Abstract
Pain is common and poorly managed in patients with advanced CKD, likely due to both under and over prescription of appropriate analgesics. Poorly managed pain contributes to patients’ poor quality of life and excessive health care use. There is tremendous variability within and between countries in prescribing patterns of analgesics, suggesting that factors other than patient characteristics account for these differences. This article discusses the pharmacologic management of acute and chronic pain in patients with advanced CKD, and the role analgesics, including opioids, play in the overall approach to pain management.
Keywords: chronic kidney disease; pain; analgesics; opioids; NSAIDs; Analgesics, Opioid; Chronic Pain; Pain Management; quality of life; Pharmacology, Clinical; Prescriptions; Renal Insufficiency, Chronic
Introduction
Pain is common in patients with advanced CKD. Over 58% of patients experience pain and approximately 49% of patients report moderate or severe pain, whether they are treated with dialysis or managed conservatively (1). It is widely recognized that pain, in particular chronic pain, is associated with psychologic distress; depressive disorders; limitations in work, family, and social life; decreased life satisfaction and quality of life (QOL); and increased hospitalizations and emergency department visits (2–5). For patients receiving hemodialysis (HD), uncontrolled pain leads to shortened or missed treatments (6). Chronic pain in the United States costs hundreds of billions of dollars annually (7).
Pain is defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (8). This definition recognizes pain as a multidimensional phenomenon with physical and psychosocial components. A unidimensional approach to pain management, especially one that relies exclusively on analgesics, is unlikely to be successful. Nonpharmacologic therapies that address the whole person in the context of their disease and personal life are vital in managing chronic pain and should augment pharmacologic treatments (i.e., multimodal therapy). These may include physical therapies such as aerobic exercise, stretching, massage, acupressure, and acupuncture; behavioral therapies such as cognitive behavioral therapy, biofeedback, relaxation techniques, counseling, guided imagery, and mindfulness-based stress reduction; as well as interventions such as nerve blocks and trigger point injections.
The focus of this article is the pharmacologic management of acute and chronic pain in patients with advanced CKD, including patients with ESKD treated with dialysis or conservative kidney management. The role analgesics play in the overall approach to pain management will be discussed, with the understanding that analgesics should not be the sole focus of treatment and should only be used when needed, in conjunction with other treatment modalities, to meet patient-specific treatment goals.
Key Considerations in the Evaluation of Pain to Guide Pharmacologic Management
Evaluation of pain requires a comprehensive patient assessment and physical examination that includes understanding the patient’s diagnosis and medical history in addition to determining the effects of the pain on the patient’s psychologic status, social functioning, functional status, and QOL. Four aspects of the evaluation essential to determining a pharmacologic approach will be discussed here. They consist of determining (1) pain intensity; (2) chronicity and possible reversible causes for the pain; (3) the type of pain—nociceptive, neuropathic, or combined; and (4) treatment goals.
Pain Intensity
Determining pain intensity helps establish the need for treatment. The experience of pain is unique to each individual and can only be measured by that individual. Listening to the patient validates the significance of their pain and their suffering and is an important part of the therapeutic intervention. Several global symptom assessment tools have been validated for use in patients with CKD. These have recently been reviewed elsewhere (1). These tools differ in format, such as numeric, visual, or verbal scales. Although each has evidence for validity, patients interpret them differently, making it difficult to compare between studies. Substantial data exist around what constitutes clinically significant pain. Most of these data are on the basis of 0–10 scales and consensus from the Initiative on Methods, Measurement and Pain Assessment in Clinical Trials was to recommend using a 0–10 numeric rating scale, at least in pain studies (9). This also helps compare findings across patient populations and studies. A score of 0–3 generally reflects mild pain, which does not usually require initiation of or change in pain management. Moderate pain with a score of 4–6 generally means therapy has to be initiated or changed because pain is managed inadequately. A score of 7–10 is described as severe pain and typically requires immediate attention to treatment.
The Edmonton Symptom Assessment System–revised: Renal is a quick, simple, and widely used global symptom assessment tool that can be used successfully in patients, even as they approach death (2). The most recent version consists of a 0–10 numeric rating scale for 12 commonly experienced symptoms, including pain (10).
Chronicity of Pain and Reversible Factors
Determining the chronicity of pain is important in determining appropriate management strategies (Table 1). With acute pain it is important to treat underlying causes to ensure long-term resolution. One should initiate analgesics promptly with moderate or severe pain because pain is more difficult to treat once the pain cycle becomes established. Although we still do not fully understand the development of chronic pain, lessons learned from patients undergoing surgery have taught us that good pain control reduces the likelihood of experiencing chronic pain after surgery. We also know that the risk of abuse is low in patients receiving opioids for acute pain after surgery if the opioids are used in a controlled manner.
Table 1.
Chronicity and type of pain
| Pain | Details |
|---|---|
| Chronicity of pain | |
| Acute pain | • Typically persists for <3 mo. |
| • Associated with tissue damage. | |
| • Usually episodic with periods without pain. | |
| • Tends to last a predictable period, have no progressive pattern and subsides as healing occurs. | |
| • Tends to respond well to pharmacologic therapy: titrating analgesics against pain intensity usually works well. | |
| Chronic pain | • Often defined as any painful condition that persists for >3 mo (8). |
| • Usually initiated by tissue injury but is perpetuated by neurophysiologic changes, which take place within the peripheral and central nervous system leading to continuation of pain once healing has occurred. | |
| • Severity is often out of proportion with the extent of the originating injury. | |
| • More likely to result in functional impairment and disability, psychologic distress, sleep deprivation, and poor QOL than acute pain. | |
| • The pain experience may be affected substantially by mood, stress, and social circumstances. | |
| • May not respond well to analgesics, including opioids, except early in the course of treatment. | |
| Recurrent pain | • Acute pain from tissue injury, which may occur over long periods of time (e.g., pain from needling fistulas, intradialytic steal syndrome, intradialytic headaches, and cramps). |
| • Patient will also be free from pain for long periods. | |
| • More intrusive on everyday life than “acute pain.” | |
| Type of pain | |
| Nociceptive pain | • Results from tissue damage in the skin, muscle, and other tissues, causing stimulation of sensory receptors. |
| • May be described as sharp or like a knife and often felt at the site of damage (e.g., joint pain from dialysis-related arthropathy). | |
| • With stimulation of visceral nociceptors, may be experienced as dull, aching, and poorly localized (e.g., gut ischemia). | |
| • Tends to respond to analgesics. | |
| Neuropathic pain | • Results from damage to the nervous system resulting in either dysfunction or pathologic change. |
| • May be felt at a site distant from its cause (e.g., in the distribution of a nerve). | |
| • Common descriptors include burning, shooting, and electrical. | |
| • May be associated with episodes of spontaneous pain, hyperalgesia, and allodynia; the presence of allodynia is pathognomonic. | |
| • Examples include peripheral neuropathy. Severe pain associated with limb ischemia and calciphylaxis tend to have substantial neuropathic components. | |
| • Responds poorly to analgesics and typically requires adjuvant therapy such as anticonvulsants (gabapentinoids or carbamazepine) and tricyclic antidepressants. |
QOL, quality of life.
Patients with chronic pain often do not have a treatable underlying cause for their pain and the somatosensory component of the pain assumes greater prominence than in acute (and, some say, cancer) pain. As the disorder progresses, the original triggers become less important and psychologic mechanisms gain importance. Pharmacologic therapy alone is unlikely to be sufficient and pain scores have not been shown to correlate with analgesic therapy, including opioid use. For patients with chronic pain, nonpharmacologic assessment and support is essential. The management of recurrent pain, such as pain from needling fistulas, intradialytic steal syndrome, intradialytic headaches, and cramps (Table 1), is more in keeping with acute pain. It focuses on strategies to minimize tissue injury and short-term pharmacologic management around the time of tissue injury if the cause cannot be avoided.
Type of Pain
The choice of initial analgesic is dependent upon the type of pain. In particular, neuropathic pain should be distinguished from nociceptive pain (Table 1). Neuropathic pain is poorly responsive to nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids or requires doses for response that are associated with unacceptable toxicity. The initial treatment of neuropathic pain is an adjuvant drug, which is a drug that has a primary indication other than pain. In contrast, nociceptive pain responds well to nonopioid and opioid analgesics, at least in the short term. Many pains experienced by patients with CKD will be of mixed type, e.g., pain associated with ischemia and calciphylaxis. It is important to target the neuropathic component first with adjuvant therapy to prevent inappropriate use of opioids.
Elucidating these three components of the pain assessment (intensity, chronicity, and type of pain) can be done through the “PQRST” approach: Provokes and Palliates, Quality, Region and Radiation, Severity, and Time, as outlined in Table 2 (11).
Table 2.
The “PQRST” approach to evaluating pain (11)
| Components of the Assessment | Questions to Explore |
|---|---|
| P=Provokes and palliates | What causes the pain? |
| What makes the pain better or worse? | |
| Q=Quality | What does the pain feel like? |
| Is it sharp? Dull? Stabbing? Burning? Crushing? | |
| R=Region and radiation | Where is the pain located? |
| Is it confined to one place? | |
| Does the pain radiate? If so, where to? | |
| S=Severity | How severe is the pain? |
| T=Time (or Temporal) | When did the pain start? |
| Is it present all of the time? | |
| Are you pain-free at night or during the day? | |
| Are you pain-free on movement? |
Treatment Goals
Developing a treatment plan includes explaining the nature of the pain condition and setting appropriate treatment goals. Because relief of all pain is generally not possible, especially with chronic pain, the goal of therapy is to relive the pain to a tolerable level, allowing for acceptable function and QOL. For most patients this is a target of ≤3 out of 10. It is important that the clinician be honest and establish realistic expectations. Management may need to be staged, initially aiming for freedom from pain at rest and at night, progressing to relief of more difficult pain such as that which is related to specific activities such as walking.
Current Status of Analgesic Use among Patients with Advanced CKD
The World Health Organization (WHO) analgesic ladder has been advocated for the management of pain, including chronic pain in patients with CKD (12). A short, 4-week study of 45 patients receiving HD with mild or moderate pain showed a substantial reduction in mean pain scores for both nociceptive and neuropathic pain by using the WHO analgesic ladder approach to management (13).
The WHO analgesic ladder was introduced initially for terminally ill cancer patients who were dying in pain. It involves the slow introduction and upward titration of analgesics, starting with nonopioids then progressing to weak then strong opioids as required for pain relief. The success of this approach led to the expansion to patients with chronic nonmalignant pain and an unprecedented increase in the prescribing and dose of opioids. Nowhere has this been more pronounced than in the United States, and it has been associated with an increase in opioid-related deaths; higher rates of addiction and social dysfunction; cognitive impairment; falls and orthopedic injuries in the elderly; and an increase in emergency department visits and in-patient hospitalizations (14,15) without clear evidence that treatment relieves chronic pain in the long term. The rates of harm correlate directly with dose, which in turn is associated with continuous use.
The substantial harms associated with opioid misuse have been reported at the population level. Those at increased risk are adults aged 18–25 years, decreasing with increasing age (although death rates from opioid overdoses are highest in 45–54-year-olds); men; those with lower educational attainment; and people with psychiatric conditions or a history of substance or sexual abuse (14,15). It is unclear how the legitimate concerns of opioid misuse pertain to patients with advanced CKD. These patients have serious chronic illness with comorbidities such as bone disease, diabetes, and peripheral vascular disease, all of which are known to be associated with ongoing tissue injury and painful conditions. Are these patients more like those with terminal cancer and could they benefit from chronic (low dose) opioid use? The answers are unknown because we lack quality evidence to optimize safe and effective management. However, current prescribing of opioids for patients on HD, at least in the United States, is associated with significantly higher risk of altered mental status, falls, or fractures in a dose-dependent manner (16).
The current situation of poorly managed pain is likely due to both under and over prescription of appropriate analgesics for patients with advanced CKD (17). The prevalence of overall analgesic and opioid use in CKD is highly variable across studies. A recent meta-analysis reports an estimated prevalence of overall analgesic use in patients with advanced CKD of 47% (95% confidence interval (95% CI), 0.35 to 0.59), opioid prevalence 22% (95% CI, 0.07 to 0.41), acetaminophen prevalence 26% (95% CI, 0.16 to 0.36), and NSAID prevalence 16% (95% CI, 0.11 to 0.21) (S.N. Davison et al., submitted article). The prevalence of opioid use is much higher in the United States compared with European countries, Canada, Australia, and New Zealand. A recent, large study across the United States showed that 64% of 153,758 patients on dialysis in 2010 received opioids: 41% of patients had a short-term prescription, whereas 23% received a chronic opioid prescription defined as ≥90 days (18). Chronic opioid prescription rates ranged from 9.5% of patients on dialysis in Hawaii to 40.6% of patients in West Virginia. Eight states had prescription rates >30%. Such high variability suggests that factors other than patient characteristics account for prescribing differences. Equally concerning was the choice of opioid used: 10.6% of patients received chronic prescriptions for opioids that are not recommended, including 1.4% of patients using propoxyphene, an opioid that has been withdrawn from the market across Europe, New Zealand, and Canada. The US Food and Drug Administration advisory panel also recommended against the use of propoxyphene, after concluding that the safety risks outweighed its limited benefit. However, the end result was a black box warning only. In addition, 11.7% of patients were prescribed hydrocodone chronically, an opioid for which there is no evidence of its safety in CKD. Only 1.9% of patients received a chronic prescription for an opioid that is currently considered safer and therefore recommended for use in these patients.
The Five Essential Principles for the Pharmacologic Management of Pain
The essential principles of pain management are summarized by five phrases that are described within the context of advanced CKD in Table 3. Of particular importance is the careful selection of analgesics. Current recommendations are based primarily on indirect pharmacologic evidence and clinical experience, i.e., “expert advice,” rather than quality clinical studies. On the basis of our current understanding, the analgesics that should be avoided in patients with advanced CKD and the evidence behind the recommendations are outlined in Table 4.
Table 3.
The five principles of pain management within the context of advanced CKD
| Principle | Description | Specific Considerations in Advanced CKD |
|---|---|---|
| “By mouth” | • Oral administration is the safest and therefore usually preferred. | • Patients on HD have easy intravenous access. However, this is to be avoided as the route of administration for analgesics to optimize safety and minimize the risk of abuse and addiction. |
| • If ingestion or absorption is uncertain, analgesics need to be given by alternative routes such transdermal, rectal, or subcutaneous. | ||
| “By the clock” | • For continuous or predictable pain, analgesics should be given regularly. Additional “breakthrough” or “rescue” medication should be available on an “as needed” basis in addition to the regular dose. | • Some patients with mild-to-moderate pain may achieve adequate pain relief with analgesic dosing post-HD only. An example would be mild-to-moderate neuropathic pain dosed with gabapentin postdialysis. |
| “By the ladder” | • Pharmacologic management proceeds stepwise from nonopioids to low-dose opioids. | • Careful selection of analgesics with gradual titration is essential (Figure 3). |
| • The drug should be used at its full tolerated dose before moving to the next level. | • Sustained-release preparations are generally not recommended, at least until the individual patient’s response to the medication has been observed, due to the narrow therapeutic window in patients with advanced CKD. There is also some evidence for increased mortality with long-acting opioids (34). | |
| “For the individual” | • The “correct” dose for strong opioids is the amount needed to relieve the pain without producing intolerable side effects. Evaluation of benefit and toxicity is essential. • If an individual finds that a particular strong opioid causes unacceptable adverse effects, an alternative must be sought. |
• Chronic pain is often experienced in the context of numerous other physical, psychosocial, and spiritual concerns, including end-of-life issues. Close attention to these other issues must not be forgotten if the pain management strategy is to be successful. |
| “Attention to detail” | • Pain changes over time; therefore, there is the need for ongoing reassessment. | • There are no studies on the long-term use of analgesics in patients with CKD. Careful attention must be paid to efficacy and safety. |
| • Side effects of opioids should be explained and managed actively; e.g., constipation and nausea with anticipatory prescribing of a bowel routine (e.g., PEG 3350) and antiemetic (e.g., Zofran 4–8 mg). | • The effect on overall symptom burden, physical function, emotional state, cognition, and QOL should be assessed routinely. |
HD, hemodialysis; QOL, quality of life.
Table 4.
Analgesics to avoid in patients with advanced CKD
| Analgesic | Details |
|---|---|
| NSAIDS (for chronic pain) (20) | The major limitation is gastrointestinal toxicity due to inhibition of cytoprotective mucus secretion and impaired platelet aggregation resulting in ulceration and bleeding. Risk increases in severity and frequency with increasing age: NSAID use increases the risk of gastrointestinal bleeding in the elderly four-fold. Concomitant use of antiplatelet drugs such as aspirin, anticoagulants, and SSRIs further increases bleeding risk. |
| Although the gastrointestinal safety profile of COX-2 inhibitors is superior to nonselective NSAIDs, nephrotoxic and cardiovascular adverse effects (myocardial infarction, thrombotic events, and stroke) remain significant. It has been shown that the long-term use of all NSAIDs increases the risk of stroke by 64% at 2 yr. COX-2 selectivity may not play a role in the increased cardiovascular risk of NSAIDs because rofecoxib was the only drug in meta-analyses to demonstrate excessive harm and skewed the data of COX-2 selective NSAIDs (35). There is insufficient evidence to confirm any NSAID to be safe in terms of cardiovascular risk. | |
| In patients with residual kidney function, NSAIDs may also cause: a reduction in GFR that can be severe and irreversible if the patient has decreased effective circulating volume; sodium and water retention, which may aggravate hypertension and hyperkalemia. | |
| The elderly may be at increased risk for NSAIDs-associated psychiatric events such as agitation, depression, anxiety, paranoia, delirium, and hallucinations. | |
| Codeine | A weak opioid that is metabolized by the enzyme CYP2D6 in the liver to its active metabolite morphine, which provides the analgesic effect. Only about 5%–10% of codeine is metabolized in this pathway, with most of the administered dose being converted to inactive metabolites. |
| The percentage of codeine converted to morphine can be much higher in individuals who have three or more active copies of the CYP2D6 gene (“ultra-rapid metabolizers”), resulting in life-threatening or fatal respiratory depression due to high plasma levels of morphine, even with trivial doses. Conversely, poor analgesic response will be seen in those who carry inactive copies of CYP2D6 (“poor metabolizers”) due to low morphine levels after administration of standard doses (25). | |
| Up to 11% of codeine is also metabolized to hydrocodone (mechanism unknown). | |
| Both codeine and its metabolites are excreted by the kidneys and accumulate in patients with kidney failure. | |
| Dextropropoxyphene | A weak opioid that has been withdrawn from the market in the United Kingdom, Europe, New Zealand, and Canada due to its weak analgesic effect, addictiveness, and its association with deaths and possible arrhythmias. In the United States it has a Black Box warning and is on the High-Risk Medications in the Elderly list. |
| Decreased elimination of dextropropoxyphene and its major active metabolite, norpropoxyphene, occurs in patients with kidney failure (36). | |
| Tramadol | A weak synthetic opioid related to codeine. |
| Extensively metabolized in the liver with one main active metabolite, M1. Both the parent drug and M1 contribute to the analgesic effect through u-opioid receptors and two nonopioid mechanisms, inhibition of serotonin and norepinephrine reuptake (37). M1 has a significantly higher affinity for opioid receptors than tramadol, whereas tramadol is a more potent inhibitor of serotonin and norepinephrine reuptake (38). | |
| The enzyme CYP2D6 catalyzes the production of M1 and other CYP enzymes (CYP2B6 and CYP3A4) catalyze the production of M2, an inactive metabolite (Figure 1). | |
| Unpredictable risk of serious overdosing or under dosing after administration of standard doses. The concentrations of tramadol may be 20% higher in “poor metabolizers” versus individuals who have multiple functional copies of the CYP2D6 gene (“ultra-rapid metabolizers”), whereas M1 concentrations may be up to 40% lower. Factors such as the concurrent use of CYP2D6 inhibitors (Figure 2) could also result in increased tramadol concentration and decreased M1 concentration. | |
| Induction of CYP3A4 may pose an added risk of seizures, even when tramadol is administered in accepted doses. This is particularly problematic in the context of neuropathic pain where several of the adjuvants are CYP3A4 inducers (Figure 2). | |
| Serotonin syndrome is a potentially life-threatening syndrome that may occur with the use of tramadol, especially if other medications such as antidepressants or other drugs that impair the metabolism of tramadol (CYP2D6 and CYP3A4 inhibitors) are used concurrently. Symptoms include changes in mental status (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile BP, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). | |
| Morphine | Metabolized primarily to the active metabolite M3G and small amounts (approximately 10%) of M6G. M3G lacks analgesic effect but may have neuroexcitatory effects contributing to adverse effects such as allodynia, myoclonus, and seizures. M6G has potent analgesic effect, more so than morphine. Although M6G is dialyzed, it diffuses out of the central nervous system slowly so may not be completely removed during dialysis. |
| M3G and M6G (more so than the parent drug morphine) accumulate in patients with advanced CKD. | |
| There is poor, inconsistent correlation between plasma levels of morphine, M3G, and M6G and clinical efficacy or adverse effects (39). The best correlation appears to be between higher levels of morphine and constipation and high levels of M3G and cognitive impairment (26). There are many reports in the literature of profound toxicity in patients with advanced CKD. | |
| Oxycodone | A semisynthetic opioid metabolized primarily by CYP3A4 to the active metabolite noroxyodone with a small amount metabolized by CYP2D6 to the active metabolite oxymorphone, the clinical relevance of which is not clear. The potential for drug interaction and unpredictable pharmacodynamic response therefore is relatively high (Figure 1). |
| Less than 10% is excreted unchanged in the urine. Despite this, both the parent drug and the active metabolites appear to accumulate in patients on dialysis (40) with reports of toxicity (41). The central opioid effects are governed by the parent drug. | |
| A recent systematic review of opioid use in patients with cancer with some degree of kidney failure found two studies that evaluated oxycodone use: higher oxycodone levels were associated with increased fatigue but the metabolite noroxycodone was not associated with any of the evaluated adverse effects (26). | |
| A single study assessed the pharmacokinetics after a single 20-mg oral dose of an abuse-deterrent formulation of extended release oxycodone in patients with mild (n=6), moderate (n=5), and severe (n=6) kidney failure (42). Cmax and AUC continued to increase with increasing severity of kidney failure and patients with severe kidney failure had a Cmax (31.6 ng/ml versus 17.6 ng/ml) and AUC (493.5 ng.h/ml versus 210.7 ng.h/ml) more than double that of those with normal kidney function. Adverse effects were experienced by 50% of patients with severe kidney failure versus 14.3% in those with normal kidney function. | |
| In a case study of a single patient on HD, oxycodone and its metabolites were reduced by dialysis, yet there was no loss of analgesia (43). More recently, knowledge about the dialyzability of oxycodone comes from a study of 20 patients on HD on stable doses of oxycodone CR (44). Dialyzability of oxycodone and noroxycodone was possible but very limited. Not surprisingly, therefore, there was no significant increase in postdialysis pain with no need for additional opioid dosing. This is not surprising because oxycodone has a relatively high volume of distribution (greater than hydromorphone), is nearly 50% protein bound, and is only moderately water soluble. | |
| Hydrocodone | A semisynthetic strong opioid synthesized from codeine: 99% of the world’s supply is consumed in the United States where it is the most commonly prescribed opioid, including for patients with CKD. |
| Primarily metabolized in the liver into several metabolites, including hydromorphone, via CYP2D6. Therefore, it might be expected to have a similar unpredictable risk of serious overdosing or under dosing after administration of standard doses as seen with codeine and tramadol (Figure 1). In clinical practice this is less clear. The production of the active metabolite of hydrocodone (hydromorphone) is reduced in CYP2D6 “poor metabolizers” but there is little evidence of a difference in analgesic effect. Ultra-rapid CYP2D6 metabolizers may have an increased response to hydrocodone with an increased risk of overdose. | |
| Approximately 26% is excreted in the urine either unchanged or as a metabolite; therefore, kidney failure is hoped to have only a minimal effect on drug clearance. However, data are extremely limited as described below. | |
| There is only a single study that has assessed the extent to which varying degrees of kidney failure can affect the pharmacokinetics of hydrocodone and this involved a single 45-mg dose of extended-release formulation over 144 h (45). All subjects received naloxone at 15 and 3 h before and 9 and 21 h post dose to minimize opioid-related adverse effects. There were eight patients with mild kidney failure (>50–80 ml/min), nine with moderate (30–50 ml/min), nine with severe (<30 ml/min), and nine on HD ≥6 mo. Systemic exposure was up to 70% greater in patients with moderate-to-severe kidney failure compared with patients with mild kidney failure but appeared to be unchanged in patients on HD. There was no consistent trend toward an increase in maximum concentration of hydrocodone with increasing severity of kidney failure. However, the incidence of adverse effects in patients on dialysis was similar to those with normal kidney function despite the concurrent use of naltrexone. | |
| Without any data to support its use and the high potential for unpredictable toxicity risk, it remains unclear the role that hydrocodone should or should not have in the pharmacologic management of pain for patients with advanced CKD. |
NSAIDs, nonsteroidal anti-inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors; COX, cyclooxygenase; CYP, cytochrome P450; M1, O-desmethyltramadol; M3G, morphine-3-glucuronide; M6G, morphine-6-glucuronide; HD, hemodialysis; Cmax, maximum concentration; AUC, area under the curve; HD, hemodialysis; CR, controlled release.
Nonopioids in Advanced CKD
Acetaminophen
Acetaminophen is an antipyretic analgesic with weak anti-inflammatory activity. In therapeutic doses it has no other important pharmacologic effects. It is metabolized extensively in the liver. Only 2%–5% of the dose is excreted unchanged in the urine and there are no clinically significant changes observed in patients with kidney failure. Recent evidence suggests that lifetime cumulative doses of acetaminophen do not have an adverse effect on CKD progression rate (19). Liver injury can be seen with acetaminophen doses of <4000 mg; therefore, the recommended maximum daily dose is 3000 mg.
NSAIDs
The American Geriatric Society recommends that the chronic use of all oral NSAIDs, including high-dose aspirin, be avoided, especially in the elderly >75 years (20). Clinicians should be cautious about their use in patients with CKD due to increased risks of bleeding, cardiovascular events, psychiatric events, and kidney-related complications in those with residual kidney function (Table 4). NSAIDs are best reserved for specific indications of acute pain, limiting their use to the lowest effective dose and shortest duration (20). Avoiding the use of NSAIDs is associated with increased opioid use in an effort to control pain. Therefore, the risk profile of NSAIDs versus low-dose opioids needs to be ascertained for any given patient. Topical NSAIDs can provide effective pain relief without the systemic adverse events associated with oral NSAIDs when used for both acute and chronic pain (21,22). Where pain is present in joints or nonulcerated skin, this may be a useful alternative to oral administration.
Opioid Metabolism and Advanced CKD
Patients with kidney failure are at increased risk for adverse effects of opioids due to reduced elimination and increased accumulation of the parent analgesic and/or active metabolites. Analgesics may also be removed by dialysis, leading to uncertain analgesic effects during treatment.
The risks of opioid toxicity, poor analgesic response, and drug interactions are determined largely by which enzyme system(s) metabolizes the opioid and the patient’s genetics factors and medical conditions (most notably, kidney or liver disease). Opioid metabolism takes place primarily in the liver, with the metabolites (and varying degrees of the parent drug) excreted by the kidneys. Opioid metabolism results in the production of both inactive and active metabolites, some of which may be more potent than the parent compound. These will accumulate to various degrees in patients with decreased kidney function and patients tend to have a narrow therapeutic window between analgesia and toxicity. Careful selection of opioids is essential, and understanding opioid metabolism is important in this determination.
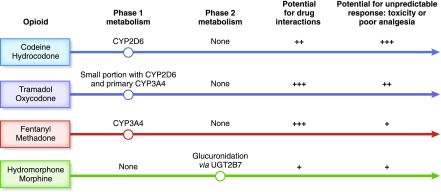
There are two forms of metabolism that occur in the liver: phase 1 metabolism, which typically subjects the drug to oxidation or hydrolysis; and phase 2 metabolism, which conjugates the drug. Opioids may undergo phase 1 metabolism, phase 2 metabolism, or both (Figure 1) (23,24). Phase 1 metabolism involves primarily the cytochrome P450 2D6 (CYP2D6) and cytochrome P450 3A4 (CYP3A4) enzymes.
Figure 1.
Metabolic pathways for opioids involve either phase 1 or phase 2 metabolism, which impacts potential for drug interaction and unpredictable clinical response. CYP2D6, cytochrome P450 2D6 enzyme; CYP3A4, cytochrome P450 3A4 enzyme; UGT2B7, UDP-glucuronosyltransferase 2B7.
There is tremendous genetic polymorphism of the CYP2D6 gene. Individuals who have three or more active copies of the CYP2D6 gene are described as “ultra-rapid metabolizers.” Conversely, those who carry inactive copies of CYP2D6 are “poor metabolizers.” An individual’s response is highly variable and can result in unpredictable toxicity with trivial doses or poor analgesic response with standard doses (25).
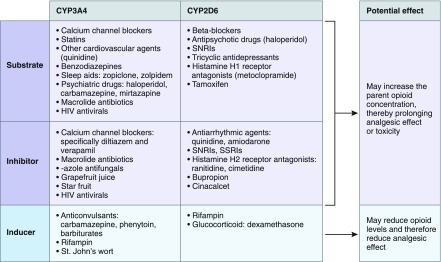
The CYP3A4 enzyme metabolizes >50% of drugs so opioids metabolized by this enzyme have a high risk of drug-drug interactions. Concomitant use of CYP3A4 substrates and inhibitors can increase the parent opioid concentration, thereby prolonging analgesic effect or toxicity. Examples can be seen in Figure 2. CYP3A4 inducers can reduce opioid levels and therefore reduce analgesic effect. The CYP2D6 enzyme metabolizes approximately 25% of drugs so is associated with a lower risk of drug-drug interactions.
Figure 2.
Commonly used classes of drugs in patients with CKD that may act as substrates, inhibitors or inducers of CYP3A4 and CYP2D6 and therefore may affect opioid metabolism and effect. CYP2D6, cytochrome P450 2D6 enzyme; CYP3A4, cytochrome P450 3A4 enzyme; SNRIs, serotonin-norepinephrine reuptake inhibitors; TCAs, tricyclic antidepressants.
Drugs that are metabolized by phase 2 glucuronidation, such hydromorphone, have minimal drug interaction potential. Although genetic variability exists, the clinical relevance is unknown and these drugs do not appear to have the same risk for unpredictable toxicity as seen with CYP2D6-mediated metabolism.
The Effect of Dialyzability of Analgesics on Pain Management
Stability of analgesia during dialysis will vary among different analgesics. Opioids that are well dialyzed will likely require supplemental dosing during or after HD and patients could be at higher risk for opioid withdrawal symptoms after dialysis. Opioids that are not well dialyzed will have more stable analgesia. However, if there is accumulation of the parent drug and/or active metabolites, the risk for toxicity increases. The ability of dialysis to remove any drug depends upon several factors. Factors that promote dialyzability include lower mol wt, lower protein binding, greater water solubility, and lower volume of distribution.
Recommended Opioids for Pain Management in Patients with Advanced CKD
The opioids felt to be the safest for patients with advanced CKD and their pharmacokinetic properties are outlined in Table 5. Given the minimal changes in kinetics in kidney failure, hydromorphone, fentanyl, methadone, and buprenorphine may be potentially useful opioids. They appear to have stable analgesic affect during HD. Hydromorphone has the advantage of undergoing no phase 1 metabolism, therefore avoiding the complications of unpredictable toxicity and drug-drug interactions seen with the CYP2D6- and CYP34A-metabolized opioids. More than 80% of 55 patients with cancer and kidney failure who experienced adverse effects, primarily with morphine, improved after a switch to hydromorphone (26). Methadone and fentanyl do not produce active metabolites. The metabolism of methadone relies on several CYP enzymes in addition to CYP3A4 so the potential for drug-drug interactions is complex. Methadone also interacts with the voltage-gated potassium channels of the myocardium and can prolong Q-T intervals. Not every patient experiences Q-T interval prolongation with methadone, but risk factors include female sex, hypokalemia, high-dose methadone, drug interactions, and underlying cardiac conditions. It is generally recommended to limit the use of methadone to experienced prescribers. Caution is required when using buprenorphine because the reversal of buprenorphine-induced respiratory depression may be delayed and inconsistent, requiring large doses of naloxone, due to the slow association and dissociation between buprenorphine and opioid receptors, which limits the ability of naloxone to displace buprenorphine from the receptors (27). Unfortunately, clinical studies are lacking to support their efficacy and safety, especially as they relate to chronic pain management.
Table 5.
The pharmacokinetics of opioids recommended for use in patients with advanced CKD
| Characteristic | Hydromorphone | Fentanyl | Methadone | Buprenorphine |
|---|---|---|---|---|
| Clinical description | A potent µ receptor agonist that is approximately 5–7 times more potent than morphine after oral administration and approximately three times more potent after intravenous administration. It may cause less pruritus, sedation, and nausea than morphine. | A potent synthetic opioid that is 50–100 times more potent than morphine. It causes less histamine release, has a lower incidence of constipation, and affords greater cardiovascular stability than morphine. | A potent synthetic opioid with activity mainly at the µ receptor. It also appears to function as an NMDA receptor antagonist and therefore may be more effective for neuropathic pain than other strong opioids, although evidence to support this remains limited. | A potent semisynthetic opioid. It is a partial µ receptor agonist and a κ receptor antagonist. |
| Oral bioavailability | Low-to-moderate: 5%–35% | Low: usually administered intravenously or transdermally | High: >80% | Low: administered effectively sublingually or transdermally. |
| Route of clearance | Extensive first-pass hepatic metabolism with little unchanged drug found in the urine. It is metabolized principally to H3G, which has no analgesic activity but possibly causes neuro-excitation, agitation, confusion, and hallucinations. Unlike morphine, which has an active analgesic 6-glucuronide metabolite, H6G is present in trace amounts only. The pharmacokinetics of the active parent compound are not substantially altered by CKD, due to the rapid conversion to H3G (46). | Hepatic metabolism with 10%–20% excreted by the kidneys. Metabolites are inactive. | Hepatic metabolism into inactive metabolites with approximately 20% excreted unchanged in the urine. In patients who are anuric, methadone is exclusively excreted in feces with no significant accumulation in plasma (47). | Extensive first-pass hepatic metabolism with little unchanged drug found in the urine (48). The two major metabolites, B3G and norbuprenorphine, are mostly excreted fecally with only 10%–30% excreted in the urine (49). B3G is inactive with no analgesic properties. Norbuprenorphine is a less potent analgesic at the µ receptor than buprenorphine; its clinical relevance is thought to be limited because it does not cross the blood-brain barrier readily. A study with ten patients on HD showed no elevated buprenorphine or norbuprenorphine plasma levels after receiving transdermal buprenorphine (median dose 52.5 μg/h) for at least 1 wk (50). |
| Plasma t1/2 | Hydromorphone: unchanged with CKD. | Unchanged with CKD. | Unchanged with CKD. However, prolonged pharmacologic action due to slow release from tissue reservoirs of up to 60 h (51). | Unchanged with CKD. |
| H3G: prolonged—33 h | ||||
| Volume of distribution | Low: 1.22 L/kg | High: 2–5 L/kg | High: 4.1–6.7 L/kg | Very high, greater than physiologic volumes. Estimated to be 188–430 L after iv administration (transdermal unknown). |
| Serum protein binding | Low: 19% | High: 79% | High: 60%–90% | High: 96% |
| Water solubility | High | Low (lipophilic): suitable for a transdermal delivery | Low (lipophilic): suitable for a transdermal delivery | High |
| mol wt | Low: 285.3 g/mol | Low: 336.5 g/mol | Low: 309.5 g/mol | Low: 467.6 g/mol |
| Removal by HD | H3G accumulates between dialysis treatments but appears to be effectively removed during HD with no significant change in pain scores post HD or a need for supplemental dosing (46,52). | Not removed to any significant degree but there is the possibility of adsorption to CT190 dialysis membranes (53).a | Parent drug and metabolites do not seem to be removed by HD: approximately 6.0%–14.9% reductions in plasma methadone (52,54,55). No significant difference in pain scores post HD and supplemental methadone is not required post HD (52). Q-T interval increased significantly: maximum 152 min after methadone intake. Remained <500 ms and was not linearly associated with serum methadone concentration—but may be exacerbated by HD reductions in serum potassium and/or magnesium (54). | HD does not appear to affect buprenorphine plasma levels, and analgesic effect is stable during HD (50). |
| Dosing recommendations | Start at 0.5 mg by mouth (or 0.2 mg subcutaneously) every 4–6 h. | Not recommended in opioid-naïve patients. When converting from hydromorphone, 6–8 mg oral hydromorphone daily can be converted to 12 μg/h transdermally every 72 h. | Start 1–2 mg every 12–24 h by mouth. Obtain a pretreatment ECG and a follow-up ECG 2–4 wk after initiation to monitor for prolonged Q-T interval. | Start at 5 μg/h transdermally every 7 d. |
NMDA, N-methyl-D-aspartate; H3G, hydromorphone-3-glucuronide; H6G, hydromorphone-6-glucuronide; B3G, buprenorphine-3-glucuronide; HD, hemodialysis; ECG, electrocardiogram.
On the basis of data from a single patient receiving maintenance hemodialysis.
Putting It All Together: A General Approach to the Pharmacologic Management of Pain for Patients with Advanced CKD
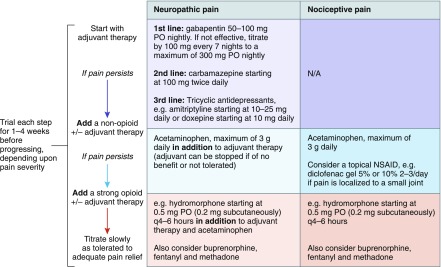
A cautious stepwise approach to the introduction and titration of analgesics is outlined in Figure 3. For patients with a neuropathic component to their pain, the first step is to introduce an adjuvant. The pharmacokinetics and pharmacodynamics of recommended adjuvants in patients with advanced CKD are outlined in Table 6.
Figure 3.
Pharmacological management of pain in patients with advanced CKD requires a cautious stepwise approach. N/A, not applicable; NSAID, nonsteroidal anti-inflammatory drug; PO, by mouth; TCAs, tricyclic antidepressants.
Table 6.
The pharmacokinetics of recommended adjuvants for the treatment of neuropathic in patients with advanced CKD
| Characteristic | Gabapentin (56,57) | Pregabalin (57,58)a | Carbamazepine (59) | Amitriptyline (60,61) | Ketamine (62,63) |
|---|---|---|---|---|---|
| Clinical description | Gabapentin is an analog of the neurotransmitter GABA with high-affinity binding to the α2δ protein. It reduces the release of excitatory neurotransmitters from the brain although does not have activity at GABA receptors. It has analgesic and anticonvulsant activity. | Pregabalin is also an analog of the neurotransmitter GABA with high-affinity binding to the α2δ protein. It reduces the release of excitatory neurotransmitters from the brain but has no activity at GABA receptors. It has analgesic, anxiolytic, and anticonvulsant activity. | Carbamazepine is an anticonvulsant used to treat seizure disorders and neuropathic pain. It is a tricyclic compound chemically related to TCAs and also functions as a mood stabilizer. | Amitriptyline is a TCA with sedative effects that is used to treat major depressive and anxiety disorders as well as migraines and neuropathic pain. | Ketamine is an anesthetic with analgesic, anti-inflammatory, and antidepressant properties when used in subanesthetic doses. It is a potent NMDA receptor channel antagonist. Its use is typically reserved for intractable neuropathic pain resistant to opioids and other adjuvants. |
| Oral bioavailability | High-to-moderate: approximately 80% at up to 300 mg daily but decreases with increasing dose, particularly with doses >900 mg daily. | High: >90% irrespective of dose | High: approximately 89% | Low-to-moderate due to extensive first-pass hepatic metabolism: approximately 33%–62% | Low due to extensive first-pass hepatic metabolism: 16%–29% |
| Peak plasma concentration | Approximately 3 h | Approximately 1 h | Approximately 6 h | Approximately 6 h | Oral: 20–120 min |
| iv: <5 min | |||||
| Route of clearance | Not appreciably metabolized and >95% is excreted unchanged by the kidneys. No inhibition of the enzyme systems responsible for the metabolism of other drugs. | Not appreciably metabolized and >95% is excreted unchanged by the kidneys. No inhibition of the enzyme systems responsible for the metabolism of other drugs. | Metabolized in the liver via phase 1 metabolism primarily by CYP3A4. The metabolites are excreted via the kidneys with approximately 20%–30% excreted via the feces. Only 3%–5% is excreted unchanged by the kidneys. | Extensively metabolized on first pass through the liver. It undergoes phase 1 metabolism primarily by CYP2D6 and CYP3A4. | Extensively metabolized on first pass through the liver. It undergoes phase 1 metabolism primarily by CYP3A4 and CYP2B6 to its two principal metabolites norketamine (has analgesic properties) and hydroxynorketamines (may have low potency antidepressant effects) before being further metabolized to mostly inactive dehydronorketamine. Metabolites are cleared by the kidneys with low levels cleared as ketamine (2%), norketamine (2%), and dehydronorketamine (16%). Most (80%) is cleared as hydroxynorketamines. |
| Plasma t1/2 | Increases linearly with decreased kidney function. | Increases linearly with decreased kidney function. | Approximately 35 h; remains unchanged with ESKD. | Highly variable at 10–28 h; remains unchanged with ESKD. | 2–4 h |
| Cl Cr>60 ml/min=9 h | |||||
| Cl Cr 30–60 ml/min=17 h | |||||
| Cl Cr 15–29 ml/min=25 h | |||||
| Cl Cr<15 ml/min=49 h | |||||
| On hemodialysis 3/wk=55 h | |||||
| Serum protein binding | Not bound to plasma proteins | Not bound to plasma proteins | 70%–80% | Highly bound to plasma and tissue proteins | 10%–50% |
| Water solubility | High | High | High | Low; highly lipophilic | High |
| Removal by HD | Well dialyzed. Approximately 50% of serum drug is removed during a 4-h session. Supplemental dosing postdialysis may be required. Gabapentin is also cleared by continuous ambulatory PD although this is a slow method to treat toxicity (31). | Well dialyzed. Approximately 50% of serum drug is removed during a 4-h session. The t1/2 during dialysis treatment is approximately 3 h. Supplemental dosing post HD may be required. | Dialyzed. Clearance in the era of low flux HD membranes is twice the endogenous plasma clearance. However, it appears that supplemental dosing post HD is not required because of the long elimination t1/2 of carbamazepine of 35 h compared with a 4-h session. | Not dialyzed with HD or PD | Not studied in dialysis |
| Dosing recommendations | Dose post HD. Below are maximum recommended doses. It may be reasonable, especially for older patients, or those with moderate rather than severe neuropathic pain, to start with doses as low as 100 mg postdialysis or 100 mg every second night in patients with stage 5 CKD managed conservatively. | Dose post HD. Below are maximum recommended doses. It may be reasonable, especially for older patients, or those with moderate rather than severe neuropathic pain, to start with doses as low as 25 mg post HD or 25 mg every second night in patients with stage 5 CKD managed conservatively. | Start at 100 mg daily or twice daily and increase by 100 mg daily to a maximum of 1200 mg daily. | Although no dose reduction is required, a low starting dose of approximately 25 mg nightly is recommended given the likelihood of anticholinergic adverse effects such as blurred vision, dry mouth, and constipation. | Given the pharmacokinetic, no dose reduction is required. |
| eGFR 50–79 ml/min: 600 mg three times per d | eGFR>30–60 ml/min: 150 mg twice per d | By mouth: 0.5 mg/kg twice daily or 2 mg/kg daily | |||
| eGFR 30–49 ml/min: 300 mg three times per d | eGFR 15–30 ml/min: 150 mg once per d | Subcutaneously: 0.05–0.15 mg/kg per h for up to 7 d | |||
| eGFR<15 ml/min: 300 mg once per d | eGFR 15–29 ml/min: 300 mg twice per d | iv: 0.15–0.25 mg/kg | |||
| To reduce the adverse effects of psychosis and tachycardia, the concurrent administration of haloperidol or midazolam is recommended. |
GABA, γ-aminobutyric acid; α2δ protein, α-2-δ protein; TCA, tricyclic antidepressant; NMDA, N-methyl-D-aspartate; CYP, cytochrome P450; Cl Cr, creatinine clearance; HD, hemodialysis; PD, peritoneal dialysis.
On the basis of a single dose of 50 mg of pregabalin in an open-label, parallel-group study.
Anticonvulsants and tricyclic antidepressants are the two classes of drugs for which there is most evidence of efficacy. Systematic reviews have found that anticonvulsants and tricyclic antidepressants are effective in reducing neuropathic pain due to diabetic peripheral neuropathy and postherpetic neuralgia (28,29). The evidence for effectiveness for other causes of neuropathic pain is too limited to provide strong conclusions, although small studies in patients on HD have shown improvement in pain and QOL scores for diverse causes of neuropathic pain using gabapentin (30). Gabapentin is structurally similar to the neurotransmitter γ-aminobutyric acid but, rather than bind to γ-aminobutyric acid receptors, its mechanism of action is thought to be through binding to calcium channels and modulating the influx of calcium. Gabapentin is almost exclusively cleared by the kidneys and substantial dose reduction is required as the GFR declines to avoid toxicity (Table 6). Adverse effects include somnolence, dizziness, peripheral edema, and gait disturbances.
Evidence suggests that carbamazepine may be as effective as gabapentin for treating neuropathic pain in the general population and may have fewer adverse effects. Unlike gabapentin, it requires no dose adjustment in CKD as outlined in Table 6 (31). Tricyclic antidepressants are effective in the management of neuropathic pain but are less well tolerated than the gabapentinoids in patients with CKD because of anticholinergic, histaminergic, and adrenergic side effects resulting in symptoms such as dry mouth, orthostatic hypotension, and somnolence. Although dose reduction of tricyclic antidepressants is not necessarily required, patients with CKD will often respond to lower doses.
Ketamine is an anesthetic agent that functions as an analgesic in subanesthetic doses. Clinically, it is reserved for intractable neuropathic pain that is resistant to basic adjuvants and opioids. An example is the management of the pain of calciphylaxis. There is no need for dose adjustment in CKD. However, adverse events such as tachycardia and psychosis may limit its use. To reduce this risk the concurrent administration of haloperidol or midazolam is recommended (Table 6).
There are insufficient data or clinical experience with selective serotonin reuptake inhibitors and selective serotonin-norepinephrine reuptake inhibitors for neuropathic pain in CKD to make a recommendation. In the general population they tend to be less effective than anticonvulsants and tricyclic antidepressants but have fewer adverse effects (28,29).
Nonopioids should be used as initial pharmacologic management for nociceptive pain and for neuropathic pain if pain persists despite maximal tolerated dose of an adjuvant. Studies have failed to show that a weak opioid has markedly superior analgesic efficacy to acetaminophen or an NSAID.
Before starting an opioid, consider completing an assessment tool such as the Screener and Opioid Assessment for Patients with Pain–Revised to assess the risk for aberrant opioid-related behavior (32). Those that are categorized as having a high risk of future abusive drug-related behavior would benefit from referral to a pain specialist for management of their pain and opioid prescribing.
Although the initial WHO analgesic ladder advocates for trialing weak opioids before starting a strong opioid, there is no evidence that weak opioids such as codeine and tramadol are less risky than strong opioids at their lowest effective dose (Table 4) (33). The response to these weak opioids varies highly from one patient to another, with an unpredictable risk of fatal overdosing with trivial doses or poor analgesic effect after administration of standard doses (Table 4) (25). The “weak” opioids also have dose-dependent adverse effects similar to the strong opioids. A recent United States study of 140,899 patients on HD showed that the highest hazards for altered mental status, falls, and fractures among all opioids prescribed were associated with codeine (16). In addition, there is no evidence that at equivalent analgesic efficacy weak opioids carry a lower risk of addiction than low-dose strong opioids. Therefore, given the risks of using weak opioids such as codeine and tramadol in patients with advanced CKD, it seems more prudent to use to a strong opioid at a low dose with careful titration when opioid therapy is required (33).
Adverse effects of opioids are common and will prevent effective analgesia if not well managed. Constipation is persistent and nearly universal and patients should have a bowel routine, e.g., PEG 3350 prescribed pre-emptively. Nausea and vomiting occur in about 50% of people, wearing off in most after 7–10 days. The central nervous system effects occur most frequently on initiating opioids and when increasing the dose, hence the need to “start low and titrate slow.” Respiratory depression is unusual if oral, short-acting preparations are used and the dose is titrated against pain and toxicity: pain is said to be the physiologic antagonist of opioids. When pain is stable, opioids can be used in long-acting preparations such as transdermal fentanyl or methadone. As a patient’s condition deteriorates, especially nearer the end of life, swallowing becomes compromised and alternate routes are required, such as subcutaneous fentanyl. Hallucinations, a very distressing adverse effect, may occur and should be managed by dose reduction, switching to an alternative opioid, or with coadministration of haloperidol.
Summary
Pain is highly complex, which is further compounded by kidney failure: a simple approach will not be sufficient. Analgesics play an important role in pain management but they should not be the sole focus of treatment, especially for patients with chronic pain where the somatosensory component of the pain tends to assume greater importance than the original trigger. Nonpharmacologic therapies that address the whole person in the context of their disease and personal life are a vital part of managing chronic pain and analgesics should only be used to augment these therapies as required to achieve adequate relief. The pharmacologic management of pain for patients with CKD requires careful selection of analgesics with close attention to efficacy and safety, keeping in mind that the overall goal is to promote function and QOL and not necessarily completely resolve the pain. To date, there are no studies that look at clinical outcomes of chronic analgesic use in patients with CKD. This will clearly need to change if we are to optimize safe and effective management of pain for our patients.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Davison SN, Koncicki H, Brennan F: Pain in chronic kidney disease: A scoping review. Semin Dial 27: 188–204, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Davison SN, Jhangri GS, Johnson JA: Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant 21: 3189–3195, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Davison SN, Jhangri GS: The impact of chronic pain on depression, sleep, and the desire to withdraw from dialysis in hemodialysis patients. J Pain Symptom Manage 30: 465–473, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Cohen SD, Patel SS, Khetpal P, Peterson RA, Kimmel PL: Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol 2: 919–925, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Davison SN, Jhangri GS: Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage 39: 477–485, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Weisbord SD, Mor MK, Sevick MA, Shields AM, Rollman BL, Palevsky PM, Arnold RM, Green JA, Fine MJ: Associations of depressive symptoms and pain with dialysis adherence, health resource utilization, and mortality in patients receiving chronic hemodialysis. Clin J Am Soc Nephrol 9: 1594–1602, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turk DC, Wilson HD, Cahana A: Treatment of chronic non-cancer pain. Lancet 377: 2226–2235, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Merskey H, Bogduk N: Classification of Chronic Pain, Seattle, International Association for the Study of Pain Press, 1994, pp 210 [Google Scholar]
- 9.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S: Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9: 105–121, 2008 [DOI] [PubMed]
- 10.Edmonton Zone Palliative Care Program and Northern Alberta Renal Program, 2016. Available at: http://www.palliative.org/tools.html. Accessed July 15, 2018
- 11.Barnard A, Gwyther E: Pain management in palliative care. S Afr Fam Pract 48: 30–33, 2006 [Google Scholar]
- 12.WHO: WHO’s cancer pain ladder for adults, 2017. Available at: http://www.who.int/cancer/palliative/painladder/en/. Accessed September 14, 2018 [Google Scholar]
- 13.Barakzoy AS, Moss AH: Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol 17: 3198–3203, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Wilkerson RG, Kim HK, Windsor TA, Mareiniss DP: The opioid epidemic in the United States. Emerg Med Clin North Am 34: e1–e23, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Rolita L, Spegman A, Tang X, Cronstein BN: Greater number of narcotic analgesic prescriptions for osteoarthritis is associated with falls and fractures in elderly adults. J Am Geriatr Soc 61: 335–340, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL: Opioid analgesics and adverse outcomes among hemodialysis patients. Clin J Am Soc Nephrol 13: 746–753, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagar VR, Birthi P, Salles S, Sloan PA: Opioid use in chronic pain patients with chronic kidney disease: A systematic review. Pain Med 18: 1416–1449, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Kimmel PL, Fwu CW, Abbott KC, Eggers AW, Kline PP, Eggers PW: Opioid prescription, morbidity, and mortality in United States dialysis patients. J Am Soc Nephrol 28: 3658–3670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans M, Fored CM, Bellocco R, Fitzmaurice G, Fryzek JP, McLaughlin JK, Nyrén O, Elinder CG: Acetaminophen, aspirin and progression of advanced chronic kidney disease. Nephrol Dial Transplant 24: 1908–1918, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J: A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis 9: 143–150, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore RA, Tramèr MR, Carroll D, Wiffen PJ, McQuay HJ: Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ 316: 333–338, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massey T, Derry S, Moore RA, McQuay HJ: Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev 16(6): CD007402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Indiana University School of Medicine: The flockhart table, 2016. Available at: http://medicine.iupui.edu/CLINPHARM/ddis/main-table. Accessed July 14, 2018
- 24.FDA: Examples of clinical index substrates for P450-mediated metabolism (for use in index clinical DDI studies), 2016. Available at: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm#table2-1. Accessed July 14, 2018
- 25.Dean L: Codeine therapy and CYP2D6 genotype. In: Medical Genetics Summaries, edited by Pratt V, Mcleod H, Dean L, Malheiro A, Rubinstein W, Bethesda, MD, National Center for Biotechnology Information (US), 2017 [PubMed] [Google Scholar]
- 26.Sande TA, Laird BJ, Fallon MT: The use of opioids in cancer patients with renal impairment-a systematic review. Support Care Cancer 25: 661–675, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Sarton E, Teppema L, Dahan A: Naloxone reversal of opioid-induced respiratory depression with special emphasis on the partial agonist/antagonist buprenorphine. Adv Exp Med Biol 605: 486–491, 2008 [DOI] [PubMed] [Google Scholar]
- 28.McQuay HJ, Tramèr M, Nye BA, Carroll D, Wiffen PJ, Moore RA: A systematic review of antidepressants in neuropathic pain. Pain 68: 217–227, 1996 [DOI] [PubMed] [Google Scholar]
- 29.CADTH Rapid Response Reports: Gabapentin for adults with neuropathic pain: A review of the clinical effectiveness, Ottawa, ON, Canadian Agency for Drugs and Technologies in Health, 2018 [PubMed] [Google Scholar]
- 30.Atalay H, Solak Y, Biyik Z, Gaipov A, Guney F, Turk S: Cross-over, open-label trial of the effects of gabapentin versus pregabalin on painful peripheral neuropathy and health-related quality of life in haemodialysis patients. Clin Drug Investig 33: 401–408, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim H, Oman Z, Schuelke M, Edwards JC: Treatment of gabapentin toxicity with peritoneal dialysis: Assessment of gabapentin clearance. Am J Kidney Dis 70: 878–880, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Varney SM, Perez CA, Araña AA, Carey KR, Ganem VJ, Zarzabal LA, Ramos RG, Bebarta VS: Detecting aberrant opioid behavior in the emergency department: A prospective study using the screener and Opioid Assessment for Patients with Pain-Revised (SOAPP®-R), Current Opioid Misuse Measure (COMM)™, and provider gestalt [published online ahead of print March 3, 2018]. Intern Emerg Med [DOI] [PubMed] [Google Scholar]
- 33.“Weak” opioid analgesics. Codeine, dihydrocodeine and tramadol: No less risky than morphine. Prescrire Int 25: 45–50, 2016 [PubMed] [Google Scholar]
- 34.Ray WA, Chung CP, Murray KT, Hall K, Stein CM: Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA 315: 2415–2423, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunter BR, Butler KA, Wallace RL, Smith SM, Harirforoosh S: Non-steroidal anti-inflammatory drug-induced cardiovascular adverse events: A meta-analysis. J Clin Pharm Ther 42: 27–38, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Gibson TP, Giacomini KM, Briggs WA, Whitman W, Levy G: Propoxyphene and norpropoxyphene plasma concentrations in the anephric patient. Clin Pharmacol Ther 27: 665–670, 1980 [DOI] [PubMed] [Google Scholar]
- 37.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL: Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 260: 275–285, 1992 [PubMed] [Google Scholar]
- 38.Smith HS: Opioid metabolism. Mayo Clin Proc 84: 613–624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quigley C, Joel S, Patel N, Baksh A, Slevin M: Plasma concentrations of morphine, morphine-6-glucuronide and morphine-3-glucuronide and their relationship with analgesia and side effects in patients with cancer-related pain. Palliat Med 17: 185–190, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Kirvela M, Lindgren L, Seppala T, Olkkola KT: The pharmacokinetics of oxycodone in uremic patients undergoing renal transplantation. J Clin Anesth 8: 13–18, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Foral PA, Ineck JR, Nystrom KK: Oxycodone accumulation in a hemodialysis patient. South Med J 100: 212–214, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Malhotra BK, Schoenhard GL, de Kater AW, Friedmann N: The pharmacokinetics of oxycodone and its metabolites following single oral doses of Remoxy®, an abuse-deterrent formulation of extended-release oxycodone, in patients with hepatic or renal impairment. J Opioid Manag 11: 157–169, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Lee MA, Leng ME, Cooper RM: Measurements of plasma oxycodone, noroxycodone and oxymorphone levels in a patient with bilateral nephrectomy who is undergoing haemodialysis. Palliat Med 19: 259–260, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Samolsky Dekel BG, Donati G, Vasarri A, Croci Chiocchini AL, Gori A, Cavallari G, Di Nino G, Mercolini L, Protti M, Mandrioli R, Melotti RM, La Manna G: Dialyzability of oxycodone and its metabolites in chronic noncancer pain patients with end-stage renal disease. Pain Pract 17: 604–615, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Darwish M, Yang R, Tracewell W, Robertson P Jr, Bond M: Effects of renal impairment and hepatic impairment on the pharmacokinetics of hydrocodone after administration of a hydrocodone extended-release tablet formulated with abuse-deterrence technology. Clin Pharmacol Drug Dev 5: 141–149, 2016 [DOI] [PubMed] [Google Scholar]
- 46. Davison SN, Mayo P: Pain management in chronic kidney disease: The pharmacokinetics and pharmacodynamics of hydromorphone and hydromorphone-3-glucuronide in hemodialysis patients. J Opioid Manag, 4: 335–336, 339–344, 2008. [PubMed]
- 47.Kreek MJ, Schecter AJ, Gutjahr CL, Hecht M: Methadone use in patients with chronic renal disease. Drug Alcohol Depend 5: 197–205, 1980 [DOI] [PubMed] [Google Scholar]
- 48.Hand CW, Sear JW, Uppington J, Ball MJ, McQuay HJ, Moore RA: Buprenorphine disposition in patients with renal impairment: Single and continuous dosing, with special reference to metabolites. Br J Anaesth 64: 276–282, 1990 [DOI] [PubMed] [Google Scholar]
- 49.Chiang CN, Hawks RL: Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug Alcohol Depend 70[Suppl 2]: S39–S47, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Filitz J, Griessinger N, Sittl R, Likar R, Schüttler J, Koppert W: Effects of intermittent hemodialysis on buprenorphine and norbuprenorphine plasma concentrations in chronic pain patients treated with transdermal buprenorphine. Eur J Pain 10: 743–748, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Dole VP, Kreek MJ: Methadone plasma level: Sustained by a reservoir of drug in tissue. Proc Natl Acad Sci USA 70: 10, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perlman R, Giladi H, Brecht K, Ware MA, Hebert TE, Joseph L, Shir Y: Intradialytic clearance of opioids: Methadone versus hydromorphone. Pain 154: 2794–2800, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Joh J, Sila MK, Bastani B: Nondialyzability of fentanyl with high-efficiency and high-flux membranes. Anesth Analg 86: 447, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Opdal MS, Arnesen M, Müller LD, Hullstein I, Sayed K, Brørs O, Kringen M, Sagedal S, Gjesdal K, Krajci P: Effects of hemodialysis on methadone pharmacokinetics and QTc. Clin Ther 37: 1594–1599, 2015 [DOI] [PubMed] [Google Scholar]
- 55.Furlan V, Hafi A, Dessalles MC, Bouchez J, Charpentier B, Taburet AM: Methadone is poorly removed by haemodialysis. Nephrol Dial Transplant 14: 254–255, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Randinitis EJ, Posvar EL, Alvey CW, Sedman AJ, Cook JA, Bockbrader HN: Pharmacokinetics of Pregabalin in Subjects with Various Degrees of Renal Function. J Clin Pharmacol 43: 277–283, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P: A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 49: 661–669, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Randinitis EJ, Posvar EL, Alvey CW, Sedman AJ, Cook JA, Bockbrader HN: Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J Clin Pharmacol 43: 277–283, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Lee CS, Wang LH, Marbury TC, Bruni J, Perchalski RJ: Hemodialysis clearance and total body elimination of carbamazepine during chronic hemodialysis. Clin Toxicol 17: 429–438, 1980 [DOI] [PubMed] [Google Scholar]
- 60.Gupta SK, Shah JC, Hwang SS: Pharmacokinetic and pharmacodynamic characterization of OROS and immediate-release amitriptyline. Br J Clin Pharmacol 48: 71–78, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandoz M, Vandel S, Vandel B, Bonin B, Hory B, St Hillier Y, Volmat R: Metabolism of amitriptyline in patients with chronic renal failure. Eur J Clin Pharmacol 26: 227–232, 1984 [DOI] [PubMed] [Google Scholar]
- 62.Bell RF: Ketamine for chronic non-cancer pain. Pain 141: 210–214, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD: Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev 70: 621–660, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]