Abstract
This study measured amyloid-beta (Aβ), interleukin-1 beta (IL-1β), and glial fibrillary acidic protein (GFAP) expression in the hippocampus of Alzheimer’s disease (AD) rat models to elucidate the mechanism of anti-inflammatory effect of ginsenoside Rb1 in AD. Eighty-four male Wistar rats were randomly divided into seven groups, learning and memory impairment was induced by Ap1-40 to establish AD rat model. Learning and memory abilities were assessed by a Morris water maze experiment. Immunohistochemistry, RT-PCR and Western blotting were used to measure IL-1β, Aβ and GFAP expression. Nissl staining and methenamine silver staining were performed to observe the morphology of neurons and Nissl Body, and to detect amyloid protein particle deposition. ELISA and LC-MS/MS were applied to detect Aβ1-42 and byproducts of S/MS were applied to IAT, VIV, ITL, VVIA, TVI, and VIT). Ginsenoside Rb1 administration could relieve cognitive deficit, and decrease expressions of IL-1β, Aβ, and GFAP. Neurons and Nissl Body were improved and plaques deposition was decreased obviously after treatment of ginsenoside Rb1, especially in medium dose of ginsenoside Rb1. Ginsenoside Rb1 can increase productions of Aβ1-42 and byproducts of β- and γ-secretase. Collected evidence supported that ginsenoside Rb1 improves learning and memory in AD rat by altering the amyloidogenic process of APP into non-amyloidogenic process, to exert its anti-inflammatory function.
Keywords: Alzheimer’s disease (AD), ginsenoside Rb1, inflammatory, interleukin-1β (IL-1β), glial fibrillary acidic protein (GFAP), amyloid protein (Aβ)
Introduction
Alzheimer’s disease (AD) is one of the most serious neurodegenerative diseases in the elderly, as well as the most common cause of dementia worldwide [1]. The predominant characteristics of AD are progressive impairments in cognition and behavior including loss of neurons, senile plaque (SP), and neurofbrillary tangles (NFT) [2]). SP, composed of Aβ peptides, is one of the major indicators of AD [3]). Aβ is generated by cleavages of β-amyloid precursor protein (APP) [4]. APP contains a KPI structure that may inhibit Aβ degradation and increase local Aβ concentrations. Evidence showed that APP can be proteolytically cleaved in two different pathways, amyloidogenic or non-amyloidogenic, which can generate secretases including α-secretase, β-secretase, and γ-secretase [5]. During amyloidogenic process, APP can be cleaved by β-secretase and γ-secretase, leading to the accumulation of peptide fragments, such as Aβ40, and Aβ42 [6]. The exact injury pathology mechanism of AD remains controversial since the pathogenesis and etiology of AD remains an enigma [7,8].
To explore the etiology of AD, many hypotheses have been proposed, including neuroinflammatory theory, which believes that inflammatory processes trigger neurodegeneration signaling pathways and exacerbate the assembly of Aβ plaques [9]. Several new approaches to AD drugs, including supplementary and alternative methods, were proposed as well [8,10]). Ginseng is a traditional cognitive drug in China, and many recent reports demonstrated that ginseng facilitates learning and memory, and improves memory disorders in animals. A study on rat model suggested that panax ginseng extract can enhance cognition in rats with alcohol-induced memory impairment, in addition to its antitumor, anti-inflammatory and antistress role [11]. Ginsenoside Rb1 is a main bioactive ingredient in ginseng [12] with nootropic effects [13]. Ginsenoside Rb1 was reported to enhance memory and cognitive function, and enjoy great popularity for treatment of dementia disease [14]. In addition, documents supported the benefits of Ginsenoside Rb1 in defending oxidative stress and neuronal apoptosis, and enhancing spatial learning ability in Aβ1-40 induced AD rat model [15,16]. Although investigations of Ginsenoside Rb1 on AD advanced our knowledge in unraveling the pathology of AD, the possible mechanism involved remains largely unknown and awaits further elucidation. We hypothesized that ginsenoside Rb1 may alter the amyloidogenic process of APP into non-amyloidogenic process, in which α-secretase and γ-secretase are generated, resulting in the inhibition of Aβ. The present study was undertaken to investigate the potential anti-neuroinflammatory effects and behavioral outcomes of ginsenoside Rb1 in Aβ1-40 induced AD rat model, and explore the possible mechanism herein.
Materials and methods
Materials
Ginsenoside Rb1 was purchased from Boyun biotechnology co., Ltd. (Shanghai, China). Aβ1-40 and DMSO were purchased from Sigma-Aldrich (St. Louis, MO, USA). Aricept was used as a positive control and was purchased from the Eisai Pharmaceutical Co., Ltd. (Suzhou, China) (Number: 100223A). Rabbit polyclonal anti-IL-1β, anti-GFAP and mouse monoclonal anti-β-Amyloid were purchased from Abcam Co., Ltd. SP immunohistochemistry kit and DAB staining kit were purchased from Bioss (Beijing, China). Trizol reagent was bought from Shanghai ShengGong Biological Engineering Co., Ltd. Reverse transcriptase and SYBR green were obtained from Bioneer (Shanghai, China). All PCR primers were obtained from Dalian Treasure Biological Engineering Co. Ltd. The BCA Protein Assay Kit was purchased from Pierce Co., Ltd.
Animal model establishment and grouping
Young male SPF Wistar rats weighing 250 ± 20 g (certificate number: SCXK2012-0001) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. Rats were housed six per cage in Wenzhou Medical Laboratory Animal Center. All rats received normal rodent food with free access to drinking water. Rats were housed at a 12-h light/dark cycle at a temperature of 21 ± 2°C with 37~42% humidity. All experimental protocols followed the ethical requirements of Wenzhou Medical University related to experimental animals (number: wydw2013-0134).
An aggregated state of Aβ1-40 was prepared according to the manufacturer’s instructions and stored at 4°C for later use. The control group received bilateral saline injections into the hippocampus based on the Brain Stereotaxic Method [17]. The rat model of AD was established via the injection of Aβ1-40. The brain was opened using a cranial drill to identify the Bregma referring to Paxinos and Watson rat brain atlas [18]. A cranial drill was used to punch two holes next to the left and right of the skull using the Bregma as the 0 point, caudally 3 mm, and 1.5 mm from the centerline. A microsyringe was inserted 3 mm intracranially through each skull hole for microinfusion. The control group received 2 μl of saline, and the AD model groups received 2 μl of aggregated Aβ1-40 at a concentration of 2.5 μg/μl (equal to 5 μg of Aβ). The fluid was injected at a speed of 0.5 μl/min, and the needle shall be remained in place for 10 min after injection. Eighty-four young male SPF Wistar rats were randomly divided into seven groups: normal group (NG), control group (CG), AD model group (AG), Aricept group (RG), low-dose ginsenoside Rb1 (LG), mid-dose ginsenoside Rb1 (MG), and high-dose ginsenoside Rb1 (HG) (n = 12 per group).
Animal treatment
Ginsenoside Rb1 (12.5 mg/kg/d, 25.0 mg/kg/d and 50.0 mg/kg/d) was intragastrically administered once every day for consecutive 2 weeks at 10 a.m. The rats in RG group received 1.67 mg/kg/d dose of Aricept. AG rats and CG rats received an equivalent dose of saline after surgery.
Morris water maze
Spatial learning and memory abilities of rats were assessed in Morris water maze model establishment. Rats were subjected to behavioral tests with a Morris water maze for spatial navigation task (escape latency) and probe trial for 5 consecutive days. The tests were assessed by two investigators who are completely blind to the rat groups. The swimming speed and location of the rats were monitored by video tracking software. From the first to fourth day, the platform was placed just below the water surface for spatial navigation test. The maximum swim time was set to 60 s for rat to find the platform. If the rats located the platform within 60 s, they shall be maintained at the platform for 10 s. If the rats failed to find the hidden platform within 60 s, they should be manually guided to the platform and stayed for 10 s before removing them from the pool. The maximum time recorded was 60 s. Each rat was subjected to four trials per day at an inter-trial interval of 10 min. For each trial, the platform shall be remained on the same place and the time spent to locate the hidden platform (escape latency) and the path length of each rat was recorded. On the 5th day, the platform was removed from the water for the probe trial. The number of times that each rat crossed the center of the quadrant (where the platform was previously located) at an interval of 1 min was recorded for evaluation of memory performance.
Animal tissue processing
After the behavior test, rats were euthanized and the heart was perfused with 4% paraformaldehyde-0.1 M phosphate buffer (pH 7.4). The brains of 6 rats from each group were removed and placed in a 4% paraformaldehyde fixation solution and stored at 4°C. The cortex and hippocampus were removed from the remaining rats in each group and stored at -80°C.
RNA preparation and RT-PCR
Whole RNA of the hippocampus samples was extracted using Trizol reagent according to the manufacturer’s procedures, and the absorbance values of RNA at OD 260 and OD 280 were detected using a UV spectrophotometer to calculate the RNA content. cDNA was synthesized using reverse transcriptase kit. Primers of IL-1β, Aβ, and GFAP mRNA were designed and configured by Shanghai Ruijing Biological Engineering Co., Ltd. The following sequences were used: IL-1β: F: 5’-TCTGTGACTCGTGGGATGATGAC-3’, R: 5’-TTGGCTTATGTTCTGTCCATTGAG-3’; Aβ: F: 5’-CTGGAGGTGCCCACTGATG-3’, R: 5’-GGGTCTGACTCCCATTTTCC-3’; GFAP: F: 5’-AGAGTGGTATCGGTCCAAGTT-3’, R: 5’-TCAAGGTCGCAGGTCAAG-3’; and β-Actin: F: 5’-CCCATCTATGAGGGTTACGC-3’; R: 5’-TTTAATGTCACGCACGATTTC-3’. The fluorescence quantitative PCR reaction was performed in 25 μl PCR buffer, 0.6 μl×2 of primers (25 pmol/μl), 0.3 μl of SYBR Green I (20X), 1.0 μl cDNA template and 22.5 μl DEPC-treated water. The amplification conditions were 4 min at 95°C for denaturation followed by 35 cycles of 20 s at 94°C, 30 s at 60°C and 30 s at 72°C. The signal was detected at 72°C.
Western blotting
Hippocampus was removed at -80°C, and 1 mM PMSF was added. The tissue was homogenized and maintained on ice for 30 min. Lysates were centrifuged at 3000×g for 5 min at 4°C. Lysates were subjected to ultrasound for 3 times, each for 5 min, and centrifuged at 12000×g for 5 min. Supernatants were stored at -80°C. The protein concentration of clarified lysates was determined using a commercial BCA kit according to the manufacturer’s instructions. Total lysate protein was separated in 10% or 12% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked for 90 min in a skim milk blocking buffer, and washed 5 times with TBS/0.1% Tween 20 before incubation with primary antibodies against IL-1β (1:1000), Aβ (1:1000) and GFAP (1:1000) overnight at 4°C. Membranes were washed 3 times with TBS/0.1% Tween 20 and incubated with secondary antibody (horseradish peroxidase-conjugated, goat-anti rabbit or mouse, 1:1000) for 60 min. An enhanced chemiluminescence kit was used to detect the bands. The gray values of IL1-β, GFAP, Aβ, and β-Actin bands were analyzed using a Quantity One software system.
Immunohistochemistry
Brains were fixed in a 4% paraformaldehyde fixation fluid and dehydrated by passing through a graded series of alcohol. Then slices were subjected to permeabilization by xylene at room temperature, and embedded into paraffin blocks. Fixed brains were sliced into 5-μm sections for immunohistochemistry. Immunohistochemistry was performed according to the manufacturers’ instructions. Six sections were chosen from each group. Five areas were randomly selected from each section, and all positive granules within the visual field were used to compute the integrated option density (IOD) and area. The optical density (OD) (IOD/area) was calculated in Image Pro-Plus (IPP) 6.0. The OD reflected the degree of quantitative positive immunohistochemistry reaction.
Nissl staining
The paraffin-embedded brain slices were dewaxed and hydrated according to standard histological procedures. After washed 1~2 min with distilled water, the brain slices were immersed in Nissl staining solution for 10 min. Then the sections were rinsed with distilled water, dehydrated in ethanol, and cleared in xylene. Neurons and Nissl Bodies in hippocampus CA1 area were captured using a light microscope (Olympus; AX70U-Photo, Japan).
Methenamine silver staining
Methenamine silver primary liquid was mixed with sodium borate (1:1). Then mixture were boiled in a microwave oven, and kept at 60°C for water bath. After sections were fully dewaxed in xylene and rehydrated through a graded series of series of ethanol, slices were placed into 0.5% periodate and 8% chromic acid for 15 min and 30 min, respectively. Subsequently, slices were washed with distilled water and processed with 1% sodium metabisulfite solution for 1 min. Pre-heated methenamine working solution was prepared and incubated with slices at 60°C for 90 min, until black particles on a brown purple background were observed. Then, 1% gold chloride aqueous solution was added for reaction for 2 min. Slices were then processed with 3% sodium thiosulfate solution for 3 min and dehydrated. Images were analyzed using a light microscopy.
ELISA
Human Aβ42 Elisa kit was used to measure the content of Aβ1-42 in hippocampal tissue sample. Standard or sample with 50 μL was added on each well and then, the regular operation was conducted according to the specification of Human Aβ42 Elisa kit. The Standard protein lines were drew by Excel and sample Aβ1-42 concentration was converted based on its measured value. The Aβ1-42 concentration in hippocampal tissue sample was calculated in line with dilution concentration of that sample.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
Prior to LC-MS/MS, supernatants were vacuumized and mixed with liquid phase [19]. APP cleavage peptides were identified and measured with application of a triple quadrupole mass spectrometer (6460; Agilent Technologies, Santa Clara, CA, USA), equipped with Agilent Jet Stream Technology and accompanied by ultra-performance LC (Agilent 1260). C18 column (300 A, 5 μM particle size, 50 mm ID×150 mm bed length) was used to separate peptides. Peptides were quantified by multiple reaction monitoring mode as previously described [20].
Statistical analysis
All data were processed using SPSS 17.0 statistical software. All data were subjected to normality tests (P > 0.1 indicates normal distribution). Data are expressed as the means ± standard deviation (x̅ ± s). Pairwise comparisons of homogeneous data were performed using LSD test. Non-homogeneous data were analyzed using Dunnett’s test. Comparisons among multiple groups were performed using one-way analysis of variance. p values less than 0.05 were considered statistically significant.
Results
Ginsenoside Rb1 improves learning and memory capability in AD rats
In the Morris water maze test, all rats were able to swim normally and find the hidden platform during the training trials. The swim speed of rats is considered to be a crucial parameter to clarify the locomotivity between wild normal rats and postoperative rats. As shown in Figure 1A, the swim speed of rats in all groups showed no differences. ANOVA revealed no statistically significant difference between normal group and control group the day after modeling (Figure 1B). The escape latency of AG rats was notably longer than any other groups (P < 0.01, P < 0.05). In contrast, the rats treated with Aricept alone or with ginsenoside Rb1 shortened the escape latency as compared with AD model group, especially rats with dose of 25 mg/kg/d showed the maximum decline in escape latency (P < 0.01). A probe task was performed on the fifth day, and the data are shown in Table 1. No difference in the times crossing the platform location was observed between NG and CG rats (P > 0.05). Statistical significant differences were observed between AG and NG, RG and NG, LG and NG, MG and NG, HG and NG rats (P < 0.05). AG rats had the least platform crossing behavior. The number of times MG rats crossed the platform location was more frequent than other groups, and there was no significant difference between LG and HG or RG and MG (P > 0.05).
Figure 1.
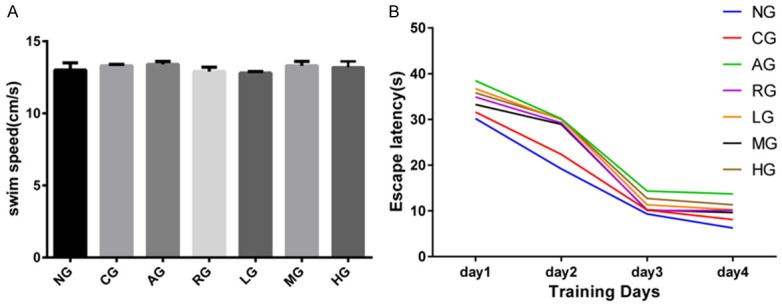
Morris water maze was applied to assess the learning and memory ability of AD rats. In a spatial navigation task, all rats had similar swimming speed (A), while rats with received Aricept or ginsenoside Rb1 had shortened escape latency (B). NG: normal group; CG: control group; AG: AD model group; RG: Aricept group; LG: low-dose ginsenoside Rb1 group; MG: mid-dose ginsenoside Rb1 group; HG: high-dose ginsenoside Rb1 group.
Table 1.
Probe task indicates ginsenoside Rb1 can enhance the spatial memory of AD rats
| Group (N = 12) | NG | CG | AG | RG | LG | MG | HG |
|---|---|---|---|---|---|---|---|
| Number of times crossing the platform | 5.09 ± 1.33 | 4.82 ± 1.21 | 2.59 ± 0.52* | 4.28 ± 0.97*,▲,# | 3.56 ± 0.94*,▲,# | 4.04 ± 1.02*,▲ | 3.99 ± 1.04*,▲ |
Results were expressed as mean ± SD.
P < 0.05 versus the normal group;
P < 0.05 versus the AD model group;
P < 0.05 versus the mid-dose ginsenoside Rb1.
NG: normal group; CG: control group; AG: AD model group; RG: Aricept group; LG: low-dose ginsenoside Rb1 group; MG: mid-dose ginsenoside Rb1 group; HG: high-dose ginsenoside Rb1 group.
Ginsenoside Rb1 decreases IL-1β, GFAP, and Aβ expressions in AD rats detected by RT-PCR and western blot
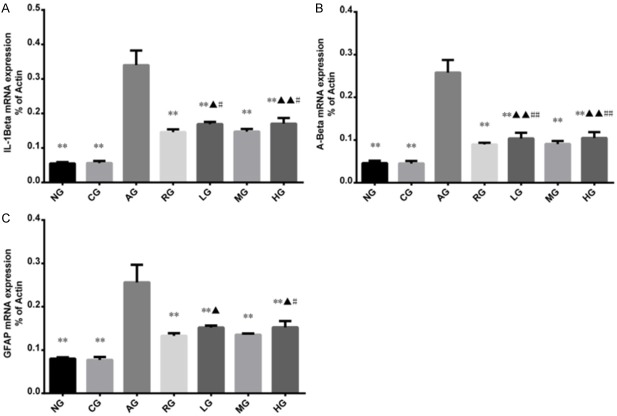
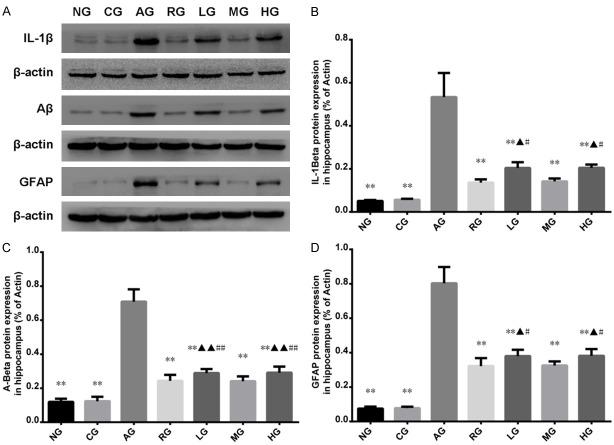
RT-PCR and western blot were applied to measure the mRNA and protein expressions of IL-1β, GFAP and Aβ in hippocampus, respectively (Figures 2 and 3). There was no significant difference regarding mRNA expressions of Aβ, IL-1β, or GFAP between CG and NG. Compared with CG, rats with injection of Aβ1-40 in the hippocampus had increased mRNA expressions of IL-1β, GFAP and Aβ (P < 0.01 or P < 0.05). Compared with rats with injection of Aβ1-40, rats with injections of Aricept or ginsenoside Rb1 had decreased mRNA expressions of IL-1β, GFAP and Aβ (P < 0.01). Western blot showed that there were significant differences in IL-1β, GFAP and Aβ protein levels in hippocampus between RG and AG, LG and AG, MG and AG, and HG and NG (P < 0.01). No statistically significant difference was found between RG and MG (P > 0.05). Among the rats with ginsenoside Rb1 treatments, rats with dose of 25.0 mg/kg per day had the highest protein expressions of IL-1β, GFAP and Aβ compared to rats with dose of 12.5 mg/kg per day or 50.0 mg/kg per day.
Figure 2.
The Effect of ginsenoside Rb1 on mRNA expressions of IL-1β, Aβ and GFAP in the hippocampus CA1 area as detected by RT-PCR. AD rats had increased mRNA expressions of IL-1β, Aβ and GFAP, while injection of Aricept or ginsenoside Rb1 could suppress the mRNA expressions of IL-1β, Aβ and GFAP. Results were expressed as mean ± SD. Compared to AD model group, *P < 0.05, **P < 0.01; compared to Aricept model group, ▲P < 0.05, ▲▲P < 0.01; Compared to mid-dose of ginsenoside Rb1 group, #P < 0.05, ##P < 0.01; NG: normal group; CG: control group; AG: AD model group; RG: Aricept group; LG: low-dose ginsenoside Rb1 group; MG: mid-dose ginsenoside Rb1 group; HG: high-dose ginsenoside Rb1 group.
Figure 3.
Western blot was used to detect the protein expressions of IL-1β, Aβ and GFAP in hippocampus. The western blots are representative of six independent experiments (A), all revealing similar results. The intensity of bands was normalized to actin for each treatment using Image-pro Plus 6.0 analysis software. Western blot showed AD rats had elevated protein expressions of IL-1β, Aβ and GFAP and rats with injection of Aricept or ginsenoside Rb1 had decreased expressions of IL-1β, Aβ and GFAP (B-D). Results were expressed as mean ± SD. *P < 0.05, **P < 0.01; compared to Aricept model group, ▲P < 0.05, ▲▲P < 0.01; Compared to mid-dose of ginsenoside Rb1 group, #P < 0.05, ##P < 0.01; NG: normal group; CG: control group; AG: AD model group; RG: Aricept group; LG: low-dose ginsenoside Rb1 group; MG: mid-dose ginsenoside Rb1 group; HG: high-dose ginsenoside Rb1 group.
Ginsenoside Rb1 suppresses Aβ, IL-1β, and GFAP expressions in AD rats detected by immunohistochemistry
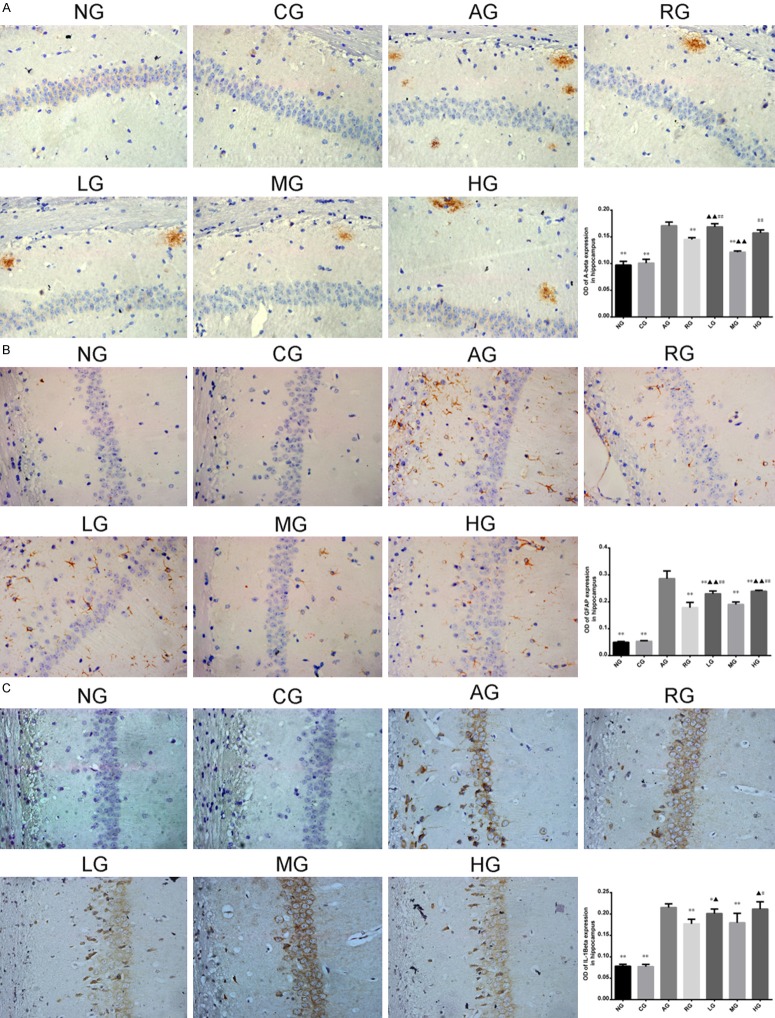
Positive staining of IL-1β, Aβ, and GFAP are shown in Figure 4. The expression levels of IL-1β, Aβ, and GFAP in hippocampus were significantly increased in AD model group in comparison to other groups (P < 0.01). After treatment with Aricept or ginsenoside Rb1, expressions of Aβ, IL-1β, and GFAP were substantially suppressed. Statistical significance was observed between CG and AG, RG and AG, LG and AG, MG and AG, HG and AG (P < 0.05, P < 0.01). But no significant difference was observed between RG and MG (P > 0.05).
Figure 4.
Positive staining of IL-1β (C), Aβ (A), and GFAP (B) were respectively detected (×400 magnification). Expressions of IL-1β, Aβ, and GFAP in hippocampus were significantly increased in AD model group (P < 0.01). After treatment with Aricept or ginsenoside Rb1, expressions of Aβ, IL-1β, and GFAP levels were decreased. Compared to AD model group, *P < 0.05, **P < 0.01; compared to Aricept model group, ▲P < 0.05, ▲▲P < 0.01; Compared to mid-dose of ginsenoside Rb1 group, #P < 0.05, ##P < 0.01. NG: normal group; CG: control group; AG: AD model group; RG: Aricept group; LG: low-dose ginsenoside Rb1 group; MG: mid-dose ginsenoside Rb1 group; HG: high-dose ginsenoside Rb1 group.
Ginsenoside Rb1 attenuates Aβ plaques deposition
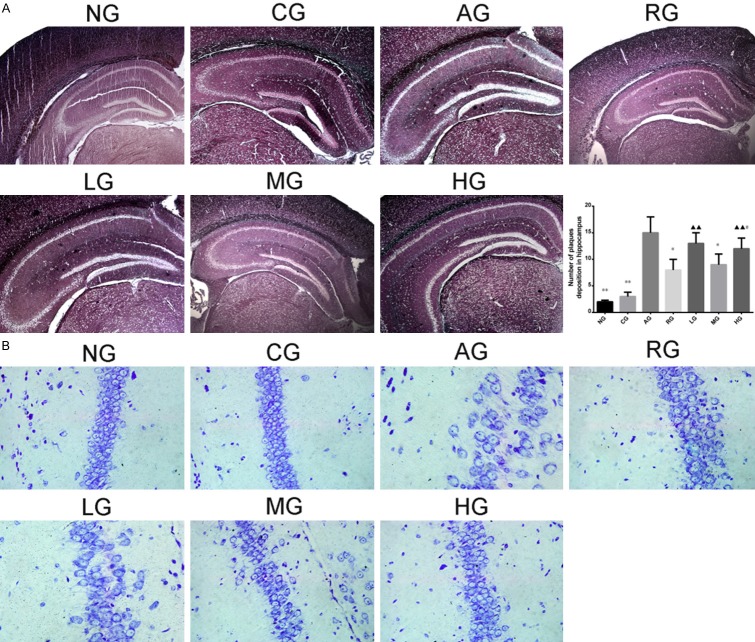
As shown in Methenamine silver staining, amyloid protein particles can be dyed black particles (Figure 5A). The number of Aβ plaques deposition in hippocampus was significantly increased in AD model group in comparison to CG (P < 0.05, P < 0.01). Black particles in rats with injection of Aricept or ginsenoside Rb1 were reduced compared to AD model group (P < 0.05, P < 0.01), especially in the mid-dose group (P < 0.01). However, there was no significant difference between mid-dose group and Aricept group (P > 0.05).
Figure 5.
Aβ plaques deposition in hippocampus was observed under a microscope after Methenamine silver staining (A) (×40 magnification). AD rats had elevated Aβ plaques deposition while rats with injection of Aricept or ginsenoside Rb1 had reduced Aβ plaques deposition. Rats with injection of Ginsenoside Rb1 had increased neurons and nissl Body (B) (×400 magnification). Results were expressed as mean ± SD. Compared to AD model group, *P < 0.05, **P < 0.01; compared to Aricept model group, ▲P < 0.05, ▲▲P < 0.01; Compared to mid-dose of ginsenoside Rb1 group, #P < 0.05, ##P < 0.01. (B). NG: normal group; CG: control group; AG: AD model group; RG: Aricept group; LG: low-dose ginsenoside Rb1 group; MG: mid-dose ginsenoside Rb1 group; HG: high-dose ginsenoside Rb1 group.
Ginsenoside Rb1 increases neurons and Nissl body in hippocampal CA1 area
Nissl Staining observed the neurons in hippocampus CA1 area of NG and CG rats were in a larger quantity, arranged in neat rows, rich in Nissl Bodies (dark blue), with pale blue nucleus and light blue background; the neurons of AD model group rats in the hippocampal CA1 area decreased in quantity, arranged disorderedly, with obscure or disappeared boundary of Nissl Bodies, karyopyknosis, or karyolysis which were shaped in elliptic or triangle and dyed dark blue; the neurons in hippocampal CA1 area of RG and MG rats increased significantly in quantity, arranged neatly, with more Nissl Bodies, little karyopyknosis, and karyolysis (Figure 5B).
Ginsenoside Rb1 changes the cleavage pattern of APP
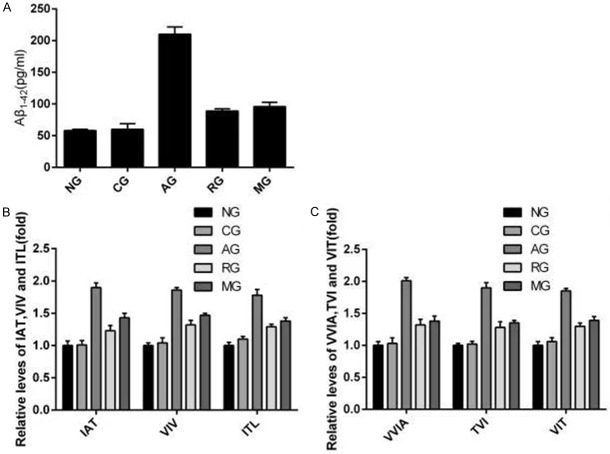
According to the previous research, there were two cleavage patterns for APP (amyloidogenic pattern and non-amyloidogenic patterns). Therefore, we supposed ginsenoside Rb1 changes the cleavage pattern of APP in AD, after that, the expression of Aβ could be decreased subsequently. The Aβ1-42 and peptides (ZAT, VIV, ITL, VVIA, TVI, and VIT) in groups of NG, CG, AG, RG and MG were respectively measured by ELISA (Figure 6A) and LC-MS/MS experiments (Figure 6B and 6C). The result demonstrated that comparing to AD rat model, the Aβ1-42 and peptideds in rats injected with Aricept or mid-dose ginsenoside were obviously reduced (P < 0.01), implying that ginsenoside Rb1 improves learning and memory ability of AD rats by changes the cleavage pattern of APP.
Figure 6.
Effect of Ginsenoside Rb1 on production of Aβ1-42 by enzyme-linked immunusorbent assay) and on long Aβ cleavage by LC-MS/MS. Hippocampal tissue secreted amyloid protein 1-42 levels were measured by ELISA in the five groups of NG, CG, AG, RG and MG (A). Relative IAT, VIV, and ITL levels in cell lysates during Aβ1-42 formation (B). Relative VVIA, TVI, and VIT levels in cell lysates during Aβ1-42 formation (C). Results are expressed as a ratio of NG. Data are expressed as mean ± SD, and analyzed by one-way analysis of variance. *P < 0.05, **P < 0.01, vs. NG. LC-MS/MS: liquid chromatography-tandem mass spectrometer. NG: normal group; CG: control group; AG: AD model group; RG: Aricept group; MG: mid-dose ginsenoside Rb1 group.
Discussion
AD is the fourth leading cause of death in the aging population worldwide. Although numerous studies supported the implication of Aβ secretion in AD, the exact pathogenesis of AD is still not clear. There are many hypotheses of AD pathogenesis, such as immune and inflammatory hypotheses [21], β-amyloid hypothesis [22], cholinergic loss hypothesis [23], Tau protein hyperphosphorylation hypothesis [24], peroxidation [25], and the imbalance of intracellular calcium homeostasis [26]. In our study, we using AD rat model confirmed the benefit of ginsenoside Rb1 on learning and memory ability by exerting its anti-inflammatory effect.
Firstly, RT-PCR and western blot were applied to measure the expressions of Aβ, IL-1β and GFAP, which showed that ginsenoside Rb1 could decrease the expressions of Aβ, IL-1β and GFAP in AD rat model. It is widely believed that inflammation has great role to play in AD pathology, despite the controversial argument whether inflammation is a cause or consequence of AD [27]. Recent studies demonstrated persistent chronic inflammation in the brains of AD patients may initiate the formation and development of other pathological features [28]. The expression of pro-inflammatory cytokines such as IL-1β, IL-6, IL-10, and TNF-β were reported to be increased in the brain and cerebrospinal fluid of AD patients [29,30]. Glial fibrillary acidic protein (GFAP), a marker of astrocytes is related to memory disorders and neuron reduction [31]. Additionally, Aβ is a commonly induced pathway in AD [32], whose abnormal deposition exhibits neurotoxicity and may lead to a series of complex reactions, including inflammatory cascade [33]. Aβ promotes inflammation and thrombosis in AD by interacting with circulating proteins factor XII and fibrinogen [34]. Our results found decreased expressions of inflammatory-related genes, Aβ, IL-1β and GFAP in AD rats after injection with ginsenoside Rb1 may indicate that ginsenoside Rb1 can attenuate the inflammation in AD. In addition, the subsequent Nissl Staining and Methenamine Silver Staining also supported the protective role of ginsenoside Rb1 in AD as evident by less Aβ plaques deposition, and increased neurons and Nissl Body in hippocampus area. This result was consistent with previous studies which support the protective role of ginsenosides Rb1 on central nervous system and neural stem cells [35,36].
To verify our hypothesis that ginsenoside Rb1 enhances learning and memory ability by changing the cleavage pattern of APP, in which α-secretase and γ-secretas are generated, resulting in the inhibition of Aβ, we applied ELISA and CC-MS/MS to detect the expressions of Aβ1-42 and byproducts of β- and γ-secretase (IAT, VIV, ITL, VVIA, TVI, and VIT). Those assays demonstrated that AD rat injected with ginsenoside Rb1 had suppressed expressions of Aβ1-42, and byproducts of β- and γ-secretase. The inflammation process in AD could stimulate and activate microglia cells to generate more pro-inflammatory cytokines, including IL-1β, which contributes to APP enhancement, consequently resulting in amyloidopathy and neuronal damage [1,37,38]. Aβ is mainly generated by APP, the latter can be cleaved into two patterns, amyloidogenic processing (β- and γ-secretase) and non-amyloidogenic processing (α- and γ-secretase) [5,39]. In the amyloidogenic pattern, β-secretase and γ-secretase were secreted with additional generation of byproduct polypeptides (IAT, VIV, ITL, VVIA, TVI, and VIT) [40]. Collectively, the amyloidogenic processing of APP may be triggered in AD pathology. The ginsenoside Rb1 may exert its anti-inflammation function in AD by alter the APP cleavage pattern from amyloidogenic pattern to non-amyloidogenic pattern, thus precludes Aβ formation. Although the experiment result in this study supported our hypothesis, due to the complexity of AD pathology, more investigations were required as additional mechanisms may also implicated in this process. In addition, further studies are needed to identify other involved protective effects and the mechanisms of ginsenoside Rb1 on AD in clinical practice.
We used several methods to evaluate the anti-inflammatory effect of ginsenoside Rb1 in AD rats. However, there are some shortcomings of this study. First, the number of specimens in animal experiments was not sufficient, and the observation period was relatively short. Second, other functions of ginsenoside Rb1, such as improving calcium metabolism, inhibiting neuronal apoptosis and enhancing the protective effect of neurotrophic factors, must be elucidated in future studies.
Taken together, our observation supported that ginsenoside Rb1 can improve learning and memory in Aβ1-40 induced AD rats by altering the amyloidogenic process of APP into non-amyloidogenic process, thus precludes inflammatory process in AD.
Acknowledgements
This work was supported by the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Scientific Research Centre, the National Natural Science Foundation of China (Grant no. 81573896), the Natural Science Foundation of Zhejiang Province (Grant no. LY15H270016), and the Wenzhou Municipal Science and Technology Bureau of the Wenzhou Municipal People’s Government (Branch Science and Technology Bureau of Wenzhou City, Grant no. Y20080106).
Disclosure of conflict of interest
None.
References
- 1.Philippens IH, Ormel PR, Baarends G, Johansson M, Remarque EJ, Doverskog M. Acceleration of amyloidosis by inflammation in the amyloid-beta marmoset monkey model of Alzheimer’s disease. J Alzheimers Dis. 2017;55:101–113. doi: 10.3233/JAD-160673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao DD, Yang WY, Li Y, Lin JW, Gao SY, Wang YR, Hu HY. Effect of Qingxin Kaiqiao Fang on hippocampus mRNA expression of the inflammation-related genes IL-1beta, GFAP, and abeta in an Alzheimer’s disease rat model. Evid Based Complement Alternat Med. 2018;2018:9267653. doi: 10.1155/2018/9267653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez-Rapp J, Rainone S, Goupil C, Dorval V, Smith PY, Saint-Pierre M, Vallee M, Planel E, Droit A, Calon F, Cicchetti F, Hebert SS. microRNA-132/212 deficiency enhances Abeta production and senile plaque deposition in Alzheimer’s disease triple transgenic mice. Sci Rep. 2016;6:30953. doi: 10.1038/srep30953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raefsky SM, Furman R, Milne G, Pollock E, Axelsen P, Mattson MP, Shchepinov MS. Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid beta-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer’s disease. Neurobiol Aging. 2018;66:165–176. doi: 10.1016/j.neurobiolaging.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tcw J, Goate AM. Genetics of beta-amyloid precursor protein in Alzheimer’s disease. Cold Spring Harb Perspect Med. 2017;7 doi: 10.1101/cshperspect.a024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin HK, van der Wel PC. How amyloid precursor protein protects itself from cleavage. Structure. 2014;22:361–362. doi: 10.1016/j.str.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 8.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Town T. Inflammation, immunity, and Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2010;9:129–131. doi: 10.2174/187152710791012008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noorbakhsh F, Overall CM, Power C. Deciphering complex mechanisms in neurodegenerative diseases: the advent of systems biology. Trends Neurosci. 2009;32:88–100. doi: 10.1016/j.tins.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhu JD, Wang JJ, Zhang XH, Yu Y, Kang ZS. Panax ginseng extract attenuates neuronal injury and cognitive deficits in rats with vascular dementia induced by chronic cerebral hypoperfusion. Neural Regen Res. 2018;13:664–672. doi: 10.4103/1673-5374.230292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Yi YS, Kim MY, Cho JY. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benishin CG, Lee R, Wang LC, Liu HJ. Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacology. 1991;42:223–229. doi: 10.1159/000138801. [DOI] [PubMed] [Google Scholar]
- 14.Zhao R, Zhang Z, Song Y, Wang D, Qi J, Wen S. Implication of phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase-3beta pathway in ginsenoside Rb1’s attenuation of beta-amyloid-induced neurotoxicity and tau phosphorylation. J Ethnopharmacol. 2011;133:1109–1116. doi: 10.1016/j.jep.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Hoang-Gia T, Wu H, Lee MR, Gu L, Wang C, Yun BS, Wang Q, Ye S, Sung CK. Ginsenoside Rb1 improves spatial learning and memory by regulation of cell genesis in the hippocampal subregions of rats. Brain Res. 2011;1382:147–154. doi: 10.1016/j.brainres.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Li Y, Yang W, Gao S, Lin J, Wang T, Zhou K, Hu H. Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer’s disease induced by Abeta1-40. Am J Transl Res. 2018;10:796–805. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YL LD, Wu QS, Yao YY, Li WP. Effect of the extract of astragalus on learning and memory and the expression of Bcl-2 and Bcl-xl protein in hippocampus neurons in rat model with Alzheimer’s disease. Acta Universitatis Medicinalis Anhui (Chin) 2007;42:299–302. [Google Scholar]
- 18.Paxinos G, Watson CR. The rat brain in stereotaxic coordinates, compact third edition (with CD-ROM) Rat Brain in Stereotaxic Coordinates. 1997;3:6. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 19.Okochi M, Tagami S, Yanagida K, Takami M, Kodama TS, Mori K, Nakayama T, Ihara Y, Takeda M. γ-secretase modulators and presenilin 1 mutants act differently on presenilin/gamma-secretase function to cleave Abeta42 and Abeta43. Cell Rep. 2013;3:42–51. doi: 10.1016/j.celrep.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. Gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29:13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojala J, Alafuzoff I, Herukka SK, van Groen T, Tanila H, Pirttila T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol Aging. 2009;30:198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Viola KL, Velasco PT, Klein WL. Why Alzheimer’s is a disease of memory: the attack on synapses by a beta oligomers (ADDLs) J Nutr Health Aging. 2008;12:51S–57S. doi: 10.1007/BF02982587. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Yamagata N, Takasaki K, Sano K, Hayakawa K, Katsurabayashi S, Egashira N, Mishima K, Iwasaki K, Fujiwara M. Decreased acetylcholine release is correlated to memory impairment in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res. 2009;1249:222–228. doi: 10.1016/j.brainres.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Reed TT, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol Dis. 2008;30:107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Cheung KH, Shineman D, Müller M, Cárdenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyss-Coray T. Inflammation in Alzheimer’s disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 28.Halliday G, Robinson SR, Shepherd C, Kril J. Alzheimer’s disease and inflammation: a review of cellular and therapeutic mechanisms. Clin Exp Pharmacol Physiol. 2000;27:1–8. doi: 10.1046/j.1440-1681.2000.03200.x. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H, Hampel H, Prvulovic D, Wallin A, Blennow K, Li R, Shen Y. Elevated CSF levels of TACE activity and soluble TNF receptors in subjects with mild cognitive impairment and patients with Alzheimer’s disease. Mol Neurodegener. 2011;6:69. doi: 10.1186/1750-1326-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mrak RE, Griffin WS. Potential inflammatory biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2005;8:369–375. doi: 10.3233/jad-2005-8406. [DOI] [PubMed] [Google Scholar]
- 31.Murphy MP, Das P, Nyborg AC, Rochette MJ, Dodson MW, Loosbrock NM, Souder TM, McLendon C, Merit SL, Piper SC, Jansen KR, Golde TE. Overexpression of nicastrin increases Abeta production. FASEB J. 2003;17:1138–1140. doi: 10.1096/fj.02-1050fje. [DOI] [PubMed] [Google Scholar]
- 32.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 33.Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- 34.Zamolodchikov D, Strickland S. A possible new role for Abeta in vascular and inflammatory dysfunction in Alzheimer’s disease. Thromb Res. 2016;141(Suppl 2):S59–61. doi: 10.1016/S0049-3848(16)30367-X. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed T, Raza SH, Maryam A, Setzer WN, Braidy N, Nabavi SF, de Oliveira MR, Nabavi SM. Ginsenoside Rb1 as a neuroprotective agent: a review. Brain Res Bull. 2016;125:30–43. doi: 10.1016/j.brainresbull.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Lu S, Yu H, Duan S, Zhao J. Baicalin and ginsenoside Rb1 promote the proliferation and differentiation of neural stem cells in Alzheimer’s disease model rats. Brain Res. 2018;1678:187–194. doi: 10.1016/j.brainres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Cuello AC, Ferretti MT, Leon WC, Iulita MF, Melis T, Ducatenzeiler A, Bruno MA, Canneva F. Early-stage inflammation and experimental therapy in transgenic models of the Alzheimer-like amyloid pathology. Neurodegener Dis. 2010;7:96–98. doi: 10.1159/000285514. [DOI] [PubMed] [Google Scholar]
- 38.Doens D, Fernandez PL. Microglia receptors and their implications in the response to amyloid beta for Alzheimer’s disease pathogenesis. J Neuroinflammation. 2014;11:48. doi: 10.1186/1742-2094-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Fu Z, Meng L, He M, Zhang Z. The early events that initiate beta-amyloid aggregation in Alzheimer’s disease. Front Aging Neurosci. 2018;10:359. doi: 10.3389/fnagi.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tagami S, Yanagida K, Kodama TS, Takami M, Mizuta N, Oyama H, Nishitomi K, Chiu YW, Okamoto T, Ikeuchi T, Sakaguchi G, Kudo T, Matsuura Y, Fukumori A, Takeda M, Ihara Y, Okochi M. Semagacestat is a pseudo-inhibitor of gamma-secretase. Cell Rep. 2017;21:259–273. doi: 10.1016/j.celrep.2017.09.032. [DOI] [PubMed] [Google Scholar]