Abstract
Steroid analysis is essential to the fields of medicine and forensics, but such analyses can present some complex analytical challenges. While chromatographic methods require long acquisition times and often provide incomplete separation, ion mobility spectrometry (IMS) as coupled to mass spectrometry (MS) has demonstrated significant promise for the separation of steroids, particularly in concert with metal adduction and multimerization. In this study, traveling wave ion mobility spectrometry (TWIMS) was employed to separate multimer steroid metal adducts of isomers in mixtures. The results show the ability to separate steroid isomers with a decrease in resolution compared to single component standards due to the formation of heteromultimers. Additionally, ion-neutral collision cross sections (CCS) of the species studied were measured in the mixtures and compared to CCSs obtained in single component standards. Good agreement between these values suggests that the CCS may aid in identification of unknowns. Furthermore, a complex mixture comprised of five sets of steroid isomers were analyzed and distinct features for each steroid component were identified. This study further demonstrated the potential of TWIMS-MS methods for the rapid and isomer-specific study of steroids in biological samples for use either in tandem with or without chromatographic separation.
Keywords: Ion mobility spectrometry, steroids, isomer separation, metal ion adduction, multimer formation
Introduction
Steroid analysis has become an important area of research due to the use of steroids in biological applications.1–2 Steroids are uniquely characterized by four fused rings, three cyclohexane and one cyclopentane, and are all biosynthesized from cholesterol. Biologically, steroids are involved in signaling and can easily permeate the cell membrane to access nuclear receptors.1, 3–4 They can serve as biomarkers for metabolic disorders, drugs for growth and performance enhancement, and contaminants in the environment.3–11 Therefore, the ability to rapidly and accurately analyze steroids in complex mixtures would be invaluable to medical diagnosis, forensic analysis, and environmental analysis and protection.
Steroid analysis presents a difficult challenge due to the high number of isomers with very different biological functions.2, 12 These compounds have been traditionally characterized by gas chromatography (GC) and mass spectrometry (MS).2, 12–15 However, due to limited resolution between some isomers and the requirement for derivatization, the field has moved to other methods including liquid chromatography (LC), nuclear magnetic resonance (NMR), and tandem MS.12, 16–20 These methods require long acquisition times and still may not provide enough resolution for accurate analysis.12 Therefore, additional techniques have been sought after for more expeditious steroid analysis.
Ion mobility spectrometry (IMS) encompasses a group of gas phase ion separation methods that spatially or temporally disperse ions with different mobilities in the presence of a drift gas and under the influence of an electric field.21–26 LC was first coupled to high field asymmetric IMS (FAIMS), a spatial separator, for steroid mixture analysis.6, 14, 20 More recently, LC was coupled to traveling wave IMS (TWIMS), a temporal separator, to analyze steroid glucuronides.15 In both cases, IMS was used to improve signal to noise ratios and to mobility select the steroids of interest, which increased confidence and sensitivity of the techniques. IMS methods in general, including TWIMS, are faster separations techniques (typically on the order of milliseconds) as compared to LC (typically on the order of minutes). While increased resolution can be realized in TWIMS through the optimization of instrumental parameters (e.g., the traveling DC wave height and velocity), experimental considerations that bring about useful gas-phase structural changes are also often advantageous. In this regard, metal ion adduction has often proven useful.27–29 By comparison, increased LC resolution can be achieved through alterations to the mobile phase gradient, mobile phase components, or stationary phase chemistry.
Lately, IMS has been studied as a stand-alone separation method in combination with MS, without the need for prior chromatographic separation for analysis. Ahonen et al. first attempted to separate steroids with TWIMS.30 Unfortunately, they were unable to separate native steroids from each other, but through derivatization with p-toluenesulfonyl isocyanate the separation of estradiols, testosterones, and androsterones in a mixture was enhanced. However, further work examining standards has been used for IMS separation of native steroids.
Chouinard et al. previously reported the formation of metal adducted steroid dimer species of androsterone and epiandrosterone.31 They furthered this work by extending it to a multitude of steroid isomer pairs and studying the resolution and ion-neutral collision cross sections (CCS) of protonated monomer, sodiated monomer, and sodiated dimer species of these steroid isomers.32 Differences were reported in androsterone and epiandrosterone as the metal adduct was changed from group I metals to transition metals. The results showed that eight pairs of steroid isomers as standards had a resolution greater than 1.5 in the optimal conditions by varying multimer, adduct, and drift gas in a drift tube IMS (DTIMS).
Previously, we have reported a continuation of this work by reporting resolutions and CCSs for five steroid isomer pairs with different group I metal adducts.33 The five steroid isomer pairs could all be separated with a resolution value above 1 by TWIMS with their optimal adducts, namely lithium and potassium dimers. These steroid isomers include α-estradiol, β-estradiol, testosterone, DHEA, epitestosterone, estriol, epiandrosterone, androsterone, corticosterone, 11-deoxycortisol, aldosterone, and cortisone, shown in Figure 1. This work reported the first native separation of the estradiol stereoisomers and the mobility separation between estriol and testosterone isomers.
Figure 1.
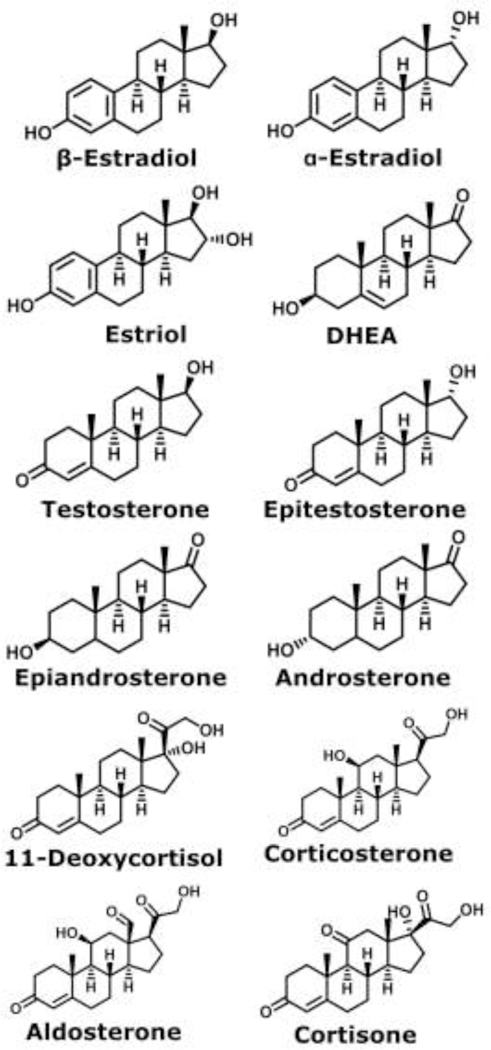
Structures for the steroids measured: α-estradiol, β-estradiol, estriol, DHEA, testosterone, epitestosterone, epiandrosterone, androsterone, 11-deoxycortisol, corticosterone, aldosterone, and cortisone.
The current study examines the use of metal adducted multimer formation to resolve steroid isomers through TWIMS in a mixture. Here we present the separation of monomer, dimer, and trimer metal adducted species in a mixture. Additionally, the presence of heterodimer formation is discussed. The resolution values were also calculated for the isomer features in a mixture. From this work, TWIMS showed potential in the separation of steroid isomers in mixtures, without prior chromatographic separation.
Experimental Methods
Solution Preparation.
Testosterone, androsterone, β-estradiol, α-estradiol, epiandrosterone, cortisone, corticosterone, 11-deoxycortisol, lithium acetate, sodium acetate, potassium acetate, and water were purchased from Sigma-Aldrich (St. Louis, MO, USA). Aldosterone, dehydroepiandrosterone (DHEA), and methanol were purchased from Fisher Scientific (Pittsburg, PA, USA). Steroid solutions contained 25 μM of each steroid for the specified mixtures with 100–150 μM of lithium acetate, sodium acetate, or potassium acetate. The complex mixture contained 10 μM of all steroids and 100 μM of lithium acetate and 100 μM of potassium acetate.
Ion Mobility Spectrometry and Mass Spectrometry.
TWIMS-MS experiments were carried out on a Waters Synapt G2-S HDMS instrument (Milford, MA, USA). All samples were introduced via nano-electrospray ionization (nESI) using a custom-built ion source interface operated in positive ion mode. The interface was designed to accommodate nESI emitters made from Pyrex melting point capillaries (Corning, NY, USA) using a vertical micropipette puller. The capillary voltage was maintained between 0.9 – 1.5 kV. Sample cone voltage and source temperature were maintained at 10 V and 80°C, respectively. TWIMS parameters were held constant for all experiments: 60 mL/min nitrogen gas flow, 40 V wave height, and 600 m/s wave velocity. The mass spectra and TWIMS arrival time distributions (ATDs) were extracted and analyzed using DriftScope 2.7 and MassLynx 4.1 (Waters), with further analysis and graphing being performed in Igor Pro 7.0 (WaveMetrics; Lake Oswego, OR, USA) and SigmaPlot 13.0 (Systat; Chicago, IL, USA).
Resolution and Collision Cross Section Calculations.
Adjacent peaks of TWIMS ATDs were fit with a Gaussian curve in Igor Pro 7.0. This yielded the centroid arrival time (t) and the width of the ATD peak at half maximum (WFWHM). The resolution (Rs) was then calculated through Equation 1, where WFWHM,avg corresponds to the average of both adjacent peak widths.
| Equation 1 |
The centroid arrival times (t) were calibrated to analyte CCSs using polyalanine ions as standards by following well-established procedures as previously described elsewhere.33 CCSs were measured four times on different days and the average and standard deviations of the measurements were calculated. For measurement of the error of the CCS, the average CCS were compared to those for previously measured single-component standards.33
Results and Discussion
Arrival Time Distributions for Isomer Mixtures.
Extracted ATDs for several metal adducted dimer species are presented in Figure 2. Corticosterone and 11-deoxycortisol were separated in a mixture as their lithiated (Rs = 0.91), sodiated (Rs = 3.08), and potassiated dimer (Rs = 2.4) species, but were optimally separated as their sodiated dimers (Figure 2a–b). Additionally, it was noted that corticosterone had lower ionization efficiency than 11-deoxycortisol in all dimer adducts, resulting in lower signal at the same concentration. Different features of α-estradiol and β-estradiol were also detected as their potassiated dimer adducts, although these features were not well resolved (Rs = 0.71) (Figure 2c). Androsterone and epiandrosterone were baseline separated as lithiated dimer adducts as standards in Rister et al.33; however, the two species form a heterodimer in a mixture, where one androsterone species and one epiandrosterone species adduct to the same metal (Figure 2d). We note here that the feature assigned as the heterodimer did not appear in pure standards for androsterone and epiandrosterone illustrated in ATDs in Rister et al.33 While androsterone was baseline resolved from epiandrosterone and the heterodimer, the heterodimer did have an effect on the resolution and analysis of epiandrosterone. The appearance of isomeric heterodimers can decrease the applicability of IMS separation of steroid multimers for quantification but should be further studied by theoretical modeling.
Figure 2.
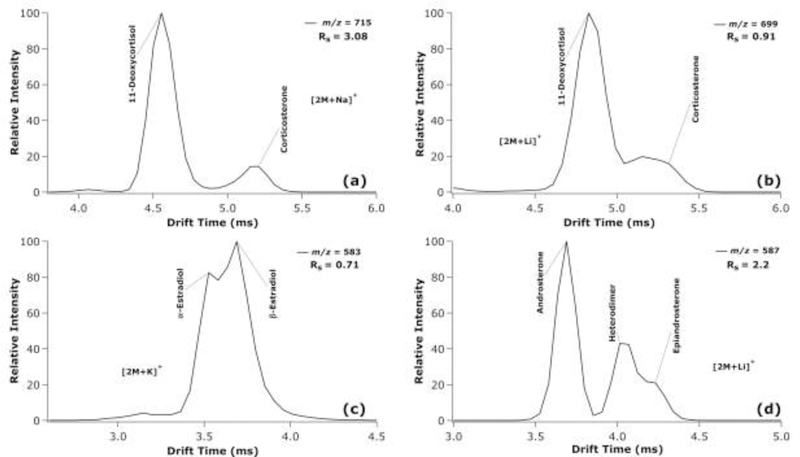
Extracted TWIMS-MS ATDs at the indicated m/z values for a mixture of corticosterone and 11-deoxycortisol as sodiated dimers (a) and as lithiated dimers (b); α-estradiol and β-estradiol as potassiated dimers (c); and androsterone and epiandrosterone as lithiated dimers (d).
The extracted ATDs for testosterone and its isomers/ isobars are illustrated in Figure 3. DHEA and epitestosterone are constitutional and stereoisomers, respectively, of testosterone. As lithiated dimers, DHEA and testosterone (Rs = 2.13) were baseline separated from each other, where sodiated dimers of the same species (Rs = 2.37) were also baseline separated, but with much lower signal observed for DHEA. In addition, epitestosterone and DHEA (Rs = 1.32) were partially separated as lithiated dimers but form a single ATD feature due to the inability for the resolution of the TWIMS analysis to distinguish their features as the sodiated dimers. Epitestosterone and testosterone were partially resolved as sodiated dimers (Rs = 1.30), but only barely distinguishable as lithiated dimers (Rs = 0.42). Compared to LC methods, Pitarch-Motellon et al. achieved baseline separation of epitestosterone and testosterone in approximately seven minutes with ultra-high performance liquid chromatography coupled to tandem mass spectrometry.34 As fully combined solutions, the same trends were observed in the ATDs, whereby lithiated dimers were best suited for separation between epitestosterone/testosterone from DHEA, while sodiated dimers were preferred for distinguishing between epitestosterone and testosterone.
Figure 3.
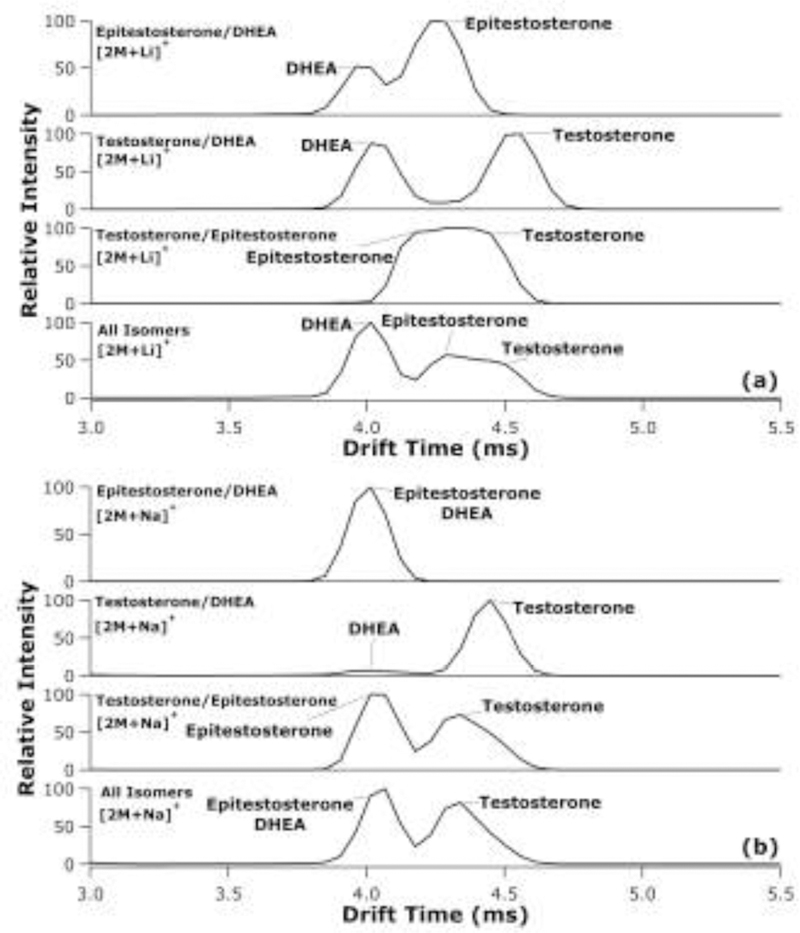
Extracted TWIMS-MS ATDs for lithiated (a) and sodiated (b) dimers from mixtures of DHEA and epitestosterone; DHEA and testosterone; testosterone and epitestosterone; and testosterone, epitestosterone, and DHEA, where two species labelled at the same peak represents an overlapping of the two ATDs.
Similar to androsterone and epiandrosterone, testosterone and epitestosterone exhibit alterations in their extracted dimer ATDs when in a mixture compared to when alone. In the lithiated dimer extracted ATDs (Figure 3a), the center of the peak for testosterone shifts to a shorter drift time when epitestosterone is present compared to the peak in the testosterone/DHEA mixture, which more closely matches the standard extracted ATDs presented in Rister et al.33 Likewise, in the sodiated dimer extracted ATDs (Figure 3b), testosterone exhibits a shift to lower drift times when mixed with epitestosterone than compared to DHEA and testosterone mixture. However, from these results, it is unclear if these are potentially heterodimer formations or alterations in the gas-phase structure formation of testosterone.
ATDs for aldosterone and cortisone as potassiated monomers, dimers, and trimers, as well as sodiated monomers and dimers are shown in Figure 4. Interestingly, aldosterone and cortisone were the only steroid isomers that could be partially separated as monomeric adducts through their potassiated adduct with Rs = 1.04 (Figure 4b). This is likely due to their more complex structures among the steroids studied, perhaps allowing more distinct adduction motifs between the two isomers. Additionally, the potassiated and sodiated aldosterone dimer had two distinct conformations, where cortisone apparently had a single conformation with a mobility falling in between the two aldosterone conformers, which is consistent with the standard ATDs shown in Rister et al.33 This could be observed in the three different features of the dimer. Aldosterone and cortisone were also the only isomer pairs exhibiting the formation of potassiated trimers, which proved to provide for the greatest separation between the isomers (Rs = 2.46). Overall, the ATDs show that the separation of steroid isomers as multimeric metal adducts varies from only partially separated features (e.g., as for estradiols or testosterone and epitestosterone) to baseline separation (e.g., as for corticosterone and deoxycortisol dimers or cortisone and aldosterone trimers).
Figure 4.
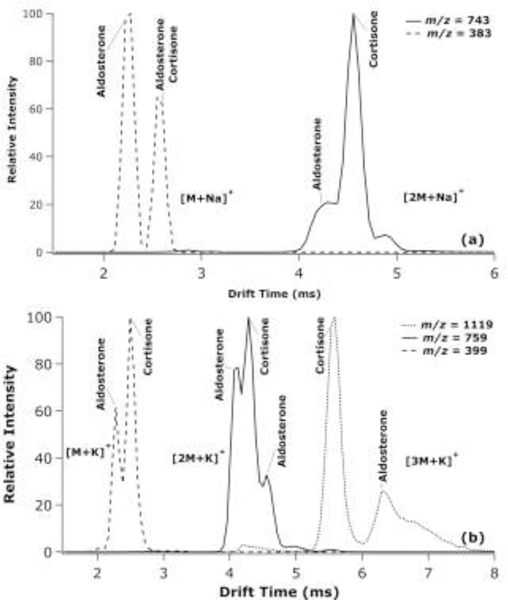
Extracted TWIMS-MS ATDs at the indicated m/z values for aldosterone and cortisone sodiated monomer and dimer species (a) and as the potassiated monomer, dimer, and trimer species (b), where two species labelled at the same peak represents an overlap of the two ATDs.
Resolution of Isomer Mixtures.
Resolution values calculated for the separation of various isomeric steroids in mixtures according to Equation 1 are shown as a function of m/z in Figure S1 of the Supporting Information. The corresponding Rs values for the various pair of isomers studied are also provided in Table S1 of the Supporting Information. Most isomeric pairs had a resolution above 1.0 for at least one adduct; the only exception was the estradiol isomers. The resolution of androsterone and epiandrosterone was the highest of the steroid isomer pairs reported by Rister et al.33; however, in a mixture, the resolution is hindered by the formation of the heterodimer, where the resolution of androsterone to the heterodimer is still greater than 2.0, but the resolution between the heterodimer and epiandrosterone falls below 1.0. Additionally, Figure S1 highlights another complication in that the best separated features of three different steroid isomer pairs have similar mass-to-charge values, each with a nominal m/z of 583. Specifically, the potassiated dimer of estradiol, the lithiated dimer of estriol, and the lithiated dimer of testosterone and its isomers have monoisotopic masses of 583.319 Da, 583.361 Da, and 583.434 Da, respectively. The isobaric nature of the optimum separation of six different steroids makes distinguishing them much more challenging, if accomplished as a single mixture.
Collision Cross Sections Measured for Isomers in Mixtures.
The nitrogen CCS and the relative error of the CCS over mass to charge range are shown in Figure 5. The values are available in tables in Table S2 of the Supporting Information and Gaussian fits overlaying the extracted ATDs are available in Figure S2–S5 of the Supporting Information. The CCS values were measured using Igor Pro 7.0 to fit a Gaussian curve to each ATD feature and determine the centroid of the peak. The ATD centroid drift time was then calibrated using standard polyalanine ions to yield nitrogen CCS values. These CCSs were compared to previously reported CCS values.33 The relative error of the CCSs for all steroids in the mixtures were below 2%. Therefore, knowledge of a previously reported CCS value obtained by analyzing a pure standard can likely be used to aid in identifying steroids in an unknown mixture.
Figure 5.
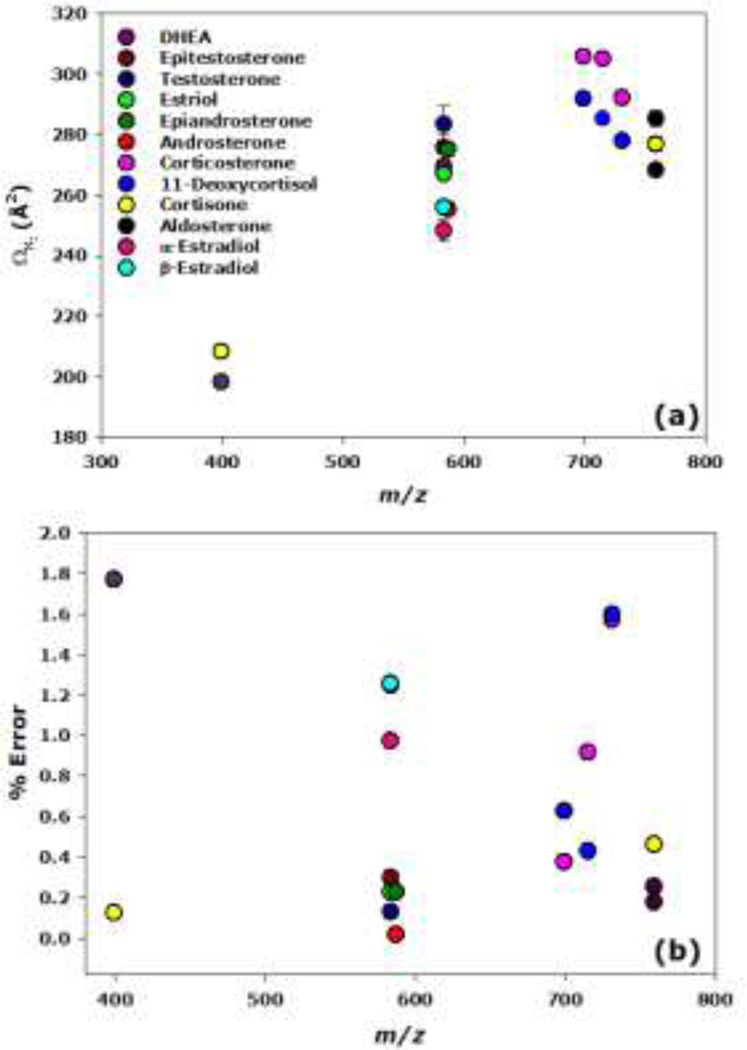
Scatter plot of nitrogen CCSs (a) and relative error in the CCSs (b) vs. mass-to-charge ratio, where error bars represent standard deviation (n = 4). The CCSs were compared to previously reported TWIMS CCS measurements.33
Analysis of a Complex Mixture.
To determine if all the above steroids could indeed be analyzed as a single mixture, all the individual steroid standards were combined. The mass spectrum and ATDs for various isomer adducts are illustrated in Figure 6. In a complex mixture, estriol could be distinguished from the testosterone isomers as the lithiated monomer at m/z 295. At m/z 399, the aldosterone and cortisone potassium adducted isomers were partially resolved. The testosterone and estradiol isomers were best resolved as their lithium and potassium dimers, respectively, at m/z 583, where DHEA is partially resolved from the features arising from epitestosterone and testosterone. Different features are also observed for the estradiol isomers, but at much lower intensity than the testosterone isomers. Then, at m/z 587, androsterone is partially resolved from the heterodimer and epiandrosterone features. Finally, although not the optimal adduct, 11-deoxycortisol and corticosterone are separated from each other as their lithium adducted dimers at m/z 699.
Figure 6.
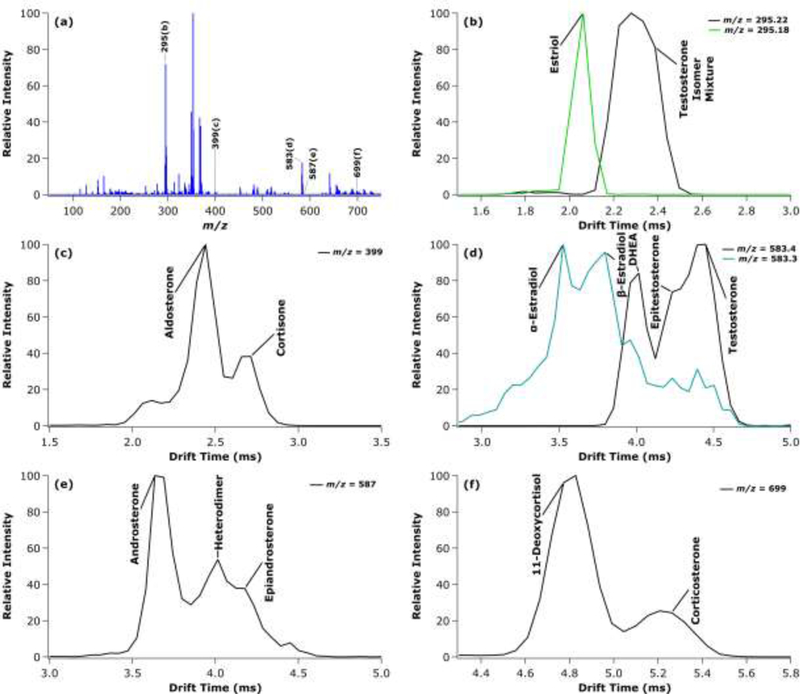
Mass spectrum of a complex steroid mixture (a) with extracted TWIMS-MS ATDs at the indicated m/z values for the estriol and testosterone isomers as lithiated monomers at m/z 295 (b); aldosterone and cortisone as potassiated monomers at m/z 399 (c); potassiated estradiol dimers and lithiated testosterone isomer dimers at m/z 583 (d); androsterone and epiandrosterone as lithiated dimers at m/z 587 (e); and 11-deoxycortisol and corticosterone as lithiated dimers at m/z 699 (f).
Unfortunately, it becomes clear from the mass spectrum in Figure 6a that the abundance of the dimers of steroid isomers (shown in the m/z between 550–750) is lower than the monomers of steroid isomers (shown in the m/z range between 250–400), due to the production of dimers by the electrospray process. Furthermore, there are additional non-isomeric heterodimer species (i.e., testosterone isomer/corticosterone isomer lithiated heterodimer at m/z 641) throughout the mass range, which could also affect quantitation through this method. As a result, the ability to perform quantitation through this TWIMS-MS method would need further investigation. Overall, this work suggests the ability to separate mixtures containing multiple groups of isomeric steroids in the millisecond time scale through TWIMS-MS without prior chromatographic analysis.
Conclusions
Compared to foregoing literature, previous works by Chouinard et al. and Rister et al. illustrated the enhanced separation of steroid metal adducted multimers using DTIMS-MS and TWIMS-MS, respectively, in single-component standards.31–33 The current study further this work by exploring the use of steroid multimers with a wider range of alkali metal adducts to enhance separation of isomers in mixtures, and without prior chromatographic separation. While TWIMS-MS has shown promise in steroid analysis, this work is the first exploring the ability of TWIMS-MS as a standalone technique to separate underivatized steroid mixtures. These findings have practical implications because the multimers studied here do indeed form in ESI-IMS-MS analysis of steroids at physiologically relevant concentrations (i.e., approximately nM range). Of course, this depends on the specific analyte and sample workup, but nonetheless presents a potentially useful analytical avenue.
In the present study, five sets of steroid isomers exhibited various degrees of separation, from the formation of distinct mobility features to baseline resolution. However, there are isomer pairs that illustrate alterations to their extracted arrival time distributions, when in a mixture compared to as standards. This can hinder the resolution and ability to quantitate these species by TWIMS-MS alone. CCSs show promise for a potential role in aiding identification of steroid isomers in unknown mixtures. Furthermore, the analysis of a mixture of multiple standards would suggest that this technique may be applicable to the separation of steroids in more complex samples. Future work can be directed in examination of the altered features / heterodimers by using isotopically labelled standards, theoretical modeling of these steroid multimer species, quantitation of steroids using TWIMS-MS, and the benefits of using multimer metal adduction with TWIMS in tandem with chromatography.
Supplementary Material
Acknowledgements
This work was supported in part by funding from the National Science Foundation, Division of Chemistry, through the Chemical Measurement and Imaging Program (award number 1507989) and through the Research Experiences for Undergraduates program (award number 1460829), which provided a summer fellowship to T.L.M. Funding from the National Institutes of Health, National Institute of General Medical Sciences, was also received through a fellowship to A.L.R. from the Molecular Mechanisms of Disease Predoctoral Training Program (award number T32GM107001). Finally, the authors thank Jessica L. Minnick and Katherine N. Schumacher for constructive comments on a draft of the manuscript.
Footnotes
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- [1].Haupt HA, Rovere GD. Anabolic steroids: A review of the literature. Am. J. Sports Med. 1984, 12, 469–484. [DOI] [PubMed] [Google Scholar]
- [2].Alda MJ, Barcelo D. Review of analytical methods for the determination of estrogens and progestogens in waste waters. Anal. Chem. 2001, 371, 437–447. [DOI] [PubMed] [Google Scholar]
- [3].Nozaki O. Steroid analysis for medical diagnosis. J. Chromatogr. A 2001, 935, 267–278. [DOI] [PubMed] [Google Scholar]
- [4].Lewis J. Steroid analysis in saliva: An overview. Clin. Biochem. Rev. 2006, 27, 139–146. [PMC free article] [PubMed] [Google Scholar]
- [5].Severi G, Morris HA, MacInnis RJ, English DR, Tilley W, Hopper JL, Boyle P, Giles G. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol., Biomarkers Prev. 2006, 15, 86–91. [DOI] [PubMed] [Google Scholar]
- [6].Guddat S, Thevis M, Kapron J, Thomas A, Schanzer W. Application of FAIMS to anabolic androgenic steroids in sport drug testing. Drug Test Anal. 2009, 1, 545–553. [DOI] [PubMed] [Google Scholar]
- [7].Morrow L, Porcu P. Neuroactive steroid biomarkers of alcohol sensitivity and alcoholism risk In The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes, (Eds.: Ritsner M), Springer Science+Business Media, Heidelberg, 2009, pp 47–57. [Google Scholar]
- [8].Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Arlt W, Biehl M, Taylor AE, Hahner S, Libe R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, Porfiri E, Opocher G, Bertherat J, Mantero F, Allolio B, Terzolo M, Nightingale P, Shackleton CH, Bertagna X, Fassnacht M, Stewart PM. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011, 96, 3775–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Van Renterghem P, Van Eenoo P, Sottas PE, Saugy M, Delbeke F. A pilot study on subject-based comprehensive steroid profiling: novel biomarkers to detect testosterone misuse in sports. Clin. Endocrinol. 2011, 75, 134–140. [DOI] [PubMed] [Google Scholar]
- [11].Gouveia MJ, Brindley PJ, Santos LL, Correia da Costa JM, Gomes P, Vale N. Mass spectrometry techniques in the survey of steroid metabolites as potential disease biomarkers: a review. Metabolism 2013, 62, 1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Settlage J, Oglesby T, Rajasekaran A, Williard C, Scott G. The importance of chromatographic resolution when analyzing steroid biomarkers. Steroids 2015, 99, 45–48. [DOI] [PubMed] [Google Scholar]
- [13].Liu R, Zhou JL, Wilding A. Simultaneous determination of endocrine disrupting phenolic compounds and steroids in water by solid-phase extraction-gas chromatography-mass spectrometry. J. Chromat. A 2004, 1022, 179–189. [DOI] [PubMed] [Google Scholar]
- [14].Kolakowski BM, Mester Z. Review of applications of high-field asymmetric waveform ion mobility spectrometry (FAIMS) and differential mobility spectrometry (DMS). Analyst 2007, 132, 842–864. [DOI] [PubMed] [Google Scholar]
- [15].Kaur-Atwal G, Reynolds JC, Mussell C, Champarnaud E, Knapman TW, Ashcroft AE, O’Connor G, Christie SD, Creaser CS. Determination of testosterone and epitestosterone glucuronides in urine by ultra performance liquid chromatography-ion mobility-mass spectrometry. Analyst 2011, 136, 3911–3916. [DOI] [PubMed] [Google Scholar]
- [16].Kirk DN, Toms HC, Douglas C, White KA, Smith LE, Latif S, Hubbard RWP. A survey of the high-field 1H NMR spectra of the steroid hormones, their hydroxylated derivatives, and related compounds. J. Chem. Soc. Perkin Trans. 2 1990, 1567–1594 [Google Scholar]
- [17].Giese RW. Measurement of endogenous estrogens: analytical challenges and recent advances. J. Chromatogr. A 2003, 1000, 401–412. [DOI] [PubMed] [Google Scholar]
- [18].Huang G, Chen H, Zhang X, Cooks RG, Ouyang Z. Rapid screening of anabolic steroids in urine by reactive desorption electrospray ionization. Anal. Chem. 2007, 79, 8327–8332. [DOI] [PubMed] [Google Scholar]
- [19].Kasal A, Budesinsky M, Griffiths WJ. Spectroscopic methods of steroid analysis In Steroid Analysis, (Eds.: Makin HLJ, Gower DB), Springer Science+Business Media, Heidelberg, 2010, pp 27–161. [Google Scholar]
- [20].Ray JA, Kushnir MM, Yost RA, Rockwood AL, Meikle AW. Performance enhancement in the measurement of 5 endogenous steroids by LC-MS/MS combined with differential ion mobility spectrometry. Clin. Chim. Acta 2015, 438, 330–336. [DOI] [PubMed] [Google Scholar]
- [21].Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH Jr. Ion mobility-mass spectrometry. J. Mass Spectrom. 2008, 43, 1–22. [DOI] [PubMed] [Google Scholar]
- [22].Shvartsburg AA, Smith RD. Fundamentals of traveling wave ion mobility spectrometry. Anal. Chem. 2008, 80, 9689–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Giles K, Williams JP, Campuzano I. Enhancements in travelling wave ion mobility resolution. Rapid Commun. Mass Spectrom. 2011, 25, 1559–1566. [DOI] [PubMed] [Google Scholar]
- [24].Campuzano I, Bush MF, Robinson CV, Beaumont C, Richardson K, Kim H, Kim HI. Structural characterization of drug-like compounds by ion mobility mass spectrometry: Comparison of theoretical and experimentally derived nitrogen collision cross sections. Anal. Chem. 2012, 84, 1026–1033. [DOI] [PubMed] [Google Scholar]
- [25].Lapthorn C, Pullen F, Chowdhry BZ. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: Separating and assigning structures to ions. Mass Spectrom. Rev. 2013, 32, 43–71. [DOI] [PubMed] [Google Scholar]
- [26].Cumeras R, Figueras E, Davis CE, Baumbach JI, Gracia I. Review on ion mobility spectrometry. Part 1: Current instrumentation. Analyst 2015, 140, 1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Clowers BH, Hill HH Jr. Influence of cation adduction on the separation characteristics of flavonoid diglycoside isomers using dual gate - ion mobility - quadrupole ion trap mass spectrometry. J. Mass Spectrom. 2006, 41, 339–351. [DOI] [PubMed] [Google Scholar]
- [28].Huang Y, Dodds ED. Ion mobility studies of carbohydrates as group I adducts: isomer specific collisional cross section dependence on metal ion radius. Anal. Chem. 2013, 85, 9728–9735. [DOI] [PubMed] [Google Scholar]
- [29].Huang Y, Dodds ED. Ion-neutral collisional cross sections of carbohydrate isomers as divalent cation adducts and their electron transfer products. Analyst 2015, 140, 6912–6921. [DOI] [PubMed] [Google Scholar]
- [30].Ahonen L, Fasciotti M, Gennas GB, Kotiaho T, Daroda RJ, Eberlin M, Kostiainen R. Separation of steroid isomers by ion mobility mass spectrometry. J. Chromatogr. A 2013, 1310, 133–137. [DOI] [PubMed] [Google Scholar]
- [31].Chouinard CD, Cruzeiro VW, Roitberg AE, Yost RA. Experimental and theoretical investigation of sodiated multimers of steroid epimers with ion mobility-mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chouinard CD, Beekman CR, Kemperman RHJ, King HM, Yost RA. Ion mobility-mass spectrometry separation of steroid structural isomers and epimers. Int. J. Ion Mobility Spectrom. 2017, 20, 31–39. [Google Scholar]
- [33].Rister AL, Martin TL, Dodds ED. Application of group I metal adduction to the separation of steroids by traveling wave ion mobility spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pitarch-Motellon J, Sancho JV, Ibanez M, Pozo O, Roig-Navarro AF. Determination of selected endogenous anabolic androgenic steroids and ratios in urine by ultra high performance liquid chromatography tandem mass spectrometry and isotope pattern deconvolution. J. Chromatogr. A 2017, 1515, 172–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


