Abstract
Objectives:
To describe the prevalence and epidemiology of antimicrobial use (AU) in nursing home residents.
Design:
One-day point prevalence survey.
Setting and participants:
Nine nursing homes in four states; 1,272 eligible residents.
Measurement:
Frequency of antimicrobials prescribed, drug name, start date, duration, route, rationale, and treatment site. AU prevalence per 100 residents overall and by resident characteristic.
Results:
AU prevalence was 11.1% (95% confidence interval, 9.4%–12.9%) and varied by resident characteristics. Most (32%) antimicrobials were given for urinary tract infection. For 38% of AU, key prescribing information was not documented.
Conclusion:
Opportunities to improve AU documentation and prescribing exist in nursing homes.
Keywords: Nursing home, surveillance, antimicrobial use, antibiotic, prescribing
Harms from antimicrobial overuse have been well documented and include adverse drug events, drug interactions, Clostridium difficile infection, and colonization or infection with resistant organisms.1,2 Improving the use of antimicrobials in health care to reduce the threat of emerging resistance is a national priority.1 Effective interventions in nursing homes (NHs) require understanding the epidemiology of antimicrobial use and prescribing practices in this setting. To build upon surveillance activities of the Centers for Disease Control and Prevention (CDC) in hospitals,3,4 we piloted a 1-day point prevalence survey of antimicrobial use (AU) in a small number of US nursing homes. The goal was to obtain primary data to inform the design and development of a larger prevalence survey effort in U.S. nursing homes.
Methods
Setting and Participants
During December 2013 to May 2014, 1-day point prevalence surveys of AU were performed in a convenience sample of nine nursing homes located within four Emerging Infections Program (EIP) sites (CT, MN, NM, and NY). To be eligible, NHs were required to be certified by the Centers for Medicare & Medicaid Services (CMS) and have ≥ 120 licensed beds. Participation was voluntary. Within participating NHs, all residents who had been in the facility for >24 hours before the prevalence survey date were eligible for inclusion.
Data Collection
Participating facilities were asked to designate a Nursing Home Team Leader (registered nurse or licensed practical nurse) to coordinate survey activities and team members to collect selected demographic and clinical data on the prevalence survey date. An EIP Team, comprising trained epidemiologists and surveillance officers with expertise in medical chart abstraction, subsequently reviewed the medical records of eligible residents to collect additional clinical and AU data. Antimicrobials were defined as systemic (oral, enteral, or parenteral) or inhaled antibacterial, antimycobacterial agents, antifungal, or antiviral agents. The AU data included the drug name, start date, duration, route, rationale, and treatment site. For rationale, the EIP Team was instructed to use specific information documented in records and not make assumptions about why an agent was being administered. Response options included “therapeutic” if intended to treat an active or suspected infection, “prophylactic” if intended to prevent the occurrence of infection in a resident without signs or symptoms of that illness, or “not documented” if documentation is not present or inadequate to make a determination. Both teams used standardized data collection forms, written instruction manuals, and underwent webinar training conducted by CDC project staff. Survey dates were required to be on Monday through Friday and selected by participating NHs and EIP staff.
Data Analysis
The characteristics of NHs and eligible residents were described. The prevalence of AU per 100 residents was calculated overall and by resident characteristics. The frequency of antimicrobials prescribed by name and indication (treatment site and rationale) was calculated. AU prescribing practices were evaluated by calculating the proportion of antimicrobials with each of the five prescribing elements documented (start date, duration/end date, route, rationale, and treatment site). The χ2 test was used to compare differences in proportions with a P < .05 considered statistically significant. Analysis was performed using SAS software version 9.3 (Cary, NC). A protocol for this pilot was submitted for review by a CDC Human Subjects Advisor and was determined to not be human subjects research. Institutional review boards at the state health departments reviewed the protocol and had the same determination or approved the survey with the requirement for facility informed consent.
Results
In the nine participating NHs, the median number of beds was 130 (range, 104–229). There were 1272 eligible residents (98% of all residents present on the survey date), 30% were male, 14% were short stay (CMS definition of expected length of stay < 100 days), and median age was 85 years (range, 21–91). In total, 141 eligible residents received 160 antimicrobials (range, 6–34 antimicrobials per NH). AU prevalence was 11.1% (95% confidence interval, 9.4%−12.9%) and highest among short-stay residents (21.2%) and those with devices (23.5%) (Table 1). Of the 160 antimicrobials administered, 66% (106) were documented for therapeutic use, 23% (37) for prophylactic use, and 11% (17) were not documented/unable to determine. The most common treatment sites documented were the urinary tract (32%, 52), skin (29%, 19), and respiratory tract (26%, 42). Oseltamivir was the most commonly used antimicrobial, with the indication primarily for prophylaxis or “not documented” (Figure 1). For therapeutic use, cephalexin (12%), doxycycline (10%), ciprofloxacin, and sulfamethoxazole/trimethoprim (both 8%) were most common. Documentation of the antimicrobial prescribing elements ranged from 73% for duration, to 89% for rationale, and 96% for start date, route, and treatment site. Overall, 62% of antimicrobials had all five prescribing elements documented, ranging from 50% to 84% per nursing home.
Table 1.
Resident Characteristics and Antimicrobial Use Prevalence per 100 Residents From a 1-Day Point Prevalence Survey in Nine Nursing Homes
| Resident Characteristic | N (%) With Characteristic | Antimicrobial Use Prevalence per 100 Residents | P Value* |
|---|---|---|---|
| Gender | .788 | ||
| Male | 375 (30) | 11.7 | |
| Female | 897 (70) | 11.2 | |
| Age, y | .2186 | ||
| ≤ 85 | 590 (46) | 12.5 | |
| > 85 | 686 (54) | 10.4 | |
| Resident Type | <.0001 | ||
| Short stay | 183 (14) | 21.2 | |
| Long stay | 1089 (86) | 9.7 | |
| Device use† | <.0001 | ||
| Yes | 102 (8) | 23.5 | |
| No | 1170 (92) | 10.3 | |
| Total residents | 1272 | 11.1 |
χ2 test P value.
Indwelling urinary catheter, vascular device, ventilator or tracheostomy, percutaneous endoscopic gastrostomy or jejunostomy tube present at the time of the prevalence survey.
Fig. 1.
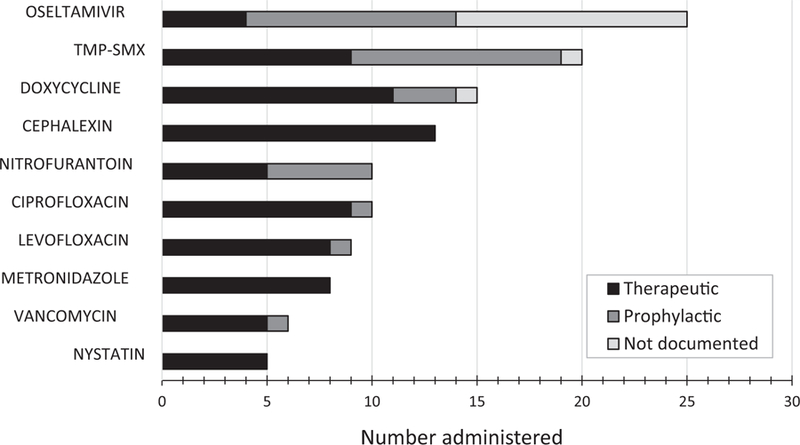
Ten most commonly administered antimicrobials (n = 121, 76%) and documented rationale for use from among 160 antimicrobials administered during a 1-day point prevalence survey in nine nursing homes. TMP-SMX, sulfamethoxazole-trimethoprim.
Conclusions
The overall prevalence of AU among NH residents was 11% but increased to 21% for short-stay residents and 24% for residents with invasive medical devices. The most commonly used drug was oseltamivir; this was attributed to confirmed influenza in one NH prompting prophylaxis of other residents. The most common treatment site was the urinary tract, with one in three antimicrobials given for the treatment or prevention of urinary tract infection (UTI). Consistent with previous reports of UTI as a major cause of antibiotic use and misuse in NH residents,5–8 our findings reinforce that AU for UTI should remain a priority target for stewardship activities in NHs. Analyses like this, that identify factors and explain variations in antimicrobial use at the NH level are necessary to develop appropriate interventions and guide stewardship activities.2,9
We found that 62% of the antimicrobials administered had all the five key prescribing elements documented, with wide variation among individual NHs noted. Adequate medical record documentation is necessary for antimicrobial stewardship programs to function.2 In 2015, the CDC released “The Core Elements of Antibiotic Stewardship for Nursing Homes,”10 outlining steps all NHs should take to improve antibiotic prescribing practices and reduce inappropriate use, including audits of the completeness of antibiotic prescribing documentation—dose, route, duration, and indication (ie, rationale and treatment site) for every course of antibiotics. Suboptimal documentation will undermine efforts to monitor and improve antimicrobial use and thus the success of antibiotic stewardship programs.
Efforts to improve the documentation of antibiotic prescribing elements are necessary, and evaluation of AU appropriateness and implementation of initiatives to reduce unnecessary use should be prioritized. These findings highlight the importance of prevalence surveys to better understand AU practices in NHs.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the Agency for Toxic Substances and Disease Registry. Funding for Emerging Infections Program sites was provided by CDC through a cooperate agreement.
An abstract of this work was presented at: Society for Healthcare Epidemiologists of America Spring Conference, 2015. Prevalence of Antimicrobial Use and Documentation Assessment in Nine U.S. Nursing Homes. Orlando, FL, May 14th, 2015.
Footnotes
All authors report no relevant financial support and conflicts of interest.
References
- 1.The White House. National Strategy for Combating Antibioticresistant Bacteria Available at: http://www.whitehouse.gov/sites/default/files/docs/carb_national_strategy.pdf; 2014. Accessed September 30, 2014.
- 2.Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs Atlanta, GA: US Department of Health and Human Services, CDC; Available at: http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html; 2014. Accessed September 30, 2014. [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health careeassociated infections. N Engl J Med 2014;370:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magill SS, Edwards JR, Bamberg W, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014;312:1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latour K, Catry B, Broex B, et al. Indications for antimicrobial prescribing in European nursing homes: Results from a point prevalence survey. Pharmacoepidemiol Drug Saf 2012;21:937–944. [DOI] [PubMed] [Google Scholar]
- 6.Rotjanapan P, Dosa D, Thomas KS. Potentially inappropriate treatment of UTI in two Rhode Island nursing homes. Arch Intern Med 2011;171:438–443. [DOI] [PubMed] [Google Scholar]
- 7.Loeb M, Bentley D, Bradley S, et al. Antibiotic use in Ontario facilities that provide chronic care. J Gen Intern Med 2001;16:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips CD, Adepoju OE, Stone ND, et al. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatrics 2012;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridkin SK, Baggs J, Fagan R, et al. Vital Signs: Improving antibiotic use among hospitalized patients. MMWR. Morbidity and Mortality Weekly Report 2014;63. [PMC free article] [PubMed]
- 10.Centers for Disease Control and Prevention. The Core Elements of Antibiotic Stewardship for Nursing Homes Available at: www.cdc.gov/longtermcare/pdfs/core-elements-antibiotic-stewardship.pdf. Accessed January 4, 2016.


