Abstract
Breast cancer is one of the most common cancers among women. Long non-coding RNAs (lncRNAs) are involved in the initiation and development of breast cancer and lncRNA H19 is a potential oncogenic factor; however, the underlying mechanisms remain unknown. In the present study, the regulatory functions of H19 in breast cancer were investigated. We found that H19 was upregulated in breast cancer tissues and cells and associated with poor prognosis. MiR-138 was downregulated in breast cancer tissues and negatively correlated with the expression of H19 and SOX4. Furthermore, SOX4 was upregulated in breast cancer tissues and positively correlated with H19. Downregulated H19 suppressed the proliferation, invasion and migration of breast cancer cells, but promoted cell cycle arrest and apoptosis. Additionally, miR-138 was identified as a direct target of H19 and SOX4; overexpression of miR-138 inhibited the proliferation, invasion and migration of MDA-MB-231 and MCF-7 cells, but promoted apoptosis, which were abrogated by SOX4 overexpression. Downregulated miR-138 induced cell proliferation, invasion and migration, but inhibited apoptosis of MDA-MB-231 and MCF-7 cells, which were promoted by SOX4 overexpression. In addition, miR-138 overexpression reversed the effects of H19 in breast cancer cells; silencing of H19 inhibited tumor growth and downregulate EMT markers in vivo. In summary, H19 was upregulated in breast cancer and associated with poor prognosis. Silencing of H19 inhibited cell proliferation, invasion and migration, but induced cell cycle arrest and apoptosis by regulating miR-138 and SOX4 in breast cancer.
Keywords: Breast cancer, long non-coding RNA H19, microRNA-181, SOX4, proliferation, invasion, migration, cell cycle, apoptosis
Introduction
Breast cancer is one of the most common types of cancer and the second prevalent and aggressive cancer, which has become a great threat against women’s health [1]. High mortality in patients with breast cancer is caused by tumor metastasis [2]. The standard first-line treatment of newly diagnosed patients involves a variety of modalities, including surgery with adjuvant radiotherapy, hormone therapy, chemotherapy and targeted biologic therapy [3]; however, these treatments exhibit side effects, and novel effective therapies could be developed. Thus, potential molecular targets for breast cancer should be identified.
Long noncoding RNAs (lncRNAs) are a novel family of non-coding RNAs which consist ~200 nucleotides and are ~200 kb in length [4]. LncRNAs are involved in various biological processes including gene expression and cell multiplication and differentiation [5]. Furthermore, lncRNAs regulate gene expression at epigenetical, transcriptional and post-transcriptional levels [6]. LncRNAs have been demonstrated to regulate numerous biological and pathological processes [7]. Previous studies have revealed aberrant expression levels of lncRNAs in cancer cells, suggesting the essential roles of lncRNAs in the initiation and development of a variety of cancers such as breast cancer [8,9]. Previous studies have reported that lncRNA H19 was upregulated in retinoblastoma, oral squamous cell carcinoma, lung cancer, hepatic carcinogenesis, endometrial carcinoma and breast cancer [10-14]. H19 has been revealed to be associated with tumorigenesis by regulating various mechanisms and pathways [15]. Additionally, previous studies have demonstrated that H19 is a putative biomarker and prognostic indicator of breast cancer [16,17]; however, the underlying molecular mechanisms have not been completely elucidated.
MicroRNAs (miRNAs), a class of endogenously small non-coding RNAs having a length of 21-23 nucleotides, are potential targets of lncRNAs [18]. Recent studies have revealed that miRNAs are dysregulated in numerous types of cancer, and could be involved in tumorigenesis and tumor development [19,20]. MiR-138 was aberrantly expressed in various cancers such as breast cancer [21]. Furthermore, upregulation of miR-138 could suppress the proliferation, invasion and migration of breast cancer cells [21]; however, the underlying mechanisms require further investigation. In the present study, the mechanism of H19-mediated miR-138/SOX4 signaling pathway in breast cancer cells were investigated, which may provide novel insight into potential therapeutic targets for the treatment of breast cancer.
Materials and methods
Patient samples
Forty patients diagnosed with breast cancer between January 2014 and January 2015 were recruited in the present study, and none of the patients had received radiotherapy, chemotherapy or other treatments prior to surgery. The patients are in the 23-75 age bracket (57.3 ± 11.7), and there were 27 patients with lymph node metastasis and 15 patients with I-II stage. Three-year follow-up of the 80 patients was carried out. Tumor and adjacent normal tissues were collected at surgery and snap-frozen in liquid nitrogen post-surgery. The present study was approved by the Ethics Committee of The First People’s Hospital of Jiaozuo City, and informed consents were obtained from all patients or their families. The animal experiments were performed with the approval of The First People’s Hospital of Jiaozuo City.
Cell culture
Four human breast cancer cell lines (LCC9, LCC2, MCF-7 and MDA-MB-231) and one normal cell line MCF-10A were obtained from the American Type Culture Collection (Rockville, MD, USA) and cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2.
Vector construction and transfection
SiRNA targeting H19 (si-H19), miR-138 mimics, miRNA control (miR-NC), miR-138 inhibitor and short hairpin RNA (shRNA)/scramble fragments targeting H19 were synthesized by GenePharma (Shanghai, China). SOX4 and H19 overexpression plasmids pcDNA3.1-SOX4 and pcDNA3.1-H19 lacking 3’-UTR were obtained from IBSbio (IBS Solutions Co. Ltd, Shanghai, China). All transfections were performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocols.
Luciferase reporter assays
The fragments of H19 and SOX4-3’UTR containing putative miR-138 binding sites were amplified by PCR and constructed into pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega Corporation, Madison, WI, USA), named as H19-WT and SOX4-3’UTR-WT reporter. QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) was used to generate H19-MUT and SOX4-3’UTR-MUT reporter containing mutant miR-138 binding sites. Then the constructed plasmids were co-transfected with miR-NC or miR-138 into competent cell DH5α. At 48 h post-transfection, luciferase assays were performed using the Firefly Luciferase Reporter Gene Assay Kit (RG005, Beyotime Biotechnology Co., Ltd., Shanghai, China) according to the manufacturer’s protocols. Luminescent signals were quantified with a luminometer (MK3, Thermo Fisher Scientific Inc., Waltham, MA, USA), and the value of firefly luciferase was normalized to that of Renilla luciferase.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tissues or cells was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocols. Reverse transcription was performed to generate the first-strand cDNA using M-MLV Reverse Transcriptase (Promega Corporation). The expression level of mRNA was detected using SYBR Premix Ex Taq II (TaKaRa, Dalian, China). Total RNA was reverse-transcribed by a miScript reverse transcription kit (Qiagen) and then amplified using SYBR Premix Ex Taq™ (TaKaRa) to quantify the miRs. Relative gene expression was analyzed using the 2-ΔΔCq method and the expression levels of mRNAs and miRs were normalized to GAPDH and U6. Each experiment was repeated in triplicate. Primer sequences were as follows: H19, 5’-ATCGGTGCCTCAGCGTTCGG-3’ (forward) and 5’-CTGTCCTCGCCGTCACACCG-3’ (reverse); miR-138, 5’-AGCTGGTGTTGTGAATC-3’ (forward) and 5’-GTGCAGGGTCCGAGGT-3’ (reverse); SOX4, 5’-GTGAGCGAGATGATCTCGGG-3’ (forward) and 5’-CAGGTTGGAGATGCTGGACTC-3’ (reverse); U6, 5’-CTCGCTTCGGCAGCACA-3’ (forward) and 5’-AACGCTTCACGAATTTGCGT-3’ (reverse) and GAPDH, 5’-ACAGTCAGCCGCATCTTCTT-3’ (forward) and 5’-GACAAGCTTCCCGTTCTCAG-3’ (reverse).
Western blot analysis
Total protein from tissues or cells was isolated using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Shanghai, China). Protein concentration was evaluated by a BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Equal amounts of protein were separated by 10% SDS-PAGE gel and transferred to nitrocellulose membrane (Beyotime Institute of Biotechnology), followed by incubation with 5% non-fat milk in Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 7.5, 150 mM NaCl) containing 0.1% Tween 20 for 2 h at room temperature. Subsequently, the membranes were incubated at 4°C overnight with primary antibodies against SOX4 (1:1,000; cat. no. ab80261), N-cad, Vi, FN and GAPDH (1:1,000; cat. no. ab 18203, ab92547, ab2413, and ab181603; Abcam, Cambridge, UK). The membranes were then rinsed with TBST and incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000; cat. no. ab6721; Abcam) at room temperature for additional 2 h. Protein bands were visualized using an enhanced chemiluminescence kit (Thermo Fisher Scientific) and detected by an ECL detection system (Thermo Fisher Scientific). Each experiment was repeated in triplicate, and GAPDH was used as a loading control.
Cell proliferation assay
Cell proliferation was examined using a Cell Counting Kit-8 (CCK8) assay (MedChemExpress, Monmouth Junction, NJ, USA), according to the manufacturer’s protocols. Briefly, MCF-7 and MDA-MB-231 cells (1 × 103 cells/well) were cultured in 96-well plates and transfected with indicated vectors at 37°C with 5% CO2, and 10 μL of CCK-8 solution was added into each well at 0, 24, 48, and 72 h, and incubated for another 3 h. The absorbance at 450 nm was measured by a microplate reader. The cell growth curves were plotted using the absorbance value at each time point.
Cell migration and invasion assays
For cell migration assay, cells were diluted in 200 μl serum-free RPMI-1640 medium and inoculated onto the upper compartments of Transwell chamber coated with Matrigel (BD Biosciences, Franklin Lakes, New Jersey, USA), while the medium containing 10% FBS was added to the lower chambers. The chambers were then incubated at 37°C for 48 h. Non-migratory cells were removed using a cotton swab following overnight incubation, while the migratory cells in the lower chamber were fixed and stained using 100% pre-cold methanol and 0.1% crystal violet. A cell invasion assay was performed in a similar manner, but 20 μl Matrigel (BD Biosciences) was added to the upper chamber. Finally, the number of migrated and invasive cells were observed and counted using an inverted light microscope (magnification × 100, Olympus Corporation, Tokyo, Japan Nikon Corporation, Tokyo, Japan). Five visual fields of each chamber insert were randomly chosen and the average number was counted.
Flow cytometry
At 48 h post-transfection, cells were centrifuged at 403 × g for 10 min and fixed with 70% pre-cooled ethanol at 4°C overnight. Fixed cells were rinsed twice with PBS and 1 ml propidium iodide (PI) (50 mg/L) containing RNAase was added into 100 μl cell suspension. Cells were filtrated using nylon mesh (300 meshes) followed by incubation in dark for 30 min. The red fluorescence of excitation wavelength at 488 nm was measured by flow cytometry to determine the cell cycle. Annexin V-fluorescein isothiocyanate (FITC)/PI staining was used to evaluate the apoptosis. Following the incubation at 37°C for 48 h, cells were resuspended with 200 μl binding buffer, and incubated with 10 μL Annexin V-FITC and 5 μL PI in dark at room temperature for 15 min, followed by the addition with 300 μl binding buffer. Data acquisition and analyses were performed on a Becton Dickinson (Franklin Lakes, NJ, USA) FACSCalibur using CellQuest software (Becton Dickinson).
In vivo nude mouse xenograft assay
Sixteen female BALB/c mice (nu/nu strain), 5-week-old, weighting of 17-22 g were obtained from Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China) and routinely housed in humidity- and temperature-controlled rooms (40-80%; 22 ± 2°C) for at least 3 days prior to the experiments. All the animals were provided with food and water and randomly assigned into two groups, NC and shRNA-H19 group. Nude mice of each group were injected subcutaneously on the right axilla with 0.1 ml of MDA-MB-231 cell suspension transfected with shRNA/scramble fragments targeting H19. Following successful transplantation, the tumor growth of nude mice was observed on day 7, 14, 21, 28, 35 and 42. The tumor volume and weight were determined routinely following inoculation using direct measurement and calculated using the formula V = π/6 × length × width × height. The mice were then sacrificed; tumor tissues were obtained and tumor weights were determined.
Statistical analysis
Statistical analysis was performed using the Graphpad Prism v5.0 (Graphpad Software Inc., La Jolla, CA, USA). All data were presented as mean ± standard deviation (SD). The differences among groups, in at least three separate experiments, were analyzed using a Student’s t-test or one-way analysis of variance. Kaplan-Meier method and log-rank test were performed to determine patient survival and the differences, as appropriate. Spearman’s correlation analysis was applied to analyze the association between miR-138 and H19 or SOX4 mRNA. P < 0.05 was considered to indicate a statistically significant difference.
Results
H19 is upregulated in breast cancer and associated with poor prognosis
The expression level of H19 in tumor and adjacent normal tissues were determined by RT-qPCR. The results revealed that H19 was significantly upregulated in breast cancer compared with the control (Figure 1A). In addition, the expression level of H19 in breast cancer with lymph node metastasis was significantly increased compared with that without metastasis (Figure 1B). Furthermore, H19 was upregulated in patients with grades III-IV breast cancer compared with those with grades I-II (Figure 1C). Kaplan-Meier survival analysis revealed that upregulation of H19 was associated with poor prognosis in patients with breast cancer. In summary, these data indicated that H19 was upregulated in breast cancer and may be associated with its development.
Figure 1.
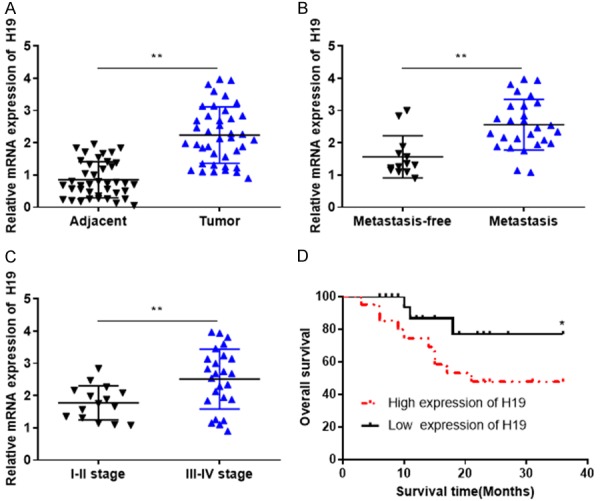
H19 is upregulated in breast cancer and associated with poor prognosis. A. RT-qPCR revealed that the expression of H19 was increased in breast cancer tissues compared with adjacent normal tissues. B. RT-qPCR demonstrated that the expression level of H19 was increased in patients with breast cancer and lymphatic metastasis compared with those without metastasis. C. RT-qPCR revealed that H19 was increased in patients with grades III-IV breast cancer compared with those with grades I-II. D. Kaplan-Meier survival analysis revealed that upregulation of H19 was associated with poor prognosis in patients with breast cancer. *P < 0.05, **P < 0.01, compared with the control group. RT-qPCR, Reverse transcription-quantitative polymerase chain reaction.
H19 is upregulated in breast cancer cells and downregulated H19 suppresses cell proliferation, invasion and migration
RT-qPCR revealed that H19 was upregulated in breast cancer cells (LCC9, LCC2, MCF-7 and MDA-MB-231) compared with normal cell MCF-10A (Figure 2A). Following the transfection of MCF-7 and MDA-MB-231 cells with si-H19, the expression level of H19 was significantly reduced compared with the scrambled control (Figure 2B). In order to investigate the regulatory functions of H19 in breast cancer, CCK-8, Transwell migration and invasion assay were used to evaluate the effects of downregulated H19 on cell proliferation, migration and invasion. The results indicated that downregulation of H19 significantly suppressed the proliferation, invasion and migration of MCF-7 and MDA-MB-231 cells (Figure 2C-H).
Figure 2.
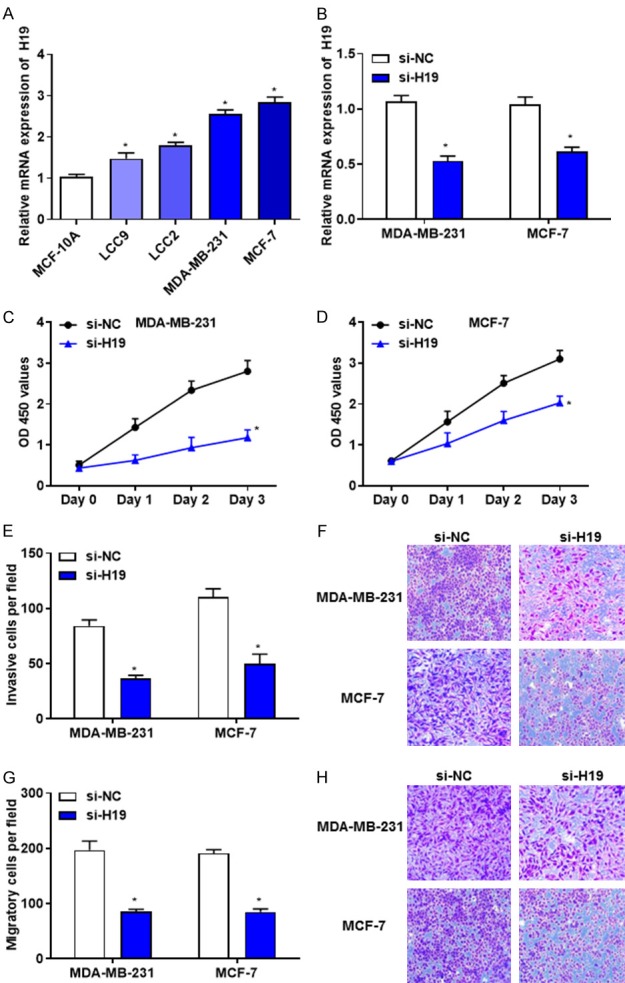
H19 is upregulated in breast cancer cells and downregulated H19 suppresses cell proliferation, invasion and migration. A. RT-qPCR revealed that the expression level of H19 was increased in breast cancer cells. B. RT-qPCR demonstrated that the expression level of H19 was decreased in MDA-MB-231 and MCF-7 cells transfected with si-H19. C and D. The proliferation activities of MDA-MB-231 and MCF-7 cells transfected with si-H19 and si-NC were determined by Cell Counting Kit-8 assay. E and F. The invasive abilities of MDA-MB-231 and MCF-7 cells transfected with si-H19 and si-NC were evaluated using a Transwell assay. G and H. The migratory abilities of MDA-MB-231 and MCF-7 cells transfected with si-H19 and si-NC were determined by a Transwell assay. *P < 0.05. RT-qPCR, Reverse transcription-quantitative polymerase chain reaction.
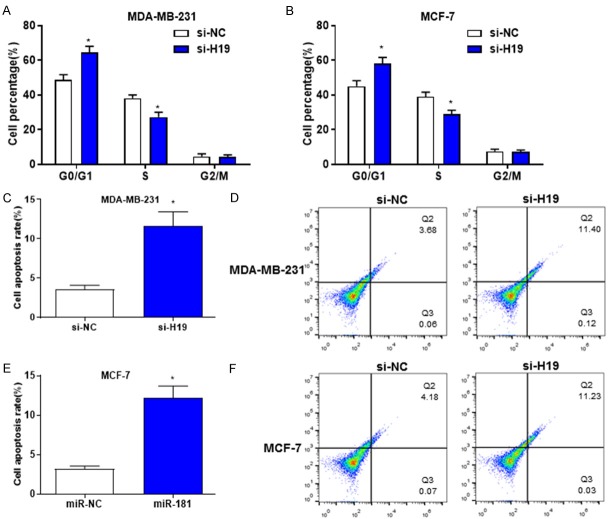
Downregulated H19 induces cell cycle arrest and apoptosis in breast cancer
In order to determine the effects of downregulated H19 on breast cancer cell cycle and apoptosis, PI staining and Annexin V/PI dual parameter test were performed to evaluate the cell cycle and apoptotic rate. The results revealed that downregulated H19 significantly prolonged G0/G1 phase (increased cell ratio at G0/G1 phase) and shortened S phase (decreased cell ratio at S phase) of MDA-MB-231 and MCF-7 cells (Figure 3A and 3B). Furthermore, downregulated H19 promoted the apoptosis of MDA-MB-231 and MCF-7 cells (Figure 3C-F). These results indicated that downregulation of H19 may induce the apoptosis and disrupt the entry of cell cycle in breast cancer cells.
Figure 3.
Downregulated H19 induces breast cancer cell cycle arrest and apoptosis. A and B. the percentage of PI-stained cells at G0/G1, S and G2/M phases in MDA-MB-231 and MCF-7 cells transfected with si-H19 and si-NC were determined by flow cytometry. C-F. The percentage of apoptotic cells in MDA-MB-231 and MCF-7 cells transfected with si-H19 and si-NC were evaluated by flow cytometry. *P < 0.05.
H19 suppresses the expression level of miR-138
Bioinformatic analysis revealed the complementary sites between H19 and miR-138 (Figure 4A). In order to determine the interaction between H19 and miR-138, Dual-luciferase reporter assay was performed, and the results suggested that overexpression of miR-138 significantly attenuated the luciferase activity of H19-WT but not H19-MUT reporter (Figure 4B). Furthermore, whether H19 could regulate the expression of miR-138 and SOX4 in breast cancer cell were investigated. RT-qPCR revealed that the mRNA levels of miR-138 were increased in MDA-MB-231 and MCF-7 cells transfected with si-H19 (Figure 4C). In addition, RT-qPCR and western blotting demonstrated that the mRNA and protein levels of SOX4 were decreased in si-H19-transfected MDA-MB-231 and MCF-7 cells compared with those transfected with si-NC (Figure 4D-F). The association between H19 and miR-138 or SOX4 mRNA was evaluated using Spearman’s correlation analysis. The results revealed that the expression levels of miR-138 and SOX4 were negatively and positively correlated with H19 in breast cancer tissues, respectively, suggesting that H19 may promote breast cancer cell growth, and induce cell cycle arrest by directly targeting miR-138.
Figure 4.
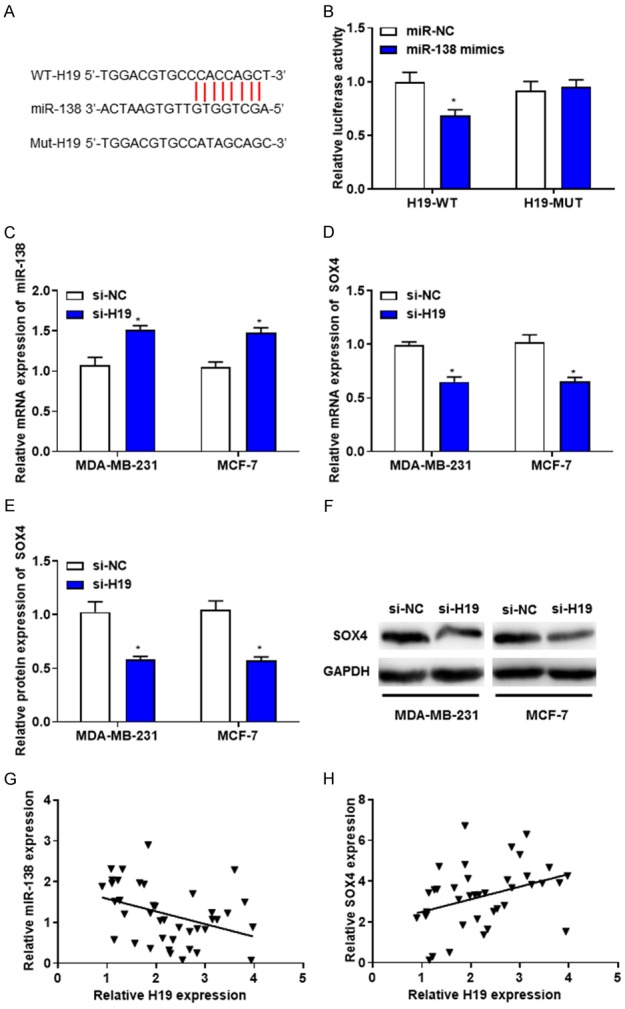
H19 suppresses the expression level of miR-138. A. The putative binding sites of H19 on miR-138. B. Luciferase reporter assays were performed following the transfection with miR-NC or miR-138. C. RT-qPCR revealed that the mRNA levels of miR-138 were increased in MDA-MB-231 and MCF-7 cells transfected with si-H19. D. RT-qPCR demonstrated that the mRNA levels of SOX4 were decreased in MDA-MB-231 and MCF-7 cells transfected with si-H19. E and F. Western blotting revealed that the protein levels of SOX4 was decreased in MDA-MB-231 and MCF-7 cells transfected with si-H19. G and H. The correlation between H19 and miR-138 (r = -0.310, P = 0.015), H19 and SOX4 (r = 0.617, P = 0.021) was evaluated by Spearman’s correlation analysis. *P < 0.05. RT-qPCR, Reverse transcription-quantitative polymerase chain reaction.
MiR-138 suppresses the expression level of SOX4
Bioinformatics analysis using TargetScan database (http://www.targetscan.org/vert_71/) revealed that SOX4 contains potential binding sites of miR-138. The complementary sequences between miR-138 and SOX4-3’UTR were identified (Figure 5A). Subsequent dual-luciferase reporter assay demonstrated that overexpression of miR-138 significantly attenuated the luciferase activity of SOX4-WT but not that of SOX4-MUT reporter (Figure 5B). Furthermore, RT-qPCR and western blotting revealed that the mRNA and protein levels of SOX4 were decreased in miR-138-transfected MDA-MB-231 and MCF-7 cells (Figure 5C-E). Additionally, spearman’s correlation analysis revealed a significantly negative correlation between miR-138 and SOX4 in breast cancer tissues (Figure 5F), suggesting that SOX4 may be a direct target gene of miR-138 in breast cancer cells.
Figure 5.
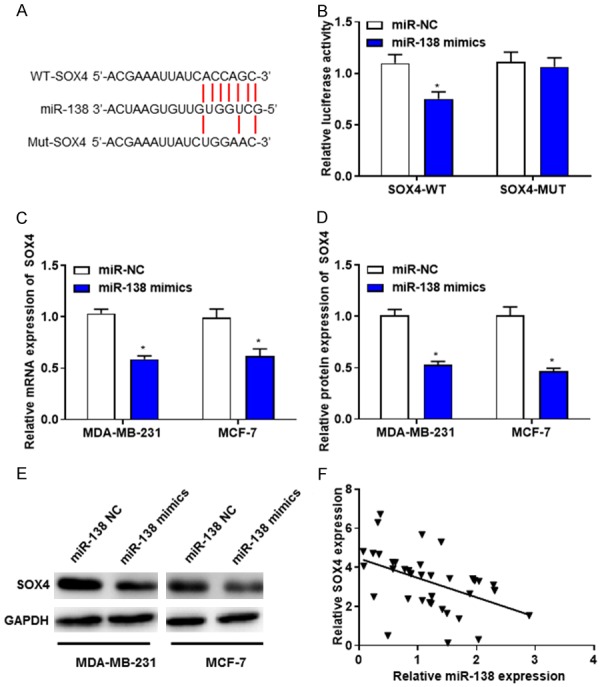
MiR-138 suppresses the expression level of SOX4. A. The putative binding sites of miR-138 on SOX4. B. Luciferase reporter assays were performed following the transfection with miR-NC or miR-138. C. RT-qPCR revealed that the mRNA levels of SOX4 were decreased in MDA-MB-231 and MCF-7 cells transfected with miR-138. D and E. Western blotting demonstrated that the protein levels of SOX4 were decreased in MDA-MB-231 and MCF-7 cells transfected with miR-138. F. The correlation between miR-138 and SOX4 (r = 0.994, P = 0.002) was determined by Spearman’s correlation analysis. *P < 0.05. RT-qPCR, Reverse transcription-quantitative polymerase chain reaction.
MiR-138 inhibits the proliferation, invasion and migration and promotes apoptosis of breast cancer cells by regulating SOX4
To investigate whether miR-138 affects breast cancer development by targeting SOX4, MDA-MB-231 and MCF-7 cells were co-transfected with miR-138 mimics and pcDNA-SOX4, or miR-138 inhibitors and pcDNA-SOX4, respectively. The results revealed that overexpression of miR-138 inhibited the proliferation (Figure 6A and 6B), invasion (Figure 6E) and migration (Figure 6G), but promoted apoptosis (Figure 6I) of MDA-MB-231 and MCF-7 cells, while these effects were abolished by SOX4 overexpression. Downregulated miR-138 induced the proliferation (Figure 6C and 6D), invasion (Figure 6F) and migration (Figure 6H), but suppressed the apoptosis (Figure 6J) of MDA-MB-231 and MCF-7 cells, while these effects were strengthened by SOX4 overexpression. These data suggested that miR-138 may inhibit the growth of breast cancer by downregulating SOX4.
Figure 6.
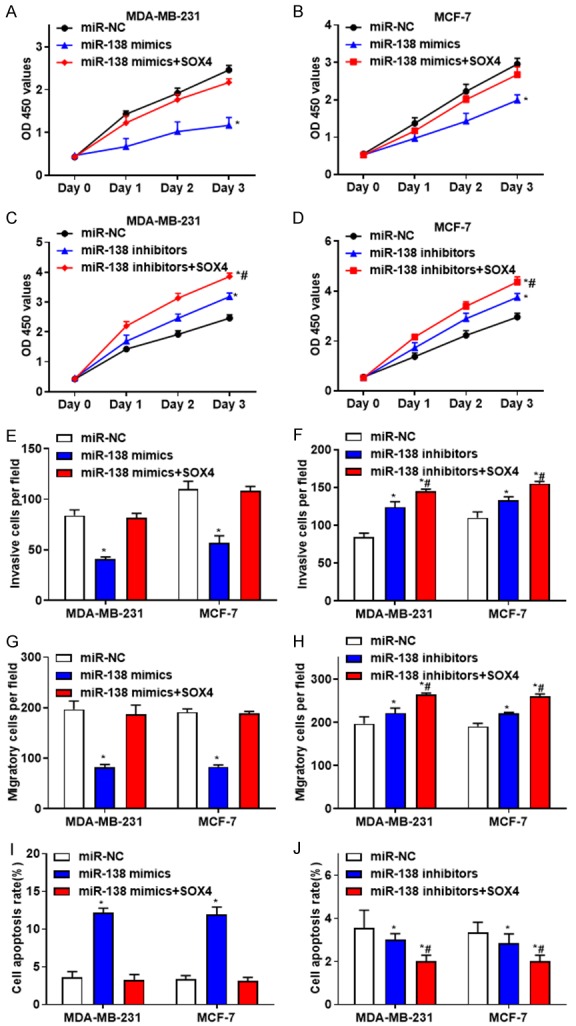
MiR-138 inhibits the proliferation, invasion and migration, but promotes apoptosis in breast cancer cells by regulating SOX4. A and B. The proliferation activity of MDA-MB-231 and MCF-7 cells transfected with miR-138 mimics, or together with pcDNA-SOX4 were determined. C and D. The proliferation activity of MDA-MB-231 and MCF-7 cells after transfected with miR-138 inhibitors, or together with pcDNA-SOX4 were evaluated. E. The invasive abilities of MDA-MB-231 and MCF-7 cells transfected with miR-138 mimics, or together with pcDNA-SOX4 were determined. F. The invasive abilities of MDA-MB-231 and MCF-7 cells transfected with miR-138 inhibitors, or together with pcDNA-SOX4 were evaluated. G. The migratory abilities of MDA-MB-231 and MCF-7 cells transfected with miR-138 mimics, or together with pcDNA-SOX4 were determined. H. The migratory abilities of MDA-MB-231 and MCF-7 cells transfected with miR-138 inhibitors, or together with pcDNA-SOX4 were evaluated. I. The percentage of apoptotic cells in MDA-MB-231 and MCF-7 cells transfected with miR-138 mimics, or together with pcDNA-SOX4 were determined. J. The percentage of apoptotic cells in MDA-MB-231 and MCF-7 cells transfected with miR-138 inhibitors, or together with pcDNA-SOX4 were evaluated. *P < 0.05, compared with the control group; #P < 0.05, compared with the miR-138 mimics or miR-138 inhibitors group.
H19 promotes the proliferation, invasion and migration and suppresses apoptosis of breast cancer cells by regulating miR-138
To investigate whether H19 affects breast cancer development by targeting miR-138, MDA-MB-231 and MCF-7 cells were co-transfected with H19 and miR-138 mimics, or si-H19 and miR-138 mimics, respectively. The results revealed that overexpression of miR-138 reversed the effects caused by downregulated H19 (Figure 7), suggesting that H19 may promote the proliferation, invasion and migration and inhibit the apoptosis of breast cancer cells by downregulating miR-138. In summary, the present study demonstrated that H19 may promote the growth of breast cancer by directly targeting miR-138 and SOX4.
Figure 7.
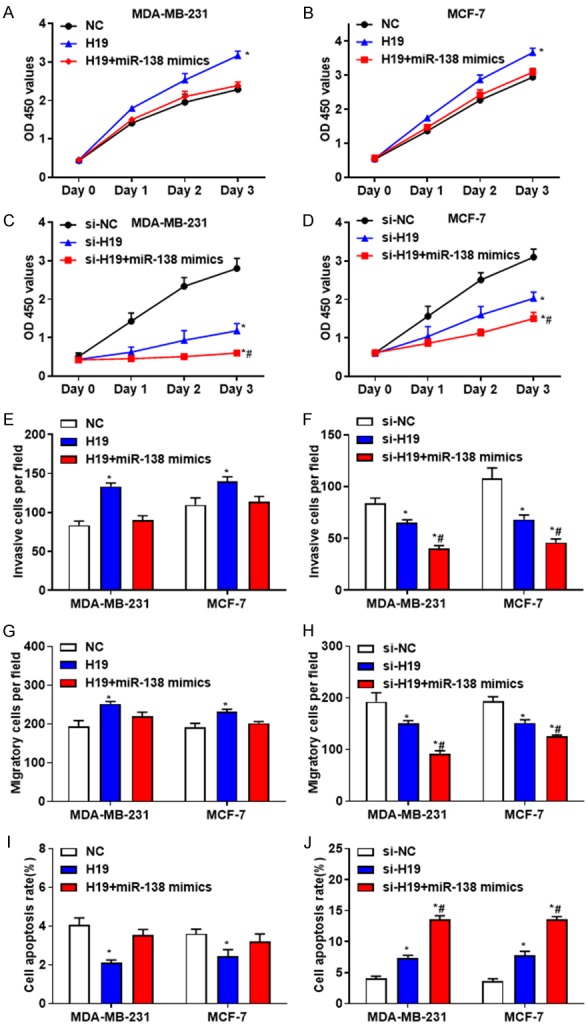
H19 promotes the proliferation, invasion and migration, but inhibits apoptosis of breast cancer cells by targeting miR-138. A and B. The proliferation activity of MDA-MB-231 and MCF-7 cells transfected with H19, or together with miR-138 mimics were determined. C and D. The proliferation activity of MDA-MB-231 and MCF-7 cells transfected with si-H19, or together with miR-138 mimics were evaluated. E. The invasive abilities of MDA-MB-231 and MCF-7 cells transfected with H19, or together with miR-138 mimics were determined. F. The invasive abilities of MDA-MB-231 and MCF-7 cells transfected with si-H19, or together with miR-138 mimics were evaluated. G. The migratory abilities of MDA-MB-231 and MCF-7 cells transfected with H19, or together with miR-138 mimics were determined. H. The migratory abilities of MDA-MB-231 and MCF-7 cells transfected with si-H19, or together with miR-138 mimics were evaluated. I. The percentage of apoptotic cells in MDA-MB-231 and MCF-7 cells transfected with H19, or together with miR-138 mimics were determined. J. The percentage of apoptotic cells in MDA-MB-231 and MCF-7 cells transfected with si-H19, or together with miR-138 mimics were evaluated. *P < 0.05, compared with the control group; #P < 0.05, compared with the H19 or si-H19 group.
Downregulated H19 induces breast cancer growth and epithelial-mesenchymal transition (EMT) markers expression in vivo
The nude mouse xenograft model was used to investigate the effects of H19 on tumor growth in vivo. The tumor volumes of shRNA-H19 group were significantly smaller compared with the NC group (Figure 8A and 8B). At day 42, the mean value of the orthotopic tumor weight of shRNA-H19 group was decreased (Figure 8C). Furthermore, RT-qPCR and western blotting revealed that the expression levels of EMT markers, including SOX4, N-cad, Vi and FN protein were reduced in shRNA-H19 group (Figure 8D and 8E), suggesting that H19 may induce the growth of breast cancer and the expression of EMT markers in vivo.
Figure 8.
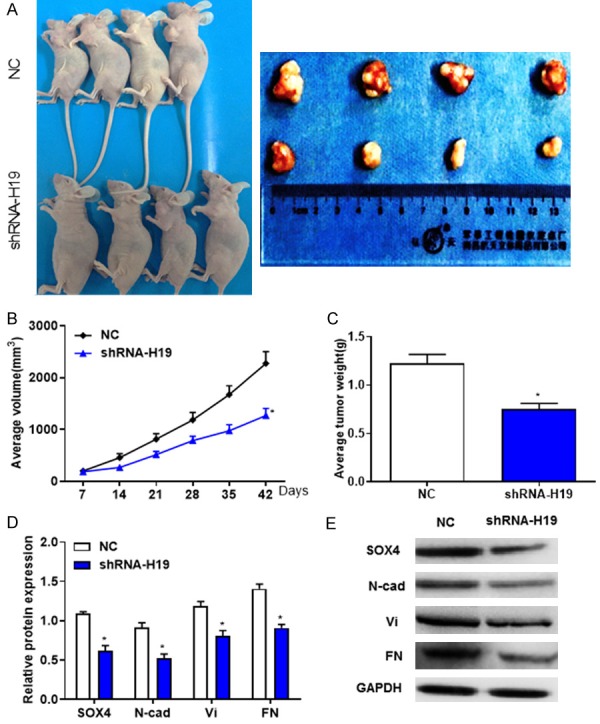
Downregulated H19 induces the growth of breast cancer and the expression of epithelial-mesenchymal transition markers in vivo. A. Representative images of nude mice and xenograft tumors derived from subcutaneous injection of MDA-MB-231 cells injection with shRNA-H19 or shRNA-NC. B. Average tumor volumes were measured following the treatment with shRNA-H19. C. Orthotopic tumor weights at day 42 post-inoculation were calculated. D and E. Western blotting revealed that the protein levels of SOX4, N-cad, Vi and FN were decreased in tumor tissues following the treatment with shRNA-H19. *P < 0.05, compared with the control group.
Discussion
Aberrant expression of lncRNAs and miRNAs have been reported in numerous types of cancer, which may be associated with its initiation and progression [22]. LncRNAs are potential tumor oncogenic and suppressive factors in breast cancer [23]; LncRNA PVT1 regulated cell proliferation, invasion, colony formation and orthotopic xenograft tumor growth in breast cancer via KLF5/beta-catenin signaling pathway [24]. Furthermore, LncRNA LINC01116 was significantly upregulated in breast cancer, which was associated with the overall survival and tumor node metastasis in patients with breast cancer [25]. LncRNA PlncRNA-1 was downregulated in breast cancer tissues, and PlncRNA-1 overexpression reduced the proliferation rate, but increased the apoptosis rate of breast cancer cells by targeting TGF-β1 and PHGDH [26]. In addition, the carcinogenicity of H19 in breast cancer has been demonstrated [27,28], but the underlying molecular mechanisms require further investigation.
In the present study, H19 was upregulated in breast cancer tissues and cells, which was associated with lymph node metastasis, tumor node metastasis stage and overall survival in the patients. The results revealed downregulated H19 significantly suppressed the proliferation, invasion and migration, but induced cell cycle arrest and apoptosis in breast cancer cells. MiRNAs function as tumor suppressors or oncogenes and are key modulators of genes expression; they could be potential targets of lncRNAs. Wu et al. [29] reported that H19 suppressed tension-induced osteogenic differentiation by regulating FAK and miR-138, and miR-138 was an important miRNA that participated in the development of numerous types of tumor [30,31].
In order to determine the interaction between H19 and miR-138, Dual-luciferase reporter assay was performed and the results revealed that overexpression of miR-138 significantly attenuated the luciferase activity of H19-WT but not H19-MUT reporter. Downregulated H19 increased the mRNA levels of miR-138. In addition, the expression levels of H19 and miR-138 were inversely correlated in breast cancer tissues. These results indicated that H19 downregulated the expression level of miR-138. Furthermore, function analysis revealed that overexpression of miR-138 reversed the effects of H19 on the proliferation, invasion, migration and apoptosis of breast cancer cells. In summary, H19 served oncogenic roles in breast cancer by downregulating miR-138. Liang et al. [21] reported that miR-138 was downregulated in breast cancer, and overexpression of miR-138 suppressed the growth of tumor in a CREPT-dependent manner; however, the underlying mechanism remain unknown.
SOX4 gene is a developmental transcriptional factor that is overexpressed in breast cancer [32]. Although SOX4 may act as a tumor suppressor, previous study suggested that SOX4 functioned as an oncogene in numerous types of cancer [33]. SOX4 could exert distinct functions depending on tumor origin, cellular context and even the stages of tumor development [34]. Furthermore, SOX4 may induce the metastasis in cancer progression, by regulating various metastasis-associated genes [35]; SOX4 also served essential roles in the progression of breast cancer by affecting EMT [36,37]. The present study revealed that SOX4 expression was positively correlated with H19, and a significantly negative correlation was detected between miR-138 and SOX4 in breast cancer tissues. Additionally, overexpression of miR-138 significantly attenuated the luciferase activity of SOX4-WT but not SOX4-MUT reporter; mRNA and protein levels of SOX4 were decreased in MDA-MB-231 and MCF-7 cells transfected with miR-138, suggesting SOX4 may be a direct target of miR-138 in breast cancer cells.
To further investigate whether miR-138 affect the proliferation, invasion, migration and apoptosis of breast cancer cells by targeting SOX4, MDA-MB-231 and MCF-7 cells were co-transfected with miR-138 mimics and pcDNA-SOX4, or miR-138 inhibitors and pcDNA-SOX4, respectively. The results revealed that overexpression of miR-138 suppressed the proliferation, invasion and migration, but promoted the apoptosis of breast cancer cells, and these effects were abolished by SOX4 overexpression. Furthermore, downregulation of miR-138 induced the proliferation, invasion and migration, but inhibited the apoptosis of breast cancer cells, and these effects were strengthened by silenced SOX4 expression, suggesting that silencing of H19 inhibited tumor growth in breast cancer via targeting miR-138 and SOX4.
The nude mouse xenograft model was used to evaluate the effects of H19 on tumor growth in vivo. The results revealed that the tumor volumes and the weight of orthotopic tumors were significantly reduced in shRNA-H19 group. In addition, the protein levels of SOX4, N-cad, Vi and FN were decreased in shRNA-H19 group, suggesting that H19 induced the growth of breast cancer and the expression of EMT markers in vivo.
In summary, the present study revealed that H19 was upregulated in breast cancer and associated with poor prognosis. Silencing of H19 inhibited cell proliferation, invasion and migration, but promoted cell cycle arrest and apoptosis in breast cancer by targeting miR-138 and SOX4. Thus, the results suggested that miR-138 may be a promising therapeutic target for the treatment of breast cancer.
Disclosure of conflict of interest
None.
References
- 1.Israel BB, Tilghman SL, Parker-Lemieux K, Payton-Stewart F. Phytochemicals: current strategies for treating breast cancer. J Oncol Lett. 2018;15:7471–7478. doi: 10.3892/ol.2018.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calaf GM, Roy D. Metastatic genes targeted by an antioxidant in an established radiation-Â and estrogen-breast cancer model. Int J Oncol. 2017;51:1590. doi: 10.3892/ijo.2017.4125. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Ye H. Screening of the prognostic targets for breast cancer based co-expression modules analysis. J Mol Med Rep. 2017;16:4038–4044. doi: 10.3892/mmr.2017.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei DM, Jiang MT, Lin P, Yang H, Dang YW, Yu Q, Liao DY, Luo DZ, Chen G. Potential ceRNA networks involved in autophagy suppression of pancreatic cancer caused by chloroquine diphosphate: a study based on differentiallyexpressed circRNAs, lncRNAs, miRNAs and mRNAs. J Int J Oncol. 2019;54:600–626. doi: 10.3892/ijo.2018.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Q, Yu T, Ou X, Cao D, Xie T, Chen X. Potential lncRNA diagnostic biomarkers for early gastric cancer. J Mol Med Rep. 2017;16:9545–9552. doi: 10.3892/mmr.2017.7770. [DOI] [PubMed] [Google Scholar]
- 6.Xing Y, Zhao Z, Zhu Y, Zhao L, Zhu A, Piao D. Comprehensive analysis of differential expression profiles of mRNAs and lncRNAs and identification of a 14-lncRNA prognostic signature for patients with colon adenocarcinoma. J Oncol Rep. 2018;39:2365–2375. doi: 10.3892/or.2018.6324. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Wang W, Chen R, Zhang Y, Zou K, Ye M, He X, Zhang F, Han J. Exosome-mediated transfer of lncRNASNHG14 promotes trastuzumab chemoresistance in breast cancer. J Int J Oncol. 2018;53:1013–1026. doi: 10.3892/ijo.2018.4467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Li X, Wang S, Li Z, Long X, Guo Z, Zhang G, Zu J, Chen Y, Wen L. The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. J Int J Biol Macromol. 2017;105:346–353. doi: 10.1016/j.ijbiomac.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 9.Heilmann K, Toth R, Bossmann C, Klimo K, Plass C, Gerhauser C. Genome-wide screen for differentially methylated long noncoding RNAs identifies Esrp2 and lncRNA Esrp2-as regulated by enhancer DNA methylation with prognostic relevance for human breast cancer. J Oncogene. 2017;36:6446–6461. doi: 10.1038/onc.2017.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Z, Yu Y, Zhang B, Miao L, Wang L, Zhao K, Ji Y, Wang R, Ma H, Chen N. Genetic variants in lncRNAH19are associated with the risk of oral squamous cell carcinoma in a Chinese population. J Oncotarget. 2018;9:23915–23922. doi: 10.18632/oncotarget.23673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Chen W, Wang Y, Tang L, Han M. Long non-coding RNA H19 regulates viability and metastasis, and is upregulated in retinoblastoma. Oncol Lett. 2018;15:8424–8432. doi: 10.3892/ol.2018.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Zhou Y, Huang T, Cheng AS, Yu J, Kang W, To KF. The interplay of lncrna-h19 and its binding partners in physiological process and gastric carcinogenesis. Int J Mol Sci. 2017;18:450. doi: 10.3390/ijms18020450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smolle MA, Bullock MD, Ling H, Pichler M, Haybaeck J. Long non-coding RNAs in endometrial carcinoma. Int J Mol Sci. 2015;16:26463–26472. doi: 10.3390/ijms161125962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matouk IJ, Halle D, Gilon M, Hochberg A. The non-coding RNAs of the H19-IGF2 imprinted loci: a focus on biological roles and therapeutic potential in lung cancer. J Transl Med. 2015;13:113. doi: 10.1186/s12967-015-0467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Si X, Zang R, Zhang E, Liu Y, Shi X, Zhang E, Shao L, Li A, Yang N, Han X, Pan B, Zhang Z, Sun L, Sun Y. LncRNA H19 confers chemoresistance in ERalpha-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. J Oncotarget. 2016;7:81452–81462. doi: 10.18632/oncotarget.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vennin C, Spruyt N, Robin YM, Chassat T, Le Bourhis X, Adriaenssens E. The long non-coding RNA 91H increases aggressive phenotype of breast cancer cells and up-regulates H19/IGF2 expression through epigenetic modifications. J Cancer Lett. 2017;385:198–206. doi: 10.1016/j.canlet.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Zhang K, Luo Z, Zhang Y, Zhang L, Wu L, Liu L, Yang J, Song X, Liu J. Circulating lncRNA H19 in plasma as a novel biomarker for breast cancer. J Cancer Biomark. 2016;17:187–194. doi: 10.3233/CBM-160630. [DOI] [PubMed] [Google Scholar]
- 18.Xie H, Fu JL, Xie C. MiR-138-5p is downregulated in patients with atrial fibrillation and reverses cardiac fibrotic remodeling via repressing CYP11B2. J Eur Rev Med Pharmacol Sci. 2018;22:4642–4647. doi: 10.26355/eurrev_201807_15523. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, Sun J, Chen Y, Su W, Shan H, Li Y, Wang Y, Zheng N, Shan H, Liang H. lncRNA PFAR promotes lung fibroblast activation and fibrosis by targeting miR-138 to regulate the YAP1-twist axis. Mol Ther. 2018;26:2206–2217. doi: 10.1016/j.ymthe.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan TB, Robert LC, Teebagy PA, Morgan SE, Beatty EW, Cicuto BJ, Nowd PK, Rieger-Christ KM, Bryan DJ. Spatiotemporal microRNA profile in peripheral nerve regeneration: miR-138 targets vimentin and inhibits Schwann cell migration and proliferation. J Neural Regen Res. 2018;13:1253–1262. doi: 10.4103/1673-5374.235073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Z, Feng Q, Xu L, Li S, Zhou L. CREPT regulated by miR-138 promotes breast cancer progression. J Biochem Biophys Res Commun. 2017;493:263–269. doi: 10.1016/j.bbrc.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Jia G, Qu Y, Q D, Liu B, Liu B. Long non-coding RNA (LncRNA) HOXA11-AS promotes breast cancer invasion and metastasis by regulating epithelial-mesenchymal transition. J Med Sci Monit. 2017;23:3393–3403. doi: 10.12659/MSM.904892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou W, Ye XL, Xu J, Cao MG, Fang ZY, Li LY, Guan GH, Liu Q, Qian YH, Xie D. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR-200b/c and let-7b. J Sci Signal. 2017;10 doi: 10.1126/scisignal.aak9557. [DOI] [PubMed] [Google Scholar]
- 24.Tang J, Li Y, Sang Y, Yu B, Lv D, Zhang W, Feng H. LncRNA PVT1 regulates triple-negative breast cancer through KLF5/beta-catenin signaling. Oncogene. 2018;37:4723–4734. doi: 10.1038/s41388-018-0310-4. [DOI] [PubMed] [Google Scholar]
- 25.Hu HB, Chen Q, Ding SQ. LncRNA LINC01116 competes with miR-145 for the regulation of ESR1 expression in breast cancer. J Eur Rev Med Pharmacol Sci. 2018;22:1987–1993. doi: 10.26355/eurrev_201804_14726. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Gao H, Zhou S, Liao Y. LncRNA PlncRNA-1 overexpression inhibits the growth of breast cancer by upregulating TGF-beta1 and downregulating PHGDH. Breast Cancer. 2018;25:619–625. doi: 10.1007/s12282-018-0858-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhu QN, Wang G, Guo Y, Peng Y, Zhang R, Deng JL, Li ZX, Zhu YS. LncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-MDR1 pathway. J Oncotarget. 2017;8:91990–92003. doi: 10.18632/oncotarget.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Wang X, Chen T, Jiang L, Yang Q. Huaier extract inhibits breast cancer progression through a LncRNA-H19/MiR-675-5p pathway. J Cell Physiol Biochem. 2017;44:581–593. doi: 10.1159/000485093. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Zhao J, Sun L, Pan Y, Wang H, Zhang WB. Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. J Bone. 2018;108:62–70. doi: 10.1016/j.bone.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Yang Q, Wang X, Tang C, Chen X, He J. H19 promotes the migration and invasion of colon cancer by sponging miR-138 to upregulate the expression of HMGA1. J Int J Oncol. 2017;50:1801–1809. doi: 10.3892/ijo.2017.3941. [DOI] [PubMed] [Google Scholar]
- 31.Ou L, Wang D, Zhang H, Yu Q, Hua F. Decreased expression of MiR-138-5p by LncRNA H19 in cervical cancer promotes tumor proliferation. Oncol Res. 2018;26:401–410. doi: 10.3727/096504017X15017209042610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuipers AJ, Middelbeek J, Vrenken K, Perez-Gonzalez C, Poelmans G, Klarenbeek J, Jalink K, Trepat X, van Leeuwen FN. TRPM7 controls mesenchymal features of breast cancer cells by tensional regulation of SOX4. J Biochim Biophys Acta. 2018;1864:2409–2419. doi: 10.1016/j.bbadis.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Bai JW, Wang X, Zhang YF, Yao GD, Liu H. MicroRNA-320 inhibits cell proliferation and invasion in breast cancer cells by targeting SOX4. J Oncol Lett. 2017;14:7145–7152. doi: 10.3892/ol.2017.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S, Nagpal N, Ghosh PC, Kulshreshtha R. P53-miR-191-SOX4 regulatory loop affects apoptosis in breast cancer. J RNA. 2017;23:1237–1246. doi: 10.1261/rna.060657.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta GA, Parker JS, Silva GO, Hoadley KA, Perou CM, Gatza ML. Amplification of SOX4 promotes PI3K/Akt signaling in human breast cancer. J Breast Cancer Res Treat. 2017;162:439–450. doi: 10.1007/s10549-017-4139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Goodarzi H, Tavazoie SF, Alarcon CR. TMEM2 is a SOX4-regulated gene that mediates metastatic migration and invasion in breast cancer. J Cancer Res. 2016;76:4994–5005. doi: 10.1158/0008-5472.CAN-15-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song GD, Sun Y, Shen H, Li W. SOX4 overexpression is a novel biomarker of malignant status and poor prognosis in breast cancer patients. J Tumour Biol. 2015;36:4167–4173. doi: 10.1007/s13277-015-3051-9. [DOI] [PubMed] [Google Scholar]