Abstract
Endoscopic full-thickness resection (EFTR) and submucosal tunneling endoscopic resection (STER) are the frontier of therapeutic endoscopic. These two methods rely on the skillset and equipment of endoscopic submucosal dissection (ESD) while going beyond the boundaries of the gastrointestinal lumen. They are both representatives of natural orifice transluminal endoscopic surgery, with STER being a direct off-shoot of peroral endoscopic myotomy (POEM). Both techniques are designed for the removal of gastrointestinal tumors originating from the muscularis propria but tend to be used in different organs and come with respective challenges. In this review we will go over the history, indication, technique and literature of these two techniques.
Keywords: Endoscopic resection, natural orifice transluminal endoscopic surgery, gastrointestinal stromal tumor (GIST), leiomyoma, therapeutic endoscopy
The field of interventional endoscopy has rapidly developed over the past decade, together with the genesis of the emerging field of Natural Orifice Transluminal Endoscopic Surgery (NOTES). Applications for gastrointestinal endoscopy is ever expanding and often go beyond the lumen of the gastrointestinal tract. Perforation, as defined by the ASGE lexicon (1), evidence of air or luminal contents outside of the GI tract, used to be a formidable word for any endoscopist but is now frequently performed during procedures such as Peroral Endoscopic Myotomy (POEM).
Treatment of gastrointestinal subepithelial lesions (SEL) is among the most exciting development of interventional endoscopy. SELs are commonly found incidentally during endoscopic procedures done for unrelated purposes. Insufficient data exists regarding their epidemiology, natural behavior or appropriate intervention. Majority of SELs detected in the upper gastrointestinal tract are gastrointestinal stromal tumors (GISTs), followed by leiomyoma, and more distantly, by other gastrointestinal mesenchymal tumors (GIMTs), neuroendocrine tumor (NET), granular cell tumor, glomus tumor, and various other pathologies. Majority of SELs detected in the rectum are SEL-mimicking epithelial tumors (oftentimes adenoma with scarring or adenocarcinoma) or NET and there is little data regarding the epidemiology of SELs in the colon due to its rarity. It is important to differentiate these pathologies, as GIST and NET have malignant potentials, for which relatively aggressive intervention or surveillance are recommended (2,3). Current NCCN guideline on GISTs recommends resection of all symptomatic lesions, any lesions that are ≥2 cm, or lesions that have high risk features under EUS. Annual surveillance is recommended for low risk GISTs (2). NET, meanwhile, has higher malignant potentials. Some investigators advocate resecting all visible lesions. The minimal approach should be to resect tumors ≥1 cm in diameter. NET require surveillance similar if not more stringent than GIST (4,5). Lesions other than GIST or NET, in comparison, have much lower malignant potentials and a more relaxed surveillance approach is favored. Despite this, it is frequently difficult to determine the nature of a SEL based on morphology alone. The most commonly used tissue acquisition method, EUS-guided needle biopsy, has highly operator-dependent result, with literature reporting a diagnostic yield of less than 20% to higher than 90% (6). Treating all tumors as a ‘potential GIST’ can lead to excessive healthcare cost and cast emotional stress onto the patients.
Because of the aforementioned limitations in SEL management, methods have been developed to remove the SELs en bloc via flexible endoscopy both for histology diagnosis and as a therapy by itself. In this review we will discuss in depth two such methods, namely, pure Endoscopic Full Thickness Resection (pure EFTR) and Submucosal Tunneling Endoscopic Resection (STER). Both these methods can be performed without assistance of laparoscopy or equipment other than those routinely stocked in an interventional endoscopy unit.
Rationales for endoscopic resection
SELs are especially appropriate for endoscopic resection due to the following reasons:
Unlike tumors of epithelial origin, SELs rarely exhibit a malignant potential when small and even when malignant, do no not metastasize via the lymph duct (7). A wide negative margin is not needed when resecting SELs (unlike when resecting epithelial tumors) as evidence from high quality trials showed no difference between survival after ‘R1’ and ‘R0’ resection in the absence of tumor rupture (8-10);
Because these tumors are frequently found in critical locations such as the esophagus, esophagogastric junction, cardia and antrum, laparoscopic wedge resection is often not possible for tumor removal and more invasive organ resection is called for (11). This leads to a disruption of the natural anatomy of the gastrointestinal tract and is too radical for small low-risk SELs;
Endoscopic removal of small SELs (less than 3–4 cm in the largest diameter) followed by purely endoscopic closure is well-tested and widely-performed in certain countries, is safe and can be done fairly quickly. ‘Standby’ of a surgical team is not routinely needed and the procedure can be performed in the endoscopic suite. Given the relatively low risk and low cost of this approach, early endoscopic removal can be favored over long-term surveillance when appropriate expertise is available in certain cases.
Development of techniques
Endoscopic full-thickness resection
The pursuit for full-thickness tumor resection followed by surgical-level closure of the gastrointestinal wall using flexible endoscopy has been long. Numerous commercially-available or custom-designed devices have been tested (12-14). A patent search using ‘gastric full thickness resection’ returned >1,000 results, majority of which never reached the clinical phase. Two devices commonly used clinically today are the Over-the-Scope-Clip (OTSC®, Ovesco Endoscopy AG, Tubingen, Germany) and its second generation, Full-Thickness-Resection Device (FTRD®). A few relatively large studies on EFRD® from Europe reported using this device on a variety of indications, including difficult colorectal adenomas (15-20), gastric subepithelial tumors (21), and duodenal tumors (22). This device is useful as it offers a quick and easy-to-learn method to manage some challenging situations, such as early carcinoma or adenomas that have been previously manipulated or involve diverticulum or the appendiceal orifice. On the other hand, this technique is limited in tumor size (mostly <1 cm in the upper GI tract and <2 cm in the colorectum) and location (sharp bending of the endoscope can lead to clip deployment failure) (8). Non-R0 resection and adverse events are other concerns (17).
In comparison, a more versatile technique of ‘pure’ EFTR using endoscopic submucosal dissection (ESD) techniques and equipment have been reported (23,24). In contrast to device-assisted EFTR, pure EFTR is a ‘cut then close’ technique that constitutes dissecting the tumor around the capsule and completely remove the tumor from its attachment to the muscularis propria prior to closing the lumen defect. Major differences exist between device-assisted EFTR and pure EFTR. In this review we will focus on pure EFTR.
Submucosal tunneling endoscopic resection
STER is a direct offshoot of the endoscopic tunneling technique (25-27) and got wide clinical adoption while POEM became standard practice. First described in humans by Japanese and Chinese pioneer POEM centers in 2012 (28,29), STER (coined Peroral Endoscopic Tumor Resection, aka POET, by the Japanese center) utilizes the mucosa flap as a safety valve to prevent extravasation of lumen contents. One advantage of STER compared to EFTR is the relative easiness of closing the tunnel entrance compared to a full-thickness defect. One study compared outcomes of STER and EFTR for gastric GIST originating from the muscularis propria and found that patients who received EFTR had a longer suture time and needed more clips to close the gastric wall defect (30). However, we should note that the anatomical location most suitable for STER and EFTR are largely non-overlapping (see below).
Choice between pure EFTR and STER
When tunneling is feasible, STER is almost always the preferred technique as the mucosa flap entrance is easier to close than a full-thickness defect. The most suitable locations for a tunnel are those reachable by endoscopy in a straight line, i.e., the middle and lower esophagus, the gastroesophageal junction and gastric cardia, and less so the gastric antrum and rectum. Tumors larger than 3–4 cm in the shortest diameter are difficult to retract through the tunnel entrance sometimes and are difficult to operate on inside a confined space. Pure EFTR has more flexibility in terms of tumor morphology and location but should be avoided in the esophagus and certain locations of limited maneuverability of the endoscope and the suturing device, i.e., the gastric fundus and duodenum, unless done by very experience operators.
Indications and contraindications
No high-quality evidence or consensus exist on the appropriate indication or contraindication for endoscopic resection of SETs. The following patient eligibility criteria are used by the writers: (I) if the lesion is symptomatic; (II) GIST or suspected GIST >2 cm or with high risk EUS features; (III) NET; (IV) adenoma in transmural scars; (V) non-GIST mesenchymal tumors (e.g., Schwannoma, leiomyoma) that are non-symptomatic but are >2–3 cm in size, have rapid growth or high risk EUS/histologic features (e.g., central necrosis, nuclear atypia, high mitotic rate); (VI) undiagnosed lesions in younger patients for whom the risk of resection might be outweighed by the benefit of avoiding long-term surveillance. Patients are considered eligible for pure EFTR or STER as opposed to endoscopic submucosal excavation (ESE) or ESD if the tumor has significant muscularis propria involvement, has extraluminal component, or if full-thickness penetration of the gastrointestinal wall is expected for complete removal of the tumor.
Contraindications for pure EFTR and STER include any contraindication for local resection (e.g., severe comorbidity or sign of metastasis). More specifically, tumor involvement and sometimes adjacency to large extra-luminal vessels is a contraindication for EFTR and STER (as compared to laparoscopic resection) as currently the ability of endoscopic hemostasis is limited by a lack of appropriate device and controlling bleeding from a large-diameter extra-luminal vessel is very difficult. There concerns aside, the size and location of the tumor that are fit for endoscopic resection has no fixed criteria but rather depends on the comfort level of the operator. However, we should note that tumors >3–4 cm in the shortest diameter often cannot be retracted from the mouth and tumors >5 cm in the longest diameter carry significantly higher risk of aggressive behavior and thus should probably be better resected surgically. Preoperative evaluation with contrast CT/ MRI for large tumors is essential to evaluate for large extra-luminal vessels and relation between tumor and extra-luminal structures.
Equipment, techniques and perioperative management
Equipment commonly used for pure EFTR and STER are the same as those used in ESD: single channel gastroscope for resection (GIF HQ 190, Olympus, MA, USA) and dual channel endoscope for suturing (CF 2T160L, Olympus, MA, USA), electrosurgical generator unit (Erbe, GA, USA), CO2 insufflator (UCR, Olympus, MA, US), injection needle (InjectorForce Max™, Olympus, MA, USA), electrosurgical knife (e.g., ITknife™, DualKnife™, Hooknife™, Olympus, MA, US; HybridKnife®, ERBE, MA, USA), hemostatic forceps (EndoJaw Hot™, Coagrasper™, Olympus, MA, USA), clips (e.g., Instinct™, Cook Medical, IN, USA; Resolution™, Boston Scientific, MA, USA), and endoscopic suturing system (OverStitch™, Apollo Endosurgery, TX, USA).
The writers (and anecdotally other US centers) routinely use the endoscopic suturing device for pure EFTR cases, whereas the Chinese operators routinely use various clip and clip-assisted techniques, such as the omentum patch (23,31) and clip-endoloop technique (31-34) to close the defect. Closure of large defect with clips tend to be more cumbersome and less secure than suturing (35) and should be reserved for cases where suturing is not possible or available.
Pure EFTR constitutes the following steps (Figure 1): (I) submucosal injection of normal saline (or hetastarch) enhanced with a blue dye to delineate the tumor; (II) mucosa incision to access the submucosal working space; (III) submucosal dissection to expose the tumor; (IV) muscle fiber dissection along the capsule; (V) complete resection and tumor removal; (VI) mucosal defect closure.
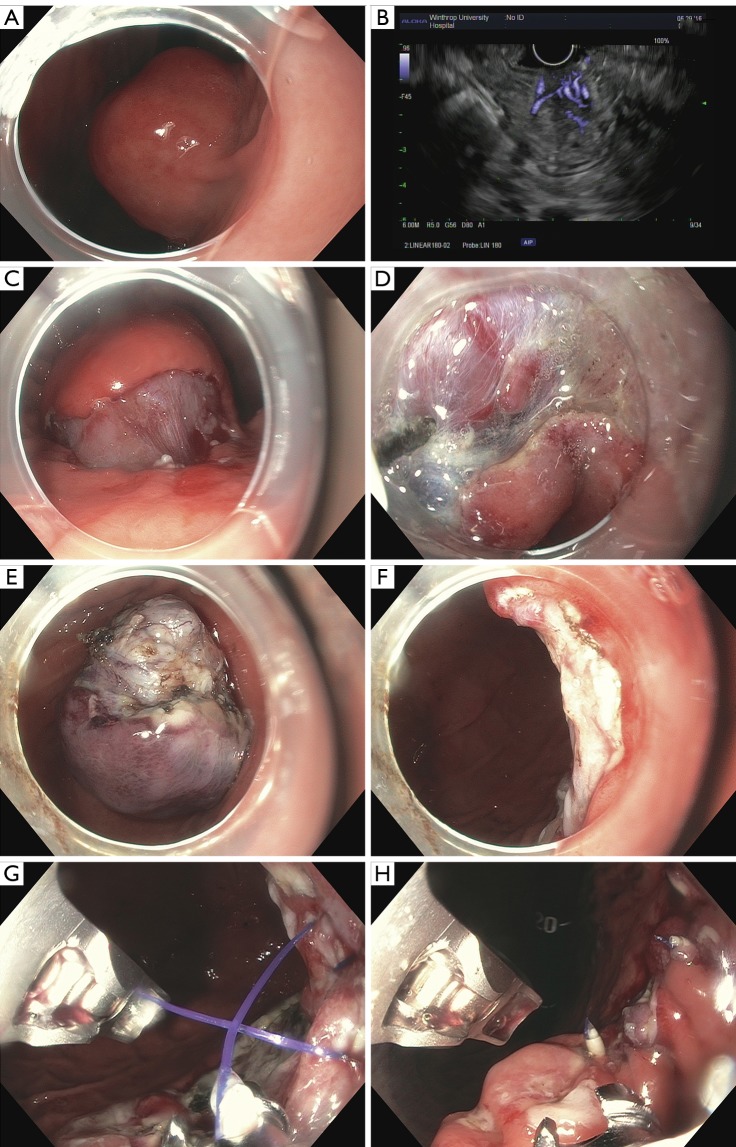
Figure 1.
Steps for EFTR. (A) A 3 cm submucosal tumor was seen in the posterior wall of the distal gastric body; (B) endoscopic ultrasound showing abundant vessels inside the tumor; (C) mucosa incision and submucosal dissection exposed one side of the tumor, which seemed to originate from the deep muscularis propria; (D) a closer view showed large-caliber vessels coiling along the tumor capsule; (E) the resected tumor lying in the gastric fundus. A ‘whirled’ growth pattern was clearly seen at the tumor base, which is typical for GIST; (F) extraluminal fatty tissues can be seen through the dissection defect; (G,H) gastric wall defect closure with OverStitch. EFTR, endoscopic full-thickness resection; GIST, gastrointestinal stromal tumor.
STER is performed according to the following steps (Figure 2): (I) submucosal injection 1–2 cm proximal to the tumor; (II) submucosal tunneling above and around the tumor to separate the tumor from its covering mucosa; (III) muscle fiber dissection around and beneath the tumor to excavate the tumor from its attachment to the muscularis propria; (IV) complete resection and removal of the tumor; (V) tunnel entrance closure.
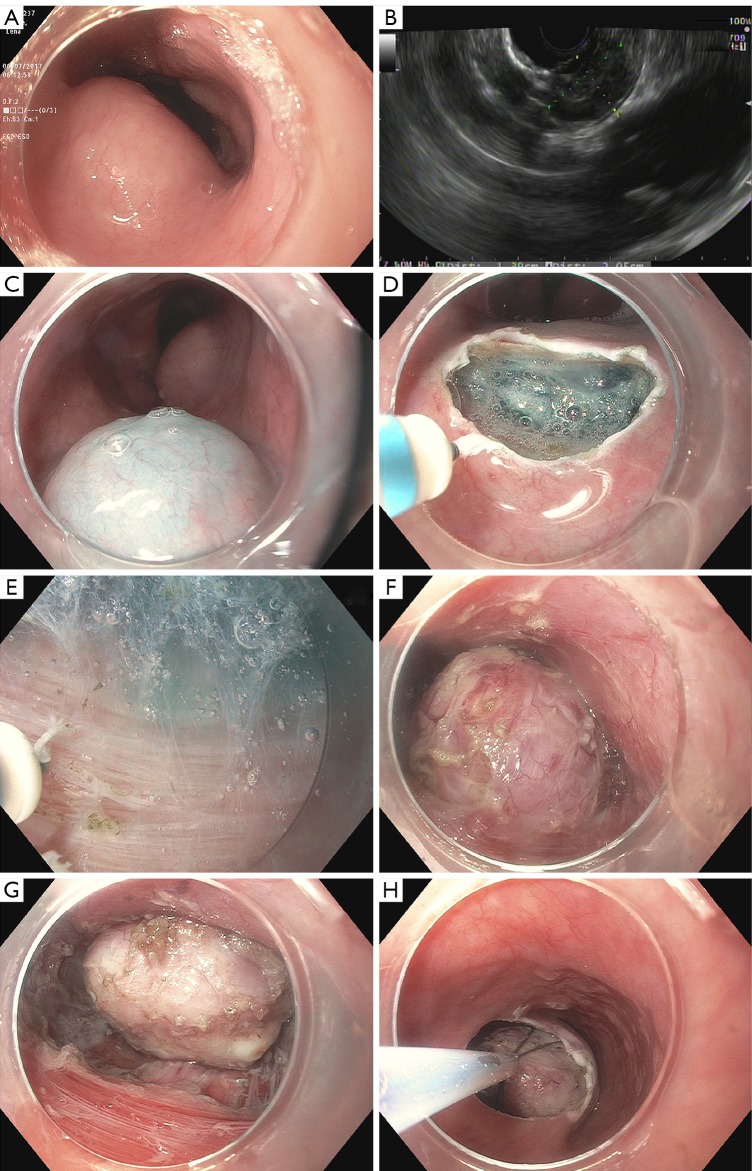
Figure 2.
Steps for STER. (A) A submucosal bulge is seen in the mid-esophagus; (B) EUS showing a 2cm homogeneous hypoechoic tumor; (C) submucosal injection 2 cm above the cephalic end of the tumor; (D) mucosa incision; (E) submucosal dissection showing circular muscle of the esophagus; (F,G) further dissection exposed a firm and muscular tumor originating from the deep muscularis propria. The mediastinum can be seen under the tumor; (H) an ERCP web basket was used to retrieve the tumor. STER, submucosal tunneling endoscopic resection; EUS, endoscopic ultrasound; ERCP, endoscopic retrograde cholangiopancreatography.
Prophylactic antibiotics covering enteric flora is administered at the start of the procedure and continued for a few days postoperatively. Cooperation with an experienced anesthesiologist is essential as once there is a full-thickness wall penetration, air tends to accumulate in the abdominal cavity, leading to a rise of the intra-abdominal pressure. Close monitoring of the peak airway pressure (as an indicator for the abdominal pressure) and prompt air venting either through the abdominal wall or by endoscopic suctioning are important. At our center, patients are kept on nil-per-mouth for at least 24 hours and undergo a barium swallow leak test prior to resuming po intake. Patients can be discharged once they can tolerate clears and if there is no symptoms or signs indicating a recovery out of ordinary.
Outcome
As of 2018 the majority of pure EFTR experience comes from China (23,24,30,33,34,36-46), reports out of China are few (31,47-49) (Table 1). Four studies that presented metrics of EFTR together with other techniques are not included in the table (24,50-52)). Almost all studies are retrospective series and universally reported a technical success rate of (near) 100% and no recurrence, few if any clinically significant adverse events, and relatively short hospital stays (mostly 3–6 days). However, most studies did not report an en bloc resection rate and described the follow up scheme very briefly.
Table 1. Literature review on EFTR series.
| Study | Study period | Tumor location | N | Tumor size, mm | Operation time, min | Closure method | Technical success | En bloc | R0 | LOS, day | csAE | Pathology | Recurrence | Follow–up, month |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhou (23), Surg Endosc, 2011 | Jul 2007–Jan 2009 | Gastric | 26 | 28 [12–45] | 105 [60–145] | Clips | 100% | 100% | NA | 5.5 [3–8] | None | 16 GIST, 6 leiomyoma, 3 glomus, 1 Schwannoma | None | 8 [6–24] |
| Xu (40), Endoscopy, 2013 | Jul 2009–Jan 2012 | Colon | 19 | 18 [12–30] | 67 [45–130] | Clips/endoloop, purse–string, lap assist | 95% | 100% | 100% | NA | 2 localized peritonitis, 1 bleeding |
9 leiomyoma, 4 GIST, 2 Schwannoma, 2 fibromatosis, 1 hamartoma, 1 granuloma | None | 18 [6–36] |
| Feng (38), J Laparoendosc Adv Surg Tech A, 2014 | Jan 2009–Oct 2012 | Gastric | 48 | 16 [5–48] | 60 [30–270] | Clips | 100% | NA | NA | [4–7] | None | 43 GIST, 4 leiomyoma, 1 Schwannoma | None | 12 [2–24] |
| Ye (33), Surg Endosc, 2014 | Jan 2009–Dec 2012 | Gastric | 51 | 24 [13–35] | 52 [30–125] | Clips, loops | 98% | NA | NA | 5.9 [3–9] | None | 30 GIST, 21 leiomyoma | None | 22.4 [1–48] |
| Huang (41), World J Gastroenterol, 2014 | Jan 2010–Sep 2013 | Gastric | 35 | 28 [20–45] | 90 [60–155] | Clips, omental patch | 100% | NA | NA | 6 [4–10] | None | 25 GIST, 7 leiomyoma, 2 Schwannoma | None | 6 |
| Guo (37), Surg Endosc, 2015 | Oct 2013–Mar 2014 | Gastric | 23 | 12 [6–20] | 40 [16–104] | Otsc | 100% | NA | NA | 3 [2–5] | 2 localized peritonitis | 19 GIST, 4 leiomyoma | None | 3 [1–6] |
| Yang (39), Surg Endosc, 2015 | Jun 2012–Apr 2014 | Gastric | 41 | 16±5.9 | 79±46 | Otsc, clips | 100% | NA | NA | 5.4±1.1 | None | 33 GIST, 4 leiomyoma, 1 NET, 1 Schwannoma, 2 benign | NA | NA |
| Wang (43), Surg Endosc, 2016 | Jan 2011–Dec 2013 | Gastric | 35 | 13±5 | 91±63 | Clips, nylon band | 100% | NA | NA | 6.7±0.9 | None | GIST | None | [1–72] |
| Lu (44), Gastrointest Endosc, 2016 | Jan 2013 –Mar 2015 | Fundus LC |
62 | [8–60] | 25–180 | Clips | 98% | NA | NA | [4–6] | NA | 44 GIST, 17 leiomyoma, 1 Schwannoma | None | [1–24] |
| Tan (30), Surg Endosc, 2017 | Apr 2011–Jun 2016 | Gastric | 32 | 15.4±6.6 | 69± 27 | Clips | 100% | 97% | NA | 6.4± 2.0 | None | GIST | None | 23.8±18.6 |
| Shi (46), Surg Endosc, 2017 | Apr 2014–Feb 2015 | Fundus | 68 | 26 [20–35] | 41 [23–118] | Clips, endoloops | 100% | 100% | NA | 5.4 [3–9] | 1 Mallory-Weiss, 1 bleeding | GIST | None | [3–13] |
| Wu (34), Medicine, 2018 | Jun 2016–May 2017 | Gastric | 25 | 17±10 [5–45] | 31±14 | Clips, purse–string | 100% | NA | NA | NA | None | 21 GIST, 2 leiomyoma, 1 neuroma, 1 calcifying fibrous tumor | None | 7 [1–11] |
| Shia (45), J Laparoendosc Adv Surg Tech A, 2018 | Jan 2015 –Dec 2016 | Fundus | 24, 24 | 8.8±3.3 | 11±3; 19±5 | Clips/loops | NA | NA | NA | 3.2±0.5; 3.2±0.5 | NA | 38 GIST, 10 leiomyoma | NA | NA |
| Andalib (47), Surg Endosc, 2018 | Dec 2014–Apr 2016 | Gastric | 12 | 24 [10–50] | 80 [17–180] | Clip, endoloops, sutures |
100% | 92% | NA | 2.08 | None | GIST | None | 12 [6.5–24] |
Summary metric is either median or mean. Range is presented in square brackets, standard deviation is presented after ±. a, with vs. without dental floss traction. NA, not available; LC, lesser curvature; csAE, clinically significant adverse events; EFTR, endoscopic full-thickness resection; LOS, length of stay; GIST, gastrointestinal stromal tumor; OTSC, Over-the-Scope-Clip.
Similar to EFTR, almost all studies on STER are retrospective case series with scant follow up data (28,29,46,53-71) (Table 2). A recent meta-analysis compiled 12 STER studies in English literature up to Jun 2016 and found a pooled complete and en bloc resection rate of 98.1% and 94.9% respectively. The pooled estimates of gas-related and inflammation-related (including pleural and abdominal effusion) adverse events rate were 21.5% and 8.4%, respectively, and the pooled estimate of delayed bleeding rate was 2.2%. One prospective, open-labeled trial randomizing 66 patients with small esophageal submucosal tumors into STER and video-assisted thoracoscopic surgery (VATS) found shorter procedure time (44.5 vs. 106.5 min), lower cost (4,499 vs. 5,137 USD), less decrease in hemoglobin level (0.16 vs. 1.47 g/dL) and lower postoperative pain scores in the STER group and comparable perioperative clinical outcomes (complete resection rate, hospital times, and adverse events) between the two groups apart from a lower en bloc resection rate of STER for SET ≥2 cm (71.4% vs. 100%) (73). Because STER is mainly used in the esophagus or gastroesophageal junction, majority of the tumors included in published series are leiomyomas. Given the low malignant potential of leiomyomas and the generally short follow up/high loss-to-follow-up rate, it is not surprising that few recurrences (68,69,72,74,75) have been reported and there are questions whether these are real recurrences or residuals of incomplete primary resection.
Table 2. Literature review on STER series.
| Study | Study period | Tumor location | N | Tumor size | Operation time, min | Closure method | Technical success | En bloc | R0 | LOS, day | csAE | Pathology | Recurrence | Follow–up, Month |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inoue (28), Endoscopy, 2012 | NA | Esophagus, cardia | 9 | 12 [12–30] | 93 [84–365] | Clips | 78% | 100% [2/9 aborted] | 100% | 4 [4–16] | None | 1 GIST, 5 leiomyoma, 1 aberrant pancreas | NA | NA |
| Gong (53), Endoscopy, 2012 | Jun 2011–Nov 2011 | Esophagus, cardia | 12 | 19 [10–40] | 48 [30–60] | Clips | 100% | 83% | NA | NA | 2 PTX | 7 GIST, 5 leiomyoma | NA | NA |
| Xu (29), Gastrointest Endosc, 2012 | Jun 2010–Mar 2011 | Esophagus, cardia, stomach | 15 | 19 [12–25] | 79 [25–130] | Clips | 100% | 100% | 100% | 3.8 [3–5] | 1 PTX | 5 GIST, 9 leiomyoma, 1 glomus tumor | None | 1–6 |
| Liu (54), Surg Endosc, 2013 | Apr 2011–Jun 2012 | Esophagus, cardia, stomach | 12 | 19 [10–30] | 78 [50–130] | Clips | 100% | 100% | 100% | NA | 4 PTX, 2 pleural effusion | 2 GIST, 9 leiomyoma, 1 Schwannoma | None | 5 [2–15] |
| Ge (55), Endosc Ultrasound, 2013 | Oct 2009–Dec 2011 | Esophagus | 17 | 24 [12–50] | 97 [60–150] | Clips | 100% | NA | NA | NA | None | 1 GIST, 16 leiomyoma | None | 7 [3–13] |
| Wang (56), Surg Endosc, 2013 | Nov 2009–Nov 2011 | Esophagus | 18 | 33 [21–45] | 68 | Clips | NA | NA | NA | 2.3 | 3 bleeding | 18 leiomyoma | None | 17 |
| Wang (57), Surg Endosc, 2014 | Jul 2010–Aug 2012 | GEJ | 57 | 22 [6–35] | 47 [15–120] | Clips | 100% | 100% | NA | 2.7 [2–6] | 5 PTX, 2 pleural effusion | 7 GIST, 46 leiomyoma, 2 Schwannoma, 1 lipoma, 1 granular cell tumor | None | 12 [6–24] |
| Ye (58), Surg Endosc, 2014 | Aug 2011–Feb 2013 | Esophagus, cardia, stomach | 85 | 19 [10–30] | 57 [30–115] | Clips | 100% | 100% | NA | 5.9 [2–14] | 6 PTX | 19 GIST, 65 leiomyoma, 1 calcifying fibrous tumor | None | 8 [2–19] |
| Lua (59), Surg Endosc, 2014 | Jan 2010–Jan 2014 | Esophagus, cardia | 45 | 12 | 84 | Clips | 100% | 98% | 98% | NA | None | 3 GIST, 42 leiomyoma | None | 9 ± 9 |
| Lu (60), Endoscopy, 2014 | Jan 2013–Apr 2014 | Fundus | 19 | 21 [8–50] | 75 [40–100] | Clips | 100% | NA | NA | NA | None | 13 GIST, 6 leiomyoma | None | 5 |
| Lu (61), PLoS One, 2015 | Jan 2012– | Stomach | 47 | 14 [5–50] | 79 [45–150] | Clips | 96% | NA | NA | NA | None | 36 GIST, 10 leiomyoma, 1 Schwannoma | None | 11 |
| Zhou (62), World J Gastroenterol, 2015 | Aug 2012–Oct 2013 | GEJ | 21 | 23 [10–40] | 63 [45–90] | Clips | 100% | 86% | NA | 4.3 [3–7] | 1 pleural effusion | 6 GIST, 15 leiomyoma | None | 6 [2–14] |
| Li (63), Surg Endosc, 2015 | Apr 2011–Mar 2014 | Cardia, lesser curvature, greater curvature | 32 | 23 [10–50] | 52 [25–125] | Clips | 100% | 100% | NA | 3.9 [2–9] | 3 PTX, 3 pleural effusion, 1 subphrenic infection | 11 GIST, 18 leiomyoma, 1 fibrous tumor, 1 glomus tumor, 1 Schwannoma | None | 6–32 |
| Zhang (64), Indian J Cancer, 2015 | Jun 2011–Jun 2014 | Esophagus, cardia, lesser curvature | 49 | 15 [8–35] | 40 [20–75] | Clips | 100% | 100% | NA | 4 [2–9] |
2 PTX, 2 pleural effusion | 49 leiomyoma | None | 18 [3–36] |
| Wang (72), Eur J Gastroenterol Hepatol, 2015 | Oct 2011–May 2014 | Esophagus, cardia | 83 | 23 [10–55] | 61 [25–160] | Clips | 100% | 98% | NA | 5.4 [3–10] |
1 PTX | 15 GIST, 68 leiomyoma | None | 10 |
| Tanb (65), Surg Endosc, 2016 | Jan 2010–Dec 2014 | Esophagus | 18 | 41 | 75 | Clips | NA | 89% | NA | 6 | None | 18 leiomyoma | NA | NA |
| Chen (66), Endoscopy, 2016 | Jan 2011–Aug 2013 | Esophagus, GEJ, stomach | 290 | 21 [10–70] | 43 [15–200] | Clips | NA | 89% | NA | 3.5 [2–22] |
29 complications requiring interventions | 53 GIST, 226 leiomyoma, 5 Schwannoma, 3 calcifying fibrous tumor, 3 glomus tumor | NA | NA |
| Tang (67), Gut Liver, 2017 | Jan 2012–Jan 2015 | Esophagus, cardia, stomach | 70 | 19 [10–40] | 49 [30–150] | Clips | NA | 96% | NA | 5.8 [3–10] |
2 PTX | 11 GIST, 59 leiomyoma | None | 18 |
| Chen (68), Ann Surg, 2017 | Jun 2011–May 2013 | Esophagus, stomach | 180 | 26 [20–50] | 45 [15–200] | Clips | NA | 90.6% | NA | 3.2 [2–22] |
10 PTX/pleural effusion, 2 bleeding, 1 fistula | 28 GIST, 146 leiomyoma, 4 Schwannoma, 2 calcifying fibrous tumor | None | 36 [28–51] |
| Mao (69), Dis Esophagus, 2017 | Jan 2012–Dec 2014 | Cardia | 56 | 18 [10–32] | 42 [20–65] | Clips | 100% | 100% | NA | 4.9 [2–9] |
8 PTX, 5 pleural effusion | 10 GIST, 45 leiomyoma, 1 fibrous tumor | None | 25 [7–42] |
| Zhouc (70), World J Gastroenterol, 2017 | Aug 2012–Dec 2015 | Esophagus, cardia, stomach | HO 34, HK 49 | HO 19.7 [10–40]; HK 19.3 [8–40] | HO 57.2 [30–150]; HK 41.3 [15–120] | Clips | NA | HO 94%; HK 100% | NA | HO 5.6 [3–10]; HK 5.8 [3–10] | 4 perf 1 bleeding | 13 GIST, 69 leiomyoma, 1 lipoma | None | HO 27±6, HK 26±4 |
| Zhang (71), Endoscopy, 2017 | 2015–2017 | Lower esophagus, fundus near cardia, GEJ | 10 | 43 [12–50] | 80 [45–150] | Clips | 100% | 90% | NA | NA | None | 1 GIST, 9 leiomyoma | NA | NA |
a, retrospective, compared to ESE; b, retrospective, compared to VATS; c, retrospective, Hook knife (HO) vs. HybridKnife (HK). Significant adverse event, gas-related adverse events including subcutaneous emphysema, pneumoperitoneum and atelectasis are not included here since they are common and mostly don’t require any intervention. Fever resolved after short-course of antibiotics is not included either. STER, submucosal tunneling endoscopic resection; LOS, length of stay; GEJ, gastroesophageal junction; PTX, pneumothorax.
In summary, current evidence supports the feasibility and safety of pure EFTR and STER as well as endorses its technical versatility (tumor size up to 6 cm; various locations such as the esophagus, gastric fundus and colon) but is not strong enough to endorse its long-term clinical success. However, we want to point out that given the low risk and slow growth of most SELs, proving long term success of these technique will be difficult since true recurrence probably takes years to detect and ‘early recurrence’ likely represents macroscopic residual of primary tumor or post-operative fibrotic changes, rather than microscopic residual secondary to a R1 margin, which is the primary concern for this type of endoscopic ‘enucleation’. In addition, given the low risk of most of these tumors, long term surveillance has questionable utility after complete tumor resection and patient compliance for follow up can be an issue.
Summary
The management of low-risk gastrointestinal subepithelial tumors is an involving field. The risk-benefit balance might shift to favor a more aggressive early-resection approach rather than long-term surveillance as new techniques and equipment got more widely used and operators get more comfortable with the new concepts. Numerous studies have shown the technical feasibility and safety of pure EFTR and STER in resecting these tumors. Future research is needed regarding the long-term result of these techniques and more tailored tools are needed were further breakthrough to be made.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Dr. Stavropoulos is a consultant for Boston Scientific and Olympus and receives an honoraria from ERBE USA; Dr. Zhang, Dr. Modayil and Dr. Criscitelli have no conflicts of interest to declare.
References
- 1.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446-54. 10.1016/j.gie.2009.10.027 [DOI] [PubMed] [Google Scholar]
- 2.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S2-4. [DOI] [PMC free article] [PubMed]
- 3.Kulke MH, Anthony LB, Bushnell DL, et al. NANETS Treatment Guidelines: Well-Differentiated Neuroendocrine Tumors of the Stomach and Pancreas. Pancreas 2010;39:735-52. 10.1097/MPA.0b013e3181ebb168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delle Fave G, Sundin A, Taal B, et al. ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology 2016;103:119-24. 10.1159/000443168 [DOI] [PubMed] [Google Scholar]
- 5.Ramage JK, De Herder W, Delle Fave G, et al. ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology 2016;103:139-43. 10.1159/000443166 [DOI] [PubMed] [Google Scholar]
- 6.Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline-Updated January 2017. Endoscopy 2017;49:695-714. 10.1055/s-0043-109021 [DOI] [PubMed] [Google Scholar]
- 7.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. 10.1053/j.semdp.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 8.Rajan E, Wong Kee Song LM. Endoscopic Full Thickness Resection. Gastroenterology 2018;154:1925-37.e2. 10.1053/j.gastro.2018.02.020 [DOI] [PubMed] [Google Scholar]
- 9.Casali PG, Jost L, Reichardt P, et al. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Annals of Oncology 2009;20:64-7. [DOI] [PubMed] [Google Scholar]
- 10.McCarter MD, Antonescu CR, Ballman KV, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg 2012;215:53-9; discussion 9-60. 10.1016/j.jamcollsurg.2012.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maranki J, Stavropoulos SN. Endoscopic Full-Thickness Resection of Subepithelial Lesions of the GI Tract. In: Clinical Gastrointestinal Endoscopy. Third Edition. Elsevier. 510-6.e2. [Google Scholar]
- 12.Kaehler G, Grobholz R, Langner C, et al. A new technique of endoscopic full-thickness resection using a flexible stapler. Endoscopy 2006;38:86-9. 10.1055/s-2005-921181 [DOI] [PubMed] [Google Scholar]
- 13.Ikeda K, Fritscher-Ravens A, Mosse CA, et al. Endoscopic full-thickness resection with sutured closure in a porcine model. Gastrointest Endosc 2005;62:122-9. 10.1016/S0016-5107(05)00517-1 [DOI] [PubMed] [Google Scholar]
- 14.Ikeda K, Mosse CA, Park P-O, et al. Endoscopic full-thickness resection: circumferential cutting method. Gastrointest Endosc 2006;64:82-9. 10.1016/j.gie.2005.12.039 [DOI] [PubMed] [Google Scholar]
- 15.Valli PV, Mertens J, Bauerfeind P. Safe and successful resection of difficult GI lesions using a novel single-step full-thickness resection device (FTRD®). Surg Endosc 2018;32:289-99. 10.1007/s00464-017-5676-9 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt A, Bauerfeind P, Gubler C, et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy 2015;47:719-25. 10.1055/s-0034-1391781 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt A, Beyna T, Schumacher B, et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut 2018;67:1280-9. 10.1136/gutjnl-2016-313677 [DOI] [PubMed] [Google Scholar]
- 18.Andrisani G, Pizzicannella M, Martino M, et al. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: A single-centre study. Dig Liver Dis 2017;49:1009-13. 10.1016/j.dld.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 19.Meier B, Caca K, Schmidt A. Hybrid endoscopic mucosal resection and full-thickness resection: a new approach for resection of large non-lifting colorectal adenomas (with video). Surg Endosc 2017;31:4268-74. 10.1007/s00464-017-5461-9 [DOI] [PubMed] [Google Scholar]
- 20.Bronzwaer MES, Bastiaansen BAJ, Koens L, et al. Endoscopic full-thickness resection of polyps involving the appendiceal orifice: a prospective observational case study. Endosc Int Open 2018;6:E1112-9. 10.1055/a-0635-0911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt A, Bauder M, Riecken B, et al. Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy 2015;47:154-8. [DOI] [PubMed] [Google Scholar]
- 22.Bauder M, Schmidt A, Caca K. Endoscopic full-thickness resection of duodenal lesions-a retrospective analysis of 20 FTRD cases. United European Gastroenterol J 2018;6:1015-21. 10.1177/2050640618773517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou PH, Yao LQ, Qin XY, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 2011;25:2926-31. 10.1007/s00464-011-1644-y [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Ren W, Fan CQ, et al. Full-thickness endoscopic resection of nonintracavitary gastric stromal tumors: a novel approach. Surg Endosc 2011;25:641-7. 10.1007/s00464-010-1189-5 [DOI] [PubMed] [Google Scholar]
- 25.Yoshizumi F, Yasuda K, Kawaguchi K, et al. Submucosal tunneling using endoscopic submucosal dissection for peritoneal access and closure in natural orifice transluminal endoscopic surgery: a porcine survival study. Endoscopy 2009;41:707-11. 10.1055/s-0029-1214959 [DOI] [PubMed] [Google Scholar]
- 26.Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointestinal endoscopy 2007;65:688-94. 10.1016/j.gie.2006.07.030 [DOI] [PubMed] [Google Scholar]
- 27.Pasricha PJ, Hawari R, Ahmed I, et al. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy 2007;39:761-4. 10.1055/s-2007-966764 [DOI] [PubMed] [Google Scholar]
- 28.Inoue H, Ikeda H, Hosoya T, et al. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy 2012;44:225-30. 10.1055/s-0031-1291659 [DOI] [PubMed] [Google Scholar]
- 29.Xu MD, Cai MY, Zhou PH, et al. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 2012;75:195-9. 10.1016/j.gie.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 30.Tan Y, Tang X, Guo T, et al. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc 2017;31:3376-82. 10.1007/s00464-016-5350-7 [DOI] [PubMed] [Google Scholar]
- 31.Inayat F, Aslam A, Grunwald MD, et al. Omental Patching and Purse-String Endosuture Closure after Endoscopic Full-Thickness Resection in Patients with Gastric Gastrointestinal Stromal Tumors. Clin Endosc 2018. [Epub ahead of print]. 10.5946/ce.2018.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Q, Chen T, Zhong YS, et al. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy 2013;45:329-34. 10.1055/s-0032-1326214 [DOI] [PubMed] [Google Scholar]
- 33.Ye LP, Yu Z, Mao XL, et al. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc 2014;28:1978-83. 10.1007/s00464-014-3421-1 [DOI] [PubMed] [Google Scholar]
- 34.Wu N, Liu S, Chen M, et al. The prepurse-string suture technique for gastric defect after endoscopic full-thickness resection (with video). Medicine 2018;97:e12118. 10.1097/MD.0000000000012118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantsevoy SV, Bitner M, Hajiyeva G, et al. Endoscopic management of colonic perforations: clips versus suturing closure (with videos). Gastrointest Endosc 2016;84:487-93. 10.1016/j.gie.2015.08.074 [DOI] [PubMed] [Google Scholar]
- 36.Zhou PH, Yao LQ, Qin XY, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 2011;25:2926-31. 10.1007/s00464-011-1644-y [DOI] [PubMed] [Google Scholar]
- 37.Guo J, Liu Z, Sun S, et al. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc 2015;29:3356-62. 10.1007/s00464-015-4076-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Y, Yu L, Yang S, et al. Endolumenal endoscopic full-thickness resection of muscularis propria-originating gastric submucosal tumors. J Laparoendosc Adv Surg Tech A 2014;24:171-6. 10.1089/lap.2013.0370 [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Wang S, Sun S, et al. Factors associated with endoscopic full-thickness resection of gastric submucosal tumors. Surg Endosc 2015;29:3588-93. 10.1007/s00464-015-4113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu M, Wang XY, Zhou PH, et al. Endoscopic full-thickness resection of colonic submucosal tumors originating from the muscularis propria: an evolving therapeutic strategy. Endoscopy 2013;45:770-3. 10.1055/s-0033-1344225 [DOI] [PubMed] [Google Scholar]
- 41.Huang LY, Cui J, Lin SJ, et al. Endoscopic full-thickness resection for gastric submucosal tumors arising from the muscularis propria layer. World J Gastroenterol 2014;20:13981-6. 10.3748/wjg.v20.i38.13981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi D, Li R, Chen W, et al. Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: a multicenter study. Surg Endosc 2017;31:837-42. 10.1007/s00464-016-5041-4 [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Feng X, Ye S, et al. A comparison of the efficacy and safety of endoscopic full-thickness resection and laparoscopic-assisted surgery for small gastrointestinal stromal tumors. Surg Endosc 2016;30:3357-61. 10.1007/s00464-015-4612-0 [DOI] [PubMed] [Google Scholar]
- 44.Lu J, Jiao T, Li Y, et al. Facilitating retroflexed endoscopic full-thickness resection through loop-mediated or rope-mediated countertraction (with videos). Gastrointest Endosc 2016;83:223-8. 10.1016/j.gie.2015.08.063 [DOI] [PubMed] [Google Scholar]
- 45.Shi Q, Li B, Qi ZP, et al. Clinical Values of Dental Floss Traction Assistance in Endoscopic Full-Thickness Resection for Submucosal Tumors Originating from the Muscularis Propria Layer in the Gastric Fundus. J Laparoendosc Adv Surg Tech A 2018;28:1261-5. 10.1089/lap.2018.0030 [DOI] [PubMed] [Google Scholar]
- 46.Shi D, Li R, Chen W, et al. Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: a multicenter study. Surg Endosc 2017;31:837-42. 10.1007/s00464-016-5041-4 [DOI] [PubMed] [Google Scholar]
- 47.Andalib I, Yeoun D, Reddy R, et al. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: methods and feasibility data. Surg Endosc 2018;32:1787-92. 10.1007/s00464-017-5862-9 [DOI] [PubMed] [Google Scholar]
- 48.Abe N, Takeuchi H, Ohki A, et al. Comparison between endoscopic and laparoscopic removal of gastric submucosal tumor. Digestive Endoscopy 2018;30:7-16. 10.1111/den.13010 [DOI] [PubMed] [Google Scholar]
- 49.Stavropoulos SN, Modayil RJ, Zhang X, et al. Sa1953 notes for subepithelial tumors: eftr and ster - a single center five year prospective series in the US. Gastrointest Endosc 2018;87:AB264 10.1016/j.gie.2018.04.1567 [DOI] [Google Scholar]
- 50.Zhang Y, Mao XL, Zhou XB, et al. Long-term outcomes of endoscopic resection for small (</= 4.0 cm) gastric gastrointestinal stromal tumors originating from the muscularis propria layer. World J Gastroenterol 2018;24:3030-7. 10.3748/wjg.v24.i27.3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu C, Liao G, Fan C, et al. Long-term outcomes of endoscopic resection of gastric GISTs. Surg Endosc 2017;31:4799-804. 10.1007/s00464-017-5557-2 [DOI] [PubMed] [Google Scholar]
- 52.Cai M, Zhou P, Lourenco LC, et al. Endoscopic Full-thickness Resection (EFTR) for Gastrointestinal Subepithelial Tumors. Gastrointest Endosc Clin N Am 2016;26:283-95. 10.1016/j.giec.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 53.Gong W, Xiong Y, Zhi F, et al. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy 2012;44:231-5. 10.1055/s-0031-1291720 [DOI] [PubMed] [Google Scholar]
- 54.Liu BR, Song JT, Kong LJ, et al. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc 2013;27:4354-9. 10.1007/s00464-013-3023-3 [DOI] [PubMed] [Google Scholar]
- 55.Ge N, Sun S, Wang S, et al. Endoscopic Ultrasound-Assisted Tunnel-Type Endoscopic Submucosal Dissection for the Treatment of Esophageal Tumors Arising in the Muscularis Propria (with video). Endosc Ultrasound 2013;2:11-5. 10.4103/2303-9027.117716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Ren W, Zhang Z, et al. Retrospective study of endoscopic submucosal tunnel dissection (ESTD) for surgical resection of esophageal leiomyoma. Surg Endosc 2013;27:4259-66. 10.1007/s00464-013-3035-z [DOI] [PubMed] [Google Scholar]
- 57.Wang XY, Xu MD, Yao LQ, et al. Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos). Surg Endosc 2014;28:1971-7. 10.1007/s00464-014-3420-2 [DOI] [PubMed] [Google Scholar]
- 58.Ye LP, Zhang Y, Mao XL, et al. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 2014;28:524-30. 10.1007/s00464-013-3197-8 [DOI] [PubMed] [Google Scholar]
- 59.Lu J, Jiao T, Zheng M, et al. Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc 2014;28:3401-7. 10.1007/s00464-014-3610-y [DOI] [PubMed] [Google Scholar]
- 60.Lu J, Zheng M, Jiao T, et al. Transcardiac tunneling technique for endoscopic submucosal dissection of gastric fundus tumors arising from the muscularis propria. Endoscopy 2014;46:888-92. 10.1055/s-0034-1377442 [DOI] [PubMed] [Google Scholar]
- 61.Lu J, Jiao T, Li Y, et al. Heading toward the right direction--solution package for endoscopic submucosal tunneling resection in the stomach. PLoS One 2015;10:e0119870. 10.1371/journal.pone.0119870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou DJ, Dai ZB, Wells MM, et al. Submucosal tunneling and endoscopic resection of submucosal tumors at the esophagogastric junction. World J Gastroenterol 2015;21:578-83. 10.3748/wjg.v21.i2.578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li QL, Chen WF, Zhang C, et al. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc 2015;29:3640-6. 10.1007/s00464-015-4120-2 [DOI] [PubMed] [Google Scholar]
- 64.Zhang C, Hu JW, Chen T, et al. Submucosal tunneling endoscopic resection for upper gastrointestinal multiple submucosal tumors originating from the muscular propria layer: a feasibility study. Indian J Cancer 2015;51 Suppl 2:e52-5. [DOI] [PubMed] [Google Scholar]
- 65.Tan Y, Tang X, Guo T, et al. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc 2017;31:3376-82. 10.1007/s00464-016-5350-7 [DOI] [PubMed] [Google Scholar]
- 66.Chen T, Zhang C, Yao LQ, et al. Management of the complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Endoscopy 2016;48:149-55. [DOI] [PubMed] [Google Scholar]
- 67.Tang X, Ren Y, Huang S, et al. Endoscopic Submucosal Tunnel Dissection for Upper Gastrointestinal Submucosal Tumors Originating from the Muscularis Propria Layer: A Single-Center Study. Gut Liver 2017;11:620-7. 10.5009/gnl15424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen T, Zhou PH, Chu Y, et al. Long-term Outcomes of Submucosal Tunneling Endoscopic Resection for Upper Gastrointestinal Submucosal Tumors. Ann Surg 2017;265:363-9. 10.1097/SLA.0000000000001650 [DOI] [PubMed] [Google Scholar]
- 69.Mao XL, Ye LP, Zheng HH, et al. Submucosal tunneling endoscopic resection using methylene-blue guidance for cardial subepithelial tumors originating from the muscularis propria layer. Dis Esophagus 2017;30:1-7. 10.1093/dote/dow023 [DOI] [PubMed] [Google Scholar]
- 70.Zhou JQ, Tang XW, Ren YT, et al. Endoscopic submucosal tunnel dissection of upper gastrointestinal submucosal tumors: A comparative study of hook knife vs hybrid knife. World J Gastroenterol 2017;23:1843-50. 10.3748/wjg.v23.i10.1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Q, Cai JQ, Xiang L, et al. Modified submucosal tunneling endoscopic resection for submucosal tumors in the esophagus and gastric fundus near the cardia. Endoscopy 2017;49:784-91. 10.1055/s-0043-111236 [DOI] [PubMed] [Google Scholar]
- 72.Wang H, Tan Y, Zhou Y, et al. Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol 2015;27:776-80. 10.1097/MEG.0000000000000394 [DOI] [PubMed] [Google Scholar]
- 73.Chai N, Du C, Gao Y, et al. Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic enucleation for esophageal submucosal tumors originating from the muscularis propria layer: a randomized controlled trial. Surg Endosc 2018;32:3364-72. 10.1007/s00464-018-6057-8 [DOI] [PubMed] [Google Scholar]
- 74.Li QY, Meng Y, Xu YY, et al. Comparison of endoscopic submucosal tunneling dissection and thoracoscopic enucleation for the treatment of esophageal submucosal tumors. Gastrointest Endosc 2017;86:485-91. 10.1016/j.gie.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 75.Du C, Ma L, Chai N, et al. Factors affecting the effectiveness and safety of submucosal tunneling endoscopic resection for esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc 2018;32:1255-64. 10.1007/s00464-017-5800-x [DOI] [PubMed] [Google Scholar]