Abstract
The superparamagnetic iron oxide tracer Sienna+® was introduced as an alternative to the radioisotope 99Tc Nanocoll to preoperatively mark sentinel lymph nodes in breast cancer. As previously reported, this tracer causes susceptibility artifacts on magnetic resonance imaging (MRI), potentially hampering the diagnostic performance of follow-up breast MRI. This short report illustrates the temporal development of these artifacts in a patient who was followed up at 6, 12, and 18 months after administration of Sienna+® with MRI systems of different magnetic field strengths (1.5 T and 3.0 T) and using an MRI protocol with sequences optimized for artifact reduction. Although the artifacts were severe and predominant at the higher magnetic strength in the early postoperative period, they diminished over time and the image quality could be further improved by adapting the sequences. These findings indicate the possible use of MRI even after administration of a superparamagnetic tracer for post-treatment monitoring in breast cancer.
Keywords: Breast neoplasms, Sentinel lymph node, Artifacts, Magnetic fields, Tc 99m nanocolloid, Superparamagnetic nanoparticles
1. Introduction
In patients without clinical signs of axillary lymph node metastasis, sentinel lymph node biopsy is performed by injecting a radioisotope (99Tc Nanocoll) either alone or combined with a blue dye. This technique was first described in the 1990s [1] and led to a significant decrease in morbidity associated with axillary lymph node dissection (e.g., lymphedema, impairment of shoulder mobility, and pain and numbness of the arm).
Although this technique is the gold standard, it has some disadvantages mostly associated with the need to use radioactivity. Therefore, ferromagnetic tracers such as Sienna+® (Endomag, Cambridge, UK) were investigated and introduced as alternatives to radioisotopes. Sienna+® is not radioactive and therefore has no environmental radiation exposure or rigorous legal requirements. Clinical trials performed across Europe have shown that use of the Sentimag magnetic probe (Endomag) and Sienna+® magnetic tracer is comparable with the gold standard [[2], [3], [4], [5], [6], [7], [8], [9]].
However, as previously described by our group [10], the superparamagnetic tracer Sienna+® impairs the image quality of magnetic resonance imaging (MRI) in patients who receive this tracer in follow-up breast MRI. Karakatsanis et al. [11] also observed these artifacts. In one case, however, MRI clearly showed a 7-mm tumor in a patient treated with lumpectomy 1 year earlier and previously marked with Sienna+®.
Both of the above-mentioned papers describe findings obtained at a single time point. In the present paper, we aim to contribute to this debate with a follow-up observation of one patient at different time points during an 18-month period following Sienna+® application, imaged at two different MRI field strengths and opting for an artifact-reduction-optimized MRI protocol.
The aim of the present work was to optimize the image quality of breast MRI in patients with artifacts due to superparamagnetic tracer injection and to observe any dynamic temporal changes of those artifacts over time. These findings will help to establish recommendations for the use of breast MRI for follow-up in this subgroup of patients.
2. Patient and methods
A 63-year-old woman with no history of breast intervention or family history of breast cancer was diagnosed with invasive ductal breast cancer (pT1c, pN0 (0/4) (sn), cM0, G2, L0, V0, Pn0, ER 100%, PR 2%, AR 100%, HER2 negative, Ki67 7%) in her right breast in 2016. She subsequently underwent breast-conserving therapy with sentinel lymph node biopsy. To mark the sentinel node, she received 2 ml of the ferromagnetic tracer Sienna+® (diluted to 5 ml in 0.9% NaCl and injected in the retroareolar region) within the context of an ongoing study. Approval from the Ethics Committee of Northwest/Central Switzerland (EKNZ BASEC 2016-00808) had been obtained at time of inclusion, and the patient provided written informed consent. She underwent follow-up MRI at 6, 12, and 18 months after surgery. As part of the protocol, she underwent native MRI at field strengths of 1.5 T and 3.0 T (MAGNETOM Area and MAGNETOM Skyrafit, respectively; Siemens Healthineers, Erlangen, Germany). The applied sequences are summarized in Table 1. To reduce the above-described susceptibility artifacts, the routinely applied three-dimensional gradient echo sequence was additionally acquired with increased bandwidth and minimal echo time at the last follow-up appointment (18 months). This sequence plays an important part in diagnostic MRI because it is used to obtain dynamic contrast-enhanced images.
Table 1.
Overview of all parameters used with the two different magnetic resonance imaging scanners.
| Parameter | Siemens Aera 1.5 T |
Siemens Skyra fit 3T |
||||
|---|---|---|---|---|---|---|
| Sequence | T2w | Single-shot EPI DWI | T1w nativ | T2w | Resolve DWI | T1w nativ |
| Repetition time (msec) | 5600 | 6000 | 4.87 | 6000 | 5920 | 4.26 |
| Echo time (msec) | 110 | 59 | 2.39 | 86 | 78 | 1.61 |
| Echo time adapt (msec) | n/a | n/a | 1.41 | n/a | n/a | 1.31 |
| Flip angle (°) | 150 | 90 | 10 | 120 | 90 | 10 |
| FOW (mm) | 360 | 340 | 360 | 360 | 360 | 360 |
| Section thickness (mm) | 3 | 4 | 1 | 3 | 5 | 1 |
| Voxel size (mm) | 0.7 × 0.7 × 3.0 | 1.7 × 1.7 × 4 | 0.8 × 0.8 × 1.0 | 0.7 × 0.7 × 3.0 | 1.3 × 1.3 × 5 | 0.8 × 0.8 × 1.0 |
| Phase oversampling (%) | 70 | 0 | 40 | 30 | 80 | 40 |
| Phase resolution (%) | 60 | 100 | 75 | 80 | 100 | 75 |
| Bandwidth (Hz/pixel) | 199 | 1470 | 340 | 391 | 760 | 380 |
| Bandwidth adapt (Hz/pixel) | n/a | n/a | 700 | n/a | n/a | 800 |
The adapted bandwidth and echo time were only applied for the T1-weighted images (T1w). DWI: diffusion weighted images. EPI: echo-planar pulse images. FOV: field of view. T2w: T2-weighted images. Hz: hertz.
Due to a technical storage problem, the diffusion-weighted images (DWI) using the 1.5 T scanner at the 12-month follow-up visit could not be saved. All other sequences acquired with the 1.5 T and 3.0 T scanners were stored by our picture archiving and communication system and visually scored by two breast radiologists. The acquired images were judged by two trained breast radiologists with 15 years (R.K.) and 10 years (S.F.) of experience. Each image received a score of 1–3, where 1 = diagnostic, 2 = impaired but still readable, and 3 = impossible to interpret.
3. Results
A small discoloration in the outer upper quadrant persisted at all clinic visits. The discoloration was approximately 3 × 5 cm at the 6-month follow-up visit and 2.5 × 4 cm at the 18-month follow-up visit.
The MRI artifacts were most severe at the first follow-up (6 months), especially on the 3.0 T scanner, and the DWI findings were impossible to interpret (Fig. 1g and h). At this time point, only the T2-weighted images from the 1.5 T scanner were evaluated as diagnostic. At the next visit (12 months), the artifacts had decreased, but the DWI findings were still impossible to interpret using the 3.0 T scanner. The T1-weighted images were scored as impaired but still readable, and there was an inter-reader agreement that the T1-weighted images from the 3.0 T scanner had fewer artifacts.
Fig. 1.
Overview of the different sequences and follow-ups. (a–f) T2-weighted images of the breasts. (g–k) Calculated apparent diffusion coefficient (ADC). (l–q) T1weighted fat-suppressed (FS) images without contrast agent.
First follow-up at 6 months using the (a, g, l) 1.5 T scanner and (b, h, m) 3.0 T scanner. All images show prominent artifact in the right breast. (h, m) This was most apparent using the 3.0 T scanner, especially in the ADC and T1-weighted FS images.
Second follow-up at 12 months using the (c, n) 1.5 T scanner and (l, o) 3.0 T scanner. (i, o) Apart from skin thickening after radiation therapy, the artifacts were still present, especially as shown by the 3.0 T scanner, but they were slightly less severe than in the previous images.
Last follow-up at 18 months using the (e, j, p) 1.5 T scanner and (f, k, q) 3.0 T scanner. Compared with the initial images, the artifacts were clearly less severe and even minor as shown by the 3.0 T scanner, (q) especially on the T1-weighted FS images.
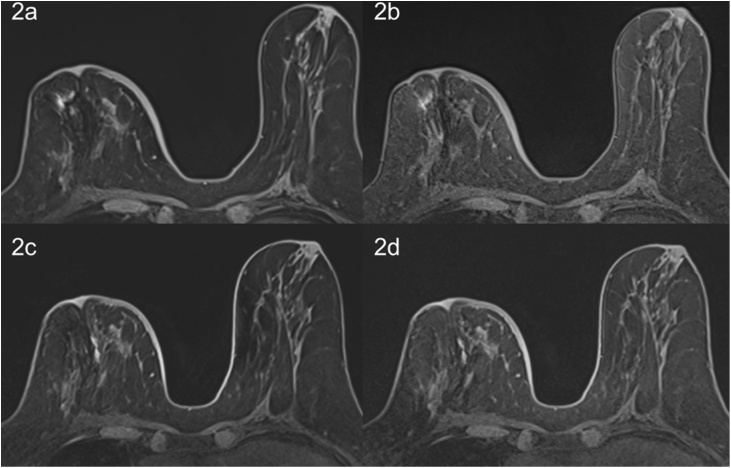
At the last visit (18 months), all images, including the DWI from the 3.0 T scanner, were evaluated impaired but still readable; additionally, the T1-weighted images from the 3.0 T were more readable than those from the 1.5 T scanner. The modified T1-weighted sequence with a high bandwidth and short echo time resulted in images that were scored as diagnostic (Fig. 2).
Fig. 2.
T1-weighted fat-suppressed images of the breast without contrast agent. Images were obtained using the (a, b) 1.5 T scanner and (c, d) 3.0 T scanner. (a, c) Images acquired with our conventional parameters. (b, d) Images acquired with a higher bandwidth and shorter echo time according to the parameters described in Table 1. The adapted sequences resulted in fewer artifacts at both field strengths.
4. Discussion
Breast MRI is an adjunct to standard diagnostic techniques of the breast. It is usually performed at magnetic field strengths of 1.5 T or even 3.0 T. Standard MRI investigation includes T2- and T1-weighted pre-contrast images and T1-weighted post-contrast dynamic sequences, which are usually gradient echo sequences repeated as rapidly as possible for 5–7 minutes after intravenous administration of a gadolinium contrast agent [12].
Ferromagnetic metals cause severe inhomogeneity in the magnetic field, resulting in distortion and a local signal intensity void in the vicinity of the metal. The degree of distortion is determined by the shape and composition of the metallic object [13]. These susceptibility effects increase as the field strength increases [14]. The predominant imaging artifacts that arise are signal loss due to dephasing, failure of fat suppression, and displacement artifacts. Dephasing effects can be almost completely avoided by the use of spin echo imaging. Still, because of the high spatial and temporal resolution, gradient echo sequences are currently standard in breast MRI.
Fat saturation (when using chemically selective saturation with a 1.5 T scanner 220 Hz above water) may fail due to the frequency shifts that occur near metallic implants. In such cases, short-TI inversion recovery imaging should be applied because it is completely independent of the resonance frequency, although the signal-to-noise ratio is low and the tissue signal is more strongly suppressed due to the shortened T1. Although T2-weighted fat-suppressed images are not standard in breast imaging, fat saturation can be omitted in the dynamic T1-weighted post-contrast sequences by using post-processing subtraction techniques.
A direct way to reduce distortion effects on gradient echo sequences is to maximize the bandwidth used both on slice selection and during readout. However, this effect is obtained at a cost of increased power deposition, which may either force a longer repetition time or fewer interleaved slices per repetition. Reducing the echo time in gradient echo sequences can reduce static effects so that there is less time for magnetization to become incoherent (or dephased) [15].
Because our protocol for the T1 gradient echo sequences already had a low TE, we could raise the bandwidth from 340 to 700 Hz/pixel on the 1.5 T scanner and from 380 to 800 Hz/pixel on the 3.0 T scanner.
To our knowledge, this is the first report of a patient who received Sienna+® to preoperatively mark the sentinel lymph nodes and was followed up with MRI for 18 months. In a previous study [10], artifacts after Sienna+® injection were seen in 76.5% of patients after a mean time of 42 months.
We also observed artifacts in the present case, but they appeared to decrease in severity as time passed, even when using the 3.0 T scanner. The other breast was not affected by artifacts. Adapting the sequences of the T1-weighted fat-suppressed images by increasing the bandwidth and shorting the echo time allowed for further reduction of the artifacts. This has also been shown in other body regions [16]. Therefore, adapted T1-weighted sequences may improve the diagnostic capability of MRI.
Although this short report is based on a single patient, this technical report still has some interesting aspects. First, it seems that the artifacts induced by the superparamagnetic iron oxide diminished over time. Second, adapting the sequences as described above may also improve the diagnostic capability of MRI. Still, this patient was followed up for 18 months, while our previous study showed one time point at 42 months after therapy; thus, whether a further decrease of artifacts will occur and whether our observation can also be confirmed in other patients remain unclear.
As suggested by Karakatsanis et al. [11], a reduction of the quantity of Sienna+® that is applied and the use of a deeper injection might reduce the artifacts because less iron will stay in the tissue. The prospective study mentioned by Karakatsanis et al. [11] (https://doi.org/10.1186/ISRCTN85167182) will hopefully determine on a larger scale whether breast MRI is feasible after application of superparamagnetic iron oxides.
Conflict of interest
We confirm that this manuscript has not been published elsewhere and is not under consideration in whole or in part by another journal. All authors have approved the manuscript and agree with submission to the European Journal of Radiology Open. The authors have no conflicts of interest to declare.
Acknowledgments
The authors thank Markus Klarhöfer for supporting and adapting the MRI sequences. The authors also thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Contributor Information
S. Forte, Email: Serafino.forte@ksb.ch.
R.A. Kubik-Huch, Email: Rahel.kubik@ksb.ch.
C. Leo, Email: Cornelia.leo@ksb.ch.
References
- 1.Giuliano A.E., Kirgan D.M., Guenther J.M., Morton D.L. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann. Surg. 1994;220(3):391–398. doi: 10.1097/00000658-199409000-00015. discussion 398-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douek M., Klaase J., Monypenny I., Kothari A., Zechmeister K., Brown D., Wyld L., Drew P., Garmo H., Agbaje O., Pankhurst Q., Anninga B., Grootendorst M., Ten Haken B., Hall-Craggs M.A., Purushotham A., Pinder S., Senti M.A.G.T.G. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann. Surg. Oncol. 2014;21(4):1237–1245. doi: 10.1245/s10434-013-3379-6. [DOI] [PubMed] [Google Scholar]
- 3.Ghilli M., Carretta E., Di Filippo F., Battaglia C., Fustaino L., Galanou I., Di Filippo S., Rucci P., Fantini M.P., Roncella M. The superparamagnetic iron oxide tracer: a valid alternative in sentinel node biopsy for breast cancer treatment. Eur. J. Cancer Care. 2017;26(4) doi: 10.1111/ecc.12385. [DOI] [PubMed] [Google Scholar]
- 4.Houpeau J.L., Chauvet M.P., Guillemin F., Bendavid-Athias C., Charitansky H., Kramar A., Giard S. Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: the French Sentimag feasibility trial. J. Surg. Oncol. 2016;113(5):501–507. doi: 10.1002/jso.24164. [DOI] [PubMed] [Google Scholar]
- 5.Pinero-Madrona A., Torro-Richart J.A., de Leon-Carrillo J.M., de Castro-Parga G., Navarro-Cecilia J., Dominguez-Cunchillos F., Roman-Santamaria J.M., Fuster-Diana C., Pardo-Garcia R., M. Grupo de Estudios Senologicos de la Sociedad Espanola de Patologia Superparamagnetic iron oxide as a tracer for sentinel node biopsy in breast cancer: a comparative non-inferiority study. Eur. J. Surg. Oncol. 2015;41(8):991–997. doi: 10.1016/j.ejso.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Rubio I.T., Diaz-Botero S., Esgueva A., Rodriguez R., Cortadellas T., Cordoba O., Espinosa-Bravo M. The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur. J. Surg. Oncol. 2015;41(1):46–51. doi: 10.1016/j.ejso.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Thill M., Kurylcio A., Welter R., van Haasteren V., Grosse B., Berclaz G., Polkowski W., Hauser N. The Central-European SentiMag study: sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast. 2014;23(2):175–179. doi: 10.1016/j.breast.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Karakatsanis A., Christiansen P.M., Fischer L., Hedin C., Pistioli L., Sund M., Rasmussen N.R., Jornsgard H., Tegnelius D., Eriksson S., Daskalakis K., Warnberg F., Markopoulos C.J., Bergkvist L. The Nordic SentiMag trial: a comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res. Treat. 2016;157(2):281–294. doi: 10.1007/s10549-016-3809-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teshome M., Wei C., Hunt K.K., Thompson A., Rodriguez K., Mittendorf E.A. Use of a magnetic tracer for sentinel lymph node detection in early-stage breast cancer patients: a meta-analysis. Ann. Surg. Oncol. 2016;23(5):1508–1514. doi: 10.1245/s10434-016-5135-1. [DOI] [PubMed] [Google Scholar]
- 10.Krischer B., Forte S., Niemann T., Kubik-Huch R.A., Leo C. Feasibility of breast MRI after sentinel procedure for breast cancer with superparamagnetic tracers. Eur. J. Surg. Oncol. 2018;44(1):74–79. doi: 10.1016/j.ejso.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Karakatsanis A., Obondo C., Abdsaleh S., Hersi A.F., Eriksson S., Warnberg F. Optimisation of breast MRI compatibility after sentinel node biopsy with paramagnetic tracers. Eur. J. Surg. Oncol. 2018;44(5):731–732. doi: 10.1016/j.ejso.2018.01.241. [DOI] [PubMed] [Google Scholar]
- 12.Mann R.M., Kuhl C.K., Kinkel K., Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur. Radiol. 2008;18(7):1307–1318. doi: 10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genson C.C., Blane C.E., Helvie M.A., Waits S.A., Chenevert T.L. Effects on breast MRI of artifacts caused by metallic tissue marker clips. AJR Am. J. Roentgenol. 2007;188(2):372–376. doi: 10.2214/AJR.05.1254. [DOI] [PubMed] [Google Scholar]
- 14.Harvey J.A., Hendrick R.E., Coll J.M., Nicholson B.T., Burkholder B.T., Cohen M.A. Breast MR imaging artifacts: how to recognize and fix them. Radiographics. 2007;27(Suppl 1):S131–45. doi: 10.1148/rg.27si075514. [DOI] [PubMed] [Google Scholar]
- 15.Hargreaves B.A., Worters P.W., Pauly K.B., Pauly J.M., Koch K.M., Gold G.E. Metal-induced artifacts in MRI. AJR Am. J. Roentgenol. 2011;197(3):547–555. doi: 10.2214/AJR.11.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viano A.M., Gronemeyer S.A., Haliloglu M., Hoffer F.A. Improved MR imaging for patients with metallic implants. Magn. Reson. Imaging. 2000;18(3):287–295. doi: 10.1016/s0730-725x(99)00135-6. [DOI] [PubMed] [Google Scholar]