Abstract
Immune dysregulation plays an important role in the pathogenesis of rheumatoid arthritis (RA). Bcl2 like protein 12 (Bcl2L12) has the ability of immune regulation. This study aims to investigate the role of Bcl2L12 in interfering with Foxp3+ regulatory T cell (Treg) development and function in RA patients. In this study, RA patients were recruited in RA clinic. The peripheral blood samples were collected from RA patients and healthy (HA) subjects. Treg status was analyzed by a variety of immune assessing approaches. We observed that the frequency of Tregs in RA patients was significantly lower than that in HA subjects. The expression of Bcl2L12 was detected in CD4+ T cells, which was markedly higher in the RA group than that in HA group. Naive CD4+ T cells from RA patients were refractory to develop as Tregs. Inhibition of Bcl2L12 in CD4+ T cells from RA patients promoted Treg generation. Tregs isolated from RA patients showed functional defects, which could be restored by knocking down of Bcl2L12. In conclusion, Bcl2L12 plays a role in suppressing Treg development and function in RA patients. Inhibition of Bcl2L12 may have therapeutic potential in the treatment of RA.
Keywords: Rheumatoid arthritis, immune regulation, Bcl2L12, regulatory T cell, inflammation
Introduction
The rheumatoid arthritis (RA) is a long-term, chronic, multifactorial immune-mediated disease in joints [1]. RA is characterized by systemic and chronic joint inflammation that leads to structural damage in joints. The pathogenesis of RA is not fully understood [2]. Although the aberrant immune responses in RA have been rather clear, many advances in RA research in the recent years, the causative factors of RA remain elusive and need to be further investigated [2-4]. The therapeutic effects of RA are quite poor currently [5].
Autoimmunity plays an important role in the development of RA [6,7]. An autoimmune disease is characterized by abnormal immune responses against normal body parts [8]. Two known antibodies, including rheumatoid factor and anti-citrullinated peptide antibody (ACPA), have been identified in the RA. These two antibodies react with common autoantigens in joints and can be detected in and outside the joints [9]. The mechanism by which autoantibodies are overproduced in the body is unclear. In general, several immune cells are involved in producing specific antibodies, including dendritic cells (DC), T helper 2 (Th2) cells, B cells and plasma cells [10,11]. The activities of these cells are tightly regulated in the body. Regulatory T cells (Treg) are one of the major components of the immune regulatory system [12]. By expressing immune regulatory molecules, such as interleukin (IL)-10 and transforming growth factor (TGF)-β, Tregs suppress other immune cell activities to maintain the immune response within proper levels [12,13]. The over production of autoantibodies suggests defects of the immune regulatory system in the body [14,15].
Recent studies indicate that the Bcl2 like protein 12 (Bcl2L12), one of the members of the Bcl2 family, has immune regulatory functions. Bcl2L12 is an anti-apoptotic protein; it inhibits p53, caspase 3 and caspase 7 to compromise the apoptotic machinery in cancer cells, such as glioma, colon cancer and carcinomas in the head and neck [16-18]. Bcl2L12 is also involved in the pathogenesis of immune inflammation. Xue et al reported that patients with allergic rhinitis had higher expression of Bcl2L12 in peripheral B cells [19]. Guo et al showed that B cells in patients with inflammatory bowel disease expressed higher levels of Bcl2L12 [20]. Li et al found that Bcl2L12 is required during development of skewed Th2-biased inflammation [21]. Based on the information above, we hypothesize that Bcl2L12 may be involved in the pathogenesis of RA by interfering with Treg activities. To test this, we collected blood samples from RA patients. The role of Bcl2L12 in Treg activities and the development of Treg was investigated. The results showed that Bcl2L12 plays a role in suppressing Treg development and function in RA patients.
Materials and methods
Reagents
Reagents and materials for RT-qPCR and Western blotting were obtained from Invitrogen (Carlsbad, CA). Reagent kits and materials for immunoprecipitation (IP) and chromatin IP (ChIP) were obtained from Sigma Aldrich (St. Louis., MO). Antibodies of Bcl2L12 antibody (ab108346), Foxp3 (ab450), RNA polymerase II (Pol II; ab240740) and transforming growth factor (TGF)-β (ab64715) were obtained from abcam (Cambridge, MA). Fluorochrome-labeled antibodies of CD3, CD4, CD25 and Foxp3 were obtained from BD Biosciences (Franklin Lakes, NJ). Bcl2L12 RNAi kits were obtained from Santa Cruz Biotech (Santa Cruz, CA). Immune cell isolation kits were obtained from Miltenyi Biotech (San Diego, CA).
Human subjects
Patients with RA at relieving period (not on medication in recent 2 months) were recruited into this study at the outpatient clinic of the Yantaishan Hospital (Yantai, China). The diagnosis and management of RA were carried out by physicians of our department. Age- and gender-matched healthy subjects were also recruited at the same hospital into this study using as controls. The demographic data of human subjects are presented in Table 1. The exclusion criteria include: Immune disorders besides RA, using immune suppressors for any reason, cancers, allergic diseases and severe organ diseases. The using human tissue in the present study was approved by the Human Ethics Committee of Yantaishan Hospital. An informed written consent was obtained from each human subject.
Table 1.
Demographic data of human subjects in this tudy
| RA (n=20) | HA (n=20) | |
|---|---|---|
| Sex (male/female) | 10/10 | 10/10 |
| Age, mean (range) | 42.5 (29-58) | 41.6 (27-57) |
| Disease duration (y), mean (range) | 6.3 (2-15) | - |
| Smoking, number (%) | 2 (10%) | 2 (10%) |
| ESR (mm), mean | 54 ± 16.6 | 15.9 ± 6.5 |
| CRP (mg/dL), mean | 3.8 ± 1.5 | 0.8 ± 0.6 |
| Positive rheumatoid factor, n (%) | 17 (85%) | 0 |
| Positive anti-CCP antibody, n (%) | 12 (60%) | 0 |
| Medication, n (%) | 0 | 0 |
RA: Rheumatoid arthritis. HA: Healthy subjects. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; anti-CCP, anti-cyclic citrullinated peptide antibodies.
Preparation of peripheral blood mononuclear cells (PBMCs)
Blood samples were collected from each human subject by an ulnar vein puncture. PBMCs were isolated from the samples by Percoll gradient density centrifugation.
Immune cell isolation
The immune cells of interest were isolated from PBMCs by magnetic cell sorting with commercial reagent kits following the manufacturer’s instructions. Purity of isolated cells was assessed by flow cytometry. If the purity was less than 90%, MACS was repeated with the cells.
Cell culture
RPMI1640 medium was used in cell culture. The medium was supplemented with 10% fetal calf serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2 mM glutamine. The medium was changed every 2-3 days. The Trypan blue exclusion assay was performed to check the viability of cells, which was greater than 99%.
Flow cytometry
To stain Foxp3, cells were stimulated with PMA (50 ng/ml), ionomycin (1 μg/ml) and BD Golgi plug (a protein transport inhibitor, BD Pharmingen) for 4 hours before harvesting cells. After cell surface staining, cells were fixed and permeabilized with Fixation/Permeabilization (Triton X-100, 0.1%) solution and intracellular Foxp3 was stained. Flow cytometric analysis was carried out using a FACSCanto II flow cytometer (BD Biosciences). The data were analyzed by Flow Jo (FlowJo, LLC).
Quantitative RT-PCR (RT-qPCR)
Cells were lysed with TRIzol reagent, and RNA was extracted from the cells. cDNAs were synthesized from 1 μg of total RNAs using a reverse transcription kit following the manufactory’s instructions. The expression levels of genes of interest were assessed by the CFX96 Touch Real-Time PCR Detection System (Bio-Rad) using SYBR Green Master Mix. Relative expression levels of target gene are presented as fold change against the housekeeping gene β-actin. The sequences of primers used in this study include Bcl2L12 (tctcctgttccaactccacc and gggccaggctctaaaccata) and TGF-β (tacagcaacaattcctggcg and gtgaacccgttgatgtccac).
Protein extraction
Cells were lysed with a lysis buffer. The lysates were centrifuged for 10 min at 10,000 g. The supernatant was collected and used as cytosolic protein. The pellets were incubated with a nuclear lysis buffer for 30 min and centrifuged for 10 min at 10,000 g. The supernatant was collected and used as nuclear protein. All the procedures were carried out at 4°C. The protein levels were determined by the BCA method.
Western blot
50 μg of protein per sample were fractioned by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were incubated with primary antibodies (1:1000) at 4°C overnight. After washing with TBST (Tris-buffered saline containing 0.1% Tween 20) for 3 times, membranes were incubated with secondary antibodies (labeled with peroxidase) for 1 h at room temperature. Immunoblots on the membrane were visualized using a chemiluminescent kit and photographed with an imaging device.
Co-immunoprecipitation (co-IP)
Proteins were precleared by incubation with Protein G agarose beads at 4°C for 60 min. The beads were removed by centrifugation. The remained samples were used for co-IP by incubating with antibodies of interest or isotype IgG at 4°C for 90 min. Immune complexes in the samples were precipitated by incubating with Protein G agarose beads at 4°C for 60 min. The beads were collected by centrifugation and washed. Proteins on the beads were eluted with an eluting buffer and analyzed by Western blotting.
Chromatin IP (ChIP)
Cells were fixed in 1% formalin for 15 min. The fixation was quenched with glycine. After rinsing with cold PBS, cells were collected and lysed. The lysates were sonicated into small pieces (200-700 base-pair DNA fragments) and treated with IP procedures as described above. Chromatin-antibody complexes on the beads were eluted. DNA in the sample was purified with a DNA recovering reagent kit following the manufacturer’s instructions. The DNA was analyzed by qPCR in the presence of primers for TGFB promoter (agggtgttgagtgacaggag and agagggtctgtcaacatggg). The results are presented as fold change against the input.
Expression of recombinant Bcl2L12
To over express Bcl2L12, HEK293 cells or CD4+ T cells were transfected with Bcl2L12-expressing plasmids (provided by Sangon Biotech; Shanghai, China) following the manufacturer’s instructions. The effects of Bcl2L12 plasmid transfection were assessed 48 h later.
RNA interference (RNAi) of Bcl2L12
To inhibit the expression of Bcl2L12, CD4+ T cells or Tregs were treated with Bcl2L12 RNAi reagent kit following the manufacturer’s instructions. The cells were collected 48 h later and analyzed by Western blotting to assess the effects of RNAi.
Generation of tregs in vitro
CD4+ CD25- T cells were isolated from HA subjects and cultured in the presence of TGFβ1 (10 ng/ml), anti-IL-4 (10 μg/ml), anti-IL-12 (10 μg/ml), and anti-IFN-γ (10 μg/ml) for 96 h. The cells were assessed by flow cytometry.
Statistical analysis
The data were analyzed using the GraphPad Prism Package version 7. The results of two independent groups were compared using the Student’s t-test. ANOVA was used in multiple comparisons followed by Dunnett’s t test or SNK test. P<0.05 was considered statistically significant.
Results
Frequency of peripheral Treg is lower RA patients
To understand the immune tolerance status in RA, peripheral blood samples were collected from RA patients and healthy (HA) subjects. Peripheral blood mononuclear cells (PBMCs) were isolated from the samples and analyzed by flow cytometry. The results showed that in the CD4+ CD25+ T cell compartment (Figure 1A), the frequency of Foxp3+ Tregs was much less in the RA group than that in HA group (Figure 1B, 1C). Since Tregs are an important component in the immune regulatory system, the data imply that the low frequency of peripheral Tregs may contribute to the immune dysregulation in RA.
Figure 1.
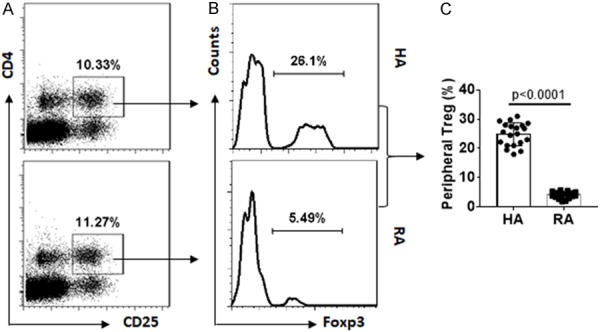
Peripheral Treg frequency is lower in RA patients. Blood samples were collected from RA patients (n=20) and HA subjects (n=20). PBMCs were isolated from the samples and analyzed by flow cytometry. (A) Gated dot plots indicate frequency of CD4+ CD25+ T cells. (B) Gated dot plots indicate frequency of Foxp3+ Tregs in the gated CD4+ CD25+ T cells of (A). (C) Summarized Treg frequency of (B).
Expression of Bcl2L12 is negatively correlated with Foxp3 expression in CD4+ T cells of RA patients
To corroborate the data, CD4+ T cells were isolated from PBMCs by magnetic cell sorting (MACS) and analyzed by RT-qPCR and Western blotting. The results showed that the expression of Foxp3 was lower, expression of Bcl2L12 was higher, in the RA group than that in HA group (Figure 2A-D). A correlation assay was performed with the data of Foxp3 and Bcl2L12. A negative correlation was identified between expression of Bcl2L12 and Foxp3 in CD4+ T cells of RA patients (Figure 2E). The results imply that the high expression of Bcl2L12 in CD4+ T cells may associate with the lower expression of Foxp3 in RA.
Figure 2.
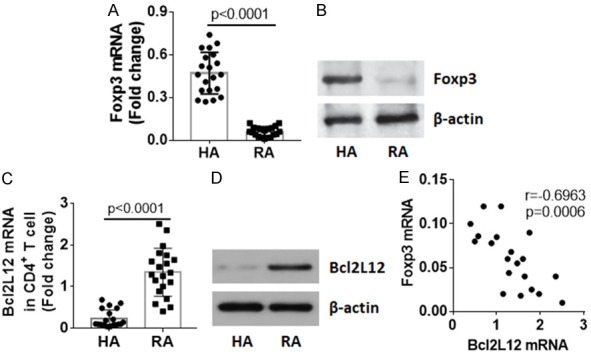
Expression of Bcl2L12 by CD4+ T cells negatively correlates with peripheral Tregs in RA patients. CD4+ T cells were isolated from PBMCs by MACS. Total RNA and proteins were extracted from the cells and analyzed by RT-qPCR and Western blotting. (A) Foxp3 mRNA levels in CD4+ T cells. (B) Foxp3 protein levels in CD4+ T cells. (C) Bcl2L12 mRNA levels in isolated CD4+ T cells. (D) Bcl2L12 protein levels in isolated CD4+ T cells. Protein extracts of 20 RA patients and HA subjects were pooled, respectively. Data of (B and D) are from one experiment represent 3 independent experiments. (E) Negative correlation between Bcl2L12 mRNA and Foxp3 mRNA in CD4+ T cells of RA patients.
Bcl2L12 forms a complex with Foxp3 in CD4+ T cells
Next, CD4+ T cells were analyzed by co-immunoprecipitation (co-IP). A complex of Bcl2L12 and Foxp3 was detected in CD4+ T cells. The amount of the complex was much more in the RA group as compared to that of the HA group (Figure 3A). Then, we confirmed the combination of Bcl2L12 and Foxp3 in HEK293 cells by co-transfecting HEK293 cells with Foxp3-expressing plasmids and Bcl2L12-expressing plasmids. A complex of recombinant (r) Bcl2L12 and rFoxp3 was detected in HEK293 cells (Figure 3B).
Figure 3.
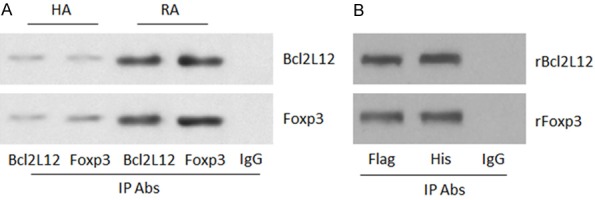
Bcl2L12 forms a complex with Foxp3 in CD4+ T cells. A. Total proteins were extracted from CD4+ T cells collected from HA subjects (n=20) and RA patients (n=20). The proteins were pooled and analyzed by co-IP. The immunoblots show a complex of Bcl2L12 and Foxp3 in CD4+ T cells. B. HEK293 cells were co-transfected with Flag-Bcl2L12-expressing plasmids and His-Foxp3-expressing plasmids. The extracts of HEK293 cells were analyzed by co-IP. The immunoblots show the complex of Bcl2L12 and Foxp3 in HEK293 cells. The data represent 3 independent experiments.
Bcl2L12 interferes with TGF-β gene transcription in CD4+ T cells
Extracts of CD4+ T cells from HA subjects and RA patients were analyzed by chromatin IP (ChIP). The results showed Foxp3 was detected in TGFB promoter locus, which was much less in the RA group as compared to HA group (Figure 4A). The results imply that Bcl2L12 may interfere with the binding between Foxp3 and TGFB promoter. To test this, naive CD4+ T cells were collected from HA subjects. The cells were transfected with Bcl2L12-expressing plasmids to make the cells over expressing Bcl2L12 (Figure 4B), and then treated with Foxp3+ Treg-generating procedures. The results showed that the overexpression of Bcl2L12 significantly suppressed binding of Foxp3 to the TGFB promoter (Figure 4C), TGF-β gene transcription activities (Figure 4D) and expression of TGF-β (Figure 4E, 4F) in CD4+ T cells. The data demonstrate that Bcl2L12 restricts TGF-β gene transcription in CD4+ T cells.
Figure 4.
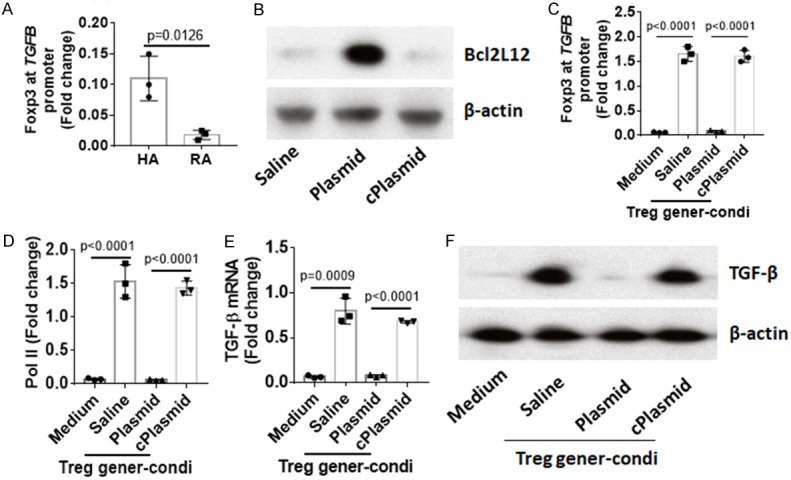
Bcl2L12 interferes with TGFB gene transcription in CD4+ T cells. (A) Nuclear proteins were extracted from CD4+ T cells collected from HA subjects (n=20) and RA patients (n=20). The proteins were pooled and analyzed by ChIP. The bars indicate Foxp3 levels at TGFB promoter locus in CD4+ T cells of human subjects. (B) The results of Bcl2L12 overexpression in CD4+ T cells. (C-F) Naive CD4+ T cells with or without Bcl2L12 overexpression were treated with Treg generation condition (Treg gener-condi). (C, D) Levels of Foxp3 (C) and Pol II (D) at TGFB promoter locus. (E, F) Expression of TGF-β in CD4+ T cells. The data represent 3 independent experiments.
Bcl2L12 interferes with Treg generation
Data reported above suggest that Bcl2L12 may interfere with the generation of Treg in RA patients. To test this, naive CD4+ T cells were collected from RA patients and HA subjects. The cells were treated with Treg generation procedures and analyzed by flow cytometry. The results showed that Foxp3+ Tregs were generated with CD4+ T cells from HA subjects, which were significantly less in CD4+ T cells from RA patients (Figure 5A, 5B). Considering CD4+ T cells from RA patients expressing high levels of Bcl2L12, which may interfere with the generation of Treg, we knocked down Bcl2L12 expression in RA CD4+ T cells by RNAi (Figure 5C); the cells were then treated with Treg generation procedures. Indeed, the generation of Treg was increased significantly after knocking down Bcl2L12 expression (Figure 5A, 5B).
Figure 5.
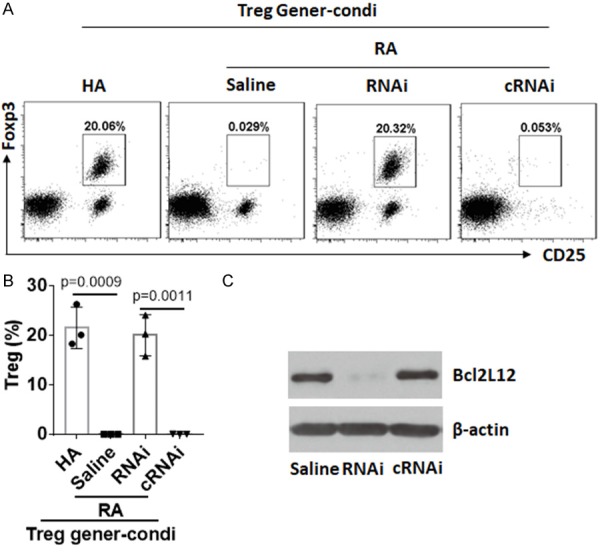
Bcl2L12 interferes with Treg generation. Naive CD4+ T cells were isolated from PBMCs collected from HA subjects and RA patients. The cells were treated with Treg generation condition (Treg gener-condi) and the procedures denoted above each dot plot panel. (A) The gated dot plots show frequency of generated Treg. (B) The bars show summarized data of generated Tregs in (A). (C) Results of Bcl2L12 RNAi. RNAi (cRNAi): RA CD4+ T cells were treated with Bcl2L12 RNAi) or control RNAi). The data represent 3 independent experiments.
Inhibition of Bcl2L12 restores RA Treg immune suppressor function
It is reported that Tregs from RA patients show incompetent immune suppressor functions [22]. In line with the reports, we also observed that Tregs isolated from RA patients showed incompetent immune suppressor function. Knockdown of Bcl2L12 by RNAi restored the immune suppressor functions (Figure 6).
Figure 6.
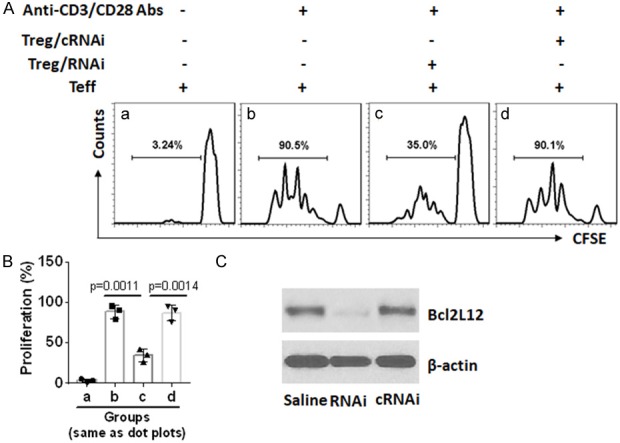
Inhibition of Bcl2L12 restores RA Treg immune suppressor function. CD4+ CD25+ CD127lo/- Tregs (RA Treg) and effector T cells (Teff; CD4+ CD25-) were isolated from RA patients. Teffs were labeled with CFSE and cultured with RA Tregs [treated with Bcl2L12 RNAi (RNAi) or control RNAi (cRNAi)] at a ratio of (5:1; Teff:Treg) in the presence of anti-CD3/CD28 antibodies for 3 days. The cells were analyzed by flow cytometry. A. The gated histograms show frequency of proliferating Teffs. B. The bars show summarized proliferating Teffs. C. Results of Bcl2L12 RNAi in RA Tregs. The data represent 3 independent experiments.
Discussion
The present data indicate that, peripheral CD4+ T cells express Bcl2L12, which is negatively correlated with peripheral Treg number in RA patients. Compared to HA subjects, peripheral Foxp3+ Tregs in RA patients are much less. Foxp3+ Tregs are one of the major components of the immune regulatory system. The normal number and function of Treg are an important premise in the maintenance of the homeostasis in the body. Insufficient number of Treg can be a sign of immune dysregulation. RA is one of the diseases with immune dysregulation. Either reduction of Treg number or Treg function defects can result in immune dysregulation. Previous reports indicate in RA patients, the functional defects of Treg are more often than the reduction of Treg number. Nie et al found that the number of Treg was not quite different between RA patients and HA subjects, while Tregs isolated from RA patients showed incompetent immune suppressor function [22]. Ji et al also reported same phenomenon [23]. Our data show both Treg number reduction and functional defects in RA patients.
The life span of immune cell is limited. T cell’s life span is from several hours to several months. Thus, new T cells need to be generated constantly in the body. The data show that naive CD4+ T cells of RA patients are refractory to be generated into Tregs. Whether this is the main reason resulting in less Tregs in the peripheral system of RA patients is to be further investigated. Our in vitro experimental data show that Bcl2L12 plays a critical role in restricting Treg generation from naive CD4+ T cells; knockdown of Bcl2L12 significantly increases Treg generation. Besides RA, the Treg number reduction was also found in other immune diseases. Such as Yentur et al found Tregs decreased markedly in patients with sclerosing panencephalitis [24]. Mao et al found a significant decrease in the percentage of circulating Foxp3+ Tregs in untreated Graves’ disease patients [25]. Wu et al observed lower frequency of Tregs in intestinal tissue of mice with immune inflammation [26]. Whether Tregs in the subjects with those immune disorders also express Bcl2L12 or whether Bcl2L12 plays a role in restricting Treg development in these disorders is worth being investigated.
The functional defects of Treg are a common phenomenon in immune disorders. As aforementioned, Tregs isolated from RA patients show incompetent immune suppressor ability [22,23]. Our data also show that RA Tregs are less capable of suppressing Teff proliferation, indicating these Tregs also have functional defects. The results reveal a novel aspect that Tregs from RA patients express high levels of Bcl2L12. This may attribute to the Treg functional defects since knockdown of Bcl2L12 can restore the immune suppressive ability of the Tregs. The underlying mechanism may be that Bcl2L12 restricts TGF-β gene transcription activities by interfering with Foxp3 binding to TGFB promoter.
In summary, the present data show less frequency of Treg in the peripheral blood system of RA patients. Tregs isolated from RA patients show functional defects. Knockdown of Bcl2L12 expression can promote Treg generation and immune suppressor ability. We conclude that Bcl2L12 in Tregs may be a therapeutic target to improve immune regulation.
Disclosure of conflict of interest
None.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Hohensinner PJ, Goronzy JJ, Weyand CM. Targets of immune regeneration in rheumatoid arthritis. Mayo Clin Proc. 2014;89:563–575. doi: 10.1016/j.mayocp.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutolo M, Nadler SG. Advances in CTLA-4-Ig-mediated modulation of inflammatory cell and immune response activation in rheumatoid arthritis. Autoimmun Rev. 2013;12:758–767. doi: 10.1016/j.autrev.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Wasserman AM. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2011;84:1245–1252. [PubMed] [Google Scholar]
- 6.Boissier MC, Semerano L, Challal S, Saidenberg-Kermanac’h N, Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun. 2012;39:222–228. doi: 10.1016/j.jaut.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Holmdahl R, Malmstrom V, Burkhardt H. Autoimmune priming, tissue attack and chronic inflammation - the three stages of rheumatoid arthritis. Eur J Immunol. 2014;44:1593–1599. doi: 10.1002/eji.201444486. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278:369–395. doi: 10.1111/joim.12395. [DOI] [PubMed] [Google Scholar]
- 9.Boissier MC, Semerano L, Challal S, Saidenberg-Kermanac’h N, Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun. 2012;39:222–8. doi: 10.1016/j.jaut.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Mahevas M, Michel M, Weill JC, Reynaud CA. Long-lived plasma cells in autoimmunity: lessons from B-cell depleting therapy. Front Immunol. 2013;4:494. doi: 10.3389/fimmu.2013.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhang Y, Gu W, He L, Sun B. Th1/Th2 cell’s function in immune system. Adv Exp Med Biol. 2014;841:45–65. doi: 10.1007/978-94-017-9487-9_3. [DOI] [PubMed] [Google Scholar]
- 12.Piccioni M, Chen Z, Tsun A, Li B. Regulatory T-cell differentiation and their function in immune regulation. Adv Exp Med Biol. 2014;841:67–97. doi: 10.1007/978-94-017-9487-9_4. [DOI] [PubMed] [Google Scholar]
- 13.Palomares O, Martin-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, Akdis CA. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-beta. Genes Immun. 2014;15:511–520. doi: 10.1038/gene.2014.45. [DOI] [PubMed] [Google Scholar]
- 14.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259:40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan X, Cheng G, Malek TR. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259:103–114. doi: 10.1111/imr.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stegh AH, DePinho RA. Beyond effector caspase inhibition: Bcl2L12 neutralizes p53 signaling in glioblastoma. Cell Cycle. 2011;10:33–38. doi: 10.4161/cc.10.1.14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geomela PA, Kontos CK, Yiotakis I, Scorilas A. Quantitative expression analysis of the apoptosis-related gene, BCL2L12, in head and neck squamous cell carcinoma. J Oral Pathol Med. 2013;42:154–161. doi: 10.1111/j.1600-0714.2012.01190.x. [DOI] [PubMed] [Google Scholar]
- 18.Tong Z, Liu N, Lin L, Guo X, Yang D, Zhang Q. miR-125a-5p inhibits cell proliferation and induces apoptosis in colon cancer via targeting BCL2, BCL2L12 and MCL1. Biomed Pharmacother. 2015;75:129–136. doi: 10.1016/j.biopha.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 19.Xue JM, Yang LT, Yang G, Geng XR, Liu ZQ, Wang S, Zhao HL, Liu ZG, Zhao CQ, Yang PC. Protease-activated receptor-2 suppresses interleukin (IL)-10 expression in B cells via upregulating Bcl2L12 in patients with allergic rhinitis. Allergy. 2017;72:1704–1712. doi: 10.1111/all.13186. [DOI] [PubMed] [Google Scholar]
- 20.Guo X, Li MG, Li SS, Liu FH, Liu ZJ, Yang PC. Tumor necrosis factor suppresses interleukin 10 in peripheral B cells via upregulating Bcl2-like protein 12 in patients with inflammatory bowel disease. Cell Biochem Funct. 2017;35:77–82. doi: 10.1002/cbf.3250. [DOI] [PubMed] [Google Scholar]
- 21.Li MG, Liu XY, Liu ZQ, Hong JY, Liu JQ, Zhou CJ, Hu TY, Xiao XJ, Ran PX, Zheng PY, Liu ZG, Yang PC. Bcl2L12 contributes to Th2-biased inflammation in the intestinal mucosa by regulating CD4(+) T cell activities. J Immunol. 2018;201:725–733. doi: 10.4049/jimmunol.1800139. [DOI] [PubMed] [Google Scholar]
- 22.Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, Liu X, Xiao L, Chen X, Wan B, Chin YE, Zhang JZ. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 23.Ji L, Geng Y, Zhou W, Zhang Z. A study on relationship among apoptosis rates, number of peripheral T cell subtypes and disease activity in rheumatoid arthritis. Int J Rheum Dis. 2016;19:167–171. doi: 10.1111/1756-185X.12211. [DOI] [PubMed] [Google Scholar]
- 24.Yentur SP, Gurses C, Demirbilek V, Adin-Cinar S, Kuru U, Uysal S, Yapici Z, Yilmaz G, Cokar O, Onal E, Gokyigit A, Saruhan-Direskeneli G. A decrease of regulatory T cells and altered expression of NK receptors are observed in subacute sclerosing panencephalitis. Viral Immunol. 2014;27:506–511. doi: 10.1089/vim.2014.0070. [DOI] [PubMed] [Google Scholar]
- 25.Mao C, Wang S, Xiao Y, Xu J, Jiang Q, Jin M, Jiang X, Guo H, Ning G, Zhang Y. Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves’ disease. J Immunol. 2011;186:4734–4743. doi: 10.4049/jimmunol.0904135. [DOI] [PubMed] [Google Scholar]
- 26.Wu W, Sun M, Zhang HP, Chen T, Wu R, Liu C, Yang G, Geng XR, Feng BS, Liu Z, Liu Z, Yang PC. Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. Gut. 2014;63:1883–1892. doi: 10.1136/gutjnl-2013-306083. [DOI] [PMC free article] [PubMed] [Google Scholar]


