Abstract
This commentary critically examines key assumptions and recommendations in the 2006 Kidney Disease Outcomes Quality Initiative vascular access guidelines, and argues that several are not relevant to the contemporary United States hemodialysis population. First, the guidelines prefer arteriovenous fistulas (AVFs) over arteriovenous grafts (AVGs), on the basis of their superior secondary survival and lower frequency of interventions and infections. However, intent-to-treat analyses that incorporate the higher primary failure of AVFs, demonstrate equivalent secondary survival of both access types. Moreover, the lower rate of AVF versus AVG infections is counterbalanced by the higher rate of catheter-related bloodstream infections before AVF maturation. In addition, AVFs with assisted maturation (interventions before successful AVF use), which account for about 50% of new AVFs, are associated with inferior secondary patency compared with AVGs without intervention before successful use. Second, the guidelines posit lower access management costs for AVFs than AVGs. However, in patients who undergo AVF or AVG placement after starting dialysis with a central venous catheter (CVC), the overall cost of access management is actually higher in patients receiving an AVF. Third, the guidelines prefer forearm over upper arm AVFs. However, published data demonstrate superior maturation of upper arm versus forearm AVFs, likely explaining the progressive increase in upper arm AVFs in the United States. Fourth, AVFs are thought to fail primarily because of aggressive juxta-anastomotic stenosis. However, recent evidence suggests that many AVFs mature despite neointimal hyperplasia, and that suboptimal arterial vasodilation may be an equally important contributor to AVF nonmaturation. Finally, CVC use is believed to result in excess mortality in patients on hemodialysis. However, recent data suggest that CVC use is simply a surrogate marker of sicker patients who are more likely to die, rather than being a mediator of mortality.
Keywords: arteriovenous fistula; arteriovenous graft; dialysis catheter; dialysis; Central Venous Catheters; Forearm; Constriction, Pathologic; Hyperplasia; Vasodilation; Catheter-Related Infections; Kidney Diseases; Decision Making; Biomarkers; Bacteremia; Arteriovenous Shunt, Surgical
The three types of vascular access available for hemodialysis are arteriovenous fistulas (AVFs), arteriovenous grafts (AVGs), and central venous catheters (CVCs), and each access type has advantages and disadvantages (1). The 2006 Kidney Disease Outcomes Quality Initiative (KDOQI) vascular access guidelines consider the outcomes and complications of each vascular access to make recommendations for the optimal selection of access type and location (2). These recommendations are on the basis of vascular access outcomes that may have been true in the past, when there was more stringent patient selection for AVF creation, but no longer apply now that the great majority of patients receive an AVF. To the extent that these recommendations are no longer relevant to the current hemodialysis population, they may lead to practices that are not in the best interest of the patients. This commentary examines several widely accepted assumptions about vascular access that are inconsistent with recently published research, and suggests the need to reexamine some existing guidelines (Table 1).
Table 1.
Summary of myths versus facts about vascular access
| Myths | Facts |
|---|---|
| AVFs have better survival than AVGs | When you include AVFs that fail to mature, secondary survival is equivalent for AVFs and AVGs. Moreover, assisted AVF maturation is associated with shorter secondary survival and more frequent interventions after maturation |
| AVFs are cheaper than AVGs | If you analyze by intent to treat, AVFs are more expensive |
| Forearm AVFs are better than upper arm AVFs | Most patients are getting upper arm AVFs because they have superior maturation rates |
| AVFs fail to mature primarily because of aggressive neointimal hyperplasia (inward remodeling) | AVF maturation reflects a balance between inward and outward remodeling |
| CVCs are a major cause of death | CVC use is a surrogate marker of sicker patients. Very few deaths in patients with CVC are directly due to CVC complications |
AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheter.
AVFs Are Better Than AVGs
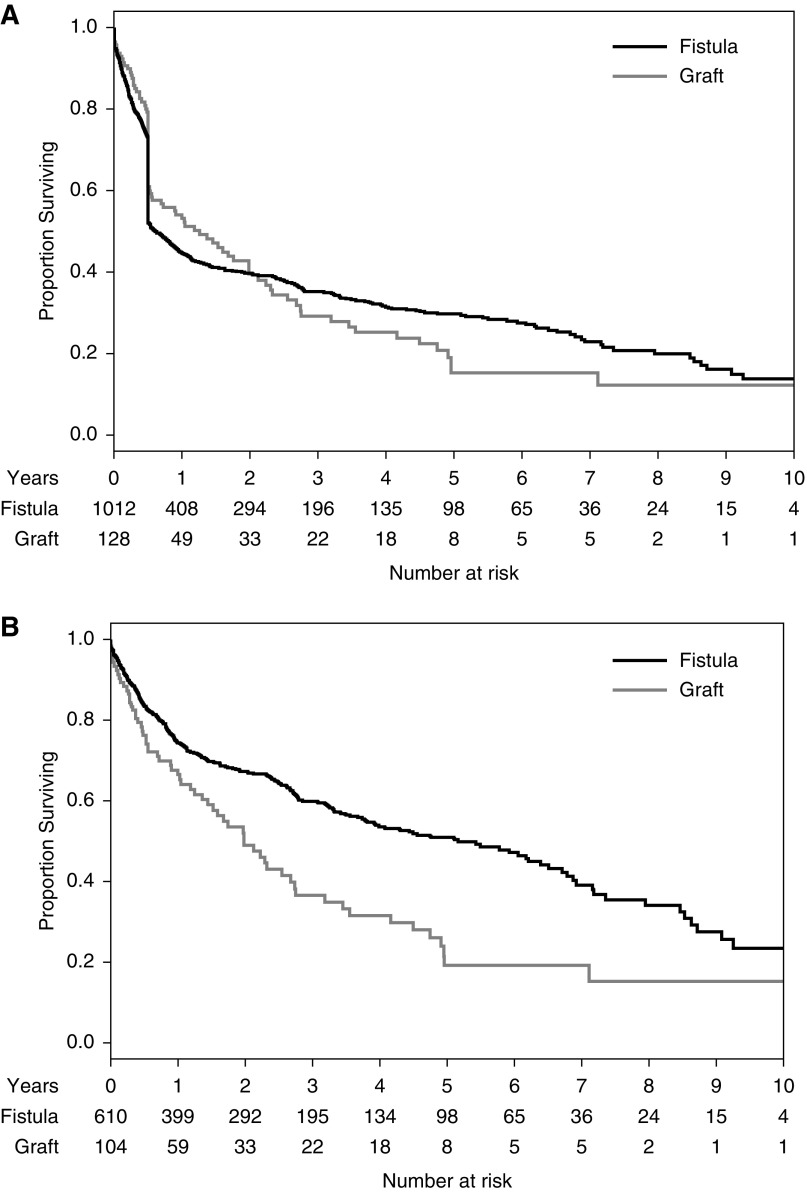
The 2006 KDOQI vascular access guidelines state that “options for fistula placement should be considered first, followed by prosthetic grafts if fistula placement is not possible” (2). The accompanying discussion acknowledges that AVFs have a higher primary failure rate (access never usable for dialysis) than AVGs. It argues, however, that AVFs are still preferred to AVGs because once they are successfully used for dialysis, AVFs have superior secondary patency, require less frequent interventions to maintain their patency, and incur fewer infections. Although each of these statements is correct, they do not adequately consider the effect of AVF nonmaturation and CVC dependence on these individual outcomes. Older publications, on which the original KDOQI guidelines were based, observed AVF nonmaturation in only 10% of patients (3–5). More recent publications, reflecting widespread AVF creation in the great majority of patients on dialysis, have reported substantially higher (30%–60%) AVF nonmaturation rates (6–8). Contemporary head-to-head comparisons of AVF and AVG outcomes at two large academic centers documented an absolute primary failure rate of AVFs that was about 20% higher than that of AVGs (9,10). Specifically, a study in Birmingham, Alabama, of 322 new AVFs and 289 new AVGs observed primary failure rates of 38% and 15%, respectively (9). Similarly, a study in Toronto, Canada, of 1012 new AVFs and 128 new AVGs documented primary failure rates of 40% and 19%, respectively (10). Both studies confirmed that secondary AVF survival was indeed superior to that of AVGs (5 versus 2 years), when the analysis was restricted to accesses successfully used for dialysis. However, in an intent-to-treat analysis that included all vascular accesses (including those with a primary failure), secondary access survival was equivalent for AVFs and AVGs (Figure 1). In fact, AVG survival was superior to that of AVFs during the first 2 years after access placement. Several smaller observational studies have arrived at similar conclusions (11–13). Thus, among elderly patients with a high comorbidity, whose median life expectancy is about 2 years, AVGs may actually be the preferred choice. In contrast, AVF placement is preferred in patients with a life expectancy >5 years. Finally, the optimal access type in patients with an expected survival of 2–5 years requires careful consideration of the relative merits and disadvantages of an AVF and AVG in that patient (14).
Figure 1.
Secondary survival of AVFs is superior to that of AVGs when primary failures are excluded, but similar when they are included. Reprinted from reference 10, with permission.
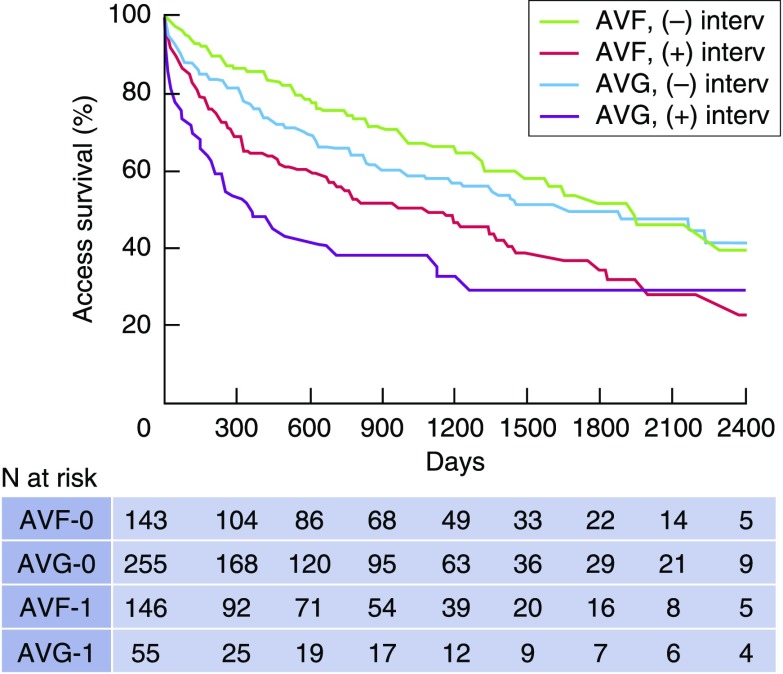
Whereas it is true that AVFs require less frequent interventions (angioplasty, thrombectomy, or surgical revision) to maintain their patency after successful use (15), they also require more frequent interventions before their successful use (assisted survival). A substantial proportion (27%–58%) of new AVFs require assisted maturation (Table 2). A small, single-center study of 110 patients undergoing placement of a new vascular access documented assisted maturation (the need for an intervention before successful use for dialysis) in 42% of patients receiving an AVF versus 16% of those with an AVG (11). Subsequently, a larger observational study of 289 AVFs and 310 AVGs from the same center observed assisted survival in 50% of AVFs, but only 18% of AVGs (16). Finally, a recent analysis of a national cohort of 9458 elderly United States patients on hemodialysis initiating dialysis with a CVC reported that assisted maturation was required in 42% of AVFs versus 23% of AVGs (17). Two recent studies associated assisted AVF maturation with a shorter secondary survival and more frequent interventions to maintain patency (16,18). In one study of 173 patients with a new AVF creation, AVF survival after maturation was 20%–30% lower among patients requiring two or more AVF interventions before maturation, compared with those with unassisted AVF maturation (18). Moreover, the frequency of AVF interventions after maturation was 1.8- and 4.6-fold higher if the AVF required one or two or more interventions to assist maturation. An accompanying editorial (19) commented that, “Although usability for dialysis is often considered an indicator of successful maturation, how the fistula got there (i.e., how it became usable) seems to be important.” A second study of 289 patients with a new AVF observed a two-fold higher likelihood of abandonment among AVFs with assisted maturation versus unassisted maturation (16). Remarkably, AVFs with assisted maturation had a secondary patency after maturation that was inferior to that of AVGs with unassisted maturation (16) (Figure 2). It is unclear whether the association of assisted maturation with inferior long-term AVF outcomes is a consequence of the intervention itself or simply a reflection of the use of poor-quality vessels that lead to impaired maturation and the need for adjuvant interventions.
Table 2.
Frequency of interventions before successful AVF use (assisted maturation) in published studies
| Reference | Patient Source | Percent of Patients with Assisted AVF Maturation |
|---|---|---|
| Falk (48) | Access ambulatory center | 58% |
| Lee et al. (18) | Two academic centers | 44% |
| Harms et al. (16) | One academic center | 50% |
| Allon et al. (8) | Seven academic centers (HFM Study) | 27% |
| Lee et al. (17) | US Renal Data System | 42% |
AVF, arteriovenous fistula; HFM, Hemodialysis Fistula Maturation.
Figure 2.
Secondary access survival after successful use is inferior if an intervention was required prior to successful use. Access patency was shorter for AVFs with prior intervention than for AVFs without interventions (P<0.001). Access patency was shorter for AVGs with prior interventions than AVGs without intervention (P<0.001). Access patency was similar for AVFs and AVGs without prior interventions (P=0.16). Access patency was worse for AVFs with prior interventions than for AVGs without prior interventions (P=0.01). Modified from reference 16.
The time from access placement to its first successful use for dialysis is substantially longer for AVFs compared with AVGs. The Dialysis Outcomes and Practice Patterns Study reported that only 2% of AVFs, but 78% of AVGs, were used for dialysis in the United States within 1 month of their placement (20). A subsequent publication found that the median time to first successful access cannulation in the United States was 82 days for AVFs and 29 days for AVGs (21). A recent US Renal Data System (USRDS) analysis of patients undergoing access surgery after dialysis initiation observed that 70% of those receiving an AVF remained CVC-dependent after 3 months, compared with 40% of those with an AVG (22). The time to successful cannulation is further prolonged if the AVF requires assisted maturation. For example, a large, single-center study observed a median interval from AVF creation to successful cannulation of 99 days for AVFs with unassisted maturation, compared with 159 days for those with assisted maturation (16). Analysis of a national cohort of elderly patients on hemodialysis reported that the median time from access placement to its successful use was 1 month for AVGs without prior intervention, 2 months for AVGs with a prior intervention, 3 months for AVFs with unassisted maturation, and 4 months for AVFs with assisted maturation (17). Among patients with vascular access placement after initiation of dialysis, such delays in successful AVF use translate into prolonged CVC dependence, with its associated risk of CVC-related bloodstream infections. In one observational study, the duration of CVC dependence until successful access use was 4 months for AVFs versus 1 month for AVGs. The proportion of patients experiencing a catheter-related bloodstream infection before access use was 44% and 24%, respectively (9). After successful access use, the annual rate of access infection was 9.7% for AVGs and 0.7% for AVFs. In other words, placement of an AVF rather than AVG entailed a trade-off between early CVC infections and late AVG infections. CVCs are also associated with a 7% risk of central vein stenosis (23), and ipsilateral CVCs have been associated with decreased secondary AVF survival (24).
AVFs Are Cheaper Than AVGs
The 2006 KDOQI vascular access guidelines state that the “costs of implantation and access maintenance are lowest for AVFs” (2). This is certainly true when the analysis is restricted to endovascular and surgical procedures required to maintain access patency after successful use (angioplasty, thrombectomy, or surgical revision). In this regard, numerous publications have reported that AVGs require a three- to seven-fold greater frequency of such interventions than AVFs (15), which would predictably translate into a higher cost for AVG maintenance. A Canadian study compared the overall cost of access management in new patients on dialysis, including 157 who received an AVF and 33 who underwent AVG surgery between 1999 and 2001 (25). The access was placed pre-ESKD in 32% of the patients. The overall median cost of access management in the first year after dialysis initiation was lower in patients receiving an AVF versus an AVG (CAN$4641 versus $8152). Remarkably, patients who dialyzed exclusively with a CVC had the lowest annual cost at CAN$3812, likely reflecting the low frequency of catheter-related bloodstream infections. When the costs were calculated per patient-year at risk, they were highest with AVGs, intermediate with CVCs, and lowest with AVFs. The costs reported in this Canadian study may not reflect contemporary costs in the United States because of differences in vascular access practice patterns over time and among countries, selection criteria for access type, the inclusion of patients with pre-ESKD access surgery, and the short patient follow-up time. It is also possible that interventions to promote AVF maturation are more likely if dialysis has already been started, because there is more pressure to accelerate AVF use.
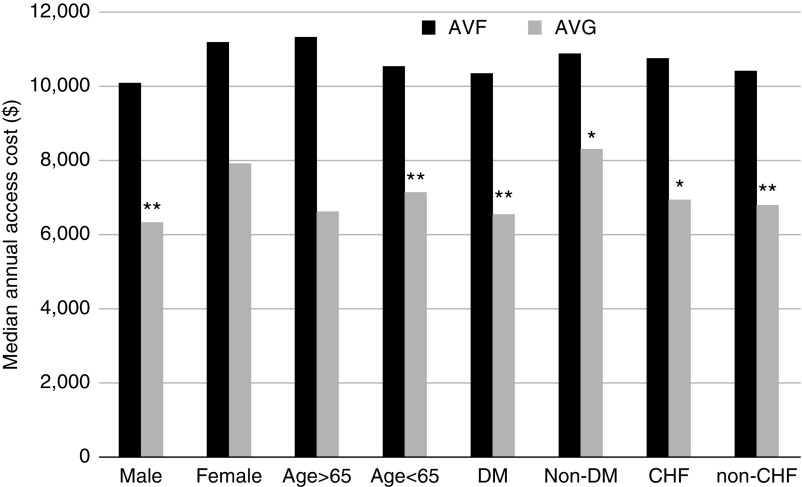
The cost calculation of vascular access management differs when one focuses exclusively on patients who initiate dialysis with a CVC, and subsequently undergo placement of an AVF or AVG, i.e., an intent-to-treat analysis. In that case, the cost includes not just procedures utilized to maintain access patency after its successful use, but also procedures required before successful access use, CVC exchanges due to dysfunction or infection, surgery to place a second access if the first one fails to mature, and hospitalizations for treatment of catheter-related bloodstream infection. An analysis from one center reported that the median annual cost of access management was twice as high in patients with an AVF that failed to mature, compared with patients in whom the AVF was successfully used for dialysis ($16,652 versus $8146) (26). Similarly, in a national cohort of elderly (age ≥66 years) patients who underwent AVF creation after starting dialysis with a CVC, the cost of access management was two- to three-fold higher if the AVF required an intervention before successful use, and four-fold higher if the AVF failed to mature (27). Given that at least one third of new AVFs fail to mature (21), this substantially increases the overall cost of patients receiving an AVF. In fact, the median annual access management cost at one center was almost $4000 greater in 295 patients receiving an AVF compared with 113 patients receiving an AVG in 2004–2012 ($10,642 versus $6810) (26). The higher cost of access management in the patients who initially received an AVF held true for multiple patient subsets (Figure 3). It was largely driven by the greater frequency of procedures to assist AVF maturation and/or to place another access if the initial AVF failed. Unlike the study by Manns et al. (25), the median overall cost of access management in patients dialyzing exclusively with CVCs was far higher, at $28,709 annually, largely driven by hospitalizations for treatment of catheter-related bloodstream infections.
Figure 3.
The median annual cost of vascular access management is greater for AVF versus AVG in patients initiating hemodialysis with a CVC and subsequently undergoing access placement. The comparisons are shown for several patient subsets, divided by sex, age, diabetes mellitus (DM) status, and congestive heart failure (CHF) status. * P<0.05; ** P<0.01. Reprinted from reference 26, with permission.
Forearm AVFs Are Better Than Upper Arm AVFs
The 2006 KDOQI vascular access guidelines recommend that “the surgeon should focus on sites distally on the extremity, reserving proximal sites for potential future access insertions should the initial access site fail” (2). This recommendation fails to consider the inferior maturation of forearm AVFs relative to upper arm AVFs, particularly among women and older patients, observed when forearm AVFs were commonly placed (28). This disparity persisted even after adoption of routine preoperative vascular mapping to ensure selection of appropriately sized vessels (29). Of note, the age and sex disparities in AVF maturation was prominent in patients receiving a forearm AVF, but markedly attenuated in those with an upper arm AVF (28,29). In the multicenter Dialysis Access Consortium study, AVF thrombosis within 6 weeks of its creation in the control arm was observed in 25% of forearm AVFs versus 13% of upper arm AVFs, and AVF nonmaturation within 6 months in 64 versus 53%, respectively (7). Recognition of the higher maturation of upper arm AVFs has led to a progressive shift from forearm to upper arm AVFs in the United States. Remarkably, over the past 20 years, the proportion of new AVFs placed in the upper arm has increased from 30% to 68% (21). It appears that nephrologists and surgeons in the United States have recognized the inferior outcomes of forearm AVFs in certain patient subsets and modified their practice patterns accordingly, in an attempt to minimize AVF nonmaturation. These efforts have been successful, such that AVF nonmaturation has decreased from approximately 60% observed in the Dialysis Access Consortium trial (2003–2007) (7) down to about 30%–35% obtained more recently in the Hemodialysis Fistula Maturation Study (2010–2013) (8) and the Dialysis Outcomes and Practice Patterns Study 4–5 (2009–2015) (21). Despite the marked decrease in the proportion of AVFs created in the forearm, indicating a high selection bias, AVF nonmaturation remains higher for forearm than upper arm AVFs (44% versus 33%) (21).
AVFs Fail to Mature Because of Aggressive Neointimal Hyperplasia
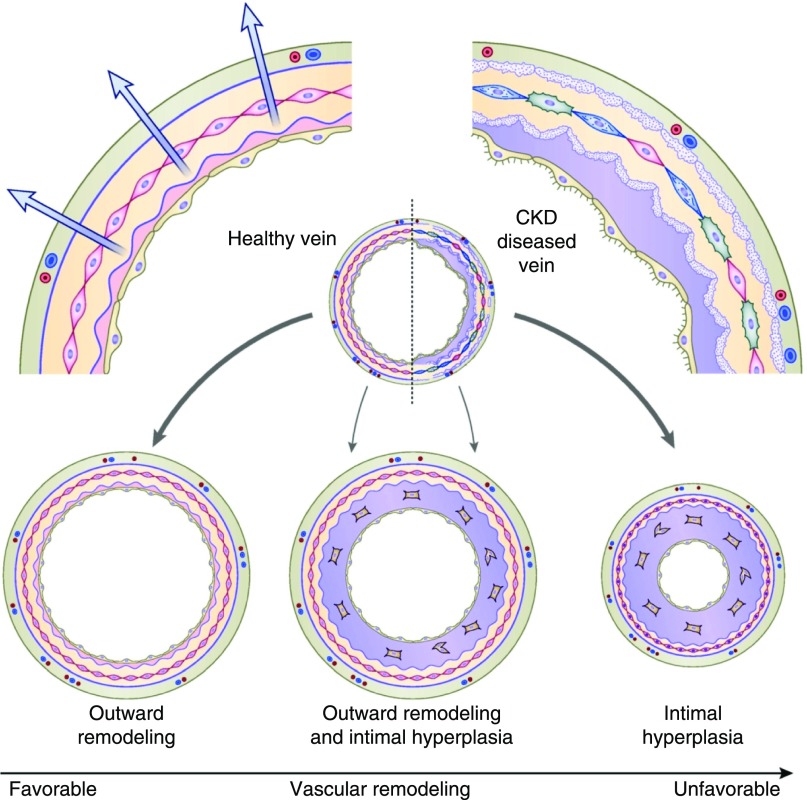
An understanding of the pathophysiology of AVF nonmaturation is critical to improving AVF maturation. Nonmaturing AVFs are frequently found to have an underlying juxta-anastomotic stenosis during imaging studies, such as a postoperative ultrasound or angiogram (30,31). In experimental models, AVFs routinely develop flow-limiting juxta-anastomotic stenosis, and the histology reveals severe neointimal hyperplasia (32,33). This observation has led to the hypothesis that aggressive neointimal hyperplasia results in focal stenosis, which in turn impairs AVF maturation. In support of this hypothesis, severe focal intimal hyperplasia was reported in four patients who underwent surgical revision of a nonmaturing AVF (34). A subsequent study described six patients in whom the native vein had minimal intimal hyperplasia, but the draining vein of the nonmaturing AVF (obtained at the time of surgical revision) exhibited severe neointimal hyperplasia (35). Taken together, these two small reports suggested a central role of neointimal hyperplasia in the pathogenesis of AVF stenosis and nonmaturation. Subsequently, a much larger study obtained draining vein samples during the second-stage transposition from 79 patients with planned two-stage AVFs (36). After excluding nonmaturing AVFs, it found no correlation between the magnitude of neointimal hyperplasia and early AVF failure, suggesting that neointimal hyperplasia was necessary, but not sufficient, for AVF nonmaturation. It is unknown, however, whether the timing of neointimal hyperplasia affects AVF maturation, i.e., whether early inward remodeling is more deleterious than later remodeling. Analysis of a large prospective cohort of patients receiving a new AVF in the Hemodialysis Fistula Maturation Study found that preexisting arterial reactivity, assessed by brachial artery nitroglycerin-mediated dilation or flow-mediated dilation, was positively correlated with the 6-week AVF diameter and blood flow (37). This observation suggested that the ability of the artery to dilate after AVF creation was an important determinant of AVF maturation. Collectively, these observations suggests that AVF maturation depends on the relative balance between neointimal hyperplasia (inward remodeling) and sustained vasodilation (outward remodeling) (38). AVF nonmaturation would occur primarily in the subset of patients with both aggressive neointimal hyperplasia and impaired vasodilation (Figure 4).
Figure 4.
AVF maturation reflects the balance between inward remodeling (intimal hyperplasia) and outward remodeling (vasodilation). Intimal hyperplasia without concurrent outward remodeling results in AVF nonmaturation. In contrast, concurrent inward and outward remodeling results in a mature AVF. Modified from reference 38.
CVCs Are a Major Cause of Death in Patients on Hemodialysis
Numerous observational studies have reported worse survival in patients dialyzing with a CVC compared with those with an AVF or AVG (39–42). Moreover, patients switching from a CVC to an AVF/AVG have better survival than those who continue to dialyze with a CVC (39,40). Unfortunately, all of these studies had a huge selection bias that could not be overcome even with sophisticated statistical adjustment for comorbidities or propensity score adjustment. Patients dialyzing with a CVC are inherently sicker than those dialyzing with an AVF or AVG. Similarly, patients who continue to dialyze with a CVC are inherently sicker than those who convert to an AVF (43,44). The challenge in comparing patient survival between patients who do or do not undergo AVF creation, is that there are important differences not easily captured in administrative databases. If a patient with advanced CKD is perceived by the nephrologist or surgeon to have a reasonable life span, it is likely that an AVF will be placed promptly. In contrast, if the patient has a poor functional status or limited life expectancy, the physician is more likely to postpone AVF creation until after the patient starts hemodialysis. Older patients are less likely to receive an AVF before initiation of dialysis. Older patients also have a higher likelihood of dying after initiating dialysis. Thus, the association between starting hemodialysis with a CVC and dying is confounded by their age. Similarly, a high comorbidity or poor functional status confound the association between CVC use and patient mortality.
Brown et al. (45) addressed this vexing statistical dilemma by designing an innovative approach. They used the USRDS database to compare the survival of three groups of elderly patients on hemodialysis: those who started hemodialysis with an AVF, those who started hemodialysis with a CVC without pre-ESKD AVF surgery, and those who started hemodialysis with a CVC after undergoing pre-ESKD AVF surgery (even if the AVF failed to mature). As expected, patients starting hemodialysis with a CVC without pre-ESKD AVF surgery had worse survival than those starting hemodialysis with an AVF. However, the group initiating hemodialysis with a CVC after an unsuccessful attempt at AVF creation had a patient survival that was more similar to those who initiated dialysis with an AVF (Figure 5). In other words, simply being selected for pre-ESKD AVF surgery was a surrogate marker for a healthier patient with a superior life expectancy.
Figure 5.
Patient survival after initiation of hemodialysis with a CVC is higher in those with versus without attempted pre-ESKD AVF creation. Group 1, patients who initiated dialysis with an AVF; group 2, patients who initiated dialysis with a CVC after undergoing pre-ESKD AVF surgery (even if the AVF failed to mature); group 3, patients who initiated dialysis with a CVC without pre-ESKD AVF creation. Patient survival in group 2 was much more similar to that of group 1 than group 3. Reprinted from reference 45, with permission.
Quinn et al. (46) evaluated cause-specific mortality in two cohorts of Canadian patients on hemodialysis dialyzing with a CVC, those with and without pre-ESKD AVF creation. In agreement with the study by Brown et al., they observed a greater mortality in the group which underwent attempted AVF placement before dialysis initiation. However, only 2.3% of deaths were adjudicated to be CVC-related. A subsequent mediational analysis by the same investigators found that patients dialyzing with a CVC were more likely to develop an access complication and to die, compared with those dialyzing with an AVF or AVG (47). However, the excess deaths in the CVC cohort could not be attributed to the excess in CVC complications.
In conclusion, since publication of the 2016 KDOQI vascular access guidelines, substantial new data has been published. This new information should be incorporated to reinform current decision making about vascular access.
Disclosures
Dr. Allon reports personal fees from CorMedix.
Acknowledgments
Dr. Allon is supported by grant 1R21DK104248-01A1 from the National Institute of Diabetes, Digestive and Kidney Diseases.
Parts of this manuscript were presented at the American Society of Nephrology Kidney Week meeting in San Diego, CA on October 24–28, 2018.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Maya ID, Allon M: Core curriculum in nephrology: Vascular access. Am J Kidney Dis 51: 702–708, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Vascular Access 2006 Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis 48: S176–S322, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kinnaert P, Vereerstraeten P, Toussaint C, Van Geertruyden J: Nine years’ experience with internal arteriovenous fistulas for haemodialysis: A study of some factors influencing the results. Br J Surg 64: 242–246, 1977 [DOI] [PubMed] [Google Scholar]
- 4.Bonalumi U, Civalleri D, Rovida S, Adami GF, Gianetta E, Griffanti-Bartoli F: Nine years’ experience with end-to-end arteriovenous fistula at the ‘anatomical snuffbox’ for maintenance haemodialysis. Br J Surg 69: 486–488, 1982 [DOI] [PubMed] [Google Scholar]
- 5.Reilly DT, Wood RFM, Bell PRF: Prospective study of dialysis fistulas: Problem patients and their treatment. Br J Surg 69: 549–553, 1982 [DOI] [PubMed] [Google Scholar]
- 6.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI; Dialysis Access Consortium Study Group: Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allon M, Imrey PB, Cheung AK, Radeva M, Alpers CE, Beck GJ, Dember LM, Farber A, Greene T, Himmelfarb J, Huber TS, Kaufman JS, Kusek JW, Roy-Chaudhury P, Robbin ML, Vazquez MA, Feldman HI; Hemodialysis Fistula Maturation (HFM) Study Group: Relationships between clinical processes and arteriovenous fistula cannulation and maturation: A multicenter prospective cohort study. Am J Kidney Dis 71: 677–689, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maya ID, O’Neal JC, Young CJ, Barker-Finkel J, Allon M: Outcomes of brachiocephalic fistulas, transposed brachiobasilic fistulas, and upper arm grafts. Clin J Am Soc Nephrol 4: 86–92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L: Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 8: 810–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee T, Barker J, Allon M: Comparison of survival of upper arm arteriovenous fistulas and grafts after failed forearm fistula. J Am Soc Nephrol 18: 1936–1941, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Oliver MJ, McCann RL, Indridason OS, Butterly DW, Schwab SJ: Comparison of transposed brachiobasilic fistulas to upper arm grafts and brachiocephalic fistulas. Kidney Int 60: 1532–1539, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Allon M, Lok CE: Dialysis fistula or graft: The role for randomized clinical trials. Clin J Am Soc Nephrol 5: 2348–2354, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Harms JC, Rangarajan S, Young CJ, Barker-Finkel J, Allon M: Outcomes of arteriovenous fistulas and grafts with or without intervention before successful use. J Vasc Surg 64: 155–162, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee T, Qian J, Thamer M, Allon M: Tradeoffs in vascular access selection in elderly patients initiating hemodialysis with a catheter. Am J Kidney Dis 72: 509–518, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, Roy-Chaudhury P: Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol 6: 575–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dember LM: Fistulas first--but can they last? Clin J Am Soc Nephrol 6: 463–464, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Saran R, Dykstra DM, Pisoni RL, Akiba T, Akizawa T, Canaud B, Chen K, Piera L, Saito A, Young EW: Timing of first cannulation and vascular access failure in haemodialysis: An analysis of practice patterns at dialysis facilities in the DOPPS. Nephrol Dial Transplant 19: 2334–2340, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Pisoni RL, Zepel L, Fluck R, Lok CE, Kawanishi H, Süleymanlar G, Wasse H, Tentori F, Zee J, Li Y, Schaubel D, Burke S, Robinson B: International differences in the location and use of arteriovenous accesses created for hemodialysis: Results from the Dialysis Outcomes and Practice patterns study (DOPPS). Am J Kidney Dis 71: 469–478, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Lee T, Thamer M, Zhang Q, Zhang Y, Allon M: Vascular access type and clinical outcomes among elderly patients on hemodialysis. Clin J Am Soc Nephrol 12: 1823–1830, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adwaney A, Lim C, Blakey S, Duncan N, Ashby DR: Central venous stenosis, access outcome and survival in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 14: 378–384, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shingarev R, Barker-Finkel J, Allon M: Association of hemodialysis central venous catheter use with ipsilateral arteriovenous vascular access survival. Am J Kidney Dis 60: 983–989, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, Radkevich V, Murphy B: Establishment and maintenance of vascular access in incident hemodialysis patients: A prospective cost analysis. J Am Soc Nephrol 16: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Al-Balas A, Lee T, Young CJ, Kepes JA, Barker-Finkel J, Allon M: The clinical and economic effect of vascular access selection in patients initiating hemodialysis with a catheter. J Am Soc Nephrol 28: 3679–3687, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thamer M, Lee TC, Wasse H, Glickman MH, Qian J, Gottlieb D, Toner S, Pflederer TA: Medicare costs associated with arteriovenous fistulas among US hemodialysis patients. Am J Kidney Dis 72: 10–18, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, Allon M: Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 56: 275–280, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Peterson WJ, Barker J, Allon M: Disparities in fistula maturation persist despite preoperative vascular mapping. Clin J Am Soc Nephrol 3: 437–441, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allon M, Robbin ML, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Litovsky S: Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol 8: 1750–1755, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung AK, Imrey PB, Alpers CE, Robbin ML, Radeva M, Larive B, Shiu YT, Allon M, Dember LM, Greene T, Himmelfarb J, Roy-Chaudhury P, Terry CM, Vazquez MA, Kusek JW, Feldman HI; Hemodialysis Fistula Maturation Study Group: Intimal hyperplasia, stenosis, and arteriovenous fistula maturation failure in the Hemodialysis Fistula Maturation Study. J Am Soc Nephrol 28: 3005–3013, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, Nath KA: Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int 74: 47–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang L, Grande JP, Hillestad ML, Croatt AJ, Barry MA, Katusic ZS, Nath KA: A new model of an arteriovenous fistula in chronic kidney disease in the mouse: Beneficial effects of upregulated heme oxygenase-1. Am J Physiol Renal Physiol 310: F466–F476, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, Samaha A, Munda R: Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis 50: 782–790, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Allon M, Litovsky S, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Robbin ML: Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis 58: 437–443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabbara M, Duque JC, Martinez L, Escobar LA, Wu W, Pan Y, Fernandez N, Velazquez OC, Jaimes EA, Salman LH, Vazquez-Padron RI: Pre-existing and postoperative intimal hyperplasia and arteriovenous fistula outcomes. Am J Kidney Dis 68: 455–464, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allon M, Greene T, Dember LM, Vita JA, Cheung AK, Hamburg NM, Imrey PB, Kaufman JS, Robbin ML, Shiu YT, Terry CM, Umphrey HR, Feldman HI; Hemodialysis Fistula Maturation Study Group: Association between preoperative vascular function and postoperative arteriovenous fistula development. J Am Soc Nephrol 27: 3788–3795, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothuizen TC, Wong C, Quax PH, van Zonneveld AJ, Rabelink TJ, Rotmans JI: Arteriovenous access failure: More than just intimal hyperplasia? Nephrol Dial Transplant 28: 1085–1092, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ: Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis 47: 469–477, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Lacson E Jr., Wang W, Lazarus JM, Hakim RM: Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis 54: 912–921, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG: Vascular access and all-cause mortality: A propensity score analysis. J Am Soc Nephrol 15: 477–486, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Quinn RR, Ravani P: Fistula-first and catheter-last: Fading certainties and growing doubts. Nephrol Dial Transplant 29: 727–730, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Murea M, Satko S: Looking beyond “fistula First” in the elderly on hemodialysis. Semin Dial 29: 396–402, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Brown RS, Patibandla BK, Goldfarb-Rumyantzev AS: The survival benefit of “Fistula First, Catheter Last” in hemodialysis is primarily due to patient factors. J Am Soc Nephrol 28: 645–652, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quinn RR, Oliver MJ, Devoe D, Poinen K, Kabani R, Kamar F, Mysore P, Lewin AM, Hiremath S, MacRae J, James MT, Miller L, Hemmelgarn BR, Moist LM, Garg AX, Chowdhury TT, Ravani P: The effect of predialysis fistula attempt on risk of all-cause and access-related death. J Am Soc Nephrol 28: 613–620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravani P, Quinn R, Oliver M, Robinson B, Pisoni R, Pannu N, MacRae J, Manns B, Hemmelgarn B, James M, Tonelli M, Gillespie B: Examining the association between hemodialysis access type and mortality: The role of access complications. Clin J Am Soc Nephrol 12: 955–964, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falk A: Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol 17: 807–813, 2006 [DOI] [PubMed] [Google Scholar]