Abstract
FOXO1, also known as FKHR, is a member of the Forkhead transcription factor family. Our previous study revealed that FOXO1 expression is significantly downregulated in pancreatic ductal adenocarcinoma (PDAC). However, our knowledge on the clinical significance of FOXO1 and its biological roles and associated mechanisms in PDAC tumorigenesis remains limited. In this study, we confirmed that FOXO1 is commonly downregulated in PDAC tissues, at both the mRNA and protein levels, compared to adjacent tissues. Furthermore, FOXO1 inhibited cell proliferation and tumor formation both in vitro and in vivo, and promoted pancreatic cancer cell invasion. Downregulation of FOXO1 resulted in enhanced Wnt/β-catenin signaling activity, thereby promoting cell proliferation and epithelial-mesenchymal transition. The highly expressed miR-27a could potentially be used to target the 3’-UTR of FOXO1 in PDAC tissues to inhibit or at least slow down the invasion and proliferation of cancerous cells. Taken together, our findings suggest that the miR-27a/FOXO1/β-catenin axis may serve as a promising therapeutic target in PDAC progression.
Keywords: FOXO1, PDAC, proliferation, invasion, miR-27a, Wnt/β-catenin signaling
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignant tumor and is the fourth leading cause of cancer-associated mortality in the USA [1]. Patients with PDAC usually present with locally advanced, unresectable, or metastatic disease, and are often in a lot of pain. Despite developments in the detection and management of PDAC, the five-year relative survival rate has not improved [2,3]. Thus, further investigation into the molecular mechanisms driving this largely incurable tumor is urgently needed.
The Forkhead transcription factor family member FOXO1 is a multifunctional transcription factor that regulates the transcription of a number of important genes involved in cell cycle arrest or apoptosis [4]. FOXO1 activation in transformed and non-transformed cells results in the upregulation of the cyclin-dependent kinase inhibitor p27KIP1 and downregulation of D-type cyclins, thereby arresting cells at the G1 phase [5]. Activated FOXO1 protein also triggers apoptosis in various cancer cell lines through the regulation of proapoptotic proteins, such as Fas ligand, TRAIL, and Bim [6]. Thus, it has been postulated that FOXO1 plays a pivotal role in cell transformation and tumorigenesis inhibition. Our previous study revealed that FOXO1 is significantly downregulated in PDAC tissues, and this downregulation correlates with cancer cell stemness in PDAC [7]. However, the detailed underlying mechanisms remain largely unknown.
The inhibitory function of FOXO1 in cell proliferation and survival is often disrupted by the overactivated phosphatidylinositol 3-kinase (PI3K) Akt pathway in cancer cells [8]. Activated Akt phosphorylates a wide range of downstream proapoptotic proteins, including FOXO1 [9]. Wnt/β-catenin signaling plays a pivotal role in maintaining cell stemness and crosstalks with the PI3K/AKT pathway [10,11].
Epigenetic alterations are involved in the inhibition of tumor suppressors in pancreatic cancer cells [12-14]. MiR-27a has been found to be highly expressed in numerous types of cancers, including breast and ovarian cancers [15,16]. However, the role of miR-27a/FOXO1 in PDAC remains unknown. This study aimed to further explore the function of FOXO1 and its epigenetic regulation in PDAC progression using in-vitro and in-vivo experiments and clinical specimens.
Materials and methods
Patient tissues and ethics statement
Fifty-seven fresh tumor and matched adjacent tissue samples were collected from patients with pathologically and clinically confirmed PDAC. Tissue microarrays (TMAs) containing 136 paired normal and adjacent PDAC tissues were obtained from Shanghai Renji Hospital (Shanghai, China). Written informed consent was obtained from patients prior to tissue sample collection. The study was conducted according to the Declaration of Helsinki. The Institutional Review Board of Shanghai General Hospital approved the use of tumor samples and animals in this study. All animal experiments were performed in accordance with The Animal Care and Use Committee of Shanghai Jiao Tong University, School of Medicine.
Immunohistochemical (IHC) staining
The TMAs were probed with a FOXO1 monoclonal antibody (#2880, Cell Signaling Technology) overnight at 4°C. After incubation with a suitable secondary antibody, the TMAs were exposed to diaminobenzidine and counterstained with hematoxylin for detection. To quantify FOXO1 protein expression, all tissues were observed and photographed using a microscope (Carl Zeiss) and scored according to the ratio and intensity of positive cells: 0-5% scored 0; 6-35% scored 1; 36-70% scored 2; 70-100% scored 3. The final expression levels of FOXO1 were categorized as “low” or “high” as follows: score 0-1, low expression; 2-3, high expression. FOXO1 expression was quantified by two independent pathologists.
Cell culture
HPNE, BxPC3, and PANC1 cells were purchased from the American Type Culture Collection (ATCC). All cells were maintained under standard culture conditions (37°C, 5% CO2) in culture medium recommended by the ATCC. All cells were characterized by DNA typing at the Shanghai Jiao Tong University Analysis Core.
RNA isolation and quantitative reverse-transcription (RT-q)PCR
Total RNA was purified from PDAC and adjacent tissues or cells using TRIzol (Invitrogen), following the manufacturer’s protocol. RNA (1 μg) was reverse-transcribed using SuperScript Reverse Transcriptase III (Invitrogen). qPCRs were run using SYBR green Supermix (ABI) on an ABI 7300 instrument. GAPDH was used as a reference gene. Primers used in this study are listed in Table 1.
Table 1.
Sequence of primers
| Name | Sequence (5’-3’) |
|---|---|
| FOXO1 | F: ACTCTGAGAAGTGCCTGATGT |
| R: TGTGGCTGACAAGACTTAACTCAA | |
| GAPDH | F: AGCCTCAAGATCATCAGCAATGCC |
| R: TGTGGTCATGAGTCCTTCCACGAT | |
| CCND1 | F: TCCTCTCCAAAATGCCAGAG |
| R: GGCGGATTGGAAATGAACTT | |
| CCNE1 | F: GAAATGGCCAAAATCGACAG |
| R: TCTTTGTCAGGTGTGGGGA | |
| MYC | F: CGTCCTCGGATTCTCTGCTC |
| R: CTTCGCTTACCAGAGTCGCT | |
| E-cadherin | F: CGAGAGCTACACGTTCACGG |
| R: GGGTGTCGAGGGAAAAATAGG | |
| Fibronectin | F: CGGTGGCTGTCAGTCAAAG |
| R: AAACCTCGGCTTCCTCCATAA | |
| ZEB1 | F: GATGATGAATGCGAGTCAGATGC |
| R: ACAGCAGTGTCTTGTTGTTGT |
Western blotting
Tissues and cells were lysed in WB/IP lysis buffer (P0013; Beyotime) and nuclear proteins were extracted using lysis buffer (P0028; Beyotime), per the manufacturer’s protocols. The cell lysates were boiled in 5× loading buffer for 10 min, resolved by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. The following antibodies were used in this study: anti-FOXO1 (#2880) and anti-β-catenin (#8480) from Cell Signaling Technology, and anti-lamin A/C (10298-1-AP) and anti-GAPDH (60004-1-Ig) from Proteintech. Immunocomplexes were visualized using an ECL kit (Thermo Scientific).
Construction of stable cell lines
To generate cell lines with stable ectopic FOXO1 expression, a vector containing full-length FOXO1 was purchased from GeneCopoeia. MiR-27a inhibitor and a negative control vector were purchased from GeneCopoeia. HEK293T cells were transfected with these vectors using Lipofectamine 2000 (Invitrogen), following the manufacturer’s instructions. Virus-containing media were centrifuged to remove cellular contaminants, and the supernatant containing the viruses was used to infect the cells. Transformed cells were selected on puromycin (2 μg/ml) for 2 weeks. FOXO1 overexpression was confirmed by western blotting prior to further analysis.
Cell viability assays
Cells were seeded into a 96-well plate at 3 × 103 cells/well in 100 µl culture medium and incubated at 37°C in the presence of 5% CO2. Ten microliters of CCK8 solution (Dojindo) was added to each well. After 1.5 h of incubation, the absorbance at 450 nm was read using a Power Wave XS microplate reader (BioTek).
Flow-cytometric cell cycle assays
Cells were seeded in 6-well plates at 2 × 105 cells/well in 100 µl culture medium and incubated at 37°C in the presence of 5% CO2. Cells were collected at 24, 48, and 72 h. The cells were washed twice with 1× PBS, resuspended, and fixed in 2 ml of 70% ethanol at -20°C. Then, the cells were stained with propidium iodide (BD), according to the manufacturer’s instructions.
Transwell cell invasion assay
For invasion assays, we used 8-μm filter-insert chambers (Millipore). Cells (4 × 104) in 100 μl of serum-free medium were placed in the upper chamber, which had been coated with 100 μl of Matrigel (BD Biosciences), and 0.7 ml of medium containing 10% FBS was placed in the lower chamber. After incubation for 24 h, cells on the upper side of the filters were wiped off with cotton-tipped swabs, and the filters were washed with PBS. Then, the cells were fixed in 2.5% glutaraldehyde for 15 min and stained with 0.5% crystal violet for 15 min. Cells on the lower side of the filters were viewed and counted under a microscope.
Animal experiment
Cells stably overexpressing FOXO1 (1 × 106) or control cells were subcutaneously injected into the right flanks of 5 BALB/c (nu/nu) mice. Tumor size was measured once a week. After 4 weeks, the mice were sacrificed for tumor burden analysis. The tumor volume was calculated as volume = (length × width2)/2. For intrahepatic metastasis assays, cells (3 × 106/mouse) were injected into the livers of nude mice.
Immunofluorescence
Coverslip-grown cells were fixed in 4% paraformaldehyde at room temperature for 15 min and rinsed with PBS three times. The cells were permeabilized in 0.3% Triton X-100/PBS for 15 min and then rinsed with PBS three times. Subsequently, the cells were blocked in 5% bovine serum albumin in PBS at 25°C for 1 h. Then, the cells were incubated overnight with primary antibodies at 1:100 dilution in staining buffer at 4°C in a humid chamber. The coverslips were subsequently washed three times. Then, the cells were incubated for 1 h with secondary antibodies labeled with Alexa Fluor 488 or Alexa Fluor 595 (Invitrogen) at 1:200 dilution in staining buffer at 37°C in a humid chamber in the dark. Prior to mounting using Vectashield with DAPI (Vector Laboratories), the coverslips were washed in PBS three times. Immunofluorescence analysis was conducted using a Carl Zeiss LSM710 Laser Scanning Microscope.
Luciferase reporter assays
Indicated cells were seeded in 96-well plates at 2 × 103 cells/well and transfected with 100 ng TCF/β-catenin reporter plasmid (Wnt/β-catenin signaling) or mutated TCF/b-catenin reporter plasmid and 10 ng Renilla, or vectors containing FOXO1 3’-UTR, FOXO1 mutant 3’-UTR, and miR-27a using Lipofectamine 2000 per the manufacturer’s protocol. After a 48-h incubation, firefly and Renilla luciferase activities in cell lysates were measured using the dual-luciferase reporter assay system (Promega, Madison, WI).
Statistics analysis
Data are expressed as the mean ± standard deviation. Correlations between FOXO1 expression and clinicopathological parameters were evaluated using the Chi-squared test. Student’s t-tests were used to compare group means. P < 0.05 was considered significant.
Results
FOXO1 is downregulated in PDAC tissues and low FOXO1 expression correlates with clinical outcome
We first measured FOXO1 mRNA expression in 57 fresh PDAC tissues and FOXO1 protein levels in three paired PDAC samples. The data indicated that FOXO1 was remarkably downregulated in PDAC malignant tissues (Figure 1A and 1B). Next, we evaluated FOXO1 mRNA and protein levels in the normal pancreatic ductal epithelial cell line HPNE and three PDAC cell lines, AsPC1, BxPC3, and PANC1. FOXO1 expression was significantly lower in PDAC malignant cell lines than in HPNE cells (Figure 1C). Furthermore, we evaluated FOXO1 expression by IHC staining using a TMA containing 136 paired PDAC and adjacent tissues, which revealed that FOXO1 was downregulated in most PDAC tissues (Figure 1D). Clinicopathological data analysis revealed that downregulation of FOXO1 was closely correlated with liver metastasis, tumor size, vascular invasion, and TNM stage (Table 2). Survival analysis indicated that patients with low FOXO1 expression have a poor prognosis (Figure 1E).
Figure 1.
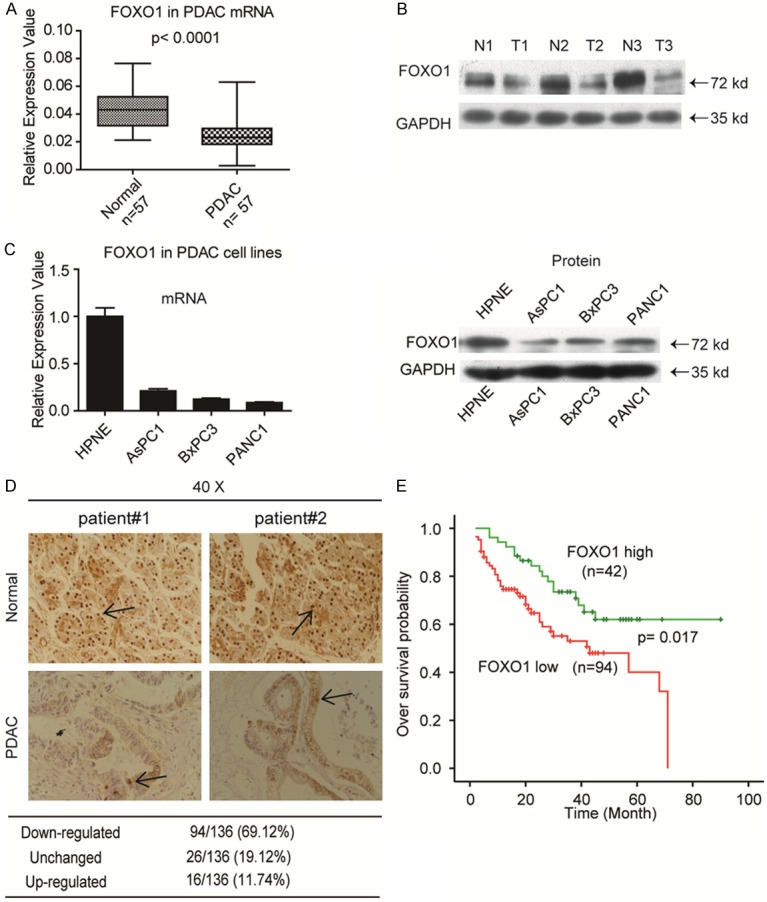
FOXO1 is downregulated and predicts poor patient prognosis in patients with PDAC. A: FOXO1 mRNA levels as detected by RT-qPCR in fresh PDAC tissues and normal tissues (n = 57). B: FOXO1 protein levels as detected by western blotting in three paired clinical normal and PDAC tissues. C: mRNA and protein levels of FOXO1 in the normal pancreatic ductal epithelial cell HPNE and in the indicated PDAC cell lines. D: IHC staining revealing FOXO1 protein expression in PDAC and adjacent tumor tissues of patients (scale bar, 50 µm). E: Patients with low FOXO1 expression have poor survival probability.
Table 2.
Correlations between FOXO1 and key clinicopathological parameters
| Variable | FOXO1 (n=136) | |||
|---|---|---|---|---|
|
| ||||
| Low (94) | High (42) | P value (χ2) | ||
| Age | ≤ 65 years | 35 | 22 | 0.098 |
| > 65 years | 59 | 20 | ||
| Gender | Female | 44 | 17 | 0.493 |
| Male | 50 | 25 | ||
| Liver metastasis | Yes | 68 | 23 | 0.044* |
| No | 26 | 19 | ||
| Tumor size | ≤ 5 cm | 45 | 31 | 0.004* |
| > 5 cm | 49 | 11 | ||
| Vascular invasion | Yes | 32 | 5 | 0.007* |
| No | 62 | 37 | ||
| TNM stage | T1-T2 | 74 | 24 | 0.001* |
| T3-T4 | 27 | 18 | ||
P < 0.05.
FOXO1 inhibits PDAC cell proliferation and invasion
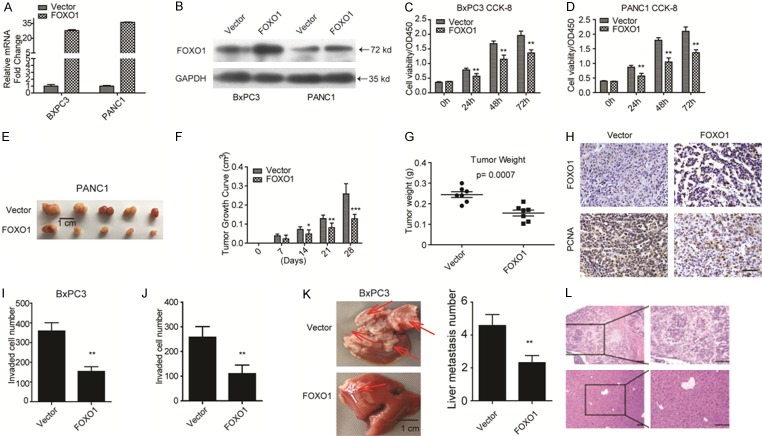
The confirmation of the clinical significance of FOXO1 prompted us to determine its biological role in PDAC progression. We ectopically expressed FOXO1 in the cell lines BxPC3 and PANC1. FOXO1 mRNA and protein levels were significantly elevated in these cell lines (Figure 2A and 2B). We then employed these stable cell lines in biological functional assays. CCK-8 cell viability assays showed that ectopic expression of FOXO1 significantly inhibited cell viability in both BxPC3 and PANC1 cell lines (Figure 2C and 2D). Subcutaneous tumor formation assays revealed that FOXO1 overexpression impaired tumor formation in vivo (Figure 2E and 2G), suggesting that FOXO1 inhibits the proliferation and growth of PDAC cells in vivo. IHC analysis revealed that the expression of FOXO1 was negatively correlated with that of PCNA, a well-accepted cell proliferation marker (Figure 2H). Together, these findings suggested that ectopic expression of FOXO1 inhibits PDAC cell proliferation and growth. As downregulation of FOXO1 was correlated with PDAC liver metastasis, we next performed Transwell invasion assays. FOXO1 overexpression remarkably inhibited the invasion ability of BxPC3 and PANC1 cells (Figure 2I and 2J). Intrahepatic metastasis assays confirmed these findings (Figure 2K and 2L).
Figure 2.
FOXO1 inhibits PDAC cell proliferation. (A, B) mRNA and protein levels of FOXO1 are significantly upregulated in FOXO1-overexpressing BXPC3 and PANC1 cell lines. (C, D) CCK-8 cell viability assays revealed that ectopic expression of FOXO1 significantly inhibits BxPC3 (C) and PANC1 (D) cell proliferation. (E) Xenograft tumor formation assays showed that overexpression of FOXO1 inhibits subcutaneous tumor formation capacity. (F, G) Tumor growth (F) and tumor weight (G) of xenografts of FOXO1-overexpressing PANC1 stable cells in nude mice. (H) IHC staining revealed that the expression of FOXO1 and PCNA in PANC1 stable cells induced tumor formation. (I, J) Matrigel-coated Transwell assays showed that overexpression of FOXO1 inhibits BxPC3 (I) and PANC1 (J) cell invasion. (K, L) Images showing intrahepatic metastasis (K) and morphological characters by HE staining (L), **P < 0.01. All assays were conducted in triplicate.
Ectopic expression of FOXO1 in PDAC cells inhibits Wnt/β-catenin signaling
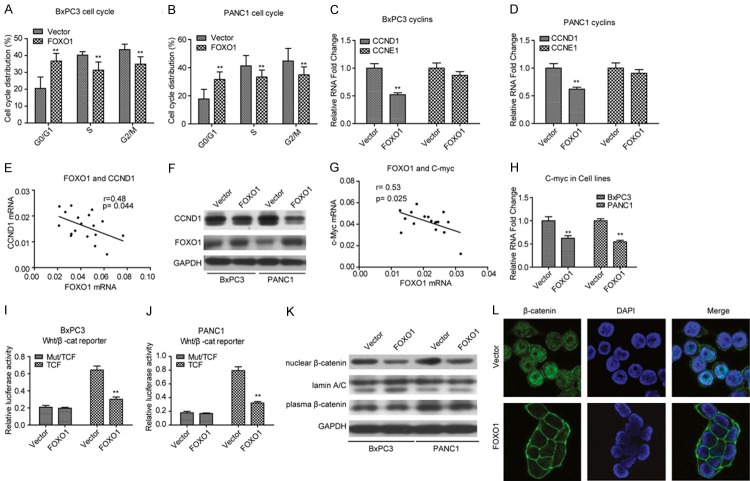
Next, we evaluated cell cycle alterations in FOXO1-expressing BxPC3 and PANC1 stable cell lines by flow cytometry. FOXO1 overexpression remarkably inhibited cell cycle transition from G0/G1 to S (Figure 3A and 3B). Therefore, we examined the expression of CCND1 and CCNE1, which play essential roles in G0/G1-to-S transition. Consistently, FOXO1 overexpression significantly inhibited CCND1, but not CCNE1 expression in PDAC cell lines (Figure 3C and 3D). To confirm these findings, we measured the expression of CCND1 in 19 fresh PDAC tissue samples. The data indicated that CCND1 expression was negatively correlated with FOXO1 expression (Figure 3E). Moreover, CCND1 protein levels were decreased in the FOXO1-expressing BxPC3 and PANC1 stable cell lines (Figure 3F). Collectively, these results indicated that FOXO1 overexpression inhibited PDAC cell cycle transition from G0/G1 to S by affecting the expression of CCND1.
Figure 3.
Ectopic expression of FOXO1 induces arrest of G0/G1-to-S transition in PDAC cell lines. (A, B) Cell cycle assay showed that overexpression of FOXO1 induces arrest of G0/G1-to-S transition BxPC3 (A) and PANC1 (B) cell lines. (C, D) mRNA levels of CCND1 and CCNE1 in FOXO1-overexpressing BxPC3 (C) and PANC1 (D) stable cell lines. (E) CCND1 expression is negatively correlated with FOXO1 expression in fresh PDAC tissues. (F) Protein levels of CCND1 in FOXO1-overexpressing BxPC3 and PANC1 cell lines. (G) The CCND1 mRNA level is negatively correlated with FOXO1 expression in fresh PDAC tissues. (H) mRNA level of MYC in FOXO1-overexpressing BxPC3 and PANC1 stable cell lines. (I, J) Dual luciferase reporter assays revealed that overexpression of FOXO1 inhibits Wnt/β-catenin signaling activity (TCF/β-catenin reporter) in BxPC3 (I) and PANC1 (J) cell lines, but not in cells carrying mutated TCF/β-catenin reporter plasmid. (K) Overexpression of FOXO1 impairs nuclear translocation of β-catenin in BxPC3 and PANC1 cell lines. (L) Immunofluorescence assays revealed that overexpression of FOXO1 impairs nuclear β-catenin translocation nuclear in BxPC3 cells. **P < 0.01. All assays were conducted in triplicate.
Previous studies have verified that CCND1 is a target of Wnt/β-catenin signaling, which strongly suggests that FOXO1 overexpression might result in inhibition of Wnt/β-catenin signaling activity. To investigate the relationship between FOXO1 and Wnt/β-catenin signaling, we examined another well-known target of Wnt/β-catenin signaling, MYC, in 19 fresh PDAC tissues. As shown in Figure 3G, the expression of MYC was similar to that of CCND1, which was negatively correlated with the expression of FOXO1. These findings prompted us to explore the correlation between FOXO1 and Wnt/β-catenin signaling. As shown in Figure 3H, MYC expression was remarkably suppressed in FOXO1-overexpressing BxPC3 and PANC1 cells. Dual luciferase reporter assays in the two stable cell lines revealed that elevated FOXO1 expression significantly inhibited Wnt/β-catenin signaling activity (Figure 3I and 3J). In addition, FOXO1 overexpression resulted in a robust decrease in nuclear β-catenin, but did not affect cytoplasmic β-catenin (Figure 3K and 3L). These results suggested that FOXO1 inhibits nuclear translocation of β-catenin and thus inhibits Wnt/β-catenin signaling.
Ectopic expression of β-catenin rescues cell proliferation and invasion impaired by FOXO1 overexpression
To confirm that FOXO1 exerts its anti-tumor effects through Wnt/β-catenin signaling, we ectopically expressed β-catenin in stably FOXO1-overexpressing BxPC3 cells. As shown in Figure 4A, overexpression of β-catenin partly rescued the inhibition of cell proliferation resulting from ectopic expression of FOXO1. Similar rescue effects were observed in both BxPC3 and PANC1 invasion assays (Figure 4B and 4C). We also found that the expression of the epithelial-mesenchymal transition (EMT) marker E-cadherin was reduced upon overexpression of β-catenin, whereas that of fibronectin and ZEB1 was increased (Figure 4D). These findings suggested that FOXO1 inhibits cell proliferation and invasion through Wnt/β-catenin signaling.
Figure 4.
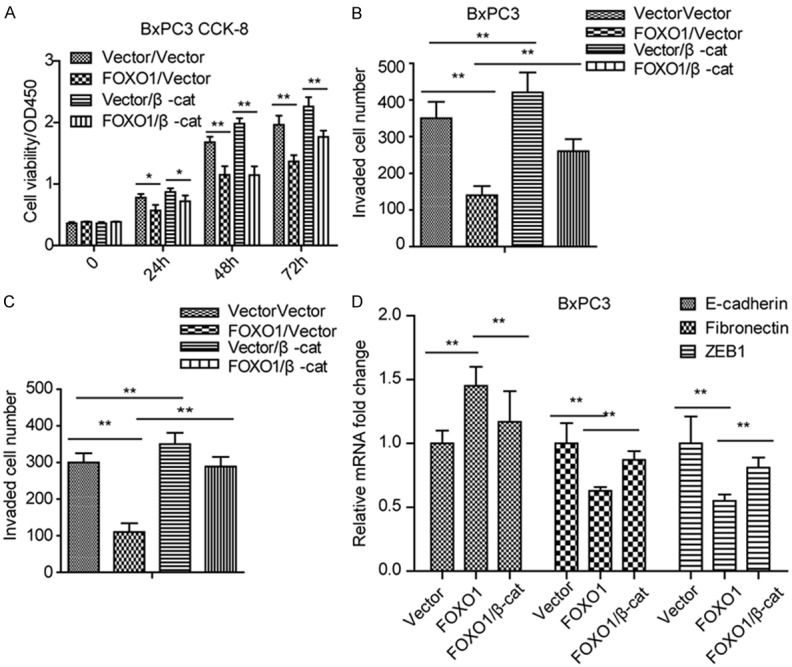
Overexpression of β-catenin signaling rescues cell proliferation and invasion under FOXO1 ectopic expression. (A) Overexpression of β-catenin rescues the inhibition of cell proliferation resulting from ectopic FOXO1 expression in BxPC3 cells. (B, C) Overexpression of β-catenin rescues the inhibition of cell invasion resulting from ectopic FOXO1 expression in BxPC3 (B) and PANC1 (C) cell lines. (D) Overexpression of β-catenin rescues the expression of EMT markers under ectopic FOXO1 expression in BxPC3 cells. *P < 0.05; **P < 0.01. All assays were conducted in triplicate.
MiR-27a targets FOXO1 in PDAC tissues and cell lines
Next, we aimed to explore the mechanisms by which FOXO1 is downregulated in PDAC tissues. DNA methylation and microRNAs can drive tumor suppressor inhibition in cancer development. We found no CpG islands in the FOXO1 promoter region (data not shown). Thus, we used TargetScan and miRBase to screen for miRNAs potentially targeting FOXO1. We found that miR-27a, miR-96, and miR-182 were commonly predicted to target FOXO1 by these two databases. We examined the expression of these three miRNAs in the 57 fresh PDAC tissues. As shown in Figure 5A-C, miR-27a, but not miR-96 and miR-182, was significantly elevated in all 57 PDAC malignant tissues. Therefore, we analyzed the correlation between miR-27a and FOXO1 in tissues and we found that FOXO1 mRNA levels were negatively correlated with miR-27a levels (Figure 5D). To validate FOXO1 as a direct target of miR-27a, fragments from the FOXO1 3’-UTR containing the putative miR-27a target sites were cloned into a luciferase reporter construct (Figure 5E). The results showed that miR-27a overexpression substantially suppressed the activity of the reporter carrying the wild-type 3’-UTR of FOXO1, but not that of the reporter carrying the mutant 3’-UTR (Figure 5F). To confirm this, we treated BxPC3 and PANC1 cells with miR-27a inhibitor and then measured FOXO1 expression. As shown in Figure 5G, inhibition of miR-27a rescued FOXO1 expression in both cell lines. Furthermore, miR-27a inhibitor significantly inhibited BxPC3 cell proliferation and PANC1 cell invasion (Figure 5H and 5I). Finally, we analyzed the correlation between miR-27a and prognosis, and we found that high miR-27a expression predicted a dismal prognosis in PDAC patients (Figure 5J). Taken together, these findings indicated that highly expressed miR-27a inhibits FOXO1 expression in PDAC tissues, thereby promoting PDAC progression.
Figure 5.
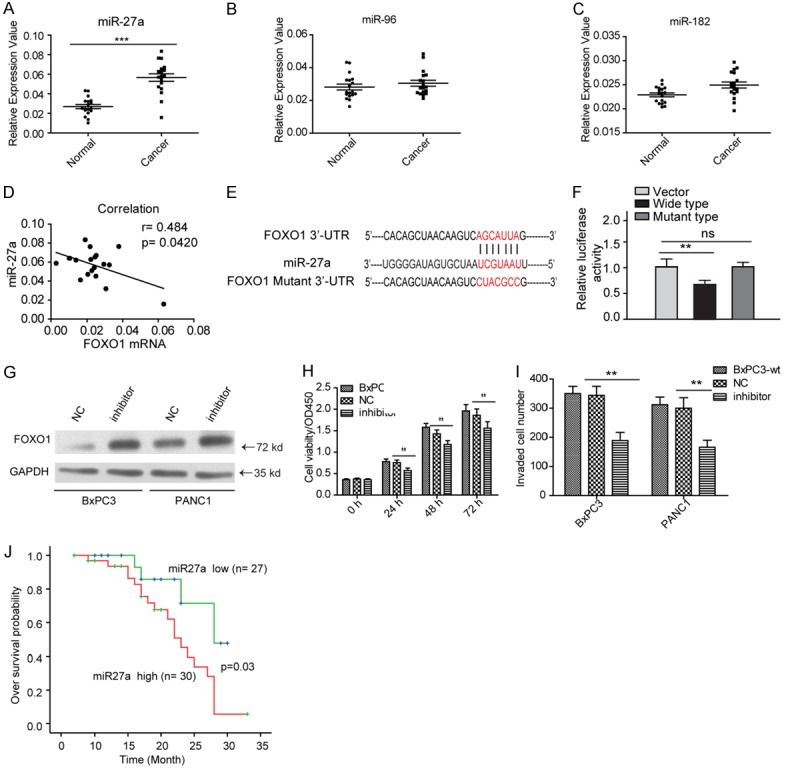
FOXO1 is directly targeted by miR-27a in PDAC. (A-C) Expression of miR-27a (A), miR-96 (B), and miR-182 (C) in fresh PDAC tissues. (D) The FOXO1 mRNA level is negatively correlated with miR-27a expression. (E) Sequence of miR-27a, and the 3’-UTR and mutant 3’-UTR of FOXO1. (F) Luciferase reporter assays showed that miR-27a specifically targets FOXO1 in BxPC3 cells. (G) Inhibition of miR-27a elevates FOXO1 expression in BxPC3 and PANC1 cell lines. “NC”, negative control. (H, I) Inhibition of miR-27a inhibits BxPC3 (H) cell proliferation and PANC1 (I) cell invasion. (J) miR-27a predicts poor prognosis in PDAC patients. “NC”, negative control. **P < 0.01. All these assays were conducted in triplicate.
Discussion
PDAC is a globally prevalent and highly aggressive malignancy. Despite the tremendous efforts put in treating PDAC, clinical outcomes remain poor. Therefore, a better understanding of the underlying molecular mechanisms from multiple perspectives is highly desirable [17,18]. Tumor initiation causes uncontrolled cell proliferation, and it is widely accepted that genetic and epigenetic alterations contribute to malignancy [18]. We previously found that FOXO1 is downregulated in PDAC tissues, and low FOXO1 expression correlates with the stemness of PDAC cells [7].
In this study, we first confirmed the expression pattern of FOXO1 in fresh PDAC tissues, and we found that FOXO1 is strongly depleted in malignant compared to adjacent tissues due to downregulation of its expression, which plays an important role in PDAC tumorigenesis. Furthermore, we found that downregulated FOXO1 not only promoted PDAC cell proliferation and growth both in vitro and in vivo, but also increased cell invasion. Aberrant cell cycle progression and invasion contribute to uncontrolled cell proliferation and metastasis [19-21]. Cancer cells employ various mechanisms, such as mutation of tumor suppressors p53 or PTEN or aberrant overexpression of cyclins, to evade cell cycle checkpoints and promote cell proliferation [22,23]. In this study, we found that CCND1 expression was inversely correlated with FOXO1 expression, suggesting that the downregulation of FOXO1 might, in part, promote cell proliferation by modulating cyclins.
Previous studies have demonstrated abnormal activation of Wnt/β-catenin signaling in numerous solid tumors, including PDAC [24,25]. Moreover, CCND1 is a downstream target of Wnt/β-catenin signaling [26]. In this study, we found that Wnt/β-catenin signaling activity was negatively correlated with the FOXO1 expression level. Additionally, the level of nuclear β-catenin, a transcription factor activated by Wnt/β-catenin signaling, decreased when FOXO1 was ectopically expressed. These findings suggested that elevated FOXO1 could in some ways promote β-catenin entry into the nucleus to regulate CCND1 expression and thus promote cell proliferation and growth. Furthermore, we found that downregulation of FOXO1 promoted PDAC cell EMT, and overexpression of β-catenin rescued this effect. Wnt/β-catenin signaling not only plays a central role in cell physiological processes, it can also target various oncogenes, such as TCF-1 and MMP7, all of which can contribute to tumor progression [27].
We found that FOXO1 expression in PDAC tissues was suppressed by highly expressed miR-27a. Moreover, miR-27a predicted poor prognosis of PDAC patients, suggesting that miR-27a targets FOXO1 and to exert aggressive effects.
In conclusion, we confirmed the expression and clinical significance of FOXO1 and explored its mechanism of action in PDAC. We found that highly expressed miR-27a targets FOXO1 to promote PDAC progression. The miR-27a/FOXO1/β-catenin axis might be a potential therapeutic target and might be useful to reduce PDAC progression in future clinical applications.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Pysz MA, Machtaler SB, Seeley ES, Lee JJ, Brentnall TA, Rosenberg J, Tranquart F, Willmann JK. Vascular endothelial growth factor receptor type 2-targeted contrast-enhanced US of pancreatic cancer neovasculature in a genetically engineered mouse model: potential for earlier detection. Radiology. 2015;274:790–799. doi: 10.1148/radiol.14140568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Q, Zhang TP, Zhao YP. Advances in early diagnosis and therapy of pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2011;10:128–135. doi: 10.1016/s1499-3872(11)60021-0. [DOI] [PubMed] [Google Scholar]
- 4.Song HM, Song JL, Li DF, Hua KY, Zhao BK, Fang L. Inhibition of FOXO1 by small interfering RNA enhances proliferation and inhibits apoptosis of papillary thyroid carcinoma cells via Akt/FOXO1/Bim pathway. Onco Targets Ther. 2015;8:3565–3573. doi: 10.2147/OTT.S95395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marfe G, Tafani M, Fiorito F, Pagnini U, Iovane G, De Martino L. Involvement of FOXO transcription factors, TRAIL-FasL/Fas, and sirtuin proteins family in canine coronavirus type II-induced apoptosis. PLoS One. 2011;6:e27313. doi: 10.1371/journal.pone.0027313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song W, Li Q, Wang L, Huang W, Wang L. FoxO1-negative cells are cancer stem-like cells in pancreatic ductal adenocarcinoma. Sci Rep. 2015;5:10081. doi: 10.1038/srep10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Zhao Y, Liao W, Yang J, Wu L, Zheng Z, Yu Y, Zhou W, Li L, Feng J, Wang H, Zhu WG. Acetylation of FoxO1 activates Bim expression to induce apoptosis in response to histone deacetylase inhibitor depsipeptide treatment. Neoplasia. 2009;11:313–324. doi: 10.1593/neo.81358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 11.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulpizio S, Franceschini N, Piattelli A, Di Sebastiano P, Innocenti P, Selvaggi F. Cathepsins and pancreatic cancer: the 2012 update. Pancreatology. 2012;12:395–401. doi: 10.1016/j.pan.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Neureiter D, Jager T, Ocker M, Kiesslich T. Epigenetics and pancreatic cancer: pathophysiology and novel treatment aspects. World J Gastroenterol. 2014;20:7830–7848. doi: 10.3748/wjg.v20.i24.7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomberk G, Blum Y, Nicolle R, Nair A, Gaonkar KS, Marisa L, Mathison A, Sun Z, Yan H, Elarouci N, Armenoult L, Ayadi M, Ordog T, Lee JH, Oliver G, Klee E, Moutardier V, Gayet O, Bian B, Duconseil P, Gilabert M, Bigonnet M, Garcia S, Turrini O, Delpero JR, Giovannini M, Grandval P, Gasmi M, Secq V, De Reynies A, Dusetti N, Iovanna J, Urrutia R. Distinct epigenetic landscapes underlie the pathobiology of pancreatic cancer subtypes. Nat Commun. 2018;9:1978. doi: 10.1038/s41467-018-04383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Ma C, Pang H, Zeng F, Cheng L, Fang B, Ma J, Shi Y, Hong H, Chen J, Wang Z, Xia J. Arsenic trioxide suppresses cell growth and migration via inhibition of miR-27a in breast cancer cells. Biochem Biophys Res Commun. 2016;469:55–61. doi: 10.1016/j.bbrc.2015.11.071. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Xiang J, Shen J, Zou X, Zhai S, Yin Y, Li P, Wang X, Sun Q. Oncogenic MicroRNA-27a is a target for genistein in ovarian cancer cells. Anticancer Agents Med Chem. 2013;13:1126–1132. doi: 10.2174/18715206113139990006. [DOI] [PubMed] [Google Scholar]
- 17.Fokas E, O’Neill E, Gordon-Weeks A, Mukherjee S, McKenna WG, Muschel RJ. Pancreatic ductal adenocarcinoma: from genetics to biology to radiobiology to oncoimmunology and all the way back to the clinic. Biochim Biophys Acta. 2015;1855:61–82. doi: 10.1016/j.bbcan.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, DePinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016;30:355–385. doi: 10.1101/gad.275776.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algul H. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai A, Matsuda M, Kiyokawa E. Activated Ras protein accelerates cell cycle progression to perturb Madin-Darby canine kidney cystogenesis. J Biol Chem. 2012;287:31703–31711. doi: 10.1074/jbc.M112.377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Gao S, Ren H, Sun W, Zhang H, Sun J, Yang S, Hao J. Hypoxia-inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-bundling protein fascin. Cancer Res. 2014;74:2455–2464. doi: 10.1158/0008-5472.CAN-13-3009. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Hui Y, Lin L, Wu Y, Zhang X, Qin X. Possible relevance of tumor-related genes mutation to malignant transformation of endometriosis. Eur J Gynaecol Oncol. 2016;37:89–94. [PubMed] [Google Scholar]
- 23.Liu G, Ren X, Gao C, Zhang W. Acylglycerol kinase promotes the proliferation and cell cycle progression of oral squamous cell carcinoma. Mol Med Rep. 2015;12:2225–2230. doi: 10.3892/mmr.2015.3602. [DOI] [PubMed] [Google Scholar]
- 24.Gough NR. Focus issue: Wnt and beta-catenin signaling in development and disease. Sci Signal. 2012;5:eg2. doi: 10.1126/scisignal.2002806. [DOI] [PubMed] [Google Scholar]
- 25.Shibayama Y, Fujimori T, Nguyen G, Hirose T, Totsune K, Ichihara A, Kitada K, Nakano D, Kobori H, Kohno M, Masaki T, Suzuki Y, Yachida S, Nishiyama A. (Pro)renin receptor is crucial for Wnt/beta-catenin-dependent genesis of pancreatic ductal adenocarcinoma. Sci Rep. 2015;5:8854. doi: 10.1038/srep08854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C, Zhang Y, Li J, Wang Y, Ren F, Zhou Y, Wu Y, Feng Y, Zhou Y, Su F, Jia B, Wang D, Chang Z. p15RS/RPRD1A (p15INK4b-related sequence/regulation of nuclear pre-mRNA domain-containing protein 1A) interacts with HDAC2 in inhibition of the Wnt/beta-catenin signaling pathway. J Biol Chem. 2015;290:9701–9713. doi: 10.1074/jbc.M114.620872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, Tang N, Haydon RC, Luu HH, He TC. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87:97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]