Visual Abstract
Keywords: ADPKD; cystic kidney; kidney volume; Polycystic Kidney, Autosomal Dominant; Follow-Up Studies; Cysts; kidney; Genotype; Mutation; Demography; Ambulatory Care
Abstract
Background and objectives
To evaluate the growth pattern of kidney cyst number and cyst volume in association with kidney size, demographics, and genotypes in autosomal dominant polycystic kidney disease.
Design, setting, participants, & measurements
Kidney cyst number and cyst volume were measured from serial magnetic resonance images, giving a maximum follow-up of 14.23 years, from 241 patients with autosomal dominant polycystic kidney disease (15–46 years old at baseline). The growth pattern was analyzed, in association with sex, age, height-adjusted total kidney volume, and genotype, using linear mixed models of repeated measurements and tests of interactions with age (as a time-dependent covariate) to assess rates of change over time. Models were also fit using Irazabal class. Genotypic groups were characterized as either (1) PKD1 truncating, PKD1 nontruncating, and PKD2 plus patients with no mutation detected; or (2) in combination with PKD1 mutation strength groups.
Results
Imaging and genetic data were collected (at least one visit) for 236 participants. The mean height-adjusted total cyst number increased exponentially over time from a baseline value of 762 to 1715 at the last clinic visit, while the mean height-adjusted total cyst volume increased exponentially from 305 to 770 ml. Height-adjusted total kidney volume, height-adjusted total cyst number, and height-adjusted total cyst volume were all highly correlated over time. Female participants and participants with larger height-adjusted total kidney volume at baseline showed smaller rates of change in the log of height-adjusted total cyst number and cyst volume. PKD1 was associated with significant increases in both cyst number and volume at a given age, but genotype did not significantly affect the rate of growth.
Conclusions
Both height-adjusted total cyst number and height-adjusted total cyst volume increased exponentially and more than doubled over 14.23 years of follow-up. Compared with PKD2 plus no mutation detected, PKD1 was associated with a greater cyst number and volume at a given age, but no significant difference in the rate of growth.
Introduction
The hallmarks of autosomal dominant polycystic kidney disease (ADPKD) are the progressive development and expansion of kidney cysts, causing life-altering symptoms and potentially lethal complications (1). The cysts, developing initially from relatively few kidney tubules, continue to grow and expand over time. In many cases, the cysts compress normally functioning kidney parenchyma and progressively impair kidney function.
The progression of ADPKD is complex and multifactorial. ADPKD is caused predominantly by mutations in two genes, PKD1 (16p13.3) and PKD2 (4q21), that encode the proteins polycystin-1 and polycystin-2 (2,3). Most patients (approximately 80%–85%) have PKD1, with PKD2 accounting for the remainder (4–6). Compared with PKD1, PKD2 is consistently a milder disease as evidenced by age at ESKD, diagnosis, and onset of hypertension (6–8). However, genetic modifying factors plus environmental factors significantly influence the course of ADPKD (9,10).
To expand our understanding of ADPKD disease progression, the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) was established. A longitudinal cohort of patients with ADPKD in the early course of their disease at baseline was followed by serial magnetic resonance imaging (MRI), kidney functional parameters, and other markers of disease progression (11). The CRISP study documented that total kidney volume measurements from MRI are more sensitive than kidney function measures in assessing yearly progression of early disease, and that the growth in kidney volume directly stems from increases in kidney cyst volume (12).
In addition to total kidney volume and cyst volumes, the progression of cyst number in each kidney also provides important information about the characteristics and progression of ADPKD. For example, differences in kidney morphology between PKD1 and PKD2 are likely due to the number of kidney cysts rather than faster volumetric growth of cysts in PKD1 kidneys (13). Although the progression of total kidney volume in ADPKD is well established and published extensively, the growth pattern of kidney cyst number and volume has rarely been reported. This paucity of research, is, in part, because the measurement of kidney cyst number and cyst volume is much more laborious than the measurement of total kidney volume, in general involving time-consuming and labor-intensive counting of cysts and measurement of cyst areas on MRI slices.
We postulate that the growth pattern of kidney cyst number and volume in ADPKD provides additional information to characterize the progression of disease. Here, we have generated unique longitudinal data, including repeated measurements of kidney cyst number and volume data from the CRISP study, to explore the nature and magnitude of the relationship between total kidney volume, demographics, and PKD genotype with progression in kidney cyst number and volume.
Materials and Methods
The study protocol for the CRISP and the baseline characteristics of the cohort have been described in previous publications (11,12,14). The study was approved by the institutional review board at each participating clinical center, and informed consent was obtained from all study participants.
Participants and MRI
In the CRISP study, which was launched in 1999 to acquire prospective, multiyear, longitudinal clinical and imaging data in a large cohort of participants with ADPKD, 241 patients with ADPKD between 15 and 46 years old with relatively intact kidney function were recruited. As part of the CRISP study protocol, standardized MRI images were obtained at four clinical centers (Mayo Clinic, Emory University, University of Kansas Medical Center, and University of Alabama at Birmingham). From the baseline visit, a total of up to 12 visits were conducted over 14.23 years. At each visit, magnetic resonance images of kidneys were acquired at 3 mm fixed slice thickness in the coronal plane, using both three-dimensional spoiled gradient interpolated T1-weighted without fat saturation and single-shot fast spin-echo T2-weighted with fat saturation sequences (11).
Measurement of the Number of Kidney Cysts
Manual counting of every kidney cyst from the entire magnetic resonance images is tremendously laborious, particularly in kidneys of severe cyst burden with numerous cysts. Therefore, cysts were counted in three selected sample slices of each magnetic resonance image set. From a series of coronal T2-weighted magnetic resonance images of the right or left kidney in each participant, we selected the midslice by reviewing and scrolling the image set. We defined the midslice as the slice whose image number corresponded to a half of the sum of the first slice and last slice image numbers in the image set (if the sum was odd, the midslice corresponded to the neighboring slice, rounding up from the mean). In addition to the midslice (i.e., the second quartile) image, we also selected two additional slices from each kidney image series: the midslice of the anterior half (the first quartile) and the midslice of the posterior half (the third quartile) of the series.
The number of cysts from the three slices in each kidney was counted manually by a radiologist. Only circular and spheroid structures ≥2 mm in diameter whose signal intensities were close to that of spinal fluid were considered cysts (Figure 1). Each identified and counted cyst was flagged by an electronic marker using an in-house cyst-labeling program. The number of cysts flagged with red marks was automatically calculated. The number of cysts counted from the three slices were divided by three to represent the number of cysts at one-slice equivalent from each magnetic resonance image dataset. The total cyst number in each kidney from the magnetic resonance image dataset was approximated using the following formula: total cyst number=average slice cyst number×total slice number×2/3. The coefficient 2/3 is on the basis of the approximation of the shape of kidney as an ellipsoid and was shown to be reasonable for approximating total kidney and cyst volume from midslice measurement in a previous study (15).
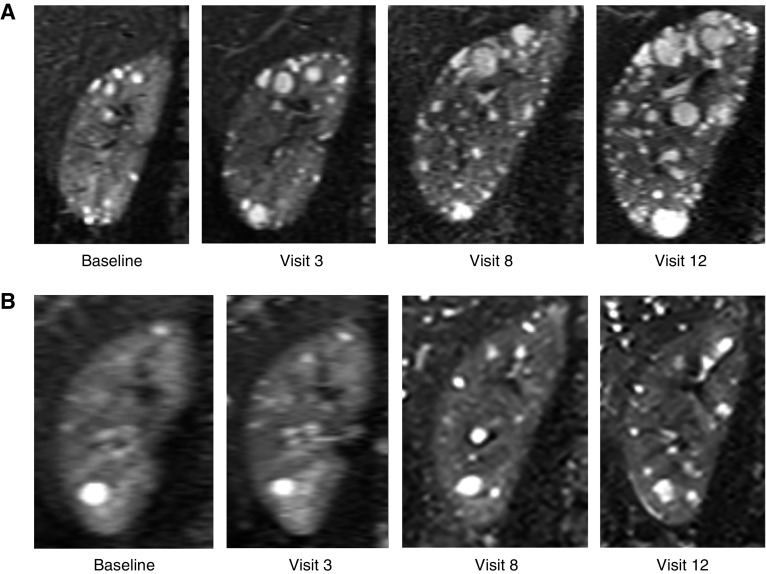
Figure 1.
Serial MR images of the right kidney from patients with PKD1 and PKD2 over 12 follow-up visits illustrate differences and changes in cyst volume and number. (A) Kidney cysts in PKD1 are more numerous and diffusely distributed than (B) those in PKD2. (A) The PKD1 participant was a male aged 15 years at baseline, with a truncating mutation and MSG1, a baseline total kidney volume of 499 ml, total cyst volume of 35 ml, and total cyst number of 456. (B) The PKD2 participant was a male aged 23 years at baseline, with a total kidney volume of 369 ml, total cyst volume of 17 mL, and total cyst number of 68.
Cyst Area Measurements Using Region-Based Method
The total volume of cysts in each kidney was measured using a region-based thresholding method on T2-weighted magnetic resonance images (11,14). Cysts that were brighter than the kidney parenchyma could be measured by summing pixels with intensity values greater than those of the background kidney parenchyma. On each kidney magnetic resonance image slice, a binary signal-intensity map was generated by determining a threshold signal intensity that visually distinguished the cyst and kidney parenchymal regions. By summing the pixels of white regions in the binary map, the cystic area was measured in every slice. The total cyst volume was calculated from each set of contiguous images by summing the products of the areas measured and the slice thickness.
Statistical Analyses
Descriptive Statistics.
Continuous variables, including height-adjusted total cyst number and height-adjusted total cyst volume, height-adjusted total kidney volume, and age were described using the mean, SD, and range (minimum, maximum). Frequencies and percentages were calculated for categories of sex and genotype. Genotype was grouped as PKD1 truncating, PKD1 nontruncating, and PKD2 plus patients with no mutation detected. Furthermore (as a secondary analysis), to evaluate the associations with PKD gene mutation strength groups (MSGs), the genotype and MSGs were grouped as (1) PKD1, MSG1+MSG2; (2) PKD1, MSG3; or (3) PKD2 plus patients with no mutation detected, as reported in a recent study (16). To investigate the correlation among the height-adjusted total kidney volume, height-adjusted total cyst volume, and height-adjusted total cyst number, Pearson correlation coefficients were calculated by visit. Histograms and q-q plots were used to show the distribution of these variables. Visits are labeled as 0 through 12. During the first 5 years after baseline (i.e., visit 0), the numbers for each visit correspond to the number of years after baseline. After the 5 years, those visits continued over a year or two (with the last clinic visit, visit 12, being measured as late as 14.23 years after baseline).
Repeated Measures Analysis of Cyst Number or Volume.
This analysis uses a linear mixed model (with a random intercept and random slope to account for within-subject correlation and individual differences) to assess relationships between genotype and natural log-transformed cyst numbers (or volumes) measured over time. This model adjusts for sex and baseline log height-adjusted total kidney volume (or with Irazabal class [17], which categorizes the estimated rate of increase in height-adjusted total kidney volume from age and a single height-adjusted total kidney volume measurement) and a time-dependent variable for patient age (at the time of the imaging measurements). The coefficients and P values for genotype (or sex or baseline height-adjusted total kidney volume) in this model thus assess the effect of genotype (other sex or baseline height-adjusted total kidney volume, holding the other factors constant) on the expected cyst number or volume at a given age. The interpretation of age, however (as a time-dependent covariate), is the effect of a 1-year increase in age on the cyst number or volume within a given participant.
Modeling the Rate of Change in Cyst Number or Volume.
In contrast to the first analysis, which assesses relationships between genotype and cyst numbers (or volumes) at a given age, this subsequent “slope analysis” assesses whether genotype (adjusted for baseline factors) is associated with the rate of change in cyst number or cyst volume. More specifically, the interaction between age (as a time-dependent covariate) and genotype were added to the above-described linear mixed models. The age-genotype interaction characterizes the differences in individual’s rate of change (in a log scale) over time between genotypes. Relationships between those slopes and genotype were then assessed using likelihood ratio test adjusted for sex, and baseline height-adjusted total kidney volume (or Irazabal class). Tests of interactions were also conducted in the same manner for sex by age interaction and baseline height-adjusted kidney volume by age interaction.
All tests were done with a two-sided significance level of 0.05, and Stata version 14 (18) was used for all statistical analyses.
Results
Descriptive Analysis
Out of 241 CRISP participants, 236 who had genotype records were included in the analysis. The baseline characteristics of the study cohort of 236 participants is shown in Table 1. At baseline, there were 95 males and 141 females (mean age, 32 years; SD 9 years; range 15–46 years). The median baseline height-adjusted total kidney volume was 503 ml (interquartile range, 350–757 ml/m). The primary categorization for genotype was PKD1 truncating (n=126), PKD1 nontruncating (n=62), and PKD2 or no mutation detected (n=31 or n=17, respectively). For the secondary analysis on the basis of MSGs (16), genotype was categorized as PKD1, MSG1 or MSG2 (n=165); PKD1, MSG3 (n=23); and PKD2 or no mutation detected (n=48). Overall, both cyst number and volume more than doubled over 14.23 years of follow-up. The trajectories of individual cyst number and cyst volume (both height-adjusted in the log scale) plotted against the participants’ age at visit was approximately linear on the log scale (Figure 2), indicating that cyst number and volume increase exponentially over time.
Table 1.
Cohort characteristics at baseline
| Characteristics | CRISP Cohort, n=236 | PKD1 Participants, n=188 | PKD2 or NMD Participants, n=48 |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 95 (40) | 73 (39) | 22 (46) |
| Female | 141 (60) | 115 (61) | 26 (54) |
| Irazabal class, n (%) | |||
| Class 1A | 14 (6) | 6 (3) | 8 (17) |
| Class 1B | 59 (25) | 35 (19) | 24 (50) |
| Class 1C | 71 (30) | 58 (31) | 13 (27) |
| Class 1D | 55 (23) | 53 (28) | 2 (4) |
| Class 1E | 37 (16) | 36 (19) | 1 (2) |
| Age, yra | 32 (9) | 32 (9) | 35 (9) |
| Baseline htTKV, ml/ma | 503 (350–757) | 580 (384–890) | 386 (269–449) |
| Genotype, n (%) | |||
| PKD1 truncating | 126 (53) | ||
| PKD1 nontruncating | 62 (26) | ||
| PKD2 or NMD | 48 (20) | ||
| Mutation strength groups, n (%) | |||
| PKD1, MSG1 or MSG2 | 165 (70) | ||
| PKD1, MSG3 | 23 (10) | ||
| PKD2 or NMD | 48 (20) |
CRISP, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease; PKD, polycystic kidney disease; NMD, no mutation detected; htTKV, height-adjusted total kidney volume; MSG, mutation strength group.
Age is summarized as mean (SD); htTKV is summarized at median (interquartile range).
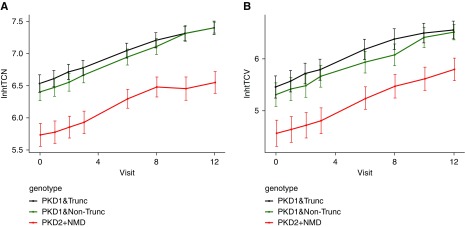
Figure 2.
Trajectory of height-adjusted total cyst number and cyst volume (both in the log scale) by genotype group shows genotype-dependent changes over time. Trajectories of (A) log height-adjusted total cyst number (htTCN) and (B) height-adjusted total cyst volume (htTCV) for PKD1 truncated (trunc), PKD1 nontruncated (nontrunc), and PKD2 plus no mutation detected (NMD) genotype group, indicate that height-adjusted cyst number and cyst volume increase exponentially over time in each genotype group.
The cohort had an average visit number of 6.1 times during 14.23 years of follow up. The characteristics of the cohort compared between the baseline and visits is summarized in Supplemental Table 1. The mean height-adjusted total cyst number increased over time from a baseline value of 762 (SD 525) to 1715 (SD 965) at the last clinic visit (Supplemental Table 2A). The mean height-adjusted total cyst volume also more than doubled across the study period (Supplemental Table 3A) from a baseline value of 305 ml (SD 298) to 770 ml (SD 646) at the last clinic visit. Reasons for missing visits included (1) reaching the end point of either death (n=3; 1.2%) or ESKD (n=47; 19.5% on dialysis, receiving a transplant, and/or reaching stage 5 CKD), (2) withdrawing from the study (n=9; 3.7%), and (3) inability to reach participants (who were originally consented before 2000) or inability of participants to come in for visits (e.g., because of work or family demands). Furthermore, to address the potential concern of participation bias (from healthier participants remaining in the study longer), the height-adjusted total cyst number and volume over time analysis was repeated in the subset of participants with at least one visit in each 5-year period (i.e., 0–5, 6–10, and over 10 years after baseline; see Supplemental Tables 2B and 3B); that analysis showed very similar results.
Correlation Analysis: Height-Adjusted Total Kidney Volume, Total Cyst Volume, and Total Cyst Number
In ADPKD, total kidney volume increases over time because of increasing total cyst volume, which is likely, in part, because of an increase in cyst number. Height-adjusted total kidney volume, total cyst volume, and total cyst number data that were right-skewed distributed, were normally distributed after log transformation (Supplemental Figure 1). Table 2 confirms that there were strong correlations between height-adjusted total kidney volume and height-adjusted total cyst volume (correlation coefficients range 0.93–0.97). The correlation coefficients ranged from 0.78 to 0.86 between height-adjusted total kidney volume and total cyst number, and from 0.83 to 0.90 between height-adjusted total cyst volume and total cyst number.
Table 2.
Pearson correlations at each visit time point
| Visit | lnhtTKV versus lnhtTCV | lnhtTKV versus lnhtTCN | lnhtTCN versus lnhtTCV |
|---|---|---|---|
| 0 | 0.93 | 0.80 | 0.85 |
| 1 | 0.94 | 0.83 | 0.87 |
| 2 | 0.93 | 0.81 | 0.88 |
| 3 | 0.94 | 0.83 | 0.88 |
| 6 | 0.95 | 0.78 | 0.83 |
| 8 | 0.97 | 0.86 | 0.90 |
| 10 | 0.95 | 0.80 | 0.87 |
| 12 | 0.97 | 0.80 | 0.84 |
All correlations were significant at P<0.001. lnhtTKV, log transformed height-adjusted total kidney volume; lnhtTCV, log transformed height-adjusted total cyst volume; lnhtTCN, log transformed height-adjusted total cyst number.
Repeated Measures Analysis: Height-Adjusted Total Cyst Number and Volume (Linear Mixed Model)
The height-adjusted total cyst number and height-adjusted total cyst volume are predicted to increase annually by 7.3% and 10.3%, respectively (Tables 3 and 4). Both PKD1 nontruncating and truncating had significantly higher height-adjusted total cyst number (1.77-fold and 2.31-fold, respectively; Table 3) relative to PKD2 or no mutation detected, as did participants with larger baseline height-adjusted total kidney volume (P<0.001 for each test), but sex was not significantly related to height-adjusted total cyst number (P=0.88). Median height-adjusted total cyst number and total cyst number breakdowns by the age group at baseline and genotype are summarized in Supplemental Table 4, A and B. Similarly, height-adjusted total cyst volume (Table 4) was also greater for PKD1 truncating (by 2.0-fold; 95% confidence interval, 1.5 to 2.7; on the basis of exponentiating the coefficient and confidence intervals in the mixed model; P<0.001) and nontruncating (1.5-fold; 95% confidence interval, 1.1 to 2.1; P=0.02) versus PKD2 or no mutation detected, and for larger baseline height-adjusted total kidney volume (P<0.001). Sex was not significantly related to height-adjusted total cyst volume (P=0.13). Trajectories of the mean log height-adjusted total cyst number and volume at each genotype group indicated that the height-adjusted total cyst number and volume increases exponentially over time (Figure 3). The linear mixed models were then used to predict height-adjusted total cyst number and volume (both in the log scale) over study visits, stratified by genotype, and adjusted for sex, age at visit, and baseline height-adjusted total kidney volume (Figure 4). The predicted trajectories for cyst number and volume follow the same pattern as the mean observed trajectories. The results from the secondary analysis for the MSG genotype and Irazabal class categorization are shown in Supplemental Tables 5, A and B and 6, A and B, respectively. The associations between the factors were very similar to those from the primary genotype categorization.
Table 3.
Linear mixed model for repeated measures of log height-adjusted total cyst number
| Category | Coef. | SEM | P Value | 95% Confidence Interval |
|---|---|---|---|---|
| Genotype | <0.001 | |||
| Reference: PKD2 or NMD | ||||
| PKD1 nontruncating | 0.57 | 0.15 | <0.001 | 0.29 to 0.86 |
| PKD1 truncating | 0.84 | 0.13 | <0.001 | 0.58 to 1.09 |
| Age at visit | 0.07 | 0.002 | <0.001 | 0.07 to 0.08 |
| Sex | ||||
| Reference: male | ||||
| Female | −0.01 | 0.10 | 0.88 | −0.20 to 0.17 |
| Baseline lnhtTKV | 0.61 | 0.09 | <0.001 | 0.43 to 0.79 |
Coef., coefficient; PKD, polycystic kidney disease; NMD, no mutation detected; lnhtTKV, log transformed height-adjusted total kidney volume (the multivariable model included all listed variables).
Table 4.
Linear mixed model for repeated measures of log height-adjusted total cyst volume
| Category | Coef. | SEM | P Value | 95% Confidence Interval |
|---|---|---|---|---|
| Genotype | <0.001 | |||
| Reference: PKD2 or NMD | ||||
| PKD1 nontruncating | 0.41 | 0.18 | 0.02 | 0.07 to 0.75 |
| PKD1 truncating | 0.70 | 0.16 | <0.001 | 0.39 to 1.00 |
| Age at visit | 0.10 | 0.00 | <0.001 | 0.09 to 0.10 |
| Sex | ||||
| Reference: male | ||||
| Female | 0.18 | 0.12 | 0.13 | −0.05 to 0.41 |
| Baseline lnhtTKV | 1.30 | 0.11 | <0.001 | 1.09 to 1.52 |
Coef., coefficient; PKD, polycystic kidney disease; NMD, no mutation detected; lnhtTKV, log transformed height-adjusted total kidney volume (the multivariable model included all listed variables).
Figure 3.
Trajectory of the mean height-adjusted total cyst number and cyst volume (both in the log scale) by genotype group shows exponential genotype-dependent changes over time. Trajectories of (A) the mean log height-adjusted total cyst number (htTCN) and (B) height-adjusted total cyst volume (htTCV) for PKD1 truncated (trunc), PKD1 nontruncated (non-trunc), and PKD2 plus no mutation detected (NMD) genotype group, indicate that height-adjusted cyst number and cyst volume increase exponentially over time in each genotype group.
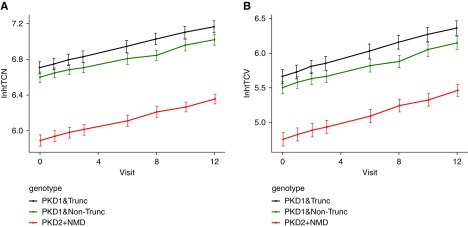
Figure 4.
Height-adjusted total cyst number and cyst volume (both in the log scale) are predicted according to genotype group. Predicted values of (A) log height-adjusted total cyst number (htTCN) and (B) total cyst volume (htTCV) for PKD1 truncated (trunc), PKD1 nontruncated (non trunc), and PKD2 plus no mutation detected (NMD) genotype group. Predictions are based on the linear mixed model adjusting for baseline height-adjusted total kidney volume, sex, and genotype.
Rates of Change Analysis: Height-Adjusted Total Cyst Number and Volume
There was no significant difference in the rate of change of log height-adjusted total cyst number between the different genotypes (P=0.24); both coefficients for the two PKD1 mutation interactions were near 0, suggesting that the difference of slopes between two PKD1 mutation groups and PKD2 plus no mutation detected was negligible (Table 5, model 1). However, female sex (P<0.001) and higher baseline log height-adjusted total kidney volume (P<0.001) were both negatively associated with the rate of change of log height-adjusted total cyst number (Table 5, models 2 and 3). Females were estimated to have 0.02 lower growth rate in log height-adjusted total kidney number compared with males, adjusting for genotype and baseline kidney volume; one-unit increase in baseline log height-adjusted total kidney volume would lead to a 0.01 decrease in the growth rate of log height adjusted total cyst number, controlling for genotype and sex. Analysis of slopes in log of height-adjusted total cyst volume showed a similar relationship (Table 6). Cyst volume growth rate were not significantly different among three genotypes (P=0.31). Both female sex (P<0.001) and higher baseline log height-adjusted total kidney volume (P=0.003) were associated with slower increase (smaller slope) of log height-adjusted total cyst volume. The models for genotype on the basis of MSG (Supplemental Table 7, A and B) and Irazabal class (Supplemental Table 8, A and B) showed very similar results.
Table 5.
Linear mixed model with interactions for slope in log height-adjusted total cyst number
| Category | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef. | 95% CI | P Value | Coef. | 95% CI | P Value | Coef. | 95% CI | P Value | |
| Age at visit | 0.07 | 0.06 to 0.08 | <0.001 | 0.08 | 0.07 to 0.09 | <0.001 | 0.16 | 0.11 to 0.22 | <0.001 |
| Genotypea | 0.002 | <0.001 | <0.001 | ||||||
| PKD1 nontruncating | 0.22 | −0.28 to 0.73 | 0.39 | 0.57 | 0.29 to 0.85 | <0.001 | 0.59 | 0.31 to 0.88 | <0.001 |
| PKD1 truncating | 0.71 | 0.27 to 1.15 | 0.002 | 0.83 | 0.58 to 1.09 | <0.001 | 0.86 | 0.60 to 1.11 | <0.001 |
| Female | −0.01 | −0.20 to 0.17 | 0.89 | 0.51 | 0.18 to 0.84 | 0.003 | −0.03 | −0.21 to 0.16 | 0.79 |
| lnhtTKV0 | 0.61 | 0.43 to 0.78 | <0.001 | 0.61 | 0.43 to 0.78 | <0.001 | 1.06 | 0.74 to 1.37 | <0.001 |
| Genotype×age | 0.24 | ||||||||
| PKD1 nontruncating | 0.01 | −0.00 to 0.02 | 0.11 | ||||||
| PKD1 truncating | 0.004 | −0.01 to 0.02 | 0.49 | ||||||
| Female×age | −0.02 | −0.03 to −0.01 | <0.001 | ||||||
| lnhtTKV0×age | −0.01 | −0.02 to −0.01 | 0.001 | ||||||
The multivariable model included all listed variables, with one interaction tested in each model. Coef. Coefficient; 95% CI, 95% confidence interval; PKD, polycystic kidney disease; lnhtTKV0, baseline log transformed height-adjusted total kidney volume.
Reference group of genotype is PKD2 and no mutation detected.
Table 6.
Linear mixed model with interactions for slope in log height-adjusted total cyst volume
| Category | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef. | 95% CI | P Value | Coef. | 95% CI | P Value | Coef. | 95% CI | P Value | |
| Age at visit | 0.09 | 0.07 to 0.10 | <0.001 | 0.12 | 0.11 to 0.13 | <0.001 | 0.21 | 0.13 to 0.28 | <0.001 |
| Genotypea | 0.19 | <0.001 | <0.001 | ||||||
| PKD1 nontruncating | 0.04 | −0.58 to 0.65 | 0.90 | 0.41 | 0.06 to 0.75 | 0.02 | 0.42 | 0.08 to 0.76 | 0.02 |
| PKD1 truncating | 0.40 | −0.13 to 0.94 | 0.14 | 0.69 | 0.39 to 1.00 | <0.001 | 0.71 | 0.40 to 1.01 | <0.001 |
| Female | 0.18 | −0.05 to 0.41 | 0.13 | 1.02 | 0.62 to 1.42 | <0.001 | 0.17 | −0.06 to 0.40 | 0.14 |
| lnhtTKV0 | 1.30 | 1.0 to 1.52 | <0.001 | 1.30 | 1.08 to 1.51 | <0.001 | 1.78 | 1.40 to 2.15 | <0.001 |
| Genotype×age | 0.31 | ||||||||
| PKD1 nontruncating | 0.01 | −0.00 to 0.03 | 0.15 | ||||||
| PKD1 truncating | 0.01 | −0.01 to 0.03 | 0.19 | ||||||
| Female×age | −0.03 | −0.04 to −0.02 | <0.001 | ||||||
| lnhtTKV0×age | −0.02 | −0.03 to −0.01 | 0.003 | ||||||
The multivariable models included all listed variables, with one interaction tested in each model. Coef. Coefficient; 95% CI, 95% confidence interval; PKD, polycystic kidney disease; lnhtTKV0, baseline log transformed height-adjusted total kidney volume.
Reference group of genotype is PKD2 and no mutation detected.
Discussion
Compared with our previous publication (13), the scope of this study was expanded including more rigorous estimate of cyst number (three slices versus a single slice), longer follow-up (14.23 versus 3 years), and more detailed analysis of PKD1 genotype (truncating versus nontruncating and MSG). In addition, we investigated the growth pattern of ADPKD kidney cyst number and volume and associations with sex, genotype, baseline height-adjusted total kidney volume, and age using linear regression analysis of slopes (to capture the rate of change) and linear mixed models of repeated measures over time. Overall, both height-adjusted total cyst number and cyst volume more than doubled over 14.23 years of follow-up, with height-adjusted total cyst volume, cyst number, and kidney volume being highly correlated. The increase in height-adjusted total cyst number and volume over time appeared exponential.
The inclusion criteria for CRISP (which enrolled participants from age 15–46 at baseline) required well preserved kidney function at the time of study recruitment in 1999 (to then study the natural history of disease). This inclusion criteria would then almost definitely exclude any older participants who were experiencing steep trajectories of cyst growth (and would thus also have poor kidney function and be ineligible for CRISP). In contrast, younger patients with the same trajectory of cyst growth and/or development would be less likely to have already reached a sufficiently low level of kidney function, thus introducing a selection bias in the association with age. This bias may explain the seemingly inconsistent result that the two oldest PKD1 truncating subgroups had smaller median height-adjusted total cyst number than the next younger subgroup, when the median height-adjusted total cyst number was broken down according to the baseline age group and genotype. The age-dependent growth pattern of rate of cyst development and height-adjusted total cyst volume increase could however also suggest that kidney cysts form early in the disease and that fewer new cysts develop in older individuals (13,19). Female sex was also associated with slower rates of cyst volume growth, consistent with the increasing evidence that ADPKD is a more severe disease in males (7,16,20–23).
The relationship between height-adjusted total cyst number and height-adjusted total cyst volume and genotype was complex. Cyst number and volume were significantly higher in PKD1 (both truncating and nontruncating) compared with PKD2 or no mutation detected at any given age, but there were no significant differences in the rate of change of cyst number or volume. The same trend was reported in a previous cross-sectional study evaluating differences in baseline cyst number and volume growth rate between PKD1 and PKD2 (13). If CRISP data could be extrapolated over the entire lifetime, this would imply that patients with PKD1 are born with more cysts than those with PKD2, but then grew at the same rate, i.e., they have different intercepts but similar slopes. However, a more likely explanation is that there is a phase of rapid cyst development in PKD1 kidneys that occurs early in life, before the observed age range of our data.
The interpretation of model coefficients is also complicated by use of ln-transformations (which is needed to satisfy assumptions of the linear and linear mixed models). To provide some explanation of the coefficients, note that the difference between the first quartile and median ln-transformed cyst number varied between 0.39 and 0.56 (across all time points); similarly, the difference between the median and third quartile varied between 0.32 and 0.48 (using the summary statistics from Supplemental Table 2A). Therefore, the coefficients for genetic mutations PKD1 nontruncating or PKD1 truncating (0.57 and 0.84, respectively; see Table 3), are highly clinically meaningful. Similar conclusions can be made regarding the coefficients in Table 4 (using the summary statistics from Supplemental Table 3A).
Caution is warranted when interpreting the linear mixed models for subject-specific slopes in the log scale (and the associated lack of significance for genotype). If for instance, we consider two trajectories, that are linear in a log scale, with a different intercept but the same slope in the log scale, they will appear as parallel lines in the log scale. However, when transforming the data back to the original scale, the trajectories will be closer together at the initial x-value or time point (e.g., age) and then separate further over time. The transformation to the log scale is required to meet assumptions of the linear mixed models, but also need to be considered in the subsequent interpretation.
The study has limitations. First, microscopic cysts in the kidney were not counted. For the measurement of cyst number, we only considered circular and spheroid structures ≥2 mm in diameter whose signal intensities were close to that of spinal fluid. As shown in a previous study (24), a vast number of microscopic cysts present in ADPKD kidneys remain undetected by clinically available imaging modalities, and the collective volume of these microscopic cysts is quantitatively insignificant compared with the overall cyst volume. Second, the total number of cysts in a kidney was computed on the basis of three sample slices in each magnetic resonance image dataset. Although it would be ideal to count all cysts from the entire magnetic resonance image slices, some large kidneys have 40–60 slices, requiring impractical amount of time and effort to count all cysts in every slice without automation of this process. On the basis of the previous study (15), we believe that the average counts from three sample slices multiplied by the number of slides of the magnetic resonance dataset provided a good approximation of the total cyst number, as evidenced by the high correlation coefficients between height-adjusted total cyst number, cyst volume, and kidney volume. Third, although we addressed the potential concern of participation bias in the analysis and found insignificant, the distribution of cohort participation between visits was uneven. For example, there was a slightly higher percentage of patients with PKD2 or no mutation detected present in the cohort at visit 12 than earlier visits, which may relate to presence of milder disease in these individuals. Finally, the findings of the study were analyzed mainly on the basis of statistical correlations and model fitting. Biologic implication and causality of the findings is yet to be determined.
In conclusion, our longitudinal study of growth pattern of kidney cyst number and volume in ADPKD showed that both cyst number and volume increased exponentially over time, and nearly doubled over 14.23 years of follow-up. The PKD1 genotype was significantly associated with both the log of height-adjusted cyst number and volume in the linear mixed model, suggesting that the PKD1 genotype (with similar relationships for MSG groups) has a significant effect on both the cyst number and volume at a given age, but they did not significantly affect the rate of growth (although there seemed to be a positive, albeit nonsignificant association). The knowledge of the growth patterns of kidney cyst number and volume may provide additional insight in the clinical management and treatment development for patients with ADPKD.
Disclosures
Dr. Bae is a consultant to Kadmon and Otsuka. Dr. Chapman is a Steering Committee member for Otsuka Pharmaceuticals. Dr. Harris reports grants from Otsuka Pharmaceuticals, outside the submitted work. Dr. Mrug reports grants, personal fees and nonfinancial support from Otsuka, grants, personal fees and nonfinancial support from Sanofi, personal fees from ClearView Healthcare Partners, personal fees from Decision Resources Group Consulting, personal fees from CLARION Healthcare, outside the submitted work; and serves as the PKD Foundation Scientific Advisory Committee Chair. Dr. Torres reports grants and other from Otsuka Pharmaceuticals, during the conduct of the study; grants and other from Palladio Biosciences, other from Mironid, grants and other from Sanofi Genzyme, other from Vertex, grants from Acceleron Pharma Inc., grants from Regulus Therapeutics, and grants from Palladio Biosciences, outside the submitted work. Dr. Yu reports other from Regulus Therapeutics and personal fees from Sanofi, outside the submitted work. Dr. Bennett, Dr. Landsittel, Dr. Shen, Dr. Tao, Dr. Wu, and Dr. Zhou have nothing to disclose.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10360818/-/DCSupplemental.
Supplemental Table 1. Characteristics of cohort compared between baseline and visits.
Supplemental Table 2. Height-adjusted total cyst number over time for all participants (A) and for participants with at least one visit in the periods 0–5, 6–10, and over 10 years after baseline (B).
Supplemental Table 3. Height-adjusted total cyst volume over time for all participants (A) and for participants with at least one visit in the periods 0–5, 6–10, and over 10 years after baseline (B).
Supplemental Table 4. Median height-adjusted total cyst number (A) and median total cyst number (B) by baseline age group and genotype.
Supplemental Table 5. Linear mixed model for repeated measures of log of height-adjusted total cyst number (A) and cyst volume (B) according to MSG.
Supplemental Table 6. Linear mixed model for repeated measures of log height-adjusted total cyst number (A) and cyst volume (B) according to PKD1 truncating or nontruncating and Irazabal class.
Supplemental Table 7. Linear mixed models with interaction by age to assess rate of change in log height-adjusted total cyst number (A) and cyst volume (B) according to MSG.
Supplemental Table 8. Linear mixed models with an interaction by age to assess rate of change in log height-adjusted total cyst number (A) and cyst volume (B) according to PKD1 truncating or nontruncating and Irazabal class.
Supplemental Figure 1. Histograms and q-q plots of height-adjusted total kidney volume (A and B), height-adjusted total cyst number (C and D) and height-adjusted total cyst volume (E and F) before and after log transformation. Histograms and q-q plots before and after log transformation are on the left and right panel, respectively. Height-adjusted total kidney volume, total cyst number, and total cyst volume that are originally right skewed become normally distributed after log transformation.
Supplementary Material
Acknowledgments
The investigators are indebted to the radiologists, radiology technologists, imaging engineers, and study coordinators in the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) study. Mr. Tiange Shi also preformed additional statistical analyses to address reviewer comments.
The CRISP study is supported by cooperative agreements from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (DK056943, DK056956, DK056957, and DK056961), and by R01 DK113111. This study was also supported in part by the NIDDK through P30 grants to the Kansas PKD Research and Translation Core Center (DK106912) and the Mayo Translational PKD Center (DK090728), by the National Center for Research Resources General Clinical Research Centers at each institution (RR000039, Emory University; RR00585, Mayo College of Medicine; RR23940, Kansas University Medical Center; RR000032, University of Alabama at Birmingham), and the National Center for Advancing Translational Sciences Clinical and Translational Science Awards at each institution (RR025008 and TR000454, Emory; RR024150 and TR000135, Mayo College of Medicine; RR033179 and TR000001, Kansas University Medical Center; RR025777, TR000165, and TR001417, University of Alabama at Birmingham; RR024153 and TR000005, University of Pittsburgh School of Medicine). D.P. Landsittel reports grants from NIDDK R01 grant to the University of Pittsburgh.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millán JL, Gamble V, Harris PC: The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10: 151–160, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S: PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Daoust MC, Reynolds DM, Bichet DG, Somlo S: Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. Genomics 25: 733–736, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Peters DJ, Sandkuijl LA: Genetic heterogeneity of polycystic kidney disease in Europe. Contrib Nephrol 97: 128–139, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Torra R, Badenas C, Darnell A, Nicolau C, Volpini V, Revert L, Estivill X: Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol 7: 2142–2151, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Torra R, Badenas C, Pérez-Oller L, Luis J, Millán S, Nicolau C, Oppenheimer F, Milà M, Darnell A: Increased prevalence of polycystic kidney disease type 2 among elderly polycystic patients. Am J Kidney Dis 36: 728–734, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Fain PR, McFann KK, Taylor MR, Tison M, Johnson AM, Reed B, Schrier RW: Modifier genes play a significant role in the phenotypic expression of PKD1. Kidney Int 67: 1256–1267, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Paterson AD, Magistroni R, He N, Wang K, Johnson A, Fain PR, Dicks E, Parfrey P, St George-Hyslop P, Pei Y: Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 16: 755–762, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF Jr, Glockner JF, Wetzel LH, Brummer ME, O’Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort : Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64: 1035–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP; CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, Baumgarten DA, King BF Jr, Wetzel LH, Kenney PJ, Brummer ME, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) : Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 1: 64–69, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Bae KT, Tao C, Wang J, Kaya D, Wu Z, Bae JT, Chapman AB, Torres VE, Grantham JJ, Mrug M, Bennett WM, Flessner MF, Landsittel DP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) : Novel approach to estimate kidney and cyst volumes using mid-slice magnetic resonance images in polycystic kidney disease. Am J Nephrol 38: 333–341, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyer CM, Sundsbak JL, Abebe KZ, Chapman AB, Torres VE, Grantham JJ, Bae KT, Schrier RW, Perrone RD, Braun WE, Steinman TI, Mrug M, Yu AS, Brosnahan G, Hopp K, Irazabal MV, Bennett WM, Flessner MF, Moore CG, Landsittel D, Harris PC; HALT PKD and CRISP Investigators : Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 2872–2884, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.StataCorp : Stata Statistical Software: Release 14, College Station, TX, StataCorp LP, 2015 [Google Scholar]
- 19.Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT: Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 5: 889–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornec-Le Gall E, Audrézet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y: Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornec-Le Gall E, Audrézet MP, Rousseau A, Hourmant M, Renaudineau E, Charasse C, Morin MP, Moal MC, Dantal J, Wehbe B, Perrichot R, Frouget T, Vigneau C, Potier J, Jousset P, Guillodo MP, Siohan P, Terki N, Sawadogo T, Legrand D, Menoyo-Calonge V, Benarbia S, Besnier D, Longuet H, Férec C, Le Meur Y: The PROPKD score: A new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 942–951, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magistroni R, He N, Wang K, Andrew R, Johnson A, Gabow P, Dicks E, Parfrey P, Torra R, San-Millan JL, Coto E, Van Dijk M, Breuning M, Peters D, Bogdanova N, Ligabue G, Albertazzi A, Hateboer N, Demetriou K, Pierides A, Deltas C, St George-Hyslop P, Ravine D, Pei Y: Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 1164–1174, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Rossetti S, Burton S, Strmecki L, Pond GR, San Millán JL, Zerres K, Barratt TM, Ozen S, Torres VE, Bergstralh EJ, Winearls CG, Harris PC: The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol 13: 1230–1237, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Grantham JJ, Mulamalla S, Grantham CJ, Wallace DP, Cook LT, Wetzel LH, Fields TA, Bae KT: Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol 7: 1087–1093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.