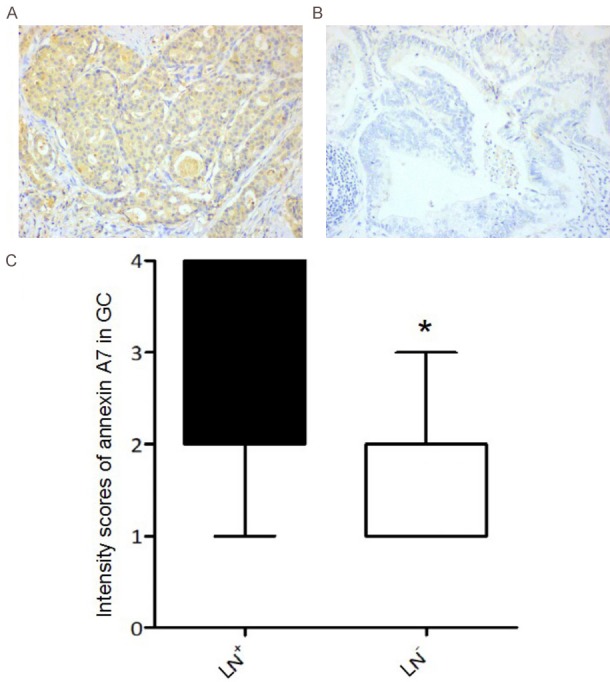
The annexin A7 protein expression in GC tissues of patients with or without lymph node metastasis. A. Representative expression of annexin A7 in gastric carcinoma tissues from patients with lymph node metastasis (LN+), B. GC patients without lymph node metastasis (LN-), measured by immunohistochemistry (× 400); C. The expression grades (grade 1-4) of Annexin A7 in LN+ and LN- gastric carcinoma patients, *, P < 0.05.
Correlation of annexin A7 expression with GC differentiation
We used western blot and real-time PCR to evaluate annexin A7 expression in the human GC adenocarcinoma cell lines MKN-28, SGC-7901, and BGC-823, which are well differentiated, moderately differentiated, and poorly differentiated, respectively. Western blots showed that the BGC-823 cells had the highest annexin A7 protein level among the cell lines, while the SGC-7901 cells had a higher annexin A7 protein level than the MKN-28 cells (Figure 2A and 2B). Likewise, the real-time PCR results showed that annexin A7 mRNA expression was highest in the BGC-823 cells and higher in the SGC-7901 than in the MKN-28 cells (Figure 2C).
Figure 2.
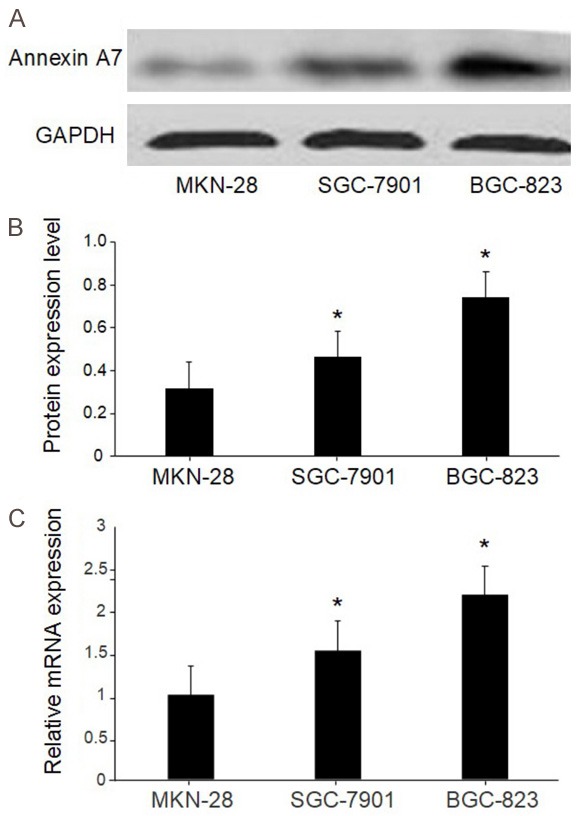
The expression of annexin A7 measured by Western blot. A. The expression of annexin A7 in different differentiation level gastric cancer cell lines. The expression of annexin A7 measured by Western blot. B. Ratio of pixel densities in different differentiated gastric cancer cell lines. C. The expression levels of Annexin A7 mRNA in different differentiated gastric cancer cell lines. *, P < 0.05, compared with MKN-28.
Effect of annexin A7 silencing by siRNA in BGC823 cells
We used siRNA techniques to further investigate the impact of annexin A7 on gastric carcinogenesis. BGC823 cells were transfected separately with three pairs of siRNAs targeting annexin A7 or with a non-targeting control siRNA. As shown in Figure 3, the three siRNAs targeting annexin A7 significantly reduced annexin A7 expression at both the mRNA level and the protein level, while the NS siRNA caused no change in annexin A7 expression. siRNA3 was the most efficient of the siRNAs for reducing annexin A7 expression in the BGC823 cells, causing approximately 90% inhibition compared with that caused by the NS siRNA. Further studies using siRNA3 showed that the amount of inhibition depended on the concentration of the siRNA. The results demonstrated that it was possible to inhibit annexin A7 expression by 95% using 80 nM siRNA3 (Figure 4).
Figure 3.
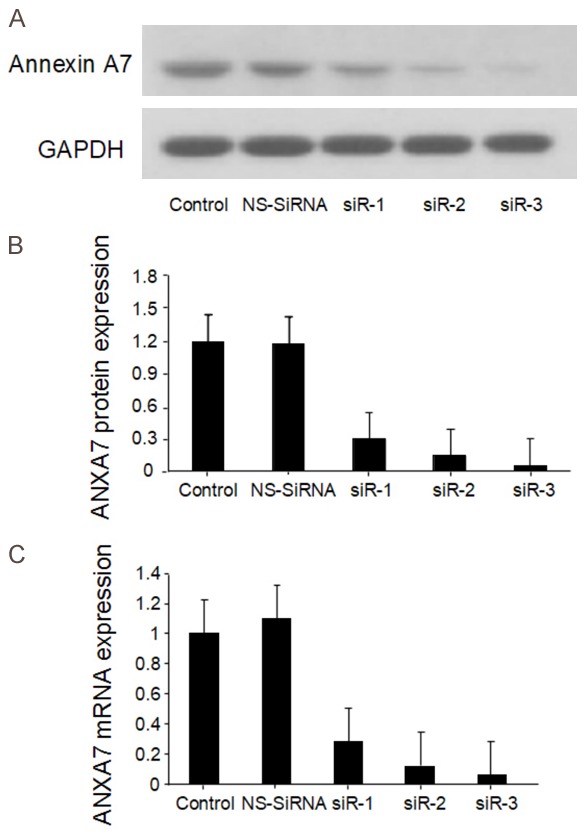
Annexin A7-siRNA down-regulated the expression of annexin A7 in BGC823 cells. A. Transfection efficiency by Western blot; B. Comparisons of Annexin A7 protein expression levels; C. Comparisons of annexin A7 mRNA expression levels.
Figure 4.
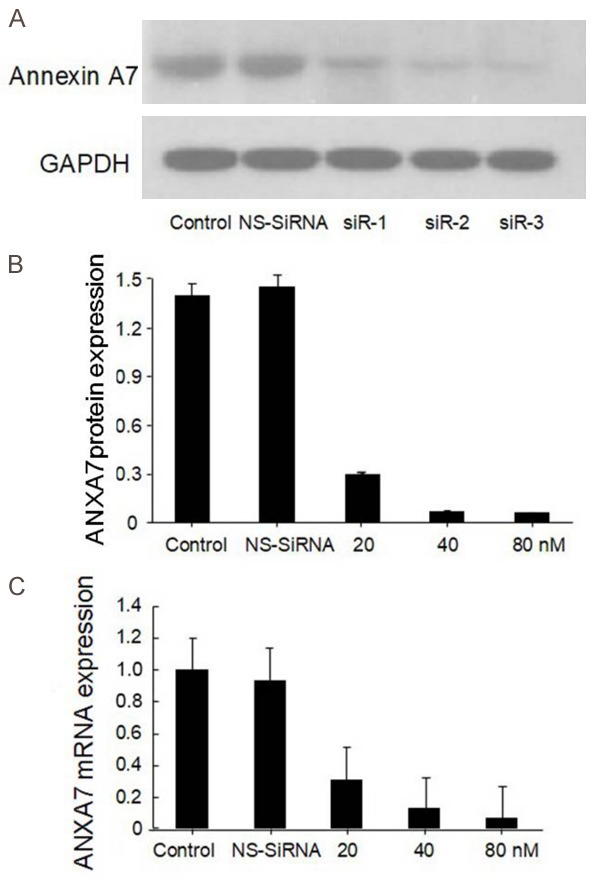
BGC823 cells were transfected with 80 nM Annexin A7 siRNA-3 for different periods of times or different concentrations (20 nM, 40 nM, 80 nM). A. Transfection efficiency by Western blot; B. Comparisons of annexin A7 protein expression levels; C. Comparisons of annexin A7 mRNA expression levels.
Effect of annexin A7 siRNA on the proliferation of BGC823 cells
We transfected BGC823 cells with 80 nM siRNA-3 at different concentrations (20 nM, 40 nM, 80 nM) for different periods of time and then performed MTT assays to measure the cells proliferation. As shown in Figure 5, siRNA3 inhibited cell proliferation in a time-dependent and dose-dependent manner (P < 0.05). The NS siRNA had no effect on cell proliferation compared with Lipofectamine alone. In addition, real-time quantitative PCR and western blot showed that compared with that in NS siRNA-treated control cells, the expression of p16, p21, and p27 was significantly upregulated whereas that of PCNA, cyclin A, cyclin D1, and cyclin E1 was significantly downregulated in BGC823 cells transfected with 80 nM siRNA3 (Figure 6). Those results suggest that the suppression of endogenous annexin A7 in BGC823 cells inhibits GC cell proliferation by promoting the expression of negative regulators of the cell cycle, including p16, p21, and p27, and by suppressing the expression of cyclin A, cyclin D1, and cyclin E1, which enhance cell proliferation by promoting G1-S cell cycle progression [22,23].
Figure 5.
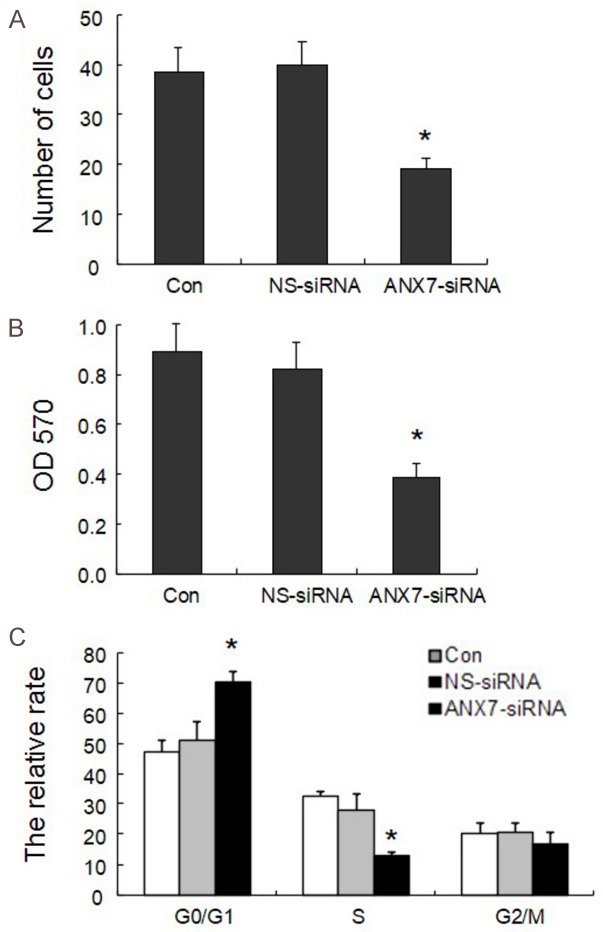
Effects of annexin A7 on proliferation activity in BGC 823 cell line: A. Cell number by cell count method; B. Results of MTT; C. The relative rate by Flow Cytometry. *P < 0.05, compared with control group.
Figure 6.
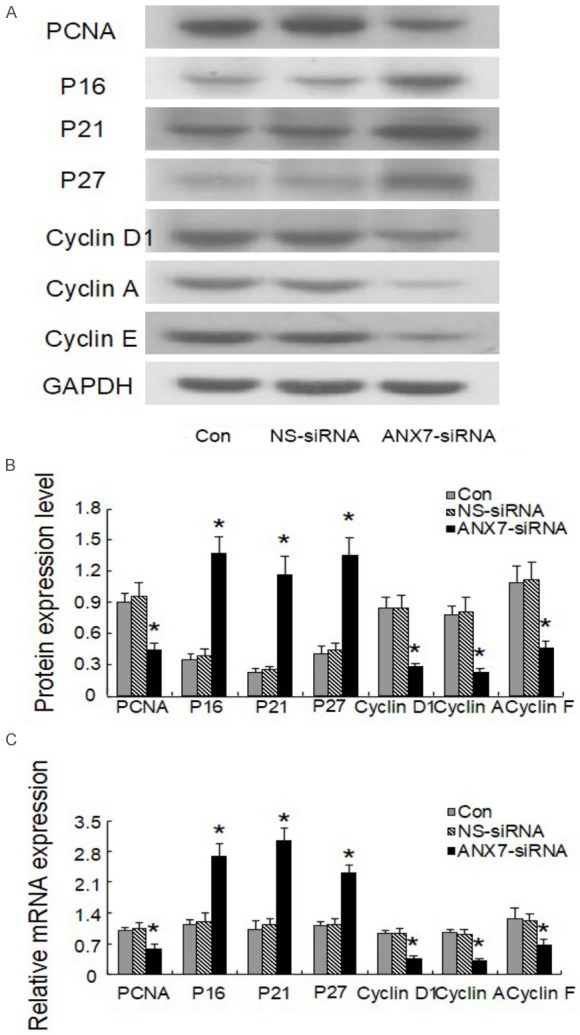
Effects of annexin A7-siRNA on the expression of proliferation related genes in gastric cancer cell BGC823. A, B. Protein expression level; C. Relative mRNA level. *P < 0.05, compared with control group.
Effect of annexin A7 siRNA on the invasion and migration abilities of BGC823 cells
We performed invasion and migration assays using cells transfected with 80 nM Annexin A7-siRNA3 or NS siRNA. As shown in Figure 7, the migration and invasion abilities of BGC823 cells treated with siRNA3 were significantly decreased compared with those of cells treated with NS siRNA (P < 0.05). NS siRNA had no significant effect on either cell invasion or migration compared with Lipofectamine alone.
Figure 7.
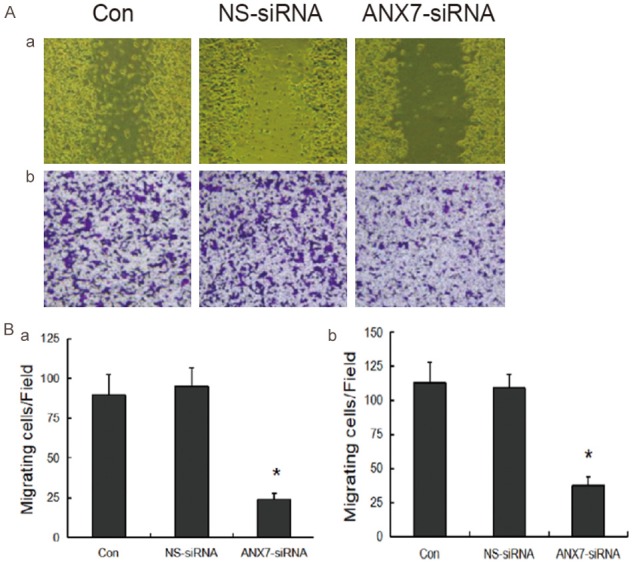
Effects of annexin A7-siRNA-3 on the invasion gastric cancer cell BGC823. a. results of scarification test; b. results of the transwell cell model. *P < 0.05, compared with the values of control group.
To explore the mechanism by which annexin A7 impacts BGC823 cell invasion and metastasis, we used western blotting to investigate the molecular changes in signaling pathways related to those functions. As shown in Figure 8, the expression of MMP-2, MMP-9, and ICAM-1 was significantly downregulated in BGC823 cells transfected with 80 nM siRNA3 compared with that in control cells treated with NS siRNA. siRNA3 had no significant effect on the expression of MMP-1, TIMP-1, or TIMP-2 (Figure 8).
Figure 8.
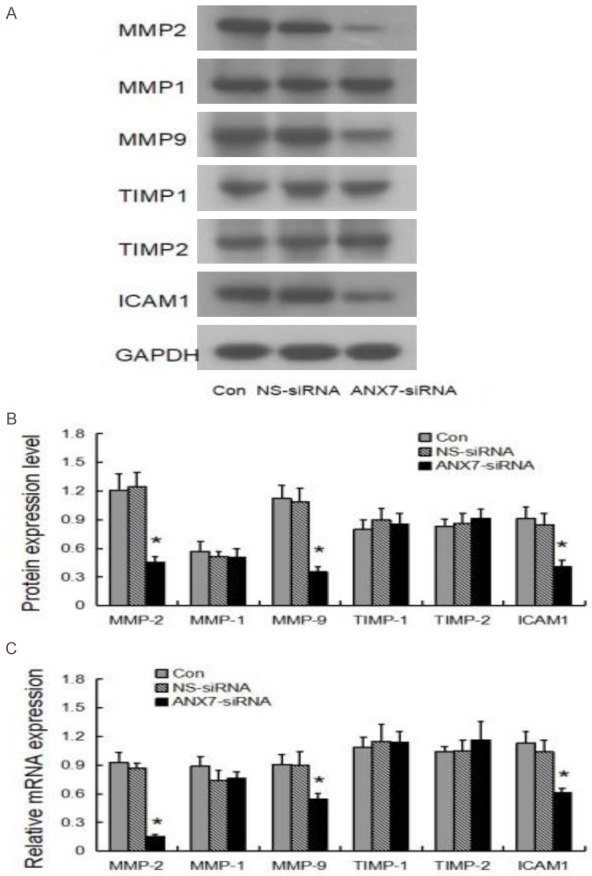
Effects of annexin A7-siRNA-3 on the expression of migration and invasion related genes in gastric cancer cell BGC823. A. Protein expressions subjected by Western blot; B. The protein expression levels subjected by Western blot. C. The mRNA expression level by qT-PCR. *P < 0.05, compared with the values of control group.
Discussion
The downregulation of annexin A7 expression decreased the proliferation activity of GC cells by promoting the expression of negative cell cycle regulators including p16, p21, and p27. The downregulation of annexin A7 also suppressed the expression of cyclin A, cyclin D1, and cyclin E1, which enhanced cell proliferation by promoting G1-S cell cycle progression. Moreover, the suppression of endogenous annexin A7 resulted in the downregulation of MMP-2, MMP-9, and ICAM-1 expression and the reduction of the invasion and migration abilities of GC cells. Those results provide evidence that can be used to further clarify GC pathogenesis and to understand how biological functions related to annexin A7 can be targeted to develop therapeutics for cancer.
Annexin A7 was the first identified annexin characterized by membrane trafficking and organization as well as by its interaction with Ca2+ homeostasis [13]. The gene encoding annexin A7 is located on human chromosome 10q21, where multiple potential tumor suppressor genes exist. Annexin A7 is a membrane-associated Ca2+-activated GTPase that also uses protein kinase C (PKC) as a substrate. Previous studies found that the annexin A7 protein has two hypotypes with molecular masses of 47 and 51 kDa [14], respectively. Those findings were confirmed in our experiments by the band that appeared with a molecular weight of 47 kDa in the GC cell lines. The annexin A7 protein, which contains a long N-terminal domain of ~200 amino acids rich in glycine, tyrosine, and proline residues, is predominantly expressed at cellular membranes, although weaker expression is also seen in the nucleus [15]. We found that annexin A7 was overexpressed both in a poorly differentiated GC cell line and in highly aggressive human GC tissues.
Annexin A7 reversibly binds to acidic phospholipids in a Ca2+-dependent manner and is involved in exocytotic secretion and the aggregation of chromaffin granules. It also causes liposome aggregation and forms classical voltage-gated Ca2+ channels in cellular and artificial membranes, which can be stabilized in an open state by GTP [16-19]. Hsu et al. found that annexin A7 expression was correlated with the TNM grade of GC and that overexpression of annexin A7 might play important roles in GC differentiation and metastasis [20]. Our results demonstrated a negative correlation between annexin A7 expression and GC differentiation, indicating that annexin A7 expression might be a candidate as a biomarker for tumor invasion.
The suppression of endogenous annexin A7 in BGC823 cells inhibited cell proliferation; promoted the expression of the negative cell cycle regulators, p16, p21 and p27; and suppressed the expression of cyclin A, cyclin D1, and cyclin E1, which enhances cell proliferation by promoting G1-S cell cycle progression [21,22]. Furthermore, expression of the cellular proliferation marker PCNA decreased after annexin A7 was suppressed by siRNA. Those results indicate that annexin A7 is involved in the proliferation of gastric cells. Similar results were also found in ovarian cancer [23]. A positive correlation between the annexin A7 level and the expression of cyclin E1 was previously shown in breast cancer metastases [24]. Annexin A7 expression can activate the phospholipid-relevant PI3K-Akt survival cascade, a critical cellular signaling pathway, especially in the cyclin E pathway. Furthermore, annexin A7 may play a role in determining the hormone-associated and phospholipid-binding ability of aggressive basal-like breast cancer cells.
Local recurrence and distant metastasis are the main causes of treatment failure in GC [3]. The development, progression, invasion, and metastasis of malignant tumors are often accompanied by changes in the expression of extracellular matrix (ECM) and its cellular surface receptors, composed of basement membrane and intercellular matrix, which represent an important tissue barrier to tumor metastasis [25]. The degradation of ECM and the destruction of basement membrane require the action of MMPs. ECM degradation by MMPs is one of the key links in the invasion and metastasis of tumor cells, and many malignant tumors display increased secretion and activity of MMPs [26]. The MMPs that are most relevant to cancer metastasis are MMP-2 and MMP-9, which can degrade type-IV collagen (the main component of the basement membrane). A previous study showed that the expression of MMP-2 and MMP-9 was closely related to GC progression and lymph node metastasis [27]. Our results showed that the reduction of annexin A7 levels significantly decreased the invasion ability and the MMP-2 and MMP-9 expression in BGC823 cells, indicating that the downregulation of MMP activity might be a mechanism by which annexin A7 suppression inhibits invasion and metastasis.
The expression of ICAM-1 was significantly downregulated in BGC823 cells treated with Annexin A7 siRNA. As a member of the immunoglobulin superfamily, ICAM-1 is a transmembrane glycoprotein with an extracellular immunoglobulin-like structure [28]. ICAM-1 exists on the surface of a variety of cells, including vascular endothelial cells, fibroblasts, and epithelioid cells. ICAM-1 promotes the occurrence and evolution of GC and can increase the metastatic potential of several types of malignant tumors [29].
We did not find annexin A7 downregulation to have any influence on TIMP-1 or TIMP-2 expression. TIMPs are the natural inhibitors of MMPs. TIMP-1, a glycoprotein with a molecular weight of 28,500 kD, is capable of inhibiting tumor invasion and metastasis [30]. TIMP-2 is mainly secreted by tumor cells and stromal cells and inhibits a wide spectrum of MMPs, excluding MMP-9. TIMP-2 can inhibit MMP-2 and its active forms in a concentration-dependent manner to protect the integrity of the ECM against tumor invasion and metastasis [31]. It might be that the Annexin A7 siRNAs failed to affect TIMP-1 and TIMP-2 expression because of the multifaceted nature of the role of annexin A7 expression in GC and the fact that the Annexin A7 siRNA treatment affects multiple signaling pathways. Previous studies demonstrated that annexin A7 impacts the progression of carcinogenesis by affecting various signaling pathways and genes related to tumor suppression, DNA repair, and apoptosis [32,33]. Further investigation will be needed to reveal the impact of annexin A7 expression on cancer progression in detail.
In conclusion, annexin A7 expression was increased in GC and was negatively correlated with GC cell differentiation. Although the detailed mechanisms underlying the effects of annexin A7 on GC cells need to be elucidated in future studies, our results suggest that annexin A7 plays an important role as an oncogene promoting the development of GC. An approach using Annexin A7 siRNA may lead to new treatment options for patients with GC.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Roukos DH. Genome-wide association studies and aggressive surgery toward individualized prevention, and improved local control and overall survival for gastric cancer. Ann Surg Oncol. 2009;16:795–798. doi: 10.1245/s10434-009-0317-8. [DOI] [PubMed] [Google Scholar]
- 3.Wu K, Nie Y, Guo C, Chen Y, Ding J, Fan D. Molecular basis of therapeutic approaches to gastric cancer. J Gastroenterol Hepatol. 2009;24:37–41. doi: 10.1111/j.1440-1746.2008.05753.x. [DOI] [PubMed] [Google Scholar]
- 4.Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895–905. doi: 10.1016/j.bpg.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 7.D‘Ugo D, Rausei S, Biondi A, Persiani R. Preoperative treatment and surgery in gastric cancer: friends or foes? Lancet Oncol. 2009;10:191–195. doi: 10.1016/S1470-2045(09)70021-X. [DOI] [PubMed] [Google Scholar]
- 8.Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388:2606. doi: 10.1016/S0140-6736(16)32226-7. [DOI] [PubMed] [Google Scholar]
- 9.Guo C, Liu S, Greenaway F, Sun MZ. Potential role of annexin A7 in cancers. Clinica Chimica Acta. 2013;423:83–89. doi: 10.1016/j.cca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Sun MZ, Liu S, Tang J. Proteomics investigation of mouse hepatocarcinoma cell lines with different lymph node metastasis capacities. Chem J Chin Univ. 2009;30:517–524. [Google Scholar]
- 11.Jimenez CR, Knol JC, Meijer GA, Fijneman RJ. Proteomics of colorectal cancer: overview of discovery studies and identification of commonly identified cancer-associated proteins and candidate CRC serum markers. J Proteomics. 2010;73:1873–1895. doi: 10.1016/j.jprot.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Yuan HF, Li Y, Zhao Q, Fan LQ, Tan BB, Ye WH. Expression of annexin A7 and its clinical significance in differentiation and metastasis of gastric carcinoma. Int J Clin Exp Pathol. 2014;7:6567–6574. [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y, Meng J, Huang Y, Wu J, Wang B, Ibrahim MM, Tang J. Guanine nucleotide-binding protein subunit beta-2-like 1, a new Annexin A7 interacting protein. Biochem Biophys Res Commun. 2014;445:58–63. doi: 10.1016/j.bbrc.2014.01.119. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava M, Bubendorf L, Nolan L, Glasman M, Leighton X, Miller G, Fehrle W, Raffeld M, Eidelman O, Kallioniemi OP, Srivastava S, Pollard HB. ANX7 as a bio-marker in prostate and breast cancer progression. Dis Markers. 2001;17:115–120. doi: 10.1155/2001/239602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torosyan Y, Dobi A, Naga S, Mezhevaya K, Glasman M, Norris C, Jiang G, Mueller G, Pollard H, Srivastava M. Distinct effects of annexin A7 and p53 on arachidonate lipoxygenation in prostate cancer cells involve 5-lipoxy-genase transcription. Cancer Res. 2006;66:9609–9616. doi: 10.1158/0008-5472.CAN-06-1574. [DOI] [PubMed] [Google Scholar]
- 16.Gerelsaikhan T, Vasa PK, Chander A. Annexin A7 and SNAP23 interactions in alveolar type II cells and in vitro: a role for Ca (2+) and PKC. Biochim Biophys Acta. 2012;1823:1796–1806. doi: 10.1016/j.bbamcr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chander A, Gerelsaikhan T, Vasa PK. Annexin A7 trafficking to alveolar type II cell surface: possible roles for protein insertion into membranes and lamellar body secretion. Biochim Biophys Acta. 2013;1833:1244–1255. doi: 10.1016/j.bbamcr.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mears D, Zimliki CL, Atwater I, Rojas E, Glassman M, Leighton X, Pollard HB, Srivastava M. The Anx7 (+/-) knockout mutation alters electrical and secretory responses to Ca (2+) -mobilizing agents in pancreatic beta-cells. Cell Physiol Biochem. 2012;29:697–704. doi: 10.1159/000186926. [DOI] [PubMed] [Google Scholar]
- 19.Taniuchi K, Yokotani K, Saibara T. BART inhibits pancreatic cancer cell invasion by PKCα inactivation through binding to ANX7. PLoS One. 2012;7:e35674. doi: 10.1371/journal.pone.0035674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu PI, Huang MS, Chen HC, Hsu PN, Lai TC, Wang JL, Lo GH, Lai KH, Tseng CJ, Hsiao M. The significance of Annexin A7 expression and its correlation with poor cellular differentiation and enhanced metastatic potential of gastric cancer. J Surg Oncol. 2008;97:609–614. doi: 10.1002/jso.21046. [DOI] [PubMed] [Google Scholar]
- 21.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 22.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo C, Liu S, Greenaway F, Sun MZ. Potential role of annexin A7 in cancers. Clinica Chimica Acta. 2013;423:83–89. doi: 10.1016/j.cca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Bera A, Leighton XM, Pollard H, Srivastava M. Cyclin E and FGF8 are downstream cell growth regulators in distinct tumor suppressor effects of ANXA7 in hormone-resistant cancer cells of breast versus prostate origin. Trends Cancer Res. 2018;13:55–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang TL, Lee LY, Wang CC, Liang Y, Huang SF, Wu CM. Claudin-4 expression is associated with tumor invasion, MMP-2 and MMP-9 expression in gastric cancer. Exp Ther Med. 2010;1:789–797. doi: 10.3892/etm.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Zhang M, Wang Y, Li M. The relationship between the expression of tumor matrix-metalloproteinase and the characteristics of magnetic resonance imaging of human gliomas. J Biomed Res. 2010;24:124–131. doi: 10.1016/S1674-8301(10)60020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon HJ, Park KS, Ku MJ, Lee MS, Jeong SH, Imbs TI, Zvyagintseva TN, Ermakova SP, Lee YH. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J Nat Prod. 2009;72:1731–1734. doi: 10.1021/np800797v. [DOI] [PubMed] [Google Scholar]
- 28.Buitrago D, Keutgen XM, Crowley M, Filicori F, Aldailami H, Hoda R, Liu YF, Hoda RS, Scognamiglio T, Jin M, Fahey TJ 3rd, Zarnegar R. Intercellular adhesion molecule-1 (ICAM-1) is upregulated in aggressive papillary thyroid carcinoma. Ann Surg Oncol. 2012;19:973–980. doi: 10.1245/s10434-011-2029-0. [DOI] [PubMed] [Google Scholar]
- 29.Janan M, Proungvitaya S, Limpaiboon T, Proungvitaya T, Roytrakul S, Wongkham C, Jearanaikoon P, Chur-in S, Wongkham S. Serum adhesion molecule-1 (ICAM-1) as a potential prognostic marker for cholangiocarcinoma patients. Asian Pac J Cancer Prev. 2012;13:107–114. [PubMed] [Google Scholar]
- 30.Kubben FJ, Sier CF, Meijer MJ, van den Berg M, van der Reijden JJ, Griffioen G, van de Velde CJ, Lamers CB, Verspaget HW. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br J Cancer. 2006;95:744–751. doi: 10.1038/sj.bjc.6603307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alakus H, Afriani N, Warnecke-Eberz U, Bollschweiler E, Fetzner U, Drebber U, Metzger R, Hölscher AH, Mönig SP. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. World J Surg. 2010;34:2853–2859. doi: 10.1007/s00268-010-0761-4. [DOI] [PubMed] [Google Scholar]
- 32.Ye W, Li Y, Fan L, Zhao Q, Yuan H, Tan B, Zhang Z. Effect of annexin A7 suppression on the apoptosis of gastric cancer cells. Mol Cell Biochem. 2017;429:33–43. doi: 10.1007/s11010-016-2934-4. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Huang S, Wang S, Zhao J, Su L, Zhao B, Zhang Y, Zhang S, Miao J. Targeting annexin A7 by a small molecule suppressed the activity of phosphatidylcholine-specific phospholipase C in vascular endothelial cells and inhibited atherosclerosis in apolipoprotein E-/-mice. Cell Death Dis. 2013;19:e806. doi: 10.1038/cddis.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]


