Abstract
Malignant pleural mesothelioma (MPM) is a rare disease with a very poor prognosis. Previous studies have indicated that women experience longer survival compared with men. We analyzed 16 267 eligible patients (21.3% females) in the National Cancer Database to evaluate which clinical factors are independently predictive of longer survival. After adjusting for all covariates, survival was significantly better in females compared with males [HRadj: 0.81, 95% confidence interval (CI): 0.77–0.85]. Other factors significantly associated with better survival were younger age at diagnosis, higher income, lower comorbidity score, epithelial histology, earlier stage and receipt of surgical or medical treatment. After propensity matching, survival was significantly better for females compared with males [hazard ratio (HR): 0.86, 95% CI: 0.80–0.94]. After propensity matching within the epithelial group, survival remained significantly better for females compared with males (HR: 0.85, 95% CI: 0.74–0.97). This study adds information to the known significant gender survival difference in MPM by disentangling the effect of gender from the effect of age and histology, two known independent factors affecting survival. Circulating estrogen, present in young but not older women, and higher expression of the estrogen receptor beta in epithelial mesothelioma have been suggested to play a role in gender survival differences. These findings may lead to exploring new therapeutic options, such as targeting estrogen receptor beta, and considering hormonal therapy including estrogens for patients with otherwise limited prognosis.
Females with malignant pleural mesothelioma survive significantly longer than males. Other factors independently significantly associated with better survival were younger age at diagnosis, higher income, lower comorbidity score, epithelial histology, earlier stage and receipt of treatment.
Introduction
Malignant pleural mesothelioma (MPM) is a rare but aggressive form of thoracic cancer linked to asbestos exposure. Patients with MPM have historically had a very poor prognosis, with modest changes in survival observed over time, despite the introduction of modern therapeutic interventions (1). Previous analyses of the Surveillance, Epidemiology, and End Results (SEER) database (2–5) and single-center studies (6–8) have suggested that females with MPM experience longer survival compared with males. However, MPM is a rare disease and only a small proportion of affected patients are female, which limits the extent of conclusions that can be drawn from single-institution databases.
Among the possible reasons for the observed longer survival experienced by women, authors suggested that they present at earlier stage (9), have tumors with more favorable histology (4), experience a different amount or type of asbestos exposure responsible for a more indolent tumor biology (10), and may benefit from protective effects of circulating estrogen (6) interacting with estrogen receptors present in their tumors (11).
Because of the aggressive nature of MPM and its poor prognosis, identifying prognostic factors and characterizing their relation with therapeutic options are critical objectives. Currently, therapeutic options are still of limited efficacy and include surgery, chemotherapy, radiation and combinations of these treatments. The suggestion that female patients with MPM survive longer than male patients and the possible association with histology, stage or the presence of estrogens and their interaction with estrogen receptors, may be key elements for identifying novel therapeutic options which could ultimately improve patient outcomes.
To date, no large population-based studies have specifically evaluated MPM outcomes and their determinants in female patients. We used the National Cancer Database (NCDB) to retrospectively evaluate survival in patients with MPM according to gender and determine which clinical factors are independently predictive of longer survival in females.
Materials and methods
Data source
The NCDB is a joint project between the American College of Surgeons and the American Cancer Society which is sourced from hospital registry data collected in more than 1500 Commission on Cancer (CoC)-accredited facilities starting in 1989. Patients who receive some element of their cancer care at a CoC-accredited facility are included in the NCDB, representing ~70% of all patients newly diagnosed with cancer nationwide (12). The NCDB offers data about cancer characteristics, patient demographics, reporting facility characteristics, first course of treatment and survival, as described previously in more detail (13,14). Because the data used in the study were derived from a de-identified NCDB file, the research was considered exempt by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai. The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methodology used, or the conclusions drawn from these data by the investigator.
Study population
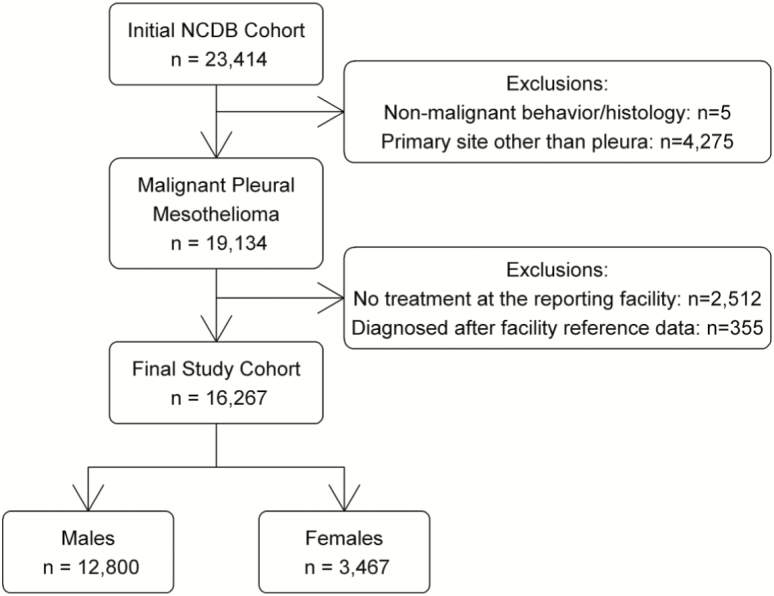
The initial NCDB dataset contained 23 414 patients diagnosed with mesothelioma between 2004 and 2013. The sample was limited to 19 134 patients with invasive pleural mesothelioma. As recommended by the NCDB, only patients (i) with at least a part of their treatment at the CoC facility that reported them and (ii) who were diagnosed after their facility’s reference date for data completeness were included in this study (n = 16 267) (Figure 1).
Figure 1.
Selection criteria.
Predictor and primary outcome
The primary predictor was gender, whereas the primary outcome was overall survival (OS) after diagnosis. The NCDB recorded the number of months of follow-up after diagnosis and the patient’s vital status at that time.
Covariates
Covariates included age at diagnosis, race/ethnicity, insurance status, percent of adults without a high school diploma in the patient’s zip code of residence, median income in the patient’s zip code of residence, Charlson Comorbidity Index, facility type, distance from the patient’s zip code to the reporting facility, histology, bilateral involvement, stage and receipt of treatment, including surgery, radiotherapy and chemotherapy. Receipt of radiotherapy was defined if the patient received 40–65 Gy of external beam radiation therapy to the chest wall, lungs or pleura as part of the first course of treatment (15).
Statistical analysis
Categorical demographic and clinical characteristics were compared between males and females using χ2 tests. Independent associations of gender with these variables were assessed using multivariable logistic regression to estimate odds ratios and 95% CIs. Patients missing any covariate information (except stage) were excluded from analysis. As ~25% of the sample had incomplete information to define stage, a missing category was created for analysis.
The Kaplan–Meier method, along with the log-rank test was used to estimate and compare univariate OS at 2 and 5 years in males and females. A multivariable Cox proportional hazards model was used to assess the association between gender and OS, adjusted for possible confounders, including year of diagnosis and US census division of the reporting facility. The association between gender and OS was also analyzed using a 1:1 propensity score matching with the greedy algorithm, matching on all covariates. A stratified Cox proportional hazard model was then used to assess the association between gender and OS. Stratification according to age at diagnosis (<50 years, ≥50 years) was done, and within each age group, the propensity score matching analysis was repeated. A similar propensity score matching analysis was also conducted among those with epithelial histology. All statistical analyses were performed using SAS software, v9.4 (SAS Institute, Cary, NC).
Results
We identified 16 267 patients with MPM who met the selection criteria of which 21.3% were female (Table 1). At diagnosis, females were significantly younger (<60 years: 17.2 versus 9.9%), less likely to be on Medicare (65.3 versus 70.6%) but more likely to be on Medicaid (3.3 versus 1.9%), had lower comorbidity scores (Charlson Comorbidity Index = 0: 70.5 versus 67.6%) and tended to live closer to their reporting facility (within 25 miles: 74.3 versus 71.9%) than males. Females had significantly more epithelial cancer (40.3 versus 35.0%), and were less likely to receive chemotherapy as a first course of treatment than males (45.9 versus 49.3%) (Table 1).
Table 1.
Characteristics of malignant pleural mesothelioma cases in the National Cancer Database (NCDB) according to gender
| Propensity-matched sample | ||||||
|---|---|---|---|---|---|---|
| Variable | Males (n = 12 800) No. (%) |
Females (n = 3467) No. (%) |
P a | Males (n = 2374) No. (%) |
Females (n = 2374) No. (%) |
P a |
| Age (years) | <0.0001 | 0.9877 | ||||
| <50 | 261 (2.0) | 176 (5.1) | 76 (3.2) | 84 (3.5) | ||
| 50–59 | 1013 (7.9) | 422 (12.2) | 286 (12.0) | 283 (11.9) | ||
| 60–69 | 3115 (24.3) | 747 (21.5) | 519 (21.9) | 510 (21.5) | ||
| 70–79 | 4669 (36.5) | 1086 (31.3) | 754 (31.8) | 748 (31.5) | ||
| ≥80 | 3742 (29.2) | 1036 (29.9) | 739 (31.1) | 749 (31.6) | ||
| Race | 0.1665 | 0.4704 | ||||
| White | 11 909 (93.0) | 3200 (92.3) | 2187 (92.1) | 2198 (92.6) | ||
| Black | 561 (4.4) | 158 (4.6) | 116 (4.9) | 113 (4.8) | ||
| Asian/other | 330 (2.6) | 109 (3.1) | 71 (3.0) | 63 (2.7) | ||
| Spanish/Hispanic origin | 0.0748 | 0.6362 | ||||
| No | 11 416 (89.2) | 3064 (88.4) | 2252 (94.9) | 2259 (95.2) | ||
| Yes | 495 (3.9) | 157 (4.5) | 122 (5.1) | 115 (4.8) | ||
| Missing/unknown | 889 (6.9) | 246 (7.1) | ||||
| % of adults in patient zip code without a HS degree (quartiles) | 0.0089 | 0.8237 | ||||
| ≥21 | 1631 (12.7) | 449 (13.0) | 331 (13.9) | 321 (13.5) | ||
| 13–20.9 | 3065 (23.9) | 841 (24.3) | 580 (24.4) | 581 (24.5) | ||
| 7–12.9 | 4576 (35.8) | 1145 (33.0) | 805 (33.9) | 818 (34.5) | ||
| <7 | 3153 (24.6) | 933 (26.9) | 658 (27.7) | 654 (27.5) | ||
| Missing/unknown | 375 (2.9) | 99 (2.9) | ||||
| Patient zip code median income ($) (quartiles) | 0.0353 | 0.4103 | ||||
| <38 000 | 1704 (13.3) | 462 (13.3) | 327 (13.8) | 304 (12.8) | ||
| 38 000–47 999 | 2925 (22.9) | 735 (21.2) | 513 (21.6) | 501 (21.1) | ||
| 48 000–62 999 | 3595 (28.1) | 950 (27.4) | 652 (27.5) | 688 (29.0) | ||
| ≥63 000 | 4197 (32.8) | 1221 (35.2) | 882 (37.2) | 881 (37.1) | ||
| Missing/unknown | 379 (3.0) | 99 (2.9) | ||||
| Insurance | <0.0001 | 0.7976 | ||||
| Uninsured | 221 (1.7) | 70 (2.0) | 40 (1.7) | 46 (1.9) | ||
| Private insurance | 2947 (23.0) | 931 (26.9) | 653 (27.5) | 648 (27.3) | ||
| Medicaid | 246 (1.9) | 113 (3.3) | 77 (3.2) | 84 (3.5) | ||
| Medicare/other government | 9033 (70.6) | 2265 (65.3) | 1604 (67.6) | 1596 (67.2) | ||
| Missing/unknown | 353 (2.8) | 88 (2.5) | ||||
| Charlson Comorbidity Index | 0.0020 | 0.2013 | ||||
| 0 | 8647 (67.6) | 2443 (70.5) | 1729 (72.8) | 1678 (70.7) | ||
| 1 | 2972 (23.2) | 755 (21.8) | 468 (19.7) | 518 (21.8) | ||
| ≥2 | 1181 (9.2) | 269 (7.8) | 177 (7.5) | 178 (7.5) | ||
| Facility type | 0.2436 | 0.5090 | ||||
| Community Cancer Program | 7384 (57.7) | 1944 (56.1) | 1390 (58.6) | 1368 (57.6) | ||
| Academic/Integrated Network | 5354 (41.8) | 1475 (42.5) | 984 (41.4) | 1006 (42.4) | ||
| Missing/unknown | 62 (0.5) | 48 (1.4) | ||||
| Distance from patient to reporting facility (miles) | 0.0117 | 0.9645 | ||||
| <25 | 9209 (71.9) | 2577 (74.3) | 1845 (77.7) | 1843 (77.6) | ||
| 25–49.9 | 1483 (11.6) | 383 (11.0) | 265 (11.2) | 267 (11.2) | ||
| ≥50 | 1741 (13.6) | 412 (11.9) | 264 (11.1) | 264 (11.1) | ||
| Missing/unknown | 367 (2.9) | 95 (2.7) | ||||
| Histology | <0.0001 | 0.3631 | ||||
| Epithelial | 4486 (35.0) | 1396 (40.3) | 979 (41.2) | 948 (39.9) | ||
| Sarcomatoid | 1766 (13.8) | 259 (7.5) | 169 (7.1) | 180 (7.6) | ||
| Biphasic | 996 (7.8) | 194 (5.6) | 153 (6.4) | 138 (5.8) | ||
| Not otherwise specified | 5552 (43.4) | 1618 (46.7) | 1073 (45.2) | 1108 (46.7) | ||
| Laterality | 0.6770 | 0.6976 | ||||
| One side | 11 857 (92.6) | 3163 (91.2) | 2320 (97.7) | 2316 (97.6) | ||
| Bilateral involvement/midline | 320 (2.5) | 81 (2.3) | 54 (2.3) | 58 (2.4) | ||
| Missing/unknown | 623 (4.9) | 223 (6.4) | ||||
| Stage | 0.1360 | 0.7998 | ||||
| Local | 5664 (44.3) | 1475 (42.5) | 1031 (43.4) | 1022 (43.0) | ||
| Regional | 2048 (16.0) | 579 (16.7) | 402 (16.9) | 406 (17.1) | ||
| Distant | 1923 (15.0) | 551 (15.9) | 359 (15.1) | 357 (15.0) | ||
| Missing/unknown | 3165 (24.7) | 862 (24.9) | 582 (24.5) | 589 (24.8) | ||
| Surgery | 0.2129 | 0.7835 | ||||
| No | 10 427 (81.5) | 2861 (82.5) | 1970 (83.0) | 1977 (83.3) | ||
| Yes | 2336 (18.3) | ≥10b | 404 (17.0) | 397 (16.7) | ||
| Missing/unknown | 37 (0.3) | <10b | ||||
| Radiotherapy | 0.3115 | 0.8175 | ||||
| No | 12 044 (94.1) | 3243 (93.5) | 2287 (96.3) | 2284 (96.2) | ||
| Yes | 432 (3.4) | 129 (3.7) | 87 (3.7) | 90 (3.8) | ||
| Missing/unknown | 324 (2.5) | 95 (2.7) | ||||
| Chemotherapy | 0.0004 | 0.9269 | ||||
| No | 6098 (47.6) | 1767 (51.0) | 1277 (53.8) | 1780 (53.9) | ||
| Yes | 6309 (49.3) | 1592 (45.9) | 1097 (46.2) | 1094 (46.1) | ||
| Missing/unknown | 393 (3.1) | 108 (3.1) |
HS, high school.
χ2 test among non-missing values.
Cells masked to protect against identification of patients.
After adjustment, females were still diagnosed at younger age, had a lower comorbidity score and were more likely to be on Medicaid compared with males (Table 2). Furthermore, females had significantly more epithelial histology and were less likely to receive chemotherapy compared with males (Table 2).
Table 2.
Demographic and clinical factors associated with gender (n = 12 759)
| Variable | Adjusted ORa (female versus male) | 95% CI | |
|---|---|---|---|
| Age (years) | |||
| <50 | 1.00 | Ref | |
| 50–59 | 0.72 | 0.54–0.97 | |
| 60–69 | 0.43 | 0.32–0.58 | |
| 70–79 | 0.43 | 0.32–0.58 | |
| ≥80 | 0.49 | 0.36–0.66 | |
| Race | |||
| White | 1.00 | Ref | |
| Black | 1.00 | 0.80–1.24 | |
| Asian/Other | 1.15 | 0.87–1.53 | |
| Spanish/Hispanic origin | |||
| Yes versus No | 0.99 | 0.79–1.2 | |
| % of adults in patient zip code without a HS degree (quartiles) | |||
| <7 | 1.00 | Ref | |
| 7–12.9 | 0.86 | 0.76–0.97 | |
| 13–20.9 | 0.96 | 0.83–1.11 | |
| ≥21 | 0.96 | 0.80–1.17 | |
| Patient zip code median income ($) (quartiles) | |||
| ≥63 000 | 1.00 | Ref | |
| 48 000–62 999 | 0.97 | 0.86–1.09 | |
| 38 000–47 999 | 0.90 | 0.78–1.04 | |
| <38 000 | 0.93 | 0.78–1.12 | |
| Insurance | |||
| Medicare/other government | 1.00 | Ref | |
| Medicaid | 1.42 | 1.08–1.89 | |
| Private insurance | 1.11 | 0.98–1.25 | |
| Uninsured | 0.98 | 0.71–1.36 | |
| Charlson Comorbidity Index | |||
| 0 | 1.00 | Ref | |
| 1 | 0.89 | 0.80–0.99 | |
| ≥2 | 0.76 | 0.65–0.90 | |
| Facility type | |||
| Community Cancer Program | 1.00 | Ref | |
| Academic/Integrated Network Cancer Program | 1.04 | 0.95–1.14 | |
| Distance from patient to reporting facility (miles) | |||
| <25 | 1.00 | Ref | |
| 25–49.9 | 0.96 | 0.84–1.11 | |
| ≥50 | 0.89 | 0.77–1.03 | |
| Histology | |||
| Epithelial | 1.00 | Ref | |
| Sarcomatoid | 0.50 | 0.42–0.59 | |
| Biphasic | 0.68 | 0.57–0.82 | |
| Not otherwise specified | 0.93 | 0.84–1.02 | |
| Laterality | |||
| Bilateral involvement/midline versus one side | 0.85 | 0.65–1.13 | |
| Stage | |||
| Local | 1.00 | Ref | |
| Regional | 1.08 | 0.96–1.23 | |
| Distant | 1.07 | 0.93–1.21 | |
| Missing/unknown | 1.05 | 0.94–1.18 | |
| Surgery | |||
| Yes versus No | 0.91 | 0.81–1.04 | |
| Radiotherapy | |||
| Yes versus No | 1.07 | 0.85–1.35 | |
| Chemotherapy | |||
| Yes versus No | 0.78 | 0.71–0.86 | |
HS, high school; OR, odds ratio.
aOdds of being female, adjusted for all other variables, year of diagnosis, and reporting facility census division.Bold values indicate statistically significant results.
OS was significantly better for females than males at 2 years (26.5 versus 16.6%), and 5 years (9.4 versus 4.2%) which remained significant after adjusting for all covariates [HRadj: 0.81, 95% confidence interval (CI): 0.77–0.85] (Table 3). Besides gender, other factors significantly associated with better survival were younger age at diagnosis, higher income at the zip-code level, lower comorbidity score, diagnosis in an academic/integrated network cancer program, epithelial histology, earlier stage and receipt of treatment (Supplementary Table 1, available at Carcinogenesis Online).
Table 3.
Univariate and multivariate overall survival according to gender
| Univariate analysis | Multivariate analysis (n = 11 388) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2-year survival | 5-year survival | |||||||
| Rate (%) | 95% CI | P | Rate (%) | 95% CI | P | Adjusted HRa | 95% CI | |
| Males | 16.6 | 15.9–17.3 | 4.2 | 3.7–4.6 | 1 | Ref | ||
| Females | 26.5 | 24.9–28.1 | <0.0001 | 9.4 | 8.2–10.5 | <0.0001 | 0.81 | 0.77–0.85 |
| Propensity-matched analysis (n = 4,748)b | ||||||||
| Males | 17.5 | 15.9–19.0 | 4.3 | 3.3–5.3 | 1 | Ref | ||
| Females | 26.0 | 24.1–27.8 | 0.0019 | 8.7 | 7.3–10.0 | 0.0005 | 0.86 | 0.80–0.94 |
Adjusted for age at diagnosis, race, Hispanic origin, % of adults in the zip code with no high school, ZIP code median income, insurance, Charlson Comorbidity Index, facility type, distance from the reporting facility, histology, laterality, stage, receipt of surgery, receipt of radiotherapy, receipt of chemotherapy, year of diagnosis and US census division of the reporting facility.
P-values and HRs obtained from stratified Cox proportional hazards models to account for matching.
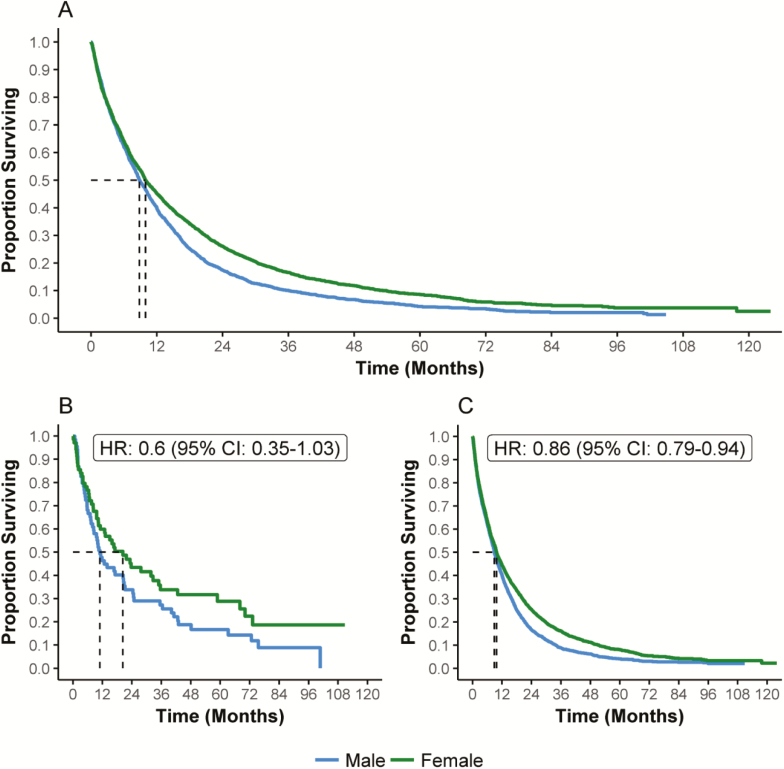
Propensity matching yielded 2374 matched cases for each gender (Table 1). Both the 2- (P = 0.0019) and 5-year (P = 0.0005) OS were better in females compared with males [hazard ratio (HR): 0.86, 95% CI: 0.80–0.94] (Table 3, Figure 2A).
Figure 2.
Overall survival according to gender in (A) propensity-matched cohort (n = 4748); (B) <50 years (n = 140); (C) ≥50 years (n = 4580).
After stratification by age at diagnosis, propensity matching within the <50 years age group yielded 70 matched female and male cases. The 2-year OS was 43.5 and 33.9% (P = 0.0772), and the 5-year OS was 28.8 and 16.7% (P = 0.0642) for females and males, respectively, with an HR of 0.6 (95% CI: 0.35–1.03) (Figure 2B).
Propensity matching within the ≥50 years age group yielded 2290 matched pairs. The 2-year OS was 25.3 and 16.7% (P = 0.0003) and the 5-year OS was 8.1 and 4.1% (P = 0.0007), for females and males, respectively, with an HR of 0.86 (95% CI: 0.79–0.94) (Figure 2C).
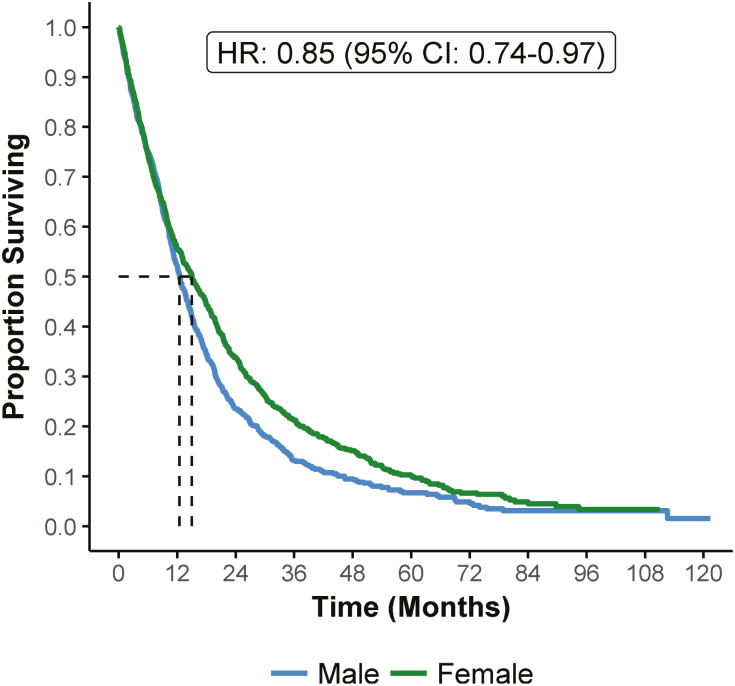
Propensity matching in the group with epithelial histology yielded two balanced groups of 945 female and male cases, and confirmed a better OS in females versus males at 2 years (P = 0.0128) and 5 years (P = 0.0152); HR: 0.85 (95% CI: 0.74–0.97) (Figure 3). Within the epithelial histology group females showed significantly better survival than males in both <50 years and ≥50 years groups (data not shown).
Figure 3.
Overall survival in epithelia histology cases (propensity-matched cohort) according to gender (n = 1890).
Discussion
This study includes more than 16 000 patients with MPM from the National Cancer Database and is one of the largest endeavors comparing survival in males and females. Our analysis shows that females with MPM survive significantly longer than males, independently from other contributing factors.
Differences in seeking medical advice have been suggested as a potential reason for the difference in survival with gender, because women tend to consult a physician earlier in the course of a disease than men (11). The current study, however, showed that stage at diagnosis was not significantly different in the two genders, as shown by the same rate of metastatic disease at diagnosis. This makes lead-time bias or earlier stage of disease at diagnosis a less likely explanation of the observed gender differences in survival.
Another factor that may affect survival is receipt of treatment. Our study showed that males and females equally received surgery or radiotherapy, and, in contrast to the survival outcome, females received chemotherapy less frequently compared with males, making treatment a less likely explanation for the difference in survival.
Recently published literature suggested that female malignant mesotheliomas have a higher frequency of germline mutations in DNA repair genes, some of which are associated with better survival, and this could in part account for the gender differences in survival observed in epidemiologic studies (16,17). Although other studies specifically focused on BAP1 polymorphism did not indicate that the frequency of the germline mutation varies with gender (18) or is lower in females than in males (19), it is possible that BAP1 is a mediator of the association between gender and survival. The dataset we used in this analysis does not include biological information or genetic testing, thus we are unable to test this hypothesis. More research on MPM germline variant in DNA repair genes and their association with gender and survival is needed.
Previous studies already identified the association of older age and male gender with shorter survival (5,8). A SEER analysis reported a better survival rate in females compared with males, however this study did not address the role of histology because this variable was missing in almost two-thirds of the cases (5). A smaller, single-institution study producing similar results on survival was also unable to fully adjust for all different histologies because of the small sample (8). Despite the fact that histology was not specified for all patients in the NCDB, we were able to study the effect of gender within the epithelial group, and confirmed the survival advantage of females.
The stratified analysis according to age found that females had higher survival at all ages, although the difference tended to be more pronounced in younger patients. These results seem to be unaffected by the tumor histology, despite the smaller sample size of this subanalysis. Circulating estrogen, present in young but not older women, and the expression of the estrogen receptor beta (ERβ) have been suggested to play a role in the survival difference between males and females. It has been shown that the ERβ has antiproliferative effects in in vitro and in vivo cancer models (20). A study analyzing 78 samples of patients with MPM showed a significantly better survival in patients with high ERβ expression compared with patients with low ERβ expression (11). A follow-up study showed that reduced ERβ expression led to a phenotypic shift to a less epithelioid phenotype and vice versa. Furthermore, ERβ expression was lost in more aggressive sarcomatoid forms of MPM (21). Therefore the potential to reverse a more aggressive biphasic phenotype by inducing the ERβ expression, as shown by Manente et al. (22), could be clinically significant. Epithelial forms of MPM have a better prognosis compared with other histology types, and the interplay with ERβ expression may be the underlying reason for better survival. Our finding that younger women have a more pronounced survival advantage further supports the theory that the presence of circulating estrogens combined with higher ERβ expression in epithelial histology may be partly responsible for the survival difference between genders. These results suggest the possibility of reversing a more aggressive histotype by targeting ERβ with a selective agonist together with estrogens as a novel treatment option, as described for prostate cancer (23), thus increasing therapeutic options for patients with MPM.
Among the limitations of this analysis is the fact that differences in survival between males and females for other histology types, such as sarcomatoid and biphasic, could not be assessed because of the small number of female patients with these MPM subtypes. Although the NCDB captures ~70% of newly diagnosed cancer cases in the USA, it is not a nationally representative population-based sample. However, cases reported to the NCDB are most likely representative at regional level (24). Another limitation is that the NCDB is used by communities and participating hospitals as a self-assessment tool to assess patterns of care and outcomes (25). The dataset might therefore lack relevant information because it was not collected. For example, a proportion of cases lacked complete information on histology; the lack of difference between males and females in the percentage of histology ‘not otherwise specified’ makes it unlikely that bias could explain the observed survival differences. Strengths related to using a large database include the large group of eligible patients with MPM, which is an otherwise rare disease. Furthermore, the results are more generalizable to the general population compared with single-institution studies.
This study adds information to the known significant survival difference between male and female patients with MPM using a large cohort of patients with MPM, by disentangling the effect of gender from the effect of age and histology, two known independent factors affecting survival. These results may provide additional evidence that ERβ expression present on epithelial-type MPM and circulating estrogens, present in younger women, positively affect survival in MPM.
Funding
This work was partially supported by the National Cancer Institute (P30CA196521).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations
- CI
confidence interval
- CoC
Commission on Cancer
- ERβ
estrogen receptor beta
- HR
hazard ratio
- MPM
malignant pleural mesothelioma
- NCDB
National Cancer Database
- OS
overall survival
References
- 1. Yang H., et al. (2008) Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr. Treat. Options Oncol., 9, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flores R.M., et al. (2010) Frequency of use and predictors of cancer-directed surgery in the management of malignant pleural mesothelioma in a community-based (Surveillance, Epidemiology, and End Results [SEER]) population. J. Thorac. Oncol., 5, 1649–1654. [DOI] [PubMed] [Google Scholar]
- 3. Spirtas R., et al. (1988) Survival patterns for malignant mesothelioma: the SEER experience. Int. J. Cancer., 41, 525–530. [DOI] [PubMed] [Google Scholar]
- 4. Milano M.T., et al. (2010) Malignant pleural mesothelioma: a population-based study of survival. J. Thorac. Oncol., 5, 1841–1848. [DOI] [PubMed] [Google Scholar]
- 5. Taioli E., et al. (2014) Women with malignant pleural mesothelioma have a threefold better survival rate than men. Ann. Thorac. Surg., 98, 1020–1024. [DOI] [PubMed] [Google Scholar]
- 6. Wolf A.S., et al. (2010) Characteristics of malignant pleural mesothelioma in women. Ann. Thorac. Surg., 90, 949–56; discussion 956. [DOI] [PubMed] [Google Scholar]
- 7. Flores R.M., et al. (2007) Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J. Thorac. Oncol., 2, 957–965. [DOI] [PubMed] [Google Scholar]
- 8. Rusch V.W., et al. (1999) Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann. Thorac. Surg., 68, 1799–1804. [DOI] [PubMed] [Google Scholar]
- 9. Yan T.D., et al. (2006) Sex difference in diffuse malignant peritoneal mesothelioma. Br. J. Surg., 93, 1536–1542. [DOI] [PubMed] [Google Scholar]
- 10. Hillerdal G. (1999) Mesothelioma: cases associated with non-occupational and low dose exposures. Occup. Environ. Med., 56, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinton G., et al. (2009) Estrogen receptor-beta affects the prognosis of human malignant mesothelioma. Cancer Res., 69, 4598–4604. [DOI] [PubMed] [Google Scholar]
- 12. Bilimoria K.Y., et al. (2009) Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J. Clin. Oncol., 27, 4177–4181. [DOI] [PubMed] [Google Scholar]
- 13. Boffa D.J., et al. (2017) Using the National Cancer Database for outcomes research: a review. JAMA Oncol., 3, 1722–1728. [DOI] [PubMed] [Google Scholar]
- 14. Steele G.D., Jr, et al. (1994) The National Cancer Data Base. a mechanism for assessment of patient care. Cancer, 73, 499–504. [DOI] [PubMed] [Google Scholar]
- 15. Ohri N, et al. (2016) Definitive radiation therapy is associated with improved survival in non-metastatic malignant pleural mesothelioma. Int. J. Radiat. Oncol. Biol. Phys., 96, S132–S133. [Google Scholar]
- 16. Hassan R, et al. (2018) Inherited predisposition to malignant mesothelioma (MM) due to mutations in DNA repair genes. J. Clin. Oncol., 15, 8504–8504. [Google Scholar]
- 17. Panou V., et al. (2018) Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J. Clin. Oncol., 36, 2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pastorino S, et al. (2018) Subset of mesotheliomas with improved survival occurring in carriers of BAP1 and other germline mutations. J. Clin. Oncol., 36, 3485–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Rienzo A., et al. (2016) Gender-specific molecular and clinical features underlie malignant pleural mesothelioma. Cancer Res., 76, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinton G., et al. (2017) Expression and therapeutic significance of estrogen receptor β in malignant pleural mesothelioma. Future Sci. OA., 3, FSO175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pinton G., et al. (2010) Estrogen receptor β exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib. PLoS One, 5, e14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manente A.G., et al. (2015) Intracellular lactate-mediated induction of estrogen receptor beta (ERβ) in biphasic malignant pleural mesothelioma cells. Oncotarget, 6, 25121–25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ho S.M., et al. (2011) Estrogens and prostate cancer: etiology, mediators, prevention, and management. Endocrinol. Metab. Clin. North Am., 40, 591–614, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fremgen A.M., et al. (1999) Clinical highlights from the National Cancer Data Base, 1999. CA. Cancer J. Clin., 49, 145–158. [DOI] [PubMed] [Google Scholar]
- 25. Menck H.R., et al. (1997) The growth and maturation of the National Cancer Data Base. Cancer, 80, 2296–2304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.