Abstract
Infectious abortions of goats in Argentina are mainly associated with brucellosis and toxoplasmosis. In this paper, we describe an abortion outbreak in goats caused by Chlamydia abortus. Seventy out of 400 goats aborted. Placental smears stained with modified Ziehl‐Neelsen stain showed many chlamydia‐like bodies within trophoblasts. One stillborn fetus was necropsied and the placenta was examined. No gross lesions were seen in the fetus, but the inter‐cotyledonary areas of the placenta were thickened and covered by fibrino‐suppurative exudate. The most consistent microscopic finding was found in the placenta and consisted of fibrinoid necrotic vasculitis, with mixed inflammatory infiltration in the tunica media. Immunohistochemistry of the placenta was positive for Chlamydia spp. The results of polymerase chain reaction targeting 23S rRNA gene performed on placenta were positive for Chlamydia spp. An analysis of 417 amplified nucleotide sequences revealed 99% identity to those of C. abortus pm225 (GenBank AJ005617) and pm112 (GenBank AJ005613) isolates. To the best of our knowledge, this is the first report of abortion associated with C. abortus in Argentina.
Keywords: Abortion, Chlamydia abortus, Goats
Introduction
Infectious abortions are among the most significant problems for goat breeding in Argentina (Samartino 2002; Rossetti et al. 2017). These abortions are caused mostly by brucellosis and toxoplasmosis. Other infectious causes of abortion have been poorly characterized (Unzaga et al. 2014).
In goats, Chlamydia abortus produces abortion mostly due to placentitis associated with placental infection (Schlafer & Miller 2007; Longbottom et al. 2013). The oro‐nasal route is the natural port of entry (Gutierrez et al. 2011), followed by blood spread of the organism and, in pregnant does, invasion of trophoblasts in the placentomes, from which bacteria spread to the inter‐cotyledonary areas. These changes in the placenta result in late‐term abortions or stillbirths (Buxton et al. 2002).
C. abortus stands for the main cause of abortion in sheep and goats at global level (Aitken & Longbottom 2007). In the United Kingdom up to 37% of abortions in sheep are caused by C. abortus (Mearns 2007). Australia and New Zealand are considered free of C. abortus infection (Longbottom & Coulter 2003). Two neighbouring countries of Argentina, i.e. Uruguay and Chile, recently confirmed the presence of abortion induced by Chlamydia sp. in goats (OIE 2012; Giannitti et al. 2016).
Abortion by Chlamydia spp. in goats has not been reported before in Argentina. This study describes an outbreak of abortions caused by C. abortus in a herd of goats from Mendoza Province, Argentina.
Materials and methods
Animals
During the kidding season in the spring, 70 does in a flock of 400 criollo goats located in the San Rafael Department of Mendoza Province aborted term fetuses or gave birth to weak kids during a 6 weeks period.
The goats of the affected flock were reared under extensive grazing conditions during the day and they were locked in a small corral every night, where they were provided water ad‐libitum. Health management included regular Fasciola hepatica treatment with triclabendazole 10% (Ganafort®, Argentina) and vaccination against brucellosis with Brucella melitensis Rev.1 vaccine (SENASA, Argentina).
Pathology
One stillborn kid and its placenta were submitted for necropsy and gross examination, respectively. Samples of liver, thymus, spleen, lung, complete brain and placenta were collected and fixed by immersion in 10%, buffered formalin, pH 7.2 and processed routinely for the production of 4 μm thick paraffin sections that were stained with haematoxylin and eosin. Selected sections of placenta were also processed by immunohistochemistry (IHC) for Toxoplasma gondii, Coxiella burnetii and Chlamydia spp., as previously described (Dilbeck & McElwain 1994; Giannitti et al. 2016). Briefly, antigen retrieval was performed in a decloaking chamber, followed by the quenching of endogenous peroxidase with 3% hydrogen peroxide. The following primary antibodies were used for T. gondii, C. burnetii and Chlamydia spp., respectively: B. Barr CAHF‐D Rbt# 58 (rabbit polyclonal; University of California, Davis, CA, US), AB‐COX‐MAB (mouse monoclonal; U.S. Department of Defense, Critical Reagents Program, Washington DC, US) and Anti‐Chlamydia lipopolysaccharide antibody (mouse monoclonal; Virostat Inc., Westbrook, ME, US). Primary antibodies were applied followed by anti‐species horseradish peroxidase (HRP)‐labelled polymer (Biocare, #GHP516, Pacheco, CA) and a detection system, with 3‐amino‐9‐ethylcarbazole (ThermoScientific, #TA‐125‐SA, Fremont, CA) as the chromogen substrate solution. Samples of caprine placentas which were PCR positive or negative for these microorganisms were used as positive and negative controls, respectively.
Bacteriology
Impression smears from cotyledons were stained using the modified Ziehl‐Neelsen stain (Poester et al. 2010). Cotyledon samples and abomasal content were cultured under aerobic and microaerophilic conditions on 5% sheep blood agar at 37°C for 72 h.
Molecular analysis
Samples of placenta were processed by a quantitative real‐time PCR to detect the 23S rRNA gene of the Chlamydiaceae family as previously described (Ehricht et al. 2006). Briefly, genomic DNA was extracted from 25 μg of placental tissue with the PureLink™ Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, USA). The samples were further examined using a nested PCR assay (Kaltenböeck et al. 1997; Sprague et al. 2009) of outer membrane protein A gene (ompA also known as omp1), the outer primer set 191CHOMP/CHOMP371 was used to amplify a 576‐bp fragment. In the second amplification round, the following inner primers sets were used: 242B577/CHOMP336s representing C. abortus (formerly known as C. psittaci serovar 1) and 204PECOR/CHOMP336s representing C. pecorum (Everett et al. 1999). In addition, 201CHOMP/CHOMP336s was used to amplify a segment in variable domains III and IV from the ompA gene of all Chlamydia spp. for sequencing (Sachse & Hotzel 2003; Sprague et al. 2009). The reactions were carried out in a thermal cycler IQ™ Multicolor Real‐Time PCR Detection System (Bio Rad, Hercules, CA, USA). Amplicons were separated on a 1.5% agarose gel by electrophoresis and observed with ethidium bromide under UV light.
The target amplification product from the primer set 201CHOMP/CHOMP336s was purified using an AccuPrep® Gel Purification Kit (Bioneer Corporation, Seoul, Korea) and subjected to a direct nucleotide sequencing reaction in both directions by a sequencing service (Unidad de Genómica, Instituto de Biotecnología, Instituto Nacional de Tecnología Agropecuaria, Castelar, Argentina).
The sequence was aligned and trimmed using ClustalX (Conway Institute UCD Dublin, Dublin, Ireland) and compared through BLASTn 2.2.19 to other Chlamydia ompA gene fragments obtained from the GenBank data bank. The sequence was found to be most closely homologous to C. abortus strains in the databases. The strain was designated as Chlamydia abortus strain CEDIVE‐G‐4661/12 and submitted to GenBank (Accession number KU728158).
Serology
Serum samples from the stillborn fetus and five aborted does were tested to detect antibodies against B. melitensis and T.gondii by plate agglutination and indirect immunofluorescence tests, respectively, as previously described (Moré et al. 2008; Unzaga et al. 2014).
Results
Grossly, the placenta was thickened, with extensive multifocal necrotic foci and diffuse fibrinopurulent exudate on the chorionic surface (Fig. 1a). These lesions were particularly evident in inter‐cotyledonary spaces. No significant gross abnormalities were observed in the fetus.
Figure 1.
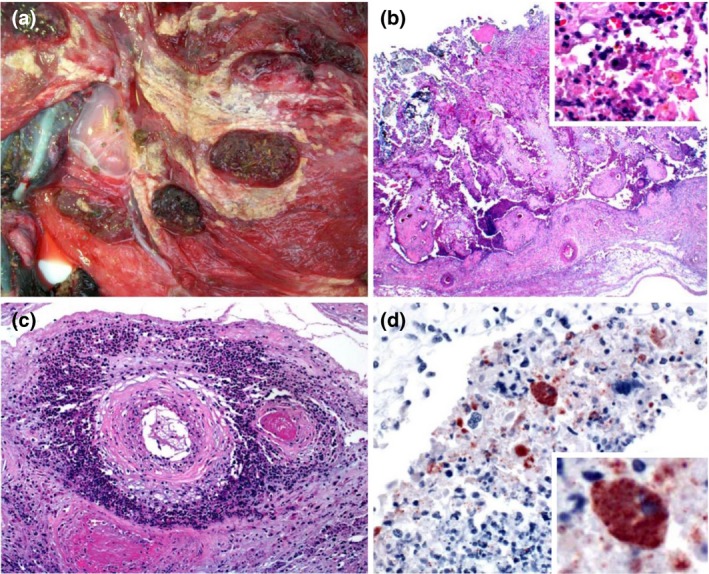
Placenta of a goat that aborted due to Chlamydia abortus infection. (a) Gross view of the chorionic side of the placenta. The membrane is thickened, with extensive multifocal to coalescing fibrinopurulent exudate. (b) Necrosis of chorioallantoic membrane, with eosinophilic cellular and karyorrhectic debris, viable and degenerate neutrophils, fewer macrophages and multifocal mineralization. Insert: higher magnification showing inflammatory cells and a trophoblast with myriad intracytoplasmic inclusions. HE. (c) Arteries within the chorioallantoic membrane showing vasculitis, thrombosis and perivascular accumulations of viable and degenerate neutrophils, macrophages, lymphocytes and plasma cells. HE. (d) Chorionic villus showing trophoblasts with myriad intracytoplasmic inclusions stained positively for Chlamydia abortus. There is also extracellular positive material. Insert: higher magnification showing positively stained trophoblasts. C. abortus immunohistochemistry.
Microscopically, the chorioallantoic membrane showed diffuse necrosis of the villus and adjacent inter‐cotyledonary epithelium, which was replaced by abundant eosinophilic karyorrhectic debris, viable and degenerate neutrophils, fewer lymphocytes, plasma cells and macrophages and multifocal mineralization. A few trophoblasts were scattered throughout the necrotic debris and were often expanded by many intracytoplasmic, basophilic, ~ 1 um diameter inclusions (Fig. 1b). Multifocally, the blood vessels of the chorioallantoic membrane were infiltrated by eosinophilic karyorrhectic debris, neutrophils, fewer lymphocytes, plasma cells and macrophages and small amounts of fibrin; the vascular lumen was occluded by fibrin thrombi. There were also perivascular accumulations of viable and degenerate neutrophils, macrophages, lymphocytes and plasma cells (Fig. 1c). In fetal brain, the Virchow‐Robin spaces were multifocally distended by moderate numbers of lymphocytes and macrophages with fewer neutrophils and necrotic debris. The endothelial cells of these blood vessels were hypertrophic and the wall of those vessels presented similar changes to those described in the chorioallantoic membrane. Surrounding these blood vessels there was spongiosis and the myelin sheaths were dilated surrounding swollen axons. A few neurons showed central chromatolysis or were necrotic. In the liver, small multifocal areas of coagulative necrosis and multifocal infiltration with lymphocytes, macrophages and plasma cells, together with lymphoplasmacytic chollangiohepatitis were observed. In the lung, diffuse lymphoplasmacytic infiltration was observed in alveolar septae.
IHC showed strong positivity mostly in the cytoplasm of the trophoblasts but also in the intercellular space (Fig. 1d). IHC for T. gondii and C. burnetii was negative.
Smears of placenta stained with modified Ziehl‐Neelsen showed a large number of red, pin point bodies free in the intercellular space and also in the cytoplasm of trophoblasts. No bacteria were isolated from the placenta.
Real‐time PCR targeting 23S rRNA gene from placental tissue was positive for the presence of Chlamydia spp. The nested PCR was positive for C. abortus and negative for C. pecorum (Kaltenböeck et al. 1997; Sprague et al. 2009).
The results of the BLASTn sequence analysis of amplified nucleotide sequences revealed 99% identity (417 nucleotides) with sequences of the C. abortus strain goat origin from China and Greece (GenBank KF130872 and GenBank HQ622433, respectively) and also with the sequence of sheep strain (GenBank KC879303) and bovine strain LW508 (GenBank M73040).
Serology for brucellosis and toxoplasmosis was negative in all animals tested.
Discussion
A presumptive diagnosis of chlamydiosis was established based on gross and microscopic changes, including the examination of the modified Ziehl‐Neelsen stained smears of placenta. Confirmation was achieved by IHC and PCR. The latter also provided confirmation that C. abortus was the species of chlamydia involved. In addition, the diagnosis was supported by the negative results of tests performed to rule out brucellosis, toxoplasmosis and C. burnetii infection, the first two being the most prevalent known causes of caprine abortion in Argentina.
In Argentina, a previous study of Chlamydia spp. responsible for infections in wild and captive birds detected Chlamydia psittaci, Chlamydia pecorum, Chlamydia gallinacea and Chlamydia pneumoniae (Frutos et al. 2015). Recently Rojas et al. (2018) detected Chlamydiaceae DNA in 12 samples of 251 bovine aborted fetuses (4.78%) and C. abortus was detected in five of the studied cases (1.99%). The mentioned study confirmed the presence of C. abortus in La Pampa Province, not far from Mendoza Province, the location of the present case. Sporadic abortions in goats from Uruguay were associated with C. pecorum (Giannitti et al. 2016). However, to the best of our knowledge, there have been no reports of ovine or caprine enzootic abortion associated with C. abortus in Argentina.
This study shows that C. abortus is a cause of caprine abortion in Argentina. This microorganism is a zoonotic agent and early diagnosis is of outmost importance to prevent its transmission to people in close contact with goats and to other goats in the herd. C. abortus should be included in the list of aetiologic agents to be investigated in cases of caprine abortion in Argentina. Presence of C. abortus in Argentina was probably overlooked; however since new diagnostic test were established in several Latin American countries recently, chlamydiosis was also reported in Uruguay (Giannitti et al. 2016), Chile (OIE 2012) and Argentina (this report). Humans are at highest risk during and immediately after abortion or parturition as large numbers of this organism are released to the environment with the fetal fluids (Seth‐Smith et al. 2017). Additional studies are required to determine the prevalence of C. abortus infection in goats in Argentina.
Source of funding
This paper was partly funded by the California Animal Health and Food Safety Laboratory, UCDavis, USA.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethics Statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered.
Contributions
Di Paolo: design, diagnosis, review Ms; Alvarado Pinedo: diagnosis, review Ms; Origlia: diagnosis, review Ms; Fernandez: diagnosis, review Ms; Uzal: diagnosis, writing the Ms; Traveria: diagnosis, writing the Ms
Acknowledgements
We thank Ms Karen Sverlow and Juliann Beingesser for excellent technical support.
References
- Aitken I.D., Longbottom D. (2007) Chlamydial abortion In: Diseases of sheep fourth edition, (eds I.D. Aitken), pp 105–112. Blackwell Scientific Ltd.: Oxford, UK. [Google Scholar]
- Buxton D., Anderson I.E., Longbottom D., Livingstone M., Wattegedera S. & Entrican G. (2002) Ovine chlamydial abortion: characterization of the inflammatory immune response in placental tissues. Journal of Comparative Pathology 127, 133–141. [DOI] [PubMed] [Google Scholar]
- Dilbeck P.M. & McElwain T.F. (1994) Immunohistochemical detection of Coxiella burnettii in formalin‐fixed placenta. Journal of Veterinary Diagnostic Investigation 6, 125–127. [DOI] [PubMed] [Google Scholar]
- Ehricht R., Slickers P., Goellner S., Hotzel H. & Sachse K. (2006) Optimized DNA microarray assay allows detection and genotyping of single PCR‐amplifiable target copies. Molecular and Cellular Probes 20, 60–63. [DOI] [PubMed] [Google Scholar]
- Everett K., Bush R. & Andersen A. (1999) Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. International Journal of Systematic Bacteriology 49, 415–440. [DOI] [PubMed] [Google Scholar]
- Frutos M.C., Monetti M.S., Vaulet L.G., Cadario M.E., Fermepin M.R., Ré V.E. & Cuffini C.G. (2015) Genetic diversity of Chlamydia among captive birds from central Argentina. Avian Pathology 44, 50–56. 10.1080/03079457.2014.993593. [DOI] [PubMed] [Google Scholar]
- Giannitti F., Anderson M., Miller M., Rowe J., Sverlow K., Vasquez M., Cantón G. (2016) Chlamydia pecorum: fetal and placental lesions in sporadic caprine abortion. Journal of Veterinary Diagnostic Investigation 2, 184–189. 10.1177/1040638715625729. [DOI] [PubMed] [Google Scholar]
- Gutierrez J., Williams E.J., O'Donovan J., Brady C., Proctor A.F., Marques P.X. et al (2011) Monitoring clinical outcomes, pathological changes and shedding of Chlamydophila abortus following experimental challenge of periparturient ewes utilizing the natural route of infection. Veterinary Microbiology 147, 119–126. 10.1016/j.vetmic.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Kaltenböeck B., Schmeer N. & Schneider R. (1997) Evidence for numerous omp1 alleles of porcine Chlamydia trachomatis and novel chlamydial species obtained by PCR. Journal of Clinical Microbiology 35, 1835–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbottom D. & Coulter L.J. (2003) Animal chlamydioses and zoonotic implications. Journal of Comparative Pathology 128, 217–244. 10.1053/jcpa.2002.0629. [DOI] [PubMed] [Google Scholar]
- Longbottom D., Entrican G., Wheelhouse N., Brough H. & Milne C. (2013) Evaluation of the impact and control of enzootic abortion of ewes. Veterinary Journal 195, 257–259. 10.1016/j.tvjl.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Mearns R. (2007) Abortion in sheep 1, investigation and principal causes. Practice 29, 40–46. [Google Scholar]
- Moré G., Basso W., Bacigalupe D., Venturini M.C. & Venturini L. (2008) Diagnosis of Sarcocystis cruzi, Neospora caninum, and Toxoplasma gondii infections in cattle. Parasitology Research 102, 671–675. [DOI] [PubMed] [Google Scholar]
- OIE . (2012) Event summary: Enzootic abortion of ewes (ovine chlamydiosis), Chile. Available at: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary?fupser=&dothis=&reportid=12046(Accessed5September2018).
- Poester F.P., Nielsen K., Samartino L.E. & Yu W.L. (2010) Diagnosis of Brucellosis. The Open Veterinary Science Journal 4, 46–60. [Google Scholar]
- Rojas M.D., Fort M., Bettermann S., Entrocassi C., Costamagna S.R., Sachse K., Rodriguez Fermepin M. (2018) [Detection of Chlamydia abortus in bovine reproductive losses in the province of La Pampa, Argentina]. Revista Argentina de Microbiología 50:269–274. S0325‐7541(17)30169‐4. 10.1016/j.ram.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Rossetti C.A., Arenas‐Gamboa A.M., Maurizio E. (2017) Caprine brucellosis: a historically neglected disease with significant impact on public health. PLoS Neglected Tropical Diseases 17, e0005692 10.1371/journal.pntd.0005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse K. & Hotzel H. (2003) Detection and differentiation of Chlamydiae by nested PCR. Methods in molecular biology 216, 123‐136. [DOI] [PubMed] [Google Scholar]
- Samartino L. (2002) Brucellosis in Argentina. Veterinary Microbiology 90, 71–80. [DOI] [PubMed] [Google Scholar]
- Schlafer D.H. & Miller R.B. (2007) Female genital system In: Jubb, Kennedy, and Palmer's pathology of domestic animals. 5th edn Vol. 3, 502–504. (ed Maxie M.G.), Elsevier: Philadelphia, PA. [Google Scholar]
- Seth‐Smith H.M., Busó L.S., Livingstone M., Sait M., Harris S.R., Aitchison K.D. et al (2017) European Chlamydia abortus livestock isolate genomes reveal unusual stability and limited diversity, reflected in geographical signatures. BMC Genomics 4, 344 10.1186/s12864-017-3657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague L.D., Schubert E., Hotzel H., Scharf S., Sachse K. (2009) The detection of Chlamydophila psittaci genotype C infection in dogs. The Veterinary Journal 181, 274–279. 10.1016/j.tvjl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Unzaga J.M., Moré G., Bacigalupe D., Rambeaud M., Pardini L., Dellarupe A. et al (2014) Toxoplasma gondii and Neospora caninum infections in goat abortions from Argentina. Parasitology International 63, 865–867. 10.1016/j.parint.2014.07.009. [DOI] [PubMed] [Google Scholar]


