Abstract
Accumulation of advanced glycation end-products (AGEs) increases inflammation and triggers processes involved in the pathogenesis of osteoarthritis (OA). As a major debilitating age-related disease, it is imperative that novel therapies for OA be sought. In the present study, we investigated the effects of the selective dipeptidyl peptidase IV (DPP-4) inhibitor sitagliptin in human primary chondrocytes exposed to insult by AGEs to elucidate the potential role of sitagliptin in the treatment of OA. Our findings show that inhibition of DPP-4 by sitagliptin could reduce oxidative stress, increase cell viability and prevent degradation of type II collagen and aggrecan by matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) induced by AGEs in human primary chondrocytes. Mechanistically, we found that sitagliptin inhibited AGEs-induced nuclear translocation of p65 protein and drastically decreased the luciferase activity of NF-κB. These findings indicate that sitagliptin may have potential as a novel therapeutic option for the treatment and prevention of OA.
Keywords: Osteoarthritis (OA), sitagliptin, dipeptidyl peptidase IV (DPP-4), advanced glycation end-products (AGEs), matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)
Introduction
Osteoarthritis (OA) is a painful debilitating disease affecting the lives of millions of people worldwide. OA was ranked as the 11th highest contributor to disability out of nearly 300 diseases reported in the 2010 Global Burden of Disease study [1]. Additionally, as the average age of the global population increases, the Centres for Disease Control (CDC) estimates that the incidence of OA will more than double by the year 2030 [2]. However, the mechanisms driving the development and progression of OA are complicated and remain poorly understood. Accumulation of advanced glycation end-products (AGEs) has been shown to play a role in the development and progression of age-related diseases such as OA by promoting the release of cytokines and chemokines, production of reactive oxygen species (ROS), and activation of the NF-κB proinflammatory signaling pathway [3,4]. Owing in part to their resilience to degradation, AGEs are commonly used a food preservative. However, once ingested, they accumulate in tissues along with AGEs produced by the body’s own process of non-enzymatic glycation, thereby further contributing to AGE-induced inflammation [5]. Additionally, chondrocytes have been shown to express the receptor for AGEs, thereby suggesting a role of AGEs in cell turnover and cartilage remodeling [6]. The two main components of articular cartilage are type II collagen and aggrecan, which are degraded by their respective enzymes, matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) [7,8]. While aggrecan has a relatively rapid rate of turnover in normal physiological conditions, type II collagen is extremely slow to regenerate, so excessive degradation of aggrecan and especially type II collagen is viewed as a major irreversible event in the pathogenesis of OA [9,10]. MMP-3 (stromelysin-1) and MMP-13 (collagenase 3) are the two major collagenases that degrade type II collagen via unwinding of the collagen triple helix and cleavage at the P4-P11’ site [11]. ADAMTS-4 and ADAMTS-5 have been cited as key aggrecanases involved in the pathogenesis of OA [12]. Upregulation of these proteolytic enzymes by AGEs coupled with AGEs-induced oxidative stress, inflammation, and apoptosis creates the perfect conditions for a sustained inflammatory response and excessive degradation of articular cartilage.
The class of drugs known as DPP-4 inhibitors, also termed gliptins, includes sitagliptin, vildagliptin, saxagliptin, teneligliptin, and alogliptin, among numerous others. While these drugs share a common mechanism of action that prevents degradation of the incretin hormone glucagon-like peptide 1 (GLP-1), they have diverse chemical properties, and thus may have a wide range of potential use cases in the treatment of various diseases [13]. Inhibition of DPP-4 reduces blood glucose levels by increasing the incretin hormones GLP-1 and gastric inhibitory polypeptide (GIP), thereby downregulating glucagon secretion, upregulating insulin secretion, and slowing gastric emptying, thus serving as an effective therapy for type II diabetes mellitus [14]. The possible involvement of DPP-4 in the development and progression of OA has recently been receiving both positive and negative attention. On one hand, inhibition of DPP-4 results in the preservation of endogenous GLP-1, which could exert beneficial actions by promoting bone formation and remodeling, suppressing bone resorption, and enhancing osteoblast differentiation [15]. On the other hand, it has been reported that the pharmacological inhibition of DPP-4 might induce the development of arthralgia [16,17]. The question of a beneficial role of DPP-4 inhibition in patients with OA has not yet been adequately answered. Therefore, in the present study, we investigated the effects of the specific DPP-4 inhibitor sitagliptin on AGEs-induced oxidative stress, cell viability, degradation of extracellular matrix and activation of the NF-κB signaling pathway in human primary chondrocytes (HPCs).
Materials and methods
Cell isolation, culture, and treatment
Human primary chondrocytes, purchased from MT-BIO Company (China), were maintained in DMEM/Ham’s F-12 medium supplemented with 10% FBS and 1% streptomycin/penicillin. Cells were treated with 100 μg/ml AGEs in the presence or absence of 100 and 200 nM sitagliptin [18] for 48 h. Human subject researches were designed following the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects.
RNA extraction and reverse transcription PCR analysis
Total RNA was extracted from cells using Qiazol (Qiagen, USA). RNA was quantified using a Nanodrop ND-1000 spectrophotometer. cDNA was generated through a reverse transcription PCR with a cDNA Synthesis Kit (Thermo Fisher Scientific, USA). The expression of target gene at the mRNA levels was measured through a real time PCR analysis using a SYBR® Green qPCR master mix on a 7500 Real-Time PCR System (Applied Biosystems, USA). Results were normalized to the expression of GAPDH using the 2-ΔΔCT method.
Western blot analysis
Human chondrocytes were scraped off of the culture plate with cell lysis buffer containing protease and phosphatase inhibitor cocktail. Protein concentration was determined using the BCA method. Proteins were separated by 8-12% SDS-PAGE and blotted onto nitrocellulose membranes. Membranes were blocked with 5% fat free milk for 2 h at room temperature. The membranes were then sequentially probed with primary antibodies overnight at 4°C and HRP-linked secondary antibodies at room temperature (RT). Blots were visualized using an enhanced chemiluminescence kit (Thermo Fisher Scientific, USA).
Measurement of intracellular reactive oxygen species (ROS)
Intracellular levels of ROS in human primary chondrocytes were assessed using DCFH-DA. Human chondrocytes were rinsed three times with PBS. DCFH-DA in phenol-free red medium at a final concentration of 10 μM was added to the cell culture plate and incubated for 30 min at 37°C to catalyze the conversion of DCFH-DA to a fluorescent DCF product. Fluorescent signals were captured using a fluorescence microscope.
Measurement of reduced glutathione (GSH)
HPCs were seeded into 6-well plates and treated with 100 μg/ml AGEs in the presence or absence of 100 and 200 nM sitagliptin for 48 h. A fluorometric assay was used to measure the reduction in the antioxidant GSH in HPCs. After treatment, cells were scraped from the culture plate and suspended in 5% ice cold meta-phosphoric acid (MPA). Cells were then sonicated and centrifugated at 14,000 rpm for 15 min. Aliquots of the supernatant were mixed with OPAME in methanol and borate buffer (Sigma-Aldrich, USA). The results were recorded at 350 nm.
Enzyme-linked immunosorbent (ELISA) assay
The secretion of HMG-1 from cytoplasm into the supernatant as well as intracellular levels of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 in HPCs were measured using ELISA kits purchased from R&D Systems. Briefly, 50 µL cell culture medium or cell lysate was collected and added into ELISA plates. After 2 h incubation, the liquid was removed. The ELISA plates were washed 3 times and incubated with 100 µL diluted detection antibody for 1 h at RT. After 3 washes, the ELISA plate was incubated with 100 µL HRP linked antibody for 30 min at RT. After 3 washes, a reaction was developed with 100 µL chromogenic substrate for 30 min in darkness. The reaction was then stopped with the stop solution. OD value was recorded at 450 nm.
Determination of lactate dehydrogenase (LDH) release
HPCs were seeded into 6-well plates and treated with 100 μg/ml AGEs in the presence or absence of 100 and 200 nM sitagliptin for 48 h. A total of 50 μl supernatant was collected and added to a fresh 96-well plate to be mixed with 50 μl of the LDH assay reagent. After incubation for 30 min in darkness, the reaction was stopped with 50 μL stop buffer. OD value at 490 nm was recorded to assess LDH release.
Measurement of cell viability
HPCs were seeded into 6-well plates and treated with 100 μg/ml AGEs in the presence or absence of 100 and 200 nM sitagliptin for 48 h. After 3 gentle washes, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in phenol-free red medium at the final concentration of 5 mg/mL was used to evaluate the cell viability of chondrocytes. After incubation for 4 h at 37°C in darkness, the product was dissolved with dimethyl sulfoxide (DMSO). OD value at 570 nm was measured to reflect the viability percentage.
Statistical analysis
Experimental data are expressed as means ± standard deviation (S.D.). Statistical analyses were performed using one-way analysis of variance (ANOVA) using the software SPSS (version 19.0). P values of less than 0.05 were considered to be statistically significant differences.
Results
Decreased oxidative stress
To determine the effects of inhibition of DPP-4 on oxidative stress in human primary chondrocytes (HPCs), we assessed the levels of ROS and the antioxidant glutathione (GSH) in HPCs stimulated with 100 ng/ml AGEs in the presence or absence of the specific DPP-4 inhibitor sitagliptin. As shown by the results of DCFH-DA staining in Figure 1A, treatment with AGEs potently increased production of ROS, which was ameliorated by sitagliptin in a dose-dependent manner. Additionally, the results in Figure 1B indicate that 100 ng/ml AGEs decreased the level of GSH by roughly half, which was also ameliorated by treatment with 100 and 200 nM sitagliptin in a dose-dependent manner. Thus, sitagliptin can improve oxidative stress in HPCs due to insult from AGEs.
Figure 1.
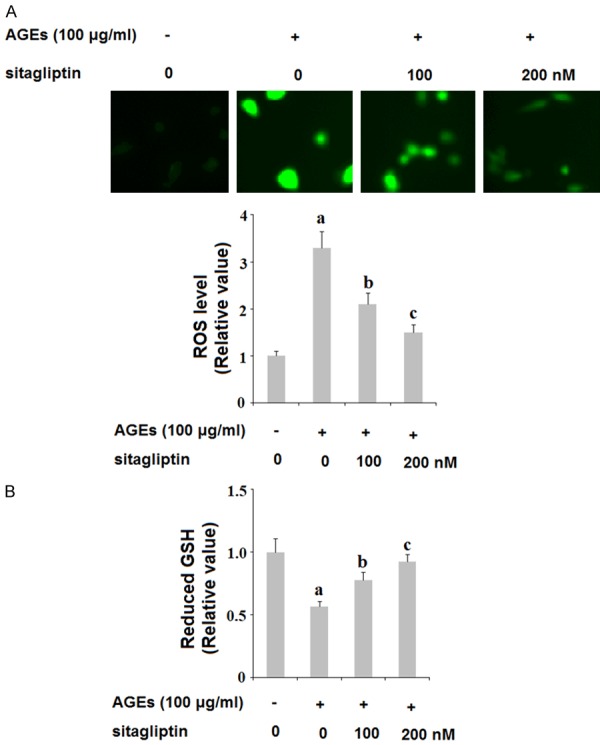
Sitagliptin ameliorates advanced glycation end-products (AGEs)-induced oxidative stress in human primary chondrocytes. Human primary chondrocytes were treated with 100 μg/ml AGEs in the presence or absence of 100, 200 nM sitagliptin for 48 h. A. Reactive oxygen species (ROS) was determined by DCFH-DA; B. Intracellular level of reduced GSH (a, b, c, P<0.01 vs. previous column group).
Downregulated expression of HMG-1 and increased cell viability
The release of the chemokine high mobility group protein 1 (HMG-1) has been associated with cell death. Here, we investigated whether treatment with sitagliptin can ameliorate cell death by downregulating expression of HMG-1 in HPCs. As shown in Figure 2, exposure to 100 ng/ml AGEs for 48 h tripled the release of HMG-1 by HPCs, which was ameliorated by treatment with 100 and 200 nM sitagliptin in a dose-dependent manner. Furthermore, we assessed the effects of inhibition of DPP-4 on AGEs-induced reduced cell viability. HPCs were exposed to 100 ng/ml AGEs in the presence or absence of 100 and 200 nM sitagliptin for 48 h. As shown by the results of lactose dehydrogenase (LDH) assay and MMT in Figure 3A and 3B, insult from AGEs drastically increased the release of LDH and reduced cell viability by more than half, both of which were ameliorated by treatment with sitagliptin in a dose-dependent manner. These findings indicate that inhibition of DPP-4 can ameliorate cell death and can increase cell viability of HPCs exposed to AGEs.
Figure 2.
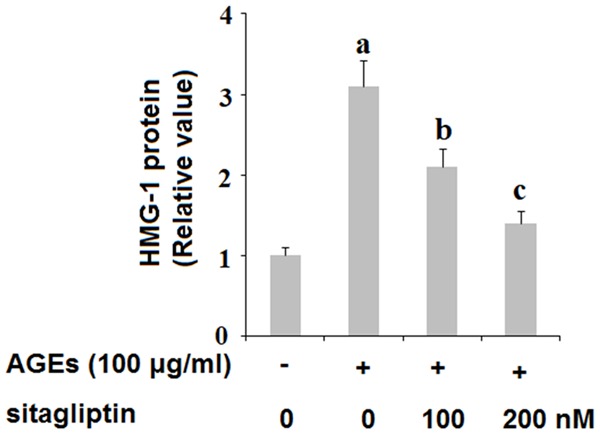
Sitagliptin mitigates advanced glycation end-products (AGEs)-induced release of high-mobility group protein 1 (HMG-1) in human primary chondrocytes. Human primary chondrocytes were treated with 100 μg/ml AGEs in the presence or absence of 100, 200 nM sitagliptin for 48 h. Release of HMG-1 was determined by ELISA assay (a, b, c, P<0.01 vs. previous column group).
Figure 3.
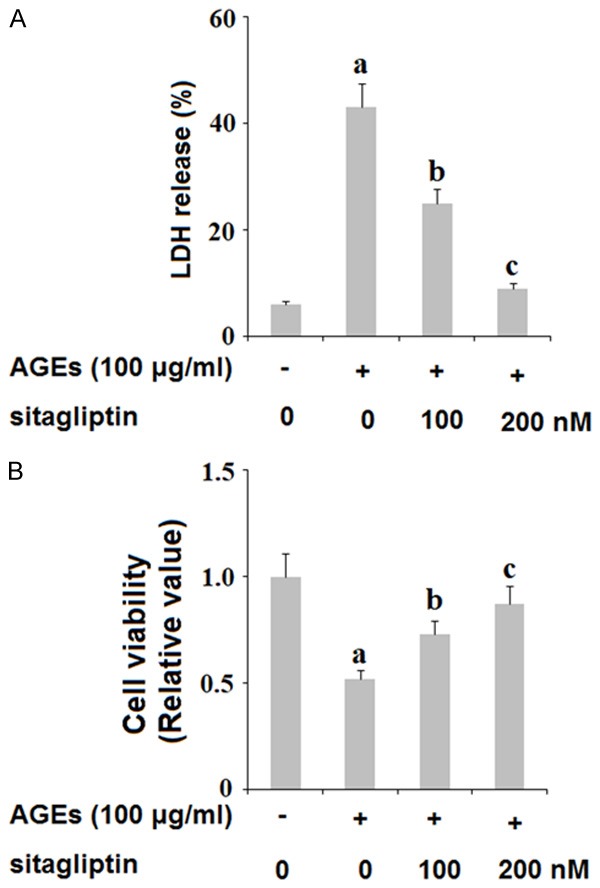
Sitagliptin attenuates advanced glycation end-products (AGEs)-induced release of lactate dehydrogenase (LDH) and reduction of cell viability in human primary chondrocytes. Human primary chondrocytes were treated with 100 μg/ml AGEs in the presence or absence of 100, 200 nM sitagliptin for 48 h. A. Release of LDH was assayed; B. Cell viability was determined by the MTT method (a, b, c, P<0.01 vs. previous column group).
Ameliorated MMP-mediated degradation of type II collagen
MMP-3 and MMP-13 are the major collagenases responsible for degradation of type II collagen, a primary component of articular cartilage. To determine the effects of inhibition of DPP-4 on AGE-induced expression of MMPs and subsequent degradation of type II collagen, we exposed HPCs to 100 ng/ml AGEs in the presence or absence of 100 and 200 nM sitagliptin for 48 h. As revealed by the results of real-time PCR and ELISA analysis shown in Figure 4, AGEs significantly increased expression of MMP-3 and MMP-13 at both the mRNA and protein levels, respectively, which was ameliorated by treatment with 100 and 200 nM sitagliptin in a dose-dependent manner. Concordantly, the results of western blot analysis in Figure 5 show that, using β-actin as a control, exposure to 100 ng/ml AGEs reduced the level of type II collagen by roughly 75%, which was ameliorated by treatment with 100 and 200 nM sitagliptin in a dose-dependent manner.
Figure 4.
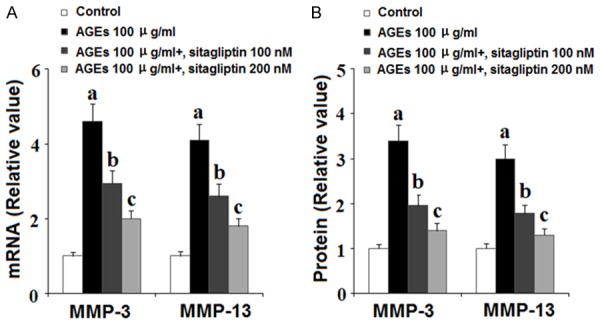
Sitagliptin suppresses advanced glycation end-products (AGEs)-induced expression of matrix metalloproteinases (MMPs) in human primary chondrocytes HPCs). HPCs were treated with 100 μg/ml AGEs in the presence or absence of 100, 200 nM sitagliptin for 48 h. A. Expression of MMP-3 and MMP-13 at the gene level was determined by real-time PCR analysis; B. Expression of MMP-3 and MMP-13 at the protein level was determined by ELISA (a, b, c, P<0.01 vs. previous column group).
Figure 5.
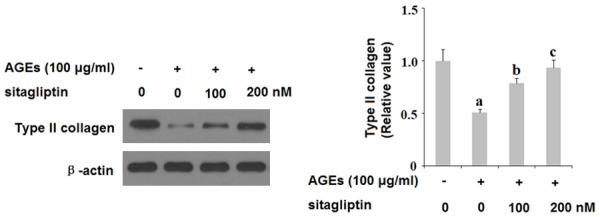
Sitagliptin inhibits advanced glycation end-products (AGEs)-induced degradation of type II collagen in human chondrocytes. Human primary chondrocytes were treated with 100 μg/ml AGEs in the presence or absence of 100, 200 nM sitagliptin for 48 h. Type II collagen was measured by western blot analysis (a, b, c, P<0.01 vs. previous column group).
Ameliorated ADAMTS-induced degradation of aggrecan
Along with degradation of type II collagen, excessive degradation of aggrecan by ADAMTS is a major event in the pathogenesis of OA. To investigate the role of DPP-4 in degradation of aggrecan, we exposed HPCs to 100 ng/ml AGEs in the presence or absence of 100 and 200 nM sitagliptin for 48 h and assessed the expression levels of ADAMTS-4 and ADAMTS-5 as well as the extent of aggrecan degradation. As shown in Figure 6, insult from AGEs quadrupled the expression of ADAMTS-4 and ADAMTS-5 at the mRNA level and tripled the expression of these two aggrecanases at the protein level, both of which were ameliorated by treatment with sitagliptin in a dose-dependent manner. To further verify the protective effects of sitagliptin against cartilage degradation, we measured the level of aggrecan expression by HPCs using β-actin as a control. As shown by the results of western blot analysis in Figure 7, exposure to AGEs significantly increased degradation of aggrecan, which was ameliorated by treatment with 100 and 200 nM sitagliptin in a dose-dependent manner. These findings implicate a potential therapeutic role of DPP-4 inhibition by sitagliptin in the treatment and prevention of OA.
Figure 6.
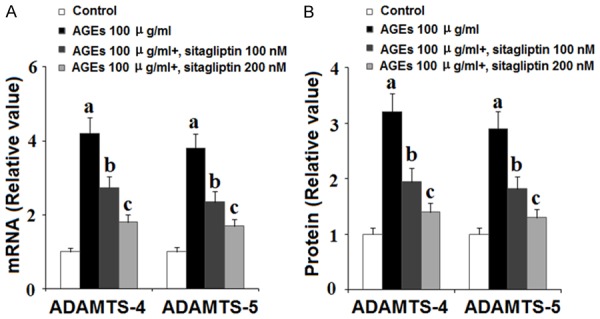
Sitagliptin suppresses advanced glycation end-products (AGEs)-induced expression of ADAMTS-4 and ADAMTS-5 in human chondrocytes. Human primary chondrocytes were treated with 100 μg/ml AGEs in the presence or absence of 100, 200 nM sitagliptin for 48 h. A. ADAMTS-4 and ADAMTS-5 at the gene levels were determined by real time PCR analysis; B. ADAMTS-4 and ADAMTS-5 at the protein levels were determined by ELISA (a, b, c, P<0.01 vs. previous column group).
Figure 7.
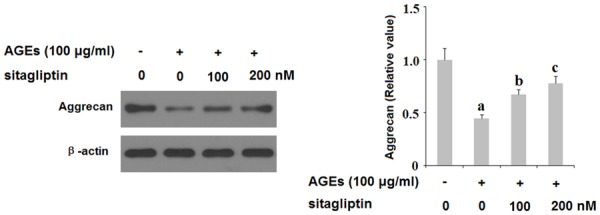
Sitagliptin suppresses advanced glycation end-products (AGEs)-induced degradation of aggrecan in human primary chondrocytes (HPCs). HPCs were treated with 100 μg/ml AGEs in the presence or absence of 100, 200 nM sitagliptin for 48 h. Expression of aggrecan was determined by western blot analysis (a, b, c, P<0.01 vs. previous column group).
Reduced activation of the NF-κB signaling pathway
Finally, we set out to determine the role of DPP-4 in activation of the NF-κB proinflammatory signaling pathway. HPCs were exposed to insult from 100 ng/ml AGEs in the presence or absence of 100 and 200 nM sitagliptin for 48 h. As shown in Figure 8, exposure to AGEs significantly increased nuclear translocation of p65 protein by 3.5-fold and drastically increased the luciferase activity of NF-κB by roughly 30-fold, which were both ameliorated by treatment with sitagliptin in a dose-dependent manner. These findings imply that inhibition of DPP-4 by sitagliptin may have a potent protective effect against activation of the proinflammatory NF-κB signaling pathway.
Figure 8.
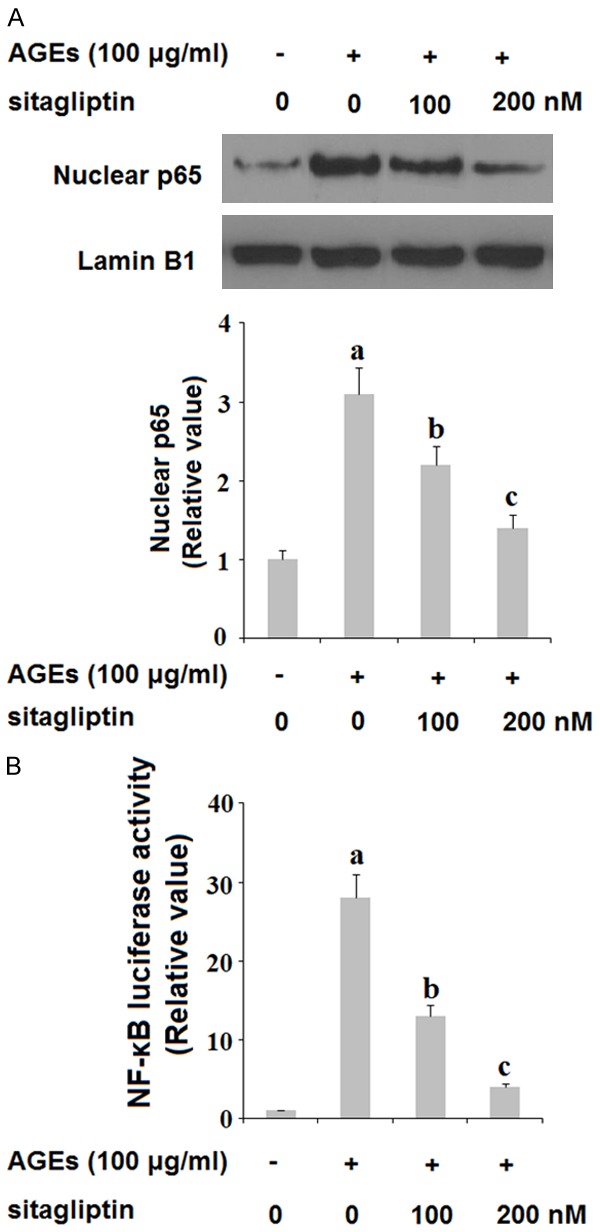
Sitagliptin inhibits the activation of the NF-κB signaling. Human primary chondrocytes (HPCs) were treated with 100 μg/ml AGEs in the presence or absence of 100, 200 nM sitagliptin for 48 h. A. Nuclear translocation of NF-κB p65; B. NF-κB luciferase activity (a, b, c, P<0.01 vs. previous column group).
Discussion
As one of the greatest immediate threats to the health of the global population, there have been numerous studies seeking safe and effective therapies to prevent, slow or reverse the pathological progression of OA. Inhibition of enzymes that facilitate cartilage degradation, such as MMPs and ADAMTS, has been widely explored as a therapeutic target for OA. However, the mechanisms driving the expression of these molecules are complicated. Recent studies have demonstrated the involvement of GLP-1 and its receptor in bone formation and remodeling, preventing bone resorption, and osteoblast differentiation [19,20]. Inhibition of DPP-4 can prevent degradation of GLP-1, thereby having an agonistic effect on the GLP-1 receptor. In the present study, we investigated the effects of inhibition of DPP-4 using the selective DPP-4 inhibitor sitagliptin on various factors related to OA. Oxidative stress is implicated in a wide range of inflammatory diseases. Recent studies have shown that sitagliptin can reduce oxidative stress in the heart, kidneys, brain, and liver [21-24] The results of our study contribute to this pool of data by demonstrating the beneficial effects of sitagliptin in preventing AGEs-induced generation of ROS and decreased levels of the antioxidant glutathione (GSH) in HPCs stimulated with AGEs (Figures 1 and 2), thereby having a potential anti-oxidative stress role in OA. High mobility group protein 1 (HMG-1) is an important proinflammatory cytokine which has been identified as a substrate of DPP-4 and which binds with high affinity to the receptor for AGEs (RAGE), thereby promoting further RAGE-mediated effects [25,26]. Here, we found that treatment with sitagliptin could reduce increased levels of HMG-1 triggered by exposure to AGEs. We also found that treatment with sitagliptin could improve cell viability of HPCs, as evidenced by reduced LDH release (Figure 3).
Previous studies have demonstrated the effects of DPP-4 inhibitors on expression of MMPs and ADAMTS. The results of a contemporary study show that treatment with vildagliptin, another DPP-4 inhibitor, could reduce expression of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5, subsequent degradation of type II collagen and aggrecan, respectively, and activation of NF-κB in HPCs stimulated with interleukin 1β [27]. Consistent with these findings, the present study demonstrates that sitagliptin can rescue degradation of type II cartilage and aggrecan via downregulation of expression of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 expression in a dose-dependent manner (Figures 4, 5, 6 and 7). Lastly, we also demonstrated the potential ability of sitagliptin to reduce inflammation via inhibition of nuclear translocation of p65 protein and activation of the NF-κB proinflammatory signaling pathway (Figure 8). The role of NF-κB in OA and other chronic inflammatory diseases has been widely researched. Recent studies have revealed the capacity of sitagliptin to reduce activation of NF-κB in diverse tissues [28-30]. To our knowledge, this is the first study to demonstrate the ability of sitagliptin to reduce AGE-induced NF-κB activation on OA chondrocytes.
The main limitation of this study is that we only investigated the protective effects of sitagliptin against AGEs-induced degradation of type II collagen and aggrecan in an in vitro primary chondrocytes culture model. It should be noticed that the pathological mechanism of OA is complex. A variety of risk factors have been associated with the pathogenesis of OA, including genetics, mechanical instability and joint injuries, ageing, obesity. Future in vivo investigations with animal models or clinical trails will be helpful for verifying the pharmacological function of sitagliptin in OA.
Taken together, our findings demonstrate that sitagliptin can ameliorate oxidative stress, improve cell viability, reduce cartilage degradation via downregulation of expression of MMPs and ADAMTS, and inhibiting activation of the NF-κB pathway in human primary chondrocytes. Therefore, administration of sitagliptin may have potential as a safe and effective therapy to prevent or halt the progression of OA. Further study is required to broaden our understanding of the mechanisms involved.
Acknowledgements
This research was supported by the National Nature Science Foundation of China (81873990, 81873991, 81672238, 81472077 and 81372018), the Jiangsu Provincial Medical Youth Talent (QNRC2016751), the Natural Science Foundation of Jiangsu province (BK20180001), and the application fundamental research program of Suzhou City (KJXW2017009, SYS2018032).
Disclosure of conflict of interest
None.
References
- 1.Allen KD, Golightly YM. Epidemiology of osteoarthritis: state of the evidence. Curr Opin Rheumatol. 2015;27:276–83. doi: 10.1097/BOR.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osteoarthritis [Online] Centers for disease control and prevention centers for disease control and prevention: 2018 [Google Scholar]
- 3.Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott C, Jacobs K, Haucke E, Santos AN, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–29. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu H, Li J, Wu L, Chen W. Trichostatin a increases the TIMP-1/MMP ratio to protect against osteoarthritis in an animal model of the disease. Mol Med Rep. 2016;14:2423–2430. doi: 10.3892/mmr.2016.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì RM, Marcu KB. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang CY, Lai KY, Hung LF, Wu WL, Liu FC, Ho LJ. Advanced glycation end products cause collagen II reduction by activating Janus kinase/signal transducer and activator of transcription 3 pathway in porcine chondrocytes. Rheumatology. 2011;50:1379–1389. doi: 10.1093/rheumatology/ker134. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 9.Ismail HM, Miotla-Zarebska J, Troeberg L, Tang X, Stott B, Yamamoto K, Nagase H, Fosang AJ, Vincent TL, Saklatvala J. Brief report: JNK-2 controls aggrecan degradation in murine articular cartilage and the development of experimental osteoarthritis. Arthritis Rheumatol. 2016;68:1165–1171. doi: 10.1002/art.39547. [DOI] [PubMed] [Google Scholar]
- 10.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siebuhr AS, He Y, Gudmann NS, Gram A, Kjelgaard-Petersen CF, Qvist P, Karsdal MA, Bay-Jensen AC. Biomarkers of cartilage and surrounding joint tissue. Biomark Med. 2014;8:713–731. doi: 10.2217/bmm.13.144. [DOI] [PubMed] [Google Scholar]
- 12.Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem. 2011;112:3507–3514. doi: 10.1002/jcb.23298. [DOI] [PubMed] [Google Scholar]
- 13.Chen XW, He ZX, Zhou ZW, Yang T, Zhang X, Yang YX, Duan W, Zhou SF. Clinical pharmacology of dipeptidyl peptidase 4 inhibitors indicated for the treatment of type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015;42:999–1024. doi: 10.1111/1440-1681.12455. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh C, Demuth H, Pospisilik J, Pederson R. Dipeptidyl peptidase IV inhibitors: how do they work as new antidiabetic agents? Regul Pept. 2005;128:159–65. doi: 10.1016/j.regpep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Iepsen EW, Lundgren JR, Hartmann B, Pedersen O, Hansen T, Jørgensen NR, Jensen JE, Holst JJ, Madsbad S, Torekov SS. GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab. 2015;100:2909–2917. doi: 10.1210/jc.2015-1176. [DOI] [PubMed] [Google Scholar]
- 16.Mascolo A, Rafaniello C, Sportiello L, Sessa M, Cimmaruta D, Rossi F, Capuano A. Dipeptidyl peptidase (DPP)-4 inhibitor-induced arthritis/arthralgia: a review of clinical cases. Drug Saf. 2016;39:401–407. doi: 10.1007/s40264-016-0399-8. [DOI] [PubMed] [Google Scholar]
- 17.Men P, He N, Song C, Zhai S. Dipeptidyl peptidase-4 inhibitors and risk of arthralgia: a systematic review and meta-analysis. Diabetes Metab. 2017;43:493–500. doi: 10.1016/j.diabet.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Gonçalves A, Almeida L, Silva AP, Fontes-Ribeiro C, Ambrósio AF, Cristóvão A, Fernandes R. The dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin ameliorates retinal endothelial cell dysfunction triggered by inflammation. Biomed Pharmacother. 2018;102:833–838. doi: 10.1016/j.biopha.2018.03.144. [DOI] [PubMed] [Google Scholar]
- 19.Iepsen EW, Lundgren JR, Hartmann B, Pedersen O, Hansen T, Jørgensen NR, Jensen JE, Holst JJ, Madsbad S, Torekov SS. GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab. 2015;100:2909–2917. doi: 10.1210/jc.2015-1176. [DOI] [PubMed] [Google Scholar]
- 20.Christensen MB, Lund A, Calanna S, Jørgensen NR, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia. J Clin Endocrinol Metab. 2018;103:288–294. doi: 10.1210/jc.2017-01949. [DOI] [PubMed] [Google Scholar]
- 21.Meng J, Ma X, Wang N, Jia M, Bi L, Wang Y, Li M, Zhang H, Xue X, Hou Z, Zhou Y, Yu Z, He G, Luo X. Activation of GLP-1 receptor promotes bone marrow stromal cell osteogenic differentiation through β-catenin. Stem Cell Rep. 2016;6:579–591. doi: 10.1016/j.stemcr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam MA, Chowdhury MRH, Jain P, Sagor MAT, Reza HM. DPP-4 inhibitor sitagliptin prevents inflammation and oxidative stress of heart and kidney in two kidney and one clip (2K1C) rats. Diabetol Metab Syndr. 2015;7:107. doi: 10.1186/s13098-015-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gault VA, Lennox R, Flat PR. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab. 2015;17:403–413. doi: 10.1111/dom.12432. [DOI] [PubMed] [Google Scholar]
- 24.Coskun ZM, Koyuturk M, Karabulut S, Bolkent S. CB-1R and GLP-1R gene expressions and oxidative stress in the liver of diabetic rats treated with sitagliptin. Pharmacol Rep. 2017;69:822–829. doi: 10.1016/j.pharep.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Tagore DM, Nolte WM, Neveu JM, Rangel R, Guzman-Rojas L, Pasqualini R, Arap W, Lane WS, Saghatelian A. Peptidase substrates via global peptide profiling. Nat Chem Biol. 2009;5:23–5. doi: 10.1038/nchembio.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Xu M, Bai J, Ge G, Guo X, Yu B, Xiao L, Geng D, Hao Y. Vildagliptin reduced extracellular matrix degradation in human primary chondrocytes. Eur J Pharmacol. 2019;844:49–55. doi: 10.1016/j.ejphar.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Liu S, Liu X, Zhang J, Liang Y, Li Y. DPP-4 (CD26) inhibitor sitagliptin exerts anti-inflammatory effects on rat insulinoma (RINm) cells via suppressing NF-κB activation. Endocrine. 2017;55:754–763. doi: 10.1007/s12020-016-1073-8. [DOI] [PubMed] [Google Scholar]
- 29.Tang ST, Su H, Zhang Q, Tang HQ, Wang CJ, Zhou Q, Wei W, Zhu HQ, Wang Y. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-κB/IκBα system through the activation of AMP-activated protein kinase. Int J Mol Med. 2016;37:1558–1566. doi: 10.3892/ijmm.2016.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang W, Wen D, Cheng Z, Yang Y, Zheng G, Yin F. Effect of sitagliptin, a DPP-4 inhibitor, against DENA-induced liver cancer in rats mediated via NF-κB activation and inflammatory cytokines. J Biochem Mol Toxicol. 2018;32:e22220. doi: 10.1002/jbt.22220. [DOI] [PubMed] [Google Scholar]


