Abstract
Introduction
Animal models of Alzheimer's disease show that exercise may modify β-amyloid (Aβ) deposition. We examined the effect of a 16-week exercise intervention on cortical Aβ in patients with mild-to-moderate Alzheimer's disease.
Methods
Thirty-six patients with Alzheimer's disease were randomized to either one hour of aerobic exercise three times weekly for 16 weeks or usual care. Pre and post intervention, 11Carbon-Pittsburgh compound B positron emission tomography was carried out to assess cortical Aβ, and quantified using standardized uptake value rations (SUVRs).
Results
The intervention showed no effect on follow-up SUVRs in a covariance analysis with group allocation, baseline intervention SUVR, age, sex, and baseline Mini–Mental State Examination as predictors. Change in SUVRs did not correlate with changes in measures of physical or aerobic fitness.
Discussion
The present findings do not support an effect of exercise on Aβ. However, the relatively short intervention period may account for a lack of efficacy. Further studies should test earlier and longer interventions.
Keywords: 11C-PiB-PET, Aerobic exercise, Alzheimer's disease, β-amyloid, Exercise, Dementia, Intervention, PET, Randomized
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disease characterized by initially asymptomatic cortical deposition of the protein β-amyloid (Aβ), followed by deposition of tau and, further downstream, by neurodegeneration and synaptic dysfunction, at which time cognitive impairment will be present or ensue as the disease progresses to dementia [1].
Several lines of evidence have emerged to support the ability of physical activity to prevent and possibly treat AD. First, epidemiological studies have found a robust association between physical activity and a reduced risk of dementia, including AD [2], [3], [4]. Second, intervention studies in mild cognitive impairment [5] and AD [6], [7], [8], [9] indicate that physical exercise improves cognitive function and other symptoms of AD. Third, animal studies have shown similar effects [10], [11], [12] and further extended these findings. Specifically, exercise seems to reduce pathological changes, such as Aβ deposition [10], [11], [13], [14], tau pathology [15], and hippocampal atrophy [11], [16] in animal models of AD. In humans, most of the studies have focused on exercise and hippocampal volume [17]. Results have been divergent, although most studies have shown a lack of association in both intervention [18], [19], [20], [21], [22] and observational studies [23], [24], [25], [26], [27], [28]. There have been discrepancies in results from observational studies examining the association between Aβ or tau and physical activity [29], [30], [31], [32], [33], [34]. In one study, Aβ measured using amyloid PET, but not cerebrospinal fluid (CSF) Aβ levels, was associated with physical activity levels [29]. In general, a relatively high concordance regarding amyloid positivity assessed by CSF- and PET-derived measures has been reported, although correlations within positive and negative groups are low between the two measures [35]. Hence, these two measures of Aβ may not be interchangeable. Amyloid PET is a measure of brain fibrillary Aβ [36], whereas CSF Aβ may instead reflect soluble species of Aβ and, as such, is only an indirect measure of fibrillary Aβ [37]. CSF Aβ has also been reported to be sensitive to variations in Aβ precursor protein processing and Aβ production [38], [39], and CSF Aβ concentrations may change earlier in the disease process [40]. These observations indicate that amyloid PET and CSF measures may reflect different aspects of Aβ biology. It may be further speculated that physical activity affects specific aspects of Aβ biology, which is best captured by one or the other biomarker (e.g., fibrillar vs. soluble Aβ), reflected in the apparently discrepant effect of physical activity on PET- versus CSF-assessed Aβ levels [29]. In a previous study using a subsample from the same randomized controlled trial (RCT) as in the present study, 16 weeks of aerobic exercise did not affect the CSF levels of the AD biomarkers, including Aβ [41]. In the present study, we examined whether aerobic exercise in a substudy population from the same RCT modulated cortical Aβ in patients with mild AD assessed using a different modality (11Carbon-Pittsburgh compound B positron emission tomography [11C-PiB-PET]). To our knowledge, this is the first study to assess the effects of an aerobic exercise intervention on Aβ assessed by PET imaging.
2. Methods
2.1. Study design and population
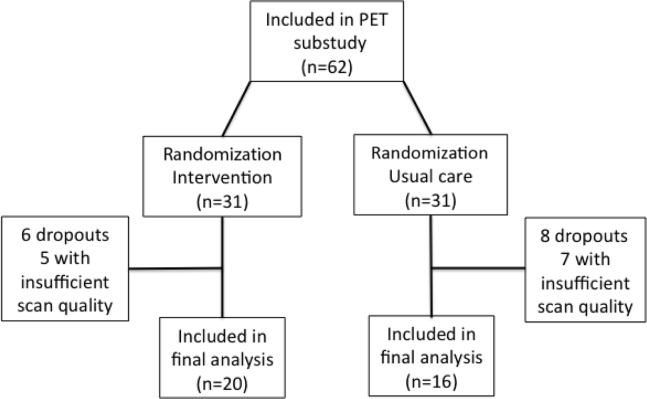
The present study reports the results of an imaging substudy from the Preserving Cognition, Quality of Life, Physical Health and Functional Ability in Alzheimer's Disease: The Effect of Physical Exercise (ADEX) study, a multicenter, single-blinded RCT of physical exercise in patients with mild AD. Details of the rationale and study design can be found elsewhere [42], as well as the main findings [7], [43]. The intervention comprised 16 weeks of moderate- to high-intensity aerobic exercise three times weekly for one hour. The exercise sessions were conducted in groups supervised by a trained physiotherapist. The first four weeks were a ramp-up period in which participants were familiarized with the exercise equipment and emphasis was put on strength exercises. This was done to avoid injuries because initial piloting indicated injuries as a potential problem [44]. For the main study, 200 participants from eight centers were recruited and underwent clinical assessment of cognitive function, activities of daily living, physical function, aerobic fitness, neuropsychiatric symptoms at baseline, and took part in a 16-week follow-up. All assessments were performed by assessors blinded to group allocation. Inclusion criteria for the main study were the following: (1) AD according to NINCDS-ADRDA Alzheimer's Criteria and DSM-IV codes; (2) between 50 and 90 years of age; (3) a Mini–Mental State Examination score of more than 19; (4) at least three months of stable doses if receiving antidementia medication or mood-stabilizing medication; and (5) informed consent. Exclusion criteria included the following: (1) severe psychiatric illness; (2) alcohol or drug abuse within the last two years; (3) participation in aerobic exercise (moderate to high intensity) more than twice weekly on a regular basis; and (4) any medical condition precluding participation in the exercise program (e.g., severe neurological or medical illness and presence of several cardiovascular risk factors). Further inclusion criteria for the imaging substudy included the absence of contraindications for undergoing magnetic resonance imaging (MRI). All participants recruited at two (Rigshospitalet, Roskilde) of the eight centers were asked to participate in the imaging substudy. They were also asked to undergo MRI and PET at baseline and at 16-week follow-up. A total of 72 participants were recruited at the two centers, 54 of whom were included in the imaging substudy and randomized. Five participants dropped out during the intervention (dropout rate 9.3 %), and the quality of scans for an additional 13 was insufficient to enable analysis, leaving a sample of 36 participants (20 intervention group; 16 usual care) for the final analysis (see Fig. 1). The ADEX trial was approved by the Committees of Biomedical Research Ethics for the Capital Region (protocol no.: H-3-2011-128) and by the Danish Data Protection Agency (file no.: 30-0718). The trial was registered at ClinicalTrials.gov (identifier: NCT01681602) on September 10, 2012.
Fig. 1.
Flowchart of patients in the study.
2.2. Cognitive tests
Cognitive assessment was carried out using Mini–Mental State Examination for global cognitive impairment [45].
2.3. Activities of Daily Living scale
For assessment of activities of daily living function the Alzheimer's Disease Cooperative Study–Activities of Daily Living scale [46] was used.
2.4. Measures of aerobic fitness and physical function
Several different tests were used to assess aerobic fitness and physical function. The 6-minute Astrand Cycle Ergometer test (Monark Ergomedic 839E; Monark Exercise AB, Sweden) was used to estimate the maximal oxygen uptake based on workload and average heart rate (HR) during the last minute of the 6-minute cycle test, corrected for age and body weight. Peak oxygen uptake (VO2max) was used as a measure of aerobic fitness [47]. For the Timed Up and Go test (TUG), which was used to assess general mobility, the amount of time it took participants to rise from a chair, walk 3 meters as quickly and safely as possible, turn around, walk back, and sit down were measured [48]. For assessment of lower extremity strength and endurance, the 30-s sit-to-stand test (STS) was used and measures the number of stands completed with the arms folded across the chest within 30 seconds [49]. Walking endurance was assessed with a 400-m walk test. The participants were instructed to walk as quickly as possible without support or sitting down, although standing breaks of up to one minute were allowed [50]. Finally, usual gait speed (m/s) was assessed with a 10-m walk test [51].
2.5. Physical activity
Level of physical activity at baseline was assessed using the Physical Activity Scale for the Elderly [52], which was filled out by the patient's caregiver.
2.6. Exercise load (attendance and intensity)
To assess attendance and intensity of training, the physiotherapist instructor at each center completed a training log. Attendance ratio was defined as the number of attended exercise sessions over total number of sessions. Exercise intensity was based on the per-session average HR recorded using continuous monitoring during exercise (including rest periods). Average HR for all sessions was calculated, with intensity defined as average HR over maximum expected HR (220 minus participant age). To obtain total exercise load, measures for attendance ratio and intensity were multiplied.
2.7. Magnetic resonance imaging
Baseline and follow-up MRI scans were performed on a 3 Tesla Siemens Trio scanner. Both baseline and follow-up scans were acquired on the same scanner. For the purpose of coregistration to the PET image, a T1-weighed magnetization-prepared rapid gradient echo sequence (TE 3.04 ms, TR 1550 ms, FoV read 256 mm, FoV phase 100%, 192 slices) was used.
The T1-weighted MRI scans were segmented into gray matter, white matter, and CSF using Statistical Parametric Mapping 5 (Wellcome Department of Cognitive Neurology, London, UK). Regions of interest were automatically delineated on each subject's MRI in a user-independent fashion with the PVElab software package (Neurobiology Research Unit, Rigshospitalet, Copenhagen, Denmark) [53].
2.8. PET imaging
PET images were acquired using either a Siemens Biograph 40 or Biograph TruePoint 64 PET/CT scanner (Siemens Healthcare, Erlangen, Germany) and the tracer 11C-PiB-PET, which binds with affinity to Aβ. Both baseline and follow-up scans were acquired on the same scanner. 11C-PIB was administered as a bolus with a mean activity of 421 MBq (183-754 MBq) per injection, with image acquisition 40–70 minutes after injection as 6 × 5-minute frames. The time window 40- to 70-minute postinjection was used because it provides stability and an effective contrast between healthy subjects and patients with AD [54].
Default random, scatter, and dead time correction and low-dose CT-based attenuation correction were applied (120 kVp, 40 mA) for all images. Image reconstruction was performed using a 2D ordered-subsets expectation maximization algorithm with 6 iterations and 16 subsets and filtered with a 5-mm (full-width, half-maximum) gaussian filter.
2.9. Image analysis
PET images were coregistered to MRI images (Statistical Parametric Mapping 5; Wellcome Department of Cognitive Neurology) and evaluated by visual inspection by the same reader trained and blinded to time of scan and group allocation. Mean voxel movement between frames (5–10 minutes) was measured using AIR 5.2.5 (LONI, UCLA, CA, USA) [55]. When movement exceeded 3 mm, movement correction was applied. The regional PET standardized uptake value (SUV) was measured by summing the data of consecutive time frames from regional gray matter voxels. The cerebellar gray matter (excluding vermis) was used as the reference region for normalization [56], [57]. The global 11C-PiB binding was expressed as the mean standardized uptake value ratio (SUVR) of six regions: lateral temporal cortex (including the superior, medial, and inferior lateral gyri), posterior cingulated gyrus, anterior cingulated gyrus, precuneus, parietal cortex, and lateral prefrontal cortex (including ventrolateral and dorsolateral areas), as previously described [58]. Furthermore, a voxel-based analysis was carried out to further confirm any findings from the initial analysis. Supplementary Material contains details about this. Analysis of the PET images was carried out blinded to the group allocation of participants.
2.10. Statistical analysis
Student's unpaired-samples t-test and the χ2 test were used to compare baseline demographic and clinical characteristics between groups. For analysis of the effects of the intervention on cortical Aβ, we performed analysis of covariance with SUVRs at follow-up as dependent variables and group allocation, baseline SUVR, age, baseline Mini–Mental State Examination, and sex as covariates as independent variables. Analysis of covariance was chosen because it has been shown to increase power in the analysis of results from RCTs [59]. To test the relationship between improvement in aerobic fitness and change in cortical Aβ, Spearman's rank correlation between change in estimated VO2max and change in SUVR was calculated. Similarly, Spearman's rank correlation was used to assess the relationship between change in physical fitness and performance as measured by changes in the TUG, STS, 10-m and 400-m walk tests, as well as a change in SUVR. Finally, correlation between exercise load and change in SUVR was assessed. All correlations were assessed in the exercise group only. Student's paired-samples t-test was used to assess change from baseline to follow-up within the exercise group. This was carried out to assess whether the intervention had improved the aerobic fitness and physical function of the participants, as has been shown in the whole study population [43].
Statistical significance was set at P < .05 (two-tailed). Statistical analysis was carried out using Intercooled Stata 9.2 for Macintosh (Stata Corporation, USA).
3. Results
3.1. Baseline characteristics
The final analysis included 36 subjects, 20 of whom were randomized to the intervention and 16 to usual care. There were no significant differences in baseline characteristics, except for baseline 11C-PiB binding, where subjects in the intervention group had a significantly higher level of binding (P = .049) (see Table 1). One patient from the intervention group (SUVR 1.16) and one patient from the usual care group (1.20) had a mean SUVR of <1.6 indicative of a PiB-negative scan. All analyses were also carried out excluding those two patients without changing the results.
Table 1.
Baseline demographics and clinical variables
| Variables | Intervention (n = 20) | Usual care (n = 16) | P value |
|---|---|---|---|
| Age, years, mean (SD) | 68.7 (7.6) | 70.4 (7.4) | 0.43 |
| Sex distribution (female/male) | 10/10 | 8/8 | 1 |
| Baseline MMSE, mean (SD) | 25.5 (3.0) | 25.8 (2.7) | 0.87 |
| Baseline ADCS-ADL, baseline mean (SD) | 68.1 (10.0) | 68.2 (7.5) | 0.76 |
| Baseline PASE, mean (SD)∗ | 83.6 (43.1) | 95.7 (39.0) | 0.26 |
| Baseline estimated VO2max† (mL/kg/min) | 24.7 (6.4) | 25.7 (6.4) | 0.49 |
| Baseline SUVR‡ | 2.35 (0.37) | 2.07 (0.46) | 0.049 |
NOTE. P values are for unpaired Student's t-test and the χ2 test comparing the two groups.
Abbreviations: ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living scale; MMSE, Mini–Mental State Examination; PASE, Physical Activity Scale for the Elderly; SD, standard deviation; SUVR, standardized uptake value ratio.
PASE was filled out by the caregiver.
n = 13 for usual care and n = 18 for the intervention.
Mean SUVR across six regions. See 2.9 Image Analysis for details regarding anatomical regions.
3.2. Effect of the intervention on cortical 11C-PiB binding
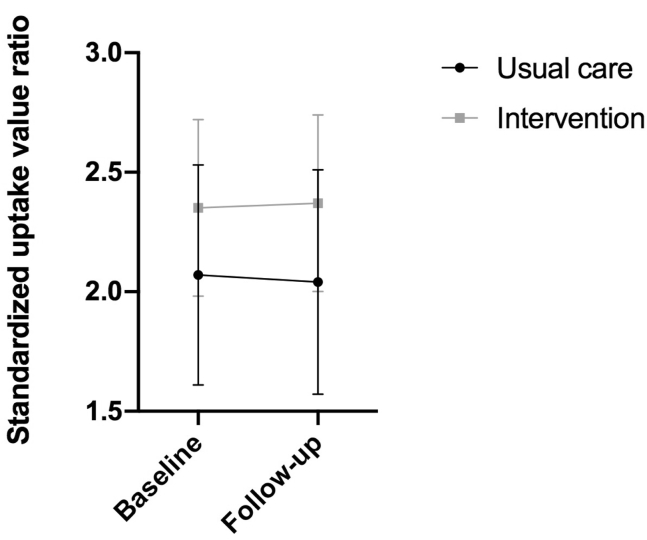
There was no significant difference within the two groups in SUVRs in the predefined region of interest from baseline to follow-up [intervention group—baseline mean (SD): 2.35 (0.37); follow-up: 2.37 (0.37) P = .46; usual care group—baseline: 2.07 (0.46); follow-up: 2.04 (0.47) P = .68] (Fig. 2). Similarly, there were no significant effects from any of the voxel-wise statistical parametric mapping analyses, even after lowering the statistical threshold.
Fig. 2.
Change from baseline in standardized uptake value ratios. The graph shows mean and standard deviation of standardized uptake value ratios for both groups at baseline and at the 16-week follow-up for six regions: lateral temporal cortex, posterior cingulated gyrus, anterior cingulated gyrus, precuneus, parietal cortex, and lateral prefrontal cortex.
3.3. Change from baseline to follow-up physical measures
Participants in the intervention group improved with regard to estimated VO2max (P < .01) and the 400-m walk. For participants in the usual care group, the STS (P < .05) score increased (Table 2), indicating improved lower leg strength.
Table 2.
Measures of physical function, aerobic fitness, and exercise load
| Variables | Usual care group |
Intervention group |
||
|---|---|---|---|---|
| Baseline | Change from baseline | Baseline | Change from baseline | |
| Timed Up and Go test (sec) | 6.3 (±1.1) | −0.1 (±0.6) | 5.7 (±1.3) | −0.5 (±1.5) |
| Sit-to-stand test∗ | 16.5 (2) | 1 (1.75)† | 13.5 (4.75) | 1 (2.75) |
| Estimated VO2max (mL/kg/min) | 26.2 (±6.4) | −0.8 (±2.5) | 23.8 (±6.5) | 2.3 (±3.5)‡ |
| 400-meter walk test (sec) | 278.5 (±54) | 2.3 (±15.3) | 270.7 (±48.9) | −17.0 (±25.6)‡ |
NOTE. Results are reported as mean (standard deviation), except for the sit-to-stand test and exercise load, which are reported as median (interquartile range). Negative change scores indicate improvements for the Timed Up and Go and 400-meter walk tests and deterioration for the sit-to-stand and estimated VO2max.
Reported as number of rises in the allotted time.
P < .05.
P < .01.
3.4. Correlations with change in physical function and aerobic capacity
There were no significant correlations between changes in the TUG, STS, 10-m and 400-m walk tests, and change in SUVR. Furthermore, exercise load did not correlate with change in SUVR.
4. Discussion
To our knowledge, this is the first human study to evaluate the effects of physical exercise on Aβ using amyloid PET. In this single-blinded RCT, we tested whether a 16-week intervention with moderate- to high-intensity aerobic exercise was able to modify the level of cortical Aβ in patients with mild AD. Our findings were twofold. First, and regarding the main objective of the study, we did not find an effect of the exercise intervention on cortical Aβ compared with usual care. Second, we did not find that change from baseline to follow-up in cortical Aβ and measures of aerobic physical fitness correlated.
As outlined previously, different lines of evidence demonstrate the positive effect of exercise on cognitive function and risk of dementia [10], [11], [12], [13], [14], [15], [16], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]. However, the biological mechanisms by which this effect may be mediated remain to be clarified. Various mechanisms, pathways, and molecular targets have been proposed and explored in animal studies [15], [60], [61], [62], [63], [64], [65], [66], but the number of human studies is small. Several animal studies have endeavored to determine the possible mechanisms by which exercise might reduce Aβ levels. Nigam et al. [60] found that exercise enhanced the activity of α-secretase, an enzyme that initiates the nonamyloidogenic processing pathways by which the amyloid precursor protein is cleaved in its intramembrane domain. This releases a large soluble protein, which may have neuroprotective properties [67], and also, importantly, hinders the production of Aβ because the cleavage site is in the amino acid sequence of the Aβ protein. Conversely, in the amyloidogenic pathway, the amyloid precursor protein is cleaved by β-secretase and γ-secretase, releasing the Aβ protein. In another study that also uses an animal model of AD, exercise was found to downregulate γ-secretase [68], indicating that exercise may both upregulate the nonamyloidogenic pathway and downregulate the amyloidogenic pathway. Another possibility is that clearance of Aβ is increased by exercise [13], [69]. From a theoretical point of view, it may be speculated that an increased clearance of Aβ may be more relevant to the patients in the present study because deposition of Aβ in sporadic AD is associated with decreased clearance [70]. On the other hand, this does not rule out a positive effect of a downregulation of Aβ production in sporadic AD.
Other aspects of Aβ biology may play a role in the negative findings of the present study such as a selective effect of exercise on soluble species of Aβ over Aβ plaques, as indicated by the findings in an observational study [29]. However, in another subpopulation from the ADEX study, exercise also failed to affect the level of Aβ in CSF, which may primarily reflect soluble forms of Aβ [37]. Furthermore, animal studies have demonstrated a direct effect of exercise on number of plaques [63] and plaque formation [71]. However, the issue of an effect on soluble versus plaque forms of Aβ ties directly into an additional perspective, namely, the timing of the intervention. Across animal studies, there seems to be a trend toward exercise being more effective in reducing Aβ and plaque formation, the earlier in the disease process it is initiated [72]. This also coincides with the pathophysiological process when soluble Aβ, but not plaques, may predominate. A move toward earlier stages of AD pathology with regard to exercise interventions would mirror the trend in studies of, e.g., monoclonal antibodies directed toward Aβ [73], [74]. To our knowledge, at least one exercise study is underway that is testing this hypothesis (ClinicalTrials.gov, identifier NCT02000583).
Another factor that is likely to play a role in the present findings is the length of the intervention, which was relatively short when considering that AD pathology may build up over years. In recent years, phase II and III trials have tested several monoclonal antibodies directed toward Aβ and drugs modulating the activity of the aforementioned secretases that have demonstrated an ability to reduce cortical Aβ, although none of them have reached clinical endpoints in phase III trials. These studies were performed over a period of one to two years [75], which may indicate the length of time that a therapy must be maintained to affect cortical Aβ. Whether this is transferable to exercise remains speculative. In animal models of AD, the length of interventions varies considerably. In one animal study, a brief 10-day intervention using wheel running led to a decrease in Aβ and APP mRNA, indicating target engagement, although the number of plaques did not decrease [76]. The shortest intervention tested in animal models that was able to modify Aβ lasted around three months [72]. The intensity of the exercise intervention may also affect the ability of the intervention to modify Aβ. In the present study, where the intensity was moderate to high, we were able to demonstrate a significant improvement in the intervention group for estimated VO2max. This is in accordance with the findings in the entire ADEX study cohort [43]. Other studies in patients with dementia have, in general, used lower-intensity exercise [6], [77], [78], [79], [80]. From a clinical point of view, it may not be feasible to carry out an exercise program of higher intensity in this patient group. Moore et al. [13], who divided a group of transgenic mice into sedentary, low-intensity, and high-intensity exercise groups, found a dose response with regard to reducing plaque formation. Two conclusions may be gleaned from these findings (1) that there is not necessarily a level of intensity below which exercise does not affect Aβ and (2) that more is better in relation to intensity. The fact that we were able to detect an improvement in estimated VO2max also indicates that the program was sufficient and that if modulation of Aβ is mediated through an improvement in aerobic fitness, this was not the cause of the negative findings.
The present study has several weaknesses. As discussed, the duration of the intervention may have led to negative findings and it is not improbable that an intervention over a longer period may have had an effect on Aβ. Moreover, the study population was relatively small partly because of dropout and insufficient quality of the acquired scans.
This study also has several strengths. First, it is well designed and has a well-characterized patient group, both regarding certainty of diagnosis and the variables included, i.e., demographics and clinical data collected from the patients. Second, the differing SUVR values between the two groups cannot be interpreted as a failure of the randomization process. Moreover, any baseline difference was accounted for in the statistical analysis by including baseline values as a covariate. In addition, we were able to monitor closely whether participants were present at training sessions, whereas pulse watches allowed us to accurately record exercise intensity. Finally, the exercise program was supervised by trained physiotherapists and performed in small groups, which may have improved adherence. This approach also allowed us to offer relatively high-intensity exercise to a comparatively fragile patient group.
In conclusion, the present findings in this exercise intervention study do not support previous findings from, primarily, animal studies of an effect of exercise on Aβ, which is in line with previous findings in the ADEX study on CSF levels of Aβ. However, the findings also do not definitively rule out an effect. Future studies should focus on longer intervention studies, in patients in earlier stages of AD pathology, e.g., patients with preclinical AD or patients without symptoms but positive AD biomarkers. In addition to exploring alternative pathways, future studies should aim to assess Aβ in CSF and by amyloid PET, as well as tau markers, to further shed light on how exercise may modulate the amyloid cascade.
Research in Context.
-
1.
Systematic Review: We searched for relevant articles in PubMed on the topic of physical exercise and its effect on Alzheimer's disease pathology. A large body of animal studies has found that physical activity may modify β-amyloid pathology, but studies in humans are lacking.
-
2.
Interpretation: Our findings do not support a disease-modifying effect of physical exercise as assessed by the effect on β-amyloid in patients with Alzheimer's disease.
-
3.
Future directions: Future studies are needed to further assess the effects of physical exercise as a possible disease-modifying strategy, specifically, ones examining longer interventions and interventions in earlier stages of the disease. Moreover, other components of Alzheimer's disease pathophysiology, such as tau deposition, may be differentially affected and should also be explored.
Acknowledgments
The authors are grateful to all of the physiotherapists, study nurses, and clinical raters for their contributions to this study. The authors also thank Jonathan Polimeni from the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, USA, for supplying gradient unwarping software. We also appreciate the language services provided by expertenglish.dk. The ADEX study is supported by the InnovationFund Denmark (J No. 10-092814).
Footnotes
Conflict of interest: None.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.04.006.
Supplementary Data
References
- 1.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu W., Wang H.F., Wan Y., Tan C.-C., Yu J.-T., Tan L. Leisure time physical activity and dementia risk: a dose-response meta-analysis of prospective studies. BMJ Open. 2017;7:e014706. doi: 10.1136/bmjopen-2016-014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarsland D., Sardahaee F.S., Anderssen S., Ballard C. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Heal. 2010;14:386–395. doi: 10.1080/13607860903586136. [DOI] [PubMed] [Google Scholar]
- 4.Sofi F., Valecchi D., Bacci D., Abbate R., Gensini G.F., Casini a. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269:107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 5.Baker L.D., Frank L.L., Foster-Schubert K., Green P.S., Wilkinson C.W., McTiernan A. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vreugdenhil A., Cannell J., Davies A., Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomized controlled trial. Scand J Caring Sci. 2012;26:12–19. doi: 10.1111/j.1471-6712.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann K., Sobol N.A., Frederiksen K.S., Beyer N., Vogel A., Vestergaard K. Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: A randomized controlled trial. J Alzheimer’s Dis. 2015;50:443–453. doi: 10.3233/JAD-150817. [DOI] [PubMed] [Google Scholar]
- 8.Venturelli M., Scarsini R., Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26:381–388. doi: 10.1177/1533317511418956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teri L., Gibbons L.E., Mccurry S.M., Logsdon R.G., Buchner D.M., Barlow W.E. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290:2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 10.Ke H., Huang H., Liang K., Hsieh-li H.M. Selective improvement of cognitive function in adult and aged APP/PS1 transgenic mice by continuous non-shock treadmill exercise. Brain Res. 2011;1403:1–11. doi: 10.1016/j.brainres.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 11.Yuede C.M., Zimmerman S.D., Dong H., Kling M.J., Bero A.W., Holtzman D.M. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimerś disease. Neurobiol Dis. 2009;35:426–432. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichol K.E., Parachikova A.I., Cotman C.W. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res. 2008;184:124–132. doi: 10.1016/j.bbr.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore K.M., Girens R.E., Larson S.K., Jones M.R., Restivo J.L., Holtzman D.M. A spectrum of exercise training reduces soluble Aβ in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;85:218–224. doi: 10.1016/j.nbd.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Adlard P., Perreau V.M., Pop V., Cotman C.W. Voluntary Exercise Decreases Amyloid Load in a Transgenic Model of Alzheimer’s Disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belarbi K., Burnouf S., Fernandez-gomez F., Laurent C., Lestavel S., Figeac M. Beneficial effects of exercise in a transgenic mouse model of Alzheimer's disease-like Tau pathology. Neurobiol Dis. 2011;43:486–494. doi: 10.1016/j.nbd.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Liu H., Zhao G., Cai K., Zhao H., Shi L. Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav Brain Res. 2011;218:308–314. doi: 10.1016/j.bbr.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Frederiksen K.S., Gjerum L., Waldemar G., Hasselbalch S.G. Effects of physical exercise on Alzheimer’s disease biomarkers: a systematic review of intervention studies. J Alzheimer’s Dis. 2017;61:359–372. doi: 10.3233/JAD-170567. [DOI] [PubMed] [Google Scholar]
- 18.Best J.R., Chiu B.K., Liang Hsu C., Nagamatsu L.S., Liu-Ambrose T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J Int Neuropsychol Soc. 2015;21:745–756. doi: 10.1017/S1355617715000673. [DOI] [PubMed] [Google Scholar]
- 19.Colcombe S.J., Erickson K.I., Scalf P.E., Kim J.S., Prakash R., McAuley E. Aerobic Exercise Training Increases Brain Volume in Aging Humans. J Gerontol A Biol Sci Med Sci. 2006;61A:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 20.Niemann C., Godde B., Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front Aging Neurosci. 2014;6:1–24. doi: 10.3389/fnagi.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura M., Nemoto K., Kawaguchi A., Kato M., Arai T., Kakuma T. Long-term mild-intensity exercise regimen preserves prefrontal cortical volume against aging. Int J Geriatr Psychiatry. 2015;30:686–694. doi: 10.1002/gps.4205. [DOI] [PubMed] [Google Scholar]
- 22.Morris J.K., Vidoni E.D., Johnson D.K., Van Sciver A., Mahnken J.D., Honea R.A. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS One. 2017;12:1–14. doi: 10.1371/journal.pone.0170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto M., Wada-Isoe K., Yamashita F., Nakashita S., Kishi M., Tanaka K. Association between exercise habits and subcortical gray matter volumes in healthy elderly people: a population-based study in Japan. eNeurologicalSci. 2017;7:1–6. doi: 10.1016/j.ensci.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith J.C., Nielson K.A., Woodard J.L., Seidenberg M., Durgerian S., Hazlett K.E. Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer’s disease. Front Aging Neurosci. 2014;6:1–7. doi: 10.3389/fnagi.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamont A.J., Mortby M.E., Anstey K.J., Sachdev P.S., Cherbuin N. Using sulcal and gyral measures of brain structure to investigate benefits of an active lifestyle. Neuroimage. 2014;91:353–359. doi: 10.1016/j.neuroimage.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Varma V.R., Chuang Y., Harris G.C., Tan E.J., Carlson M.C. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2015;25:605–615. doi: 10.1002/hipo.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makizako H., Liu-Ambrose T., Shimada H., Doi T., Park H., Tsutsumimoto K. Moderate-intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. J Gerontol A Biol Sci Med Sci. 2015;70:480–486. doi: 10.1093/gerona/glu136. [DOI] [PubMed] [Google Scholar]
- 28.Vidoni E.D., Honea R., Billinger S., Swerdlow R.H., Burns J.M. Cardiorespiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiol Aging. 2012;33:1624–1632. doi: 10.1016/j.neurobiolaging.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown B.M., Sohrabi H.R., Taddei K., Gardener S.L., Rainey-Smith S.R., Peiffer J.J. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimer’s Dement. 2017;13:1197–1206. doi: 10.1016/j.jalz.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Head D., Bugg J.M., Goate A.M., Fagan A.M., Mintun M.A., Benzinger T. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69:636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz S.A., Boots E.A., Almeida R.P., Oh J.M., Einerson J., Korcarz C.E. Cardiorespiratory fitness attenuates the influence of amyloid on cognition. J Int Neuropsychol Soc. 2015;21:841–850. doi: 10.1017/S1355617715000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang K.Y., Mintun M.A., Fagan A.M., Goate A.M., Bugg J.M., Holtzman D.M. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker L.D., Bayer-Carter J.L., Skinner J., Montine T.J., Cholerton B a, Callaghan M. High-intensity physical activity modulates diet effects on cerebrospinal amyloid-β levels in normal aging and mild cognitive impairment. J Alzheimer’s Dis. 2012;28:137–146. doi: 10.3233/JAD-2011-111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz S.A., Boots E.A., Darst B.F., Zetterberg H., Blennow K., Edwards D.F. Cardiorespiratory fitness alters the influence of a polygenic risk score on biomarkers of AD. Neurology. 2017;88:1650–1658. doi: 10.1212/WNL.0000000000003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zwan M., van Harten A., Ossenkoppele R., Bouwman F., Teunissen C., Adriaanse S. Concordance between cerebrospinal fluid biomarkers and [11C]PIB PET in a memory clinic cohort. J Alzheimers Dis. 2014;41:801–807. doi: 10.3233/JAD-132561. [DOI] [PubMed] [Google Scholar]
- 36.Ikonomovic M.D., Klunk W.E., Abrahamson E.E., Mathis C.A., Price J.C., Tsopelas N.D. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattsson N., Insel P.S., Donohue M., Landau S., Jagust W.J., Shaw L.M. Independent information from cerebrospinal fluid amyloid-β and florbetapir imaging in Alzheimer's disease. Brain. 2015;138:772–783. doi: 10.1093/brain/awu367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattsson N., Tosun D., Insel P.S., Simonson A., Jack C.R., Beckett L.A. Association of brain amyloid-β with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain. 2014;137:1550–1561. doi: 10.1093/brain/awu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May P.C., Dean R.A., Lowe S.L., Martenyi F., Sheehan S.M., Boggs L.N. Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci. 2011;31:16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen C.S., Portelius E., Siersma V., Høgh P., Wermuth L., Blennow K. Cerebrospinal fluid amyloid beta and tau concentrations are not modulated by 16 weeks of moderate- to high-intensity physical exercise in patients with Alzheimer disease. Dement Geriatr Cogn Disord. 2016;42:146–158. doi: 10.1159/000449408. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann K., Frederiksen K.S., Sobol N.A., Beyer N., Vogel A., Simonsen A.H. Preserving Cognition, quality of life, physical health and functional ability in Alzheimer’s disease: the effect of physical exercise (ADEX Trial): rationale and design. Neuroepidemiology. 2013;41:198–207. doi: 10.1159/000354632. [DOI] [PubMed] [Google Scholar]
- 43.Sobol N.A., Hoffmann K., Frederiksen K.S., Vogel A., Vestergaard K., Braendgaard H. Effect of aerobic exercise on physical performance in patients with Alzheimer’s disease. Alzheimers Dement. 2016;12:1207–1215. doi: 10.1016/j.jalz.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Frederiksen K.S., Sobol N., Beyer N., Hasselbalch S., Waldemar G. Moderate-to-high intensity aerobic exercise in patients with mild to moderate Alzheimer’s disease: a pilot study. Int J Geriatr Psychiatry. 2014;29:1242–1248. doi: 10.1002/gps.4096. [DOI] [PubMed] [Google Scholar]
- 45.Folstein M., Folstein S. “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 46.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11:S33–S39. [PubMed] [Google Scholar]
- 47.Cink R., Thomas T. Validity of the Astrand-Ryhming nomogram for predicting maximal oxygen intake. Br J Sport Med. 1981;15:182–185. doi: 10.1136/bjsm.15.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Podsiadlo D., Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 49.Eggermont L.H., Gavett B.E., Volkers K.M., Blankevoort C.G., Scherder E.J., Jefferson A.L. Lower-Extremity Function in Cognitively Healthy Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. Arch Phys Med Rehabil. 2010;91:584–588. doi: 10.1016/j.apmr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolland Y.M., Cesari M., Miller M.E., Penninx B.W., Atkinson H.H., Pahor M. Reliability of the 400-M usual-pace walk test as as assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52:972–976. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- 51.Schwenk M., Zieschang T., Oster P., Hauer K. Dual-task performances can be improved in patients with dementia: A randomized controlled trial. Neurology. 2010;74:1961–1968. doi: 10.1212/WNL.0b013e3181e39696. [DOI] [PubMed] [Google Scholar]
- 52.Washburn R., Smith K., Jette A.M., Janney C.A. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 53.Svarer C., Madsen K., Hasselbalch S.G., Pinborg L.H., Haugbøl S., Frøkjaer V.G. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 54.McNamee R., Yee S., Price J., Klunk W., Rosario B., Weissfeld L. Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J Nucl Med. 2009;50:348–355. doi: 10.2967/jnumed.108.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woods R.P., Cherry S.R., Mazziotta J.C. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 56.Lopresti B.J., Klunk W.E., Mathis C.A., Hoge J.A., Ziolko S.K., Lu X. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–1972. [PubMed] [Google Scholar]
- 57.Pike K.E., Savage G., Villemagne V.L., Ng S., Moss S.A., Maruff P. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 58.Madsen K., Hasselbalch B.J., Frederiksen K.S., Haahr M.E., Gade A., Law I. Lack of association between prior depressive episodes and cerebral [(11)C]PiB binding. Neurobiol Aging. 2012;33:2334–2342. doi: 10.1016/j.neurobiolaging.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Van Breukelen G.J.P. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. J Clin Epidemiol. 2006;59:920–925. doi: 10.1016/j.jclinepi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Nigam S.M., Xu S., Kritikou J.S., Marosi K., Brodin L., Mattson M.P. Exercise and BDNF reduce Aβ production by enhancing α-secretase processing of APP. J Neurochem. 2017;142:286–296. doi: 10.1111/jnc.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Um H., Kang E., Koo J., Kim H., Kim E., Yang C. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer ’ s disease. Neurosci Res. 2011;69:161–173. doi: 10.1016/j.neures.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Koo J.H., Kang E.B., Oh Y.S., Yang D.S., Cho J.Y. Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp Neurol. 2017;288:142–152. doi: 10.1016/j.expneurol.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Zhao G., Liu H.L., Zhang H., Tong X.J. Treadmill exercise enhances synaptic plasticity, but does not alter β-amyloid deposition in hippocampi of aged APP/PS1 transgenic mice. Neuroscience. 2015;298:357–366. doi: 10.1016/j.neuroscience.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 64.Nichol K., Deeny S.P., Seif J., Camaclang K., Cotman C.W. Exercise improves cognition and hippocampal plasticity in APOE 34 mice. Alzheimer's Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nichol K.E., Poon W.W., Parachikova A.I., Cribbs D.H., Glabe C.G., Cotman C.W. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naylor A.S., Bull C., Nilsson M.K.L., Zhu C., Björk-Eriksson T., Eriksson P.S. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105:14632–14637. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furukawa K., Sopher B., Rydel R., Begley J., Pham D., Martin G. Increased activity-regulating and neuroprotective efficacy of α-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 68.Alkadhi K.A., Dao A.T. Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Mol Cell Neurosci. 2018;86:25–29. doi: 10.1016/j.mcn.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 69.Lin T., Shih Y., Chen S., Lien C., Chang C., Huang T. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol Learn Mem. 2015;118:189–197. doi: 10.1016/j.nlm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ziegler-Waldkirch S., D'Errico P., Sauer J., Erny D., Savanthrapadian S., Loreth D. Seed-induced Aβ deposition is modulated by microglia under environmental enrichment in a mouse model of Alzheimer’s disease. EMBO J. 2017;37:e201797021. doi: 10.15252/embj.201797021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryan S.M., Kelly Á.M. Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer’s disease. Ageing Res Rev. 2016;27:77–92. doi: 10.1016/j.arr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 Study: Stopping AD Before Symptoms Begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bateman R.J., Benzinger T.L., Berry S., Clifford D.B., Duggan C., Fagan A.M. The DIAN-TU Next Generation Alzheimer’s prevention trial: Adaptive design and disease progression model. Alzheimer’s Dement. 2017;13:8–19. doi: 10.1016/j.jalz.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rygiel K. Novel strategies for Alzheimer’s disease treatment: An overview of anti-amyloid beta monoclonal antibodies. Indian J Pharmacol. 2016;48:629. doi: 10.4103/0253-7613.194867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirochnic S., Wolf S., Staufenbiel M., Kempermann G. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus. 2009;1018:1008–1018. doi: 10.1002/hipo.20560. [DOI] [PubMed] [Google Scholar]
- 77.Rolland Y., Pillard F., Klapouszczak A., Reynish E., Thomas D., Andrieu S. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55:158–165. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 78.Pitkala K.H., Raivio M.M., Laakkonen M.L., Tilvis R.S., Kautiainen H., Strandberg T.E. Exercise rehabilitation on home-dwelling patients with Alzheimer’s disease-a randomized, controlled trial. Study Protocol Trials. 2010;11:92. doi: 10.1186/1745-6215-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santana-Sosa E., Barriopedro M.I., López-Mojares L.M., Pérez M., Lucia A. Exercise Training is beneficial for Alzheimer’s patients. Int J Sport Med. 2008;29:845–850. doi: 10.1055/s-2008-1038432. [DOI] [PubMed] [Google Scholar]
- 80.Eggermont L.H.P., Swaab D.F., Hol E.M., Scherder E.J. Walking the line: a randomised trial on the effects of a short term walking programme on cognition in dementia. J Neurol Neurosurg Psychiatry. 2009;80:802–804. doi: 10.1136/jnnp.2008.158444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.