Abstract
Researchers have used dogs with neurological sequelae caused by distemper as an experimental model for multiple sclerosis, owing to the similarities of the neuropathological changes between distemper virus-induced demyelinating leukoencephalitis and multiple sclerosis in humans. However, little is known about the role of mesenchymal stem cells in treating such clinical conditions. Therefore, we investigated the use of mesenchymal stem cells in four dogs with neurological lesions caused by the distemper virus. During the first year after cellular therapy, the animals did not demonstrate significant changes in their locomotive abilities. However, the intense (Grade V) myoclonus in three animals was reduced to a moderate (Grade IV) level. At one year after the mesenchymal stem cell infusions, three animals regained functional ambulation (Grade I), and all four dogs started to move independently (Grades I and II). In two animals, the myoclonic severity had become mild (Grade III). It was concluded that the use of mesenchymal stem cells could improve the quality of life of dogs with neurological sequelae caused by canine distemper, thus presenting hope for similar positive results in human patients with multiple sclerosis.
Keywords: Cell biology, Neuroscience, Mesenchymal stem cells, Canine distemper, Multiple sclerosis, Demyelination
1. Introduction
Demyelinating leukoencephalitis is the major aggravating factor and cause of mortality from canine distemper [1]. It commonly represents the neurological stage of this disease, where inflammation of the central nervous system, demyelination, and axonal injury occur [1, 2]. However, similar to other morbilliviruses, the canine distemper virus behaves as a lymphotropic and immunosuppressive agent, rendering the animals highly susceptible to opportunistic infections and resulting in a variety of clinical forms that characterize distemper [3]. Dogs that survive this stage sustain disabling sequelae that are often incompatible with life [4]. Furthermore, owing to the morphological similarities to the neuropathological changes associated with human multiple sclerosis, canine distemper represents one of the few spontaneous occurrences in animals that can be applied as a model for the study of the pathogenesis of myelin loss [2, 3].
Multiple sclerosis, a complex human disease with unknown etiology and pathophysiology, manifests primarily as a result of an aberrant response of the immune system cells to the autoantigens of the myelin sheath of neurons. This condition results in multiple areas of scarring (sclerosis) and is also characterized by inflammation, demyelination, and axonal degeneration, which occur in canine distemper as well [5]. Because multiple sclerosis and similar degenerative myelopathy in domesticated animals require treatments that aim to recover the myelin sheath and repair the damaged neuronal tissue, their therapy and cure remain major challenges in both the human and veterinary medical fields [1].
Several research groups have investigated the therapeutic use of mesenchymal stem cells (MSCs) for demyelinating diseases [5, 6, 7, 8]. Some results have suggested that MSCs could promote endogenous repair and exert positive immunomodulatory effects to reduce demyelination, increase neuroprotection, modulate inflammation, and promote the differentiation of neural MSCs into oligodendrocytes (myelin-producing cells in the central nervous system) [9]. In addition, some clinical trials have shown promising results in the use of MSCs in multiple sclerosis [5, 10, 11, 12, 13, 14].
MSCs, which are considered a somatic stem cell line, are present in the perivascular regions of adult tissues that are responsible for cell regeneration and homeostasis [15]. These cells have already been isolated from a variety of tissues (e.g., bone marrow, umbilical cord blood, skin, dental pulp, etc.), among which adipose tissue stands out as a common source owing to a higher rate of isolation and yield [16]. Thus, the present study aimed to evaluate the therapeutic potential of MSCs in inducing the recovery from neurological sequelae in dogs naturally affected by demyelinating leukoencephalitis, assessing signs of neurological changes that may represent hope for human patients with multiple sclerosis.
2. Materials and methods
The dogs used in this study were from the Medical Clinic Sector of the Veterinary Hospital “Prof. Mário Dias Teixeira” at the Federal Rural University of Amazonia (UFRA). The animal protocol was approved by the UFRA Committee on Ethics in the Use of Animals (Protocol No. 053/2015).
2.1. Treatment protocol and evaluation parameters
We selected four dogs (designated c1, c2, c3, and c4) with evident signs of demyelinating leukoencephalitis. The diagnosis of distemper was confirmed from the clinical signs and through laboratory tests. After treatment of the multisystemic clinical symptomatology, neurological sequelae compatible with those caused by the disease still remained, however, without alterations in laboratory tests, including the polymerase chain reaction (PCR) - negative for the distemper virus.
The dogs were given a complete neurological examination, which consisted of evaluations of their mental state, locomotion, cranial nerves, postural reactions, spinal reflexes, sensory perception, and muscle tone. For analytical purposes, the neurological record was rated as 0 for absence, 1 for decrease, 2 for normality, and 3 for increase of the evaluated signal. Two neurological scales that were created by Santos [17] were used for evaluating the sequelae of distemper. One scale was for locomotion, with the following grades: (I) functional ambulation; (II) ataxic animal – walks with incoordination; (III) tetraparetic animal – stays in station, but does not get up; (IV) tetraparetic animal – does not stay in station or stand up; and (V) tetraplegic animal – without deep pain and with signs of Grade IV. The other scale was for myoclonus, with the following grades: (I) absent; (II) only at moments of agitation; (III) present – mild; (IV) present – moderate; and (V) present – intense.
The MSCs were extracted from the flank adipose tissue of each canine patient of this study through enzymatic digestion according to the protocol of Zuk et al. [18]. Three separate doses of 1 × 107 cells at passages P3 or P4 were injected into the dogs through the femoral artery at 30-day intervals, and monthly neurological examinations before each application as well as one final evaluation one year later were carried out.
2.2. Clinical conditions of the selected animals
Prior to MSC treatment, all animals were conscious and the neurological changes at the first visit were related to locomotion, postural reactions, spinal reflexes, muscle tone, and myoclonus. c1 presented with monoparesis of the right pelvic limb, with decreased conscious proprioception and hypertonia of this limb, besides motor incoordination and spontaneous falls. c2 presented with monoparesis of the right pelvic limb, with decreased conscious proprioception, patellar hyperreflexia, and hypertonia of this limb, as well as motor incoordination and spontaneous falls. c3 presented with functional deambulation, without changes in the neurological examination. c4 presented with tetraparesis, the absence of conscious proprioception, and hypertonia in the four limbs, as well as cervical stiffness. The four dogs had myoclonus of several muscular groups, with a noticeably greater incidence in the masticatory muscles. The myoclonus was classified as intense for c1, c2, and c4 and moderate for c3.
2.3. MSC cultivation, cryopreservation, and phenotype analysis
After isolation, the MSCs were maintained in cultures at 37 °C with 5% CO2 in growth media complete (Dulbecco's modified Eagle's medium, with 20% fetal bovine serum), with a medium change every 2–3 days. The cultures were cryopreserved at the P0 and P1 passages. After thawing, the viability of the cells at each passage was tested using the trypan blue exclusion dye (0.4%) test (Sigma, USA). For intra-arterial administration, the MSCs were thawed and maintained in culture for an average of 7 days for the expansion needed to reach the determined amount of cells (1 × 107 cells).
For phenotype analysis by immunofluorescence, the cells were plated and incubated with primary anti-CD105 (1:25), anti-CD34 (1: 100), and anti-CD45 (1: 100) antibodies from Abcam (USA), and goat anti-CD73 (1:25) and anti-vimentin (1:25) antibodies from Santa Cruz Biotechnology (USA). Following further processing, they were analyzed under a Nikon 80i fluorescence microscope.
For phenotype analysis by flow cytometry, the cells were first incubated with the primary antibodies (CD105, CD73, CD90, CD34, CD45, and CD79) for 45 min at 4 °C. After washing in phosphate-buffered saline, they were incubated with phycoerythrin- or fluorescein isothiocyanate-conjugated secondary antibodies for 30 min. Following this, 10,000 events were acquired on the FACSCalibur flow cytometer and FlowJo software was used to analyze the data obtained.
2.4. Gene expression by RT-qPCR
Total RNA was extracted using TRIzol Reagent (Life Technologies, USA) and reverse transcribed into cDNA using SuperScript III (Invitrogen, USA), following the manufacturers’ protocols. The cDNA was then subjected to quantitative PCR (qPCR) using SYBR Green Supermix (Bio-Rad, USA). Each sample was run in triplicate. Primers for specific genes were synthesized using Primer3 software (v. 0.4.0) or were available from the Harvard Primer Bank online. The conditions of the PCR cycles were as follows: 30 s at 95 °C, 30 s at 95 °C, 30 s at 60 °C, and 45 s at 72 °C for 50 cycles. Melting curve analysis was then conducted to verify the amplification specificity. All analyses were done by absolute quantification, with the levels of the target genes normalized to that of the GAPDH gene as a reference control, using standard curves.
2.5. MSC differentiation potential
To determine the osteogenic differentiation potential of the MSCs, 5 × 103 MSCs/mL were cultured in osteogenic differentiation induction medium (STEMPRO Osteogenesis Kit; Gibco, USA), with a change of the medium on every other day for 14 days, according to the manufacturer's recommendations. The cells were then stained with 2% Alizarin Red S (Sigma-Aldrich, USA) for 5 min.
For observation of their adipogenic differentiation potential, 1 × 104 MSCs/mL were cultured in induction medium (STEMPRO Adipogenesis Kit, Gibco, USA), with a change of the medium on every other day for 14 days. The cells were then evaluated by staining with 1.25% Oil Red O (Sigma-Aldrich, USA) for 5 min.
For determination of their chondrogenic differentiation potential, the MSCs were cultured in a conical tube at high cell density (5.7 × 107 cells/mL) in a micromass system. After centrifugation and disposal of the maintenance medium, chondrogenic differentiation induction medium (STEMPRO Chondrogenesis Kit; Gibco, USA) was added, followed by homogenization and further centrifugation. The tube was maintained in an oven at 37 °C with 5% CO2, with change of the differentiation medium on every other day for 21 days, following the manufacturer's recommendations. For fixation of the micromass cells, 4% paraformaldehyde was added. The cells were then dehydrated in serial dilutions of ethanol, embedded in paraffin blocks, and further processed according to routine histological protocols. The blocks were cut into 5-μm-thickness sections that were then stained with 1% Alcian Blue solution for 10 min.
2.6. Chromosomal stability analysis
The numerical chromosomal stability of cells of the P4, P6, and P8 passages was analyzed. To obtain the cells at metaphase, 100 μL of 0.016% colchicine solution (Gibco, USA) was added to 5 mL of the culture and the cells were kept in a 37 °C oven for 1 h. The cells were then dissociated with trypsin and transferred to a conical tube for centrifugation at 556 g for 10 min. The supernatant was discarded and the cell pellet was resuspended in a 0.075 M hypotonic solution (KCl) and kept in a 37 °C oven for 10 min. Subsequently, Carnoy's fixative solution (methanol:acetic acid, 3:1) was added, and the mixture was homogenized and centrifuged; this process was repeated two more times. Finally, the pellet was resuspended in 2 mL of the fixative and the cells were stored under 6 °C refrigeration. For visualization of the chromosomes, slides containing the cells were stained for 3 min with 10% Wright solution diluted in phosphate buffer (pH 6.8), and 15 metaphase cells of each passage were analyzed under a Leica DM1000 optical microscope. Images of the best metaphase cells were captured using the GenASIs platform (Applied Spectral Imaging, USA), which is also used for karyotyping.
2.7. Statistical analysis
Descriptive statistics were used as appropriate. The Friedman test at 5% significance was applied to determine the median treatment effect for the locomotion and myoclonus scores before and after three infusions and after one year.
3. Results
After 24 h, it was possible to observe the adherence of some of the MSCs to the plastic surface of the culture flask, and the cell confluency reached 80% after 48 h (Fig. 1A, B). After thawing, the cells propagated rapidly, maintaining a fibroblastic morphology in all the passages analyzed (P1–P8) (Fig. 1C), with a mean viability of 94.7% (Fig. 2A). The mean viability of the infused cells was 97.5% (P3) and 98.5% (P4).
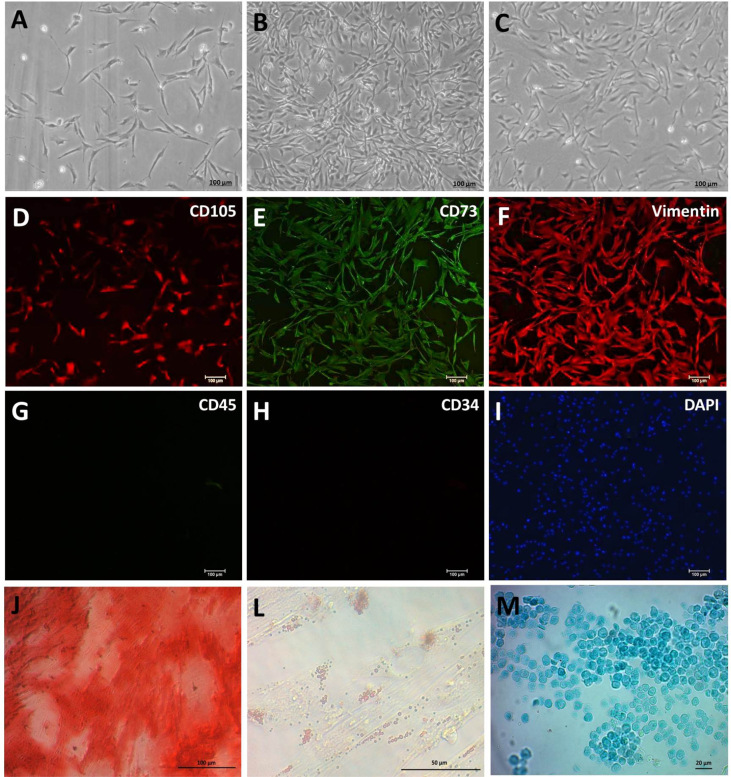
Fig. 1.
A, B, and C: Photomicrography of the canine mesenchymal stem cell (MSC) culture after two days of isolation, after six days at cell confluence, and at the P1 passage after thawing, respectively. Scale bar: 100 μm (5×). D–I: Immunocytochemical characterization of MSCs, with positive labeling for the mesenchymal markers CD105, CD73, and vimentin, and negative labeling for the hematopoietic markers CD45 and CD34, as evidenced by the DAPI-stained cell nucleus. Scale bar: 100 μm. J: Osteogenic differentiation, demonstrated by Alizarin Red coloration of the extracellular calcium matrix. L: Adipogenic differentiation, demonstrated by Oil Red O coloration of the lipid droplets. M: Chondrogenic differentiation, demonstrated by Alcian Blue staining of the proteoglycans. Scale bar: 100 μm (5×).
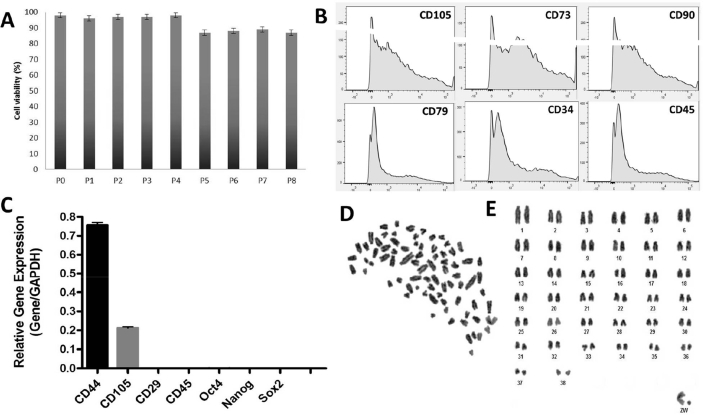
Fig. 2.
A: Trypan blue dye exclusion test of cell viability in passages P1–P8. B: Phenotype analysis by flow cytometry of mesenchymal stem cells (MSCs) positive for markers CD105, CD73, and CD90, and negative for CD79, CD34, and CD45. C: RT-qPCR analysis of MSCs with CD44 and CD105 expression, and absence of CD29, CD45, Oct4, Nanog, and Sox2 expression. D: Canine MSCs at the end of metaphase. E: Karyotype of the canine MSCs, showing 2n = 78.
In the immunocytochemical evaluation, the analyzed samples were positive for the mesenchymal labels CD105, CD73, and vimentin, and negative for the hematopoietic cell markers CD34 and CD45 (Fig. 1D–I). In the flow cytometric analysis, the cells showed positivity for the CD105, CD73, and CD90 markers, whereas the CD79, CD34, and CD45 hematopoietic cell markers were undetectable (Fig. 2B).
As determined by RT-qPCR, the analyzed samples did not express pluripotency-related genes (Nanog, Oct4, and Sox2), as expected. However, they showed CD44 and CD105 expression (mesenchymal cell markers) and had no expression of CD29 and CD45 (hematopoietic cell markers) (Fig. 2C).
With regard to the cell differentiation processes, the MSC culture produced an extracellular calcium matrix in the osteogenic differentiation medium, revealing cells with osteogenic characteristics (Fig. 1J). In the adipogenic differentiation medium, the cells had a rounded shape and accumulation of lipid droplets in their cytoplasm, indicative of adipogenic differentiation (Fig. 1L). Histologically, the micromass that had formed under the induction of the chondrogenic differentiation medium showed rounded cells surrounded by a glycosaminoglycan matrix (Fig. 1M).
The numerical chromosomal stability of the MSC cultures was successfully demonstrated. Cells in the analyzed passages (P4, P6, and P8) maintained the diploid number of 78 chromosomes for the domestic dog (Fig. 2D, E).
In the evaluation after the first autologous infusion of MSCs, the c1 and c4 animals presented changes in the neurological examinations when compared with the initial examination and at the time of admission of the canine patients to the study. That is, c1 presented normal locomotion of the right pelvic limb and the absence of both motor incoordination when walking and spontaneous falls. The c2 and c3 animals did not present changes in the neurological analysis, whereas the c4 tetraparetic animal showed the absence of cervical rigidity, being able to support its head, which alleviated the initial difficulty in eating and allowed self-feeding without the help of the owner.
After the second MSC infusion, although the c2 animal maintained paresis and hypertonia of the right pelvic limb, it presented with normal conscious proprioception and normal patellar reflex of this limb. Despite that it still had motor incoordination while walking, the animal showed improvement in relation to spontaneous falls.
The c1 and c3 animals did not present any changes in this second neurological examination. In contrast, although the c4 animal remained tetraparetic, it presented with normal muscle tone in all limbs and normal conscious proprioception in the thoracic limbs, and maintained decreased proprioception in the pelvic limbs. In the neurological examination after the third MSC infusion, no changes were observed in relation to the previous evaluations in all four patients.
At one year after the last MSC infusion, the c1 and c3 dogs did not present changes in their neurological examination. The c2 animal presented with functional ambulation without incoordination and without hypertonia of the right pelvic limb. The c4 animal presented with normal proprioception of the pelvic limbs, and started to stay in station and to walk with considerable incoordination.
With regard to the myoclonus, improvement occurred after the first infusion of MSCs in the c1 animal and after the third administration in the c2 and c4 animals. During the treatment, the c3 animal showed no changes in its myoclonus scale. After one year, the myoclonus of animals c1 and c3 had decreased to a mild intensity (Grade III), whereas that of animals c2 and c4 remained at a moderate degree (Table 1).
Table 1.
Evolution of the neurological conditions in dogs following mesenchymal stem cell therapy.
| Evaluation | Clinical signs Pre-infusion | 30 days after 1st infusion | 30 days after 2nd infusion | 30 days after 3rd infusion | 1 year after infusions | |
|---|---|---|---|---|---|---|
| C1 | Locomotion | Monoparesia TM-R (II) | Normal (I) | Normal (I) | Normal (I) | Normal (I) |
| Myoclonus | Intense (V) | Intense (V) | Moderate (IV) | Moderate (IV) | Mild (III) | |
| Muscle tone | 3 PL-R | 2 PL-R | 2 PL-R | 2 PL-R | 2 PL-R | |
| Proprioception | 1 PL-R | 2 PL-R | 2 PL-R | 2 PL-R | 2 PL-R | |
| C2 | Locomotion | Monoparesia TM-R (II) | Monoparesia TM-R (II) | Monoparesia TM-R (II) | Monoparesia TM-R (II) | Normal (I) |
| Myoclonus | Intense (V) | Intense (V) | Intense (V) | Moderate (IV) | Moderate (IV) | |
| Muscle tone | 3 PL-R | 3 PL-R | 3 PL-R | 3 PL-R | 2 PL-R | |
| Proprioception | 1 PL-R | 1 PL-R | 2 PL-R | 2 PL-R | 2 PL-R | |
| Patellar reflex | 3 PL-R | 3 PL-R | 2 PL-R | 2 PL-R | 2 PL-R | |
| C3 | Locomotion | Normal (I) | Normal (I) | Normal (I) | Normal (I) | Normal (I) |
| Myoclonus | Moderate (IV) | Moderate (IV) | Moderate (IV) | Moderate (IV) | Mild (III) | |
| C4 | Locomotion | Tetraparesia (IV) | Tetraparesia (IV) | Tetraparesia (IV) | Tetraparesia (IV) | Ataxia (II) |
| Myoclonus | Intense (V) | Intense (V) | Intense (V) | Moderate (IV) | Moderate (IV) | |
| Muscle tone | 3 TL-L 3 TL-R 3 PL-L 3 PL-R |
3 TL-L 3 TL-R 3 PL-L 3 PL-R |
2 TL-L 2 TL-R 2 PL-L 2 PL-R |
2 TL-L 2 TL-R 2 PL-L 2 PL-R |
2 TL-L 2 TL-R 2 PL-L 2 PL-R |
|
| Proprioception | 0 TL-L 0 TL-R 0 PL-L 0 PL-R |
0 TL-L 0 TL-D 0 PL-L 0 PL-R |
2 TL-L 2 TL-R 1 PL-L 1 PL-R |
2 TL-L 2 TL-R 1 PL-L 1 PL-R |
2 TL-L 2 TL-R 2 PL-L 2 PL-R |
TL = thoracic limb; PL = pelvic limb; L = left; R = right; 0 = absent; 1 = decreased; 2 = normal; 3 = increased.
On the basis of the neurological evaluation scale for distemper [12], we can conclude that the use of MSCs was successful when the animal was at Grade I for locomotion and Grade I, II, or III for myoclonus. The three infusions of MSCs at 30-day intervals were successful in one animal only in terms of locomotive improvement, whereas they were successful in changing the myoclonus from Grade V (intense) to Grade IV (moderate) in three animals. However, in the evaluation at one year post treatment, three animals regained functional ambulation (Grade I), and all four animals were able to move independently (Grades I and II). Moreover, two animals presented with Grade III (mild) myoclonus.
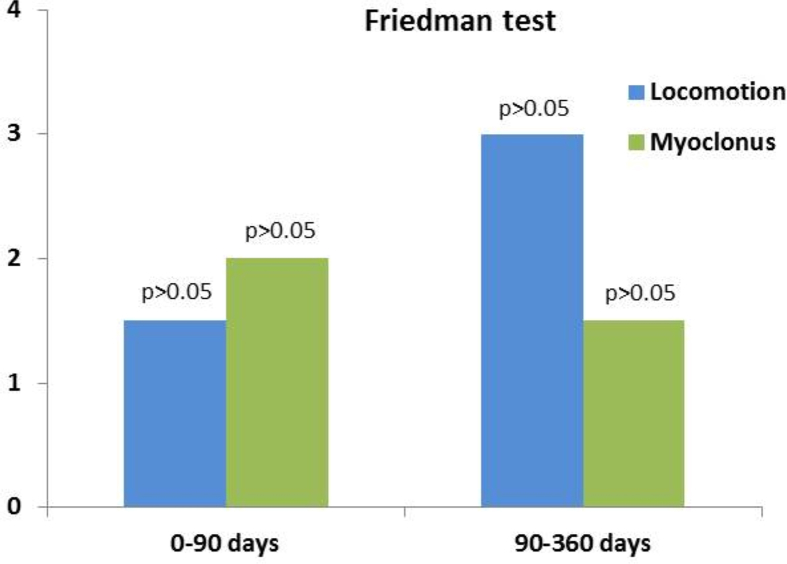
Finally, the Friedman test was applied to compare the median treatment effect in the group for the locomotive and myoclonic degrees. The results revealed that there were no significant differences before and after the three infusions, and before and after one year of treatment (Fig. 3).
Fig. 3.
Friedman test at 5% significance, indicating no significant differences between the degrees of locomotion and myoclonus in the group before and after three infusions, and before and after one year of therapy.
4. Discussion
We emphasize that depending on the virulence of the virus strain, and the age and immune status of the dog, distemper can be fatal in many cases [2]. This justifies the low number of animals used in this study, since we aimed to select only dogs with sequelae of neurological lesions (demyelinating leukoencephalitis) caused by the distemper virus, all of which had already been treated conservatively and conventionally but did not show recovery of their motor integrity. The dogs of this study did not present with multisystemic clinical symptoms and had no changes in their laboratory test findings, such blood counts and negative RT-qPCR for the virus, in accord with the animals recommended by Gebara et al. [19] and Nelson and Couto [20].
In this context, the literature highlights that the most evident signs of demyelinating leukoencephalitis in distemper are behavioral disorders, convulsions, ataxia, tetraparesis, tetraplegia, proprioception and cranial nerve dysfunctions, muscular atrophy, hyperesthesia, myoclonus, deficits or abnormal reflexes in the spine, and urinary incontinence, regardless of the evolution phase of the disease [3]. The neurological changes observed in the animals selected for this study were related to locomotion (ataxia and monoparesis), postural reactions (absent or diminished proprioception), spinal reflexes (hyperesthesia), muscle tone (hypertonia), and myoclonus of various muscle groups, in accord with the literature [3, 20]. Moreover, myoclonus is the most common sign of this condition, being present without other neurological signs [21], as was the case in one of the selected animals. Demyelinating leukoencephalitis, the neurological phase of distemper, has been suggested as a suitable naturally occurring model for the study of the pathogenesis of myelin loss associated with immune-mediated mechanisms, such as that which occurs in multiple sclerosis [1, 2, 22, 23]. Multiple sclerosis causes a deficiency in motor conduction, with a decrease or blockage of the nerve signals that control muscle coordination, strength, sensitivity, and vision [24]. In addition, when considering an experimental model, researchers search for an attractive species for translational studies, such as dogs, since they are large, long-living, and genetically diverse, and share many biochemical and physiological similarities with humans [25].
Unfortunately, there is still no known cure for multiple sclerosis [6]. However, the literature has encouraging data suggesting the use of MSCs as a treatment option for demyelinating diseases, as was demonstrated in rodent models of multiple sclerosis where MSCs elicited strong antioxidative and neuroprotective effects resulting from the release of antiapoptotic molecules and neurotrophins, which led to an improvement in the clinical evolution of the disease and reductions of both demyelination and axonal loss [5, 26]. However, the mechanisms underlying the therapeutic effects are still unknown and may involve one or more of the following possibilities, according to Rivera and Aigner [26]: transdifferentiation of MSCs in mature neurons and/or functional oligodendrocytes (plasticity); immunoregulatory effect on host-derived immunoreactive cells (immunomodulation); protective effect on the survival of damaged neurons and/or oligodendrocytes (neuroprotection); and induction of the differentiation and maturation of neural precursor cells or oligodendrocyte progenitor cells present at the lesion site (remyelination).
In the case of dogs, most studies use adipose tissue as the source of stem cells, where they are collected by non-invasive procedures such as liposuction or lipectomy, as was done in this study [27]. The protocol for the isolation and culture of MSCs from this tissue has been described by several groups [13, 27, 28], and meets the International Society for Cell Therapy recommendations regarding the characterization of MSCs, which establish a minimum of three criteria: adherence to the plastic surface in culture; expression of surface antigen markers (CD73, CD90, and CD105) and absence of hematopoietic cell markers (CD11b, CD14, CD19, CD29a, CD34, CD45, and HLA-DE); and differentiation into at least three lineages [29]. In the present study, after the enzymatic isolation of adipose tissue, the canine MSCs adhered to the plastic surface of the culture bottle, with a fibroblastoid morphology; maintained chromosomal integrity up to the last analyzed passage (P8); presented potential for osteogenic, adipogenic, and chondrogenic differentiation; and were phenotypically and functionally similar to human and canine MSCs from other previous studies [13, 27, 28, 30].
On the basis of rodent assays, as mentioned above, and considering the advantages of using the dog as an experimental model, Pinheiro et al. [31] evaluated the use of MSCs derived from the fetal olfactory epithelium of dogs and delivered intravenously in animals with acute distemper associated with symptomatic therapy, but obtained negative results in relation to the improvement of systemic and neurological clinical signs. In a randomized study, Brito [32] evaluated the use of bone marrow MSCs, delivered intravenously into dogs, in the neurological phase of distemper with motor signals that affected ambulation, and obtained a positive result in relation to the control group. Recently, Monteiro [33] assessed the effects of allogenic adipose tissue-derived MSCs on neurological abnormalities in dogs in the chronic phase of distemper, and observed that 13 of the 30 animals had a reduced neurological scale score, albeit most remained tetraparetic. Despite promising results after a single administration of MSCs, both Brito [32] and Monteiro [33] reported a poor health status of some dogs during the study, which may be related to the acute stage of clinical symptomatology, which was different from that of our dogs. Similar to our study, Gonçalves et al. [34] recently used dogs with neurological sequelae only after treatment of the multisystemic symptoms of distemper, and performed three intravenous infusions of adipose tissue-derived MSCs, but theirs were allogenic. In that study, based on the numerical scale proposed by the authors for evaluation, attenuation of the clinical signs was observed after 15 days and was maintained throughout the 180 days of observation, but with statistical differences only for the urinary incontinence and fecal incontinence variables.
In our present uncontrolled clinical trial, the autologous use of adipose tissue-derived MSCs by the intra-arterial route on every 30 days, totaling three infusions, resulted in improvement of the neurological status of the animals, with one dog regaining functional ambulation and achieving a reduction of the myoclonic intensity to Grade IV (moderate). However, consistent improvements were observed in the evaluation after one year post treatment, where three animals regained functional ambulation (Grade I), all animals moved independently (Grades I and II), and two animals presented Grade III myoclonus (mild).
The route of MSC administration is an important variable that can define the success of a transplant by interfering directly with the efficient delivery of cells to the site of interest [35]. The venous system, being the least invasive route, has been the one most often used. However, in addition to a lack of knowledge regarding the actual cellular concentration required to reach the desired lesion area, studies have shown that MSCs accumulate rapidly in the lungs, spleen, and liver after administration [35, 36]. However, by bypassing the initial uptake by the lungs, administration through the arterial system results in a greater availability of cells to ischemic sites, but may lead to a greater probability of microvascular occlusions [37]. In our study, no side effects related to short-term and long-term intra-arterial MSC administrations were observed, and choice for the femoral artery was considered due to easier access compared to the carotid artery or intrathecal route.
We believe that the indiscriminate commercialization of stem cells as a form of “treatment” of various diseases (including for the recovery of canine distemper sequelae) is unacceptable in both veterinary medicine, since it cannot be stated categorically that this therapy does in fact lead to the healing of patients. This is a current concern in many countries owing to the lack of regulations and control for the clinical use of stem cells in veterinary medicine, allowing for the increasing offer of the service by private companies and resulting in the implementation of therapies that lack proven effectiveness either in vitro or in preclinical animal studies [38, 39]. The US Food and Drug Administration's Center for Veterinary Medicine was the only legislative body to formally publish specific definitions and recommendations for stem cell use through guidelines [40], where cell-based products must follow the same legal requirements that apply to other animal drugs, forcing the industry to prove efficacy and manufacturing quality and safety prior to commercialization [38].
Despite the promising results regarding the alleviation of the severity of the disabling lesions of demyelinating leukoencephalitis caused by distemper (considered irreversible and often incompatible with animal life), our findings are considered limited because of the small sample size, and future studies should involve a greater number of animals. In addition, both in vivo and in vitro studies should be performed to determine the mechanisms underlying the therapeutic effects of MSCs in dog with neurological sequelae of distemper. In this context, the technology of induced pluripotent stem cells, from genetically modified and reprogrammed adult cells [41], would be a powerful tool in basic research, tissue differentiation research, and disease modeling, as well as being promising for future clinical applications.
5. Conclusions
Our results indicate that the strategy of three intra-arterial infusions of 1 × 107 MSCs, with a 30-day interval in between administrations, appears to be safe in dogs with demyelinating leukoencephalitis caused by the distemper virus, and presents moderate efficacy for the rehabilitation of neurological signs after recovery from a multisystemic infection, with considerable improvements in the neurological status of the animals after one year of cell therapy. However, further extensive investigations are needed for a better understanding of the mechanism of action of these MSCs on the injured nervous tissue and the time of recovery, in future studies that include a larger number of animals, placebo group and investigation of other routes of administration.
Declarations
Author contribution statement
Luane Lopes Pinheiro: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Ana Rita de Lima: Analyzed and interpreted the data.
Danielli Martinelli Martins, Michel Platini C. Souza, Carla Maria Figueiredo de Carvalho Miranda: Performed the experiments.
Edivaldo Herculano C. de Oliveira, Patrícia Cristina Baleeiro Beltrão-Braga, Fabiele Baldino Russo, Graciela Conceição Pignatari, Ednaldo da Silva Filho: Contributed reagents, materials, analysis tools or data.
Érika Branco: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brasil (Finance Code 001).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the Coordination of Improvement of Higher Education Personnel (CAPES) for financial support; the Laboratory of Tissue Culture and Cytogenetics of the Environment Section of the Evandro Chagas Institute; and the Veterinary Hospital “Prof. Mário Dias Teixeira” (UFRA) for supporting this study.
Appendix ASupplementary data
The following is the supplementary data related to this article:
Video
References
- 1.Lempp C., Spitzbarth I., Puff C. New aspects of the pathogenesis of canine distemper leukoencephalitis. Viruses. 2014;6:2571–2601. doi: 10.3390/v6072571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulrich R., Puff C., Wewetzer K. Transcriptional changes in canine distemper virus-induced demyelinating leukoencephalitis favor a biphasic mode of demyelination. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beineke A., Puff C., Seehusen F., Baumgärtner W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009;127:1–18. doi: 10.1016/j.vetimm.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Silva M.C., Fighera R.A., Brum J.S. Aspectos clinicospatológicos de 620 casos neurológicos de cinomose em cães. Pesqui. Vet. Bras. 2007;27:215–220. http://www.scielo.br/pdf/pvb/v27n5/a06v27n5.pdf [Google Scholar]
- 5.Dulamea A. Mesenchymal stem cells in multiple sclerosis – translation to clinical trials. J. Med. Life. 2015;8:24–27. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4397514 [PMC free article] [PubMed] [Google Scholar]
- 6.Marin-Bañasco C., Benabdellah K., Melero-Jerez C. Gene therapy with mesenchymal stem cells expressing IFN-ß ameliorates neuroinflammation in experimental models of multiple sclerosis. Br. J. Pharmacol. 2017;174:238–253. doi: 10.1111/bph.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kean T.J., Lin P., Caplan A.I. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cell. Int. 2013;2013:732742. doi: 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Martínez P., González-Granero S., Molina-Navarro M.M. Intraventricular injections of mesenchymal stem cells activate endogenous functional remyelination in a chronic demyelinating murine model. Cell Death Dis. 2017;7:e2223. doi: 10.1038/cddis.2016.130. https://www.nature.com/articles/cddis2016130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadasz J.J., Kremer D., Gottle P. Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman M.S., Bar-Or A., Atkins H.L. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult. Scler. 2010;16:503–510. doi: 10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- 11.Karussis D., Karageorgiou C., Vaknin-Dembinsky A. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamout B., Hourani R., Salti H. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J. Neuroimmunol. 2010;227:185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Bonab M.M., Sahraian M.A., Aghsaie A. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr. Stem Cell Res. Ther. 2012;7:407–414. doi: 10.2174/157488812804484648. [DOI] [PubMed] [Google Scholar]
- 14.Llufriu S., Sepulveda M., Blanco Y. Randomized placebo- controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;20:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Kokai L.E., Marra K., Rubin J.P. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014;163:399–408. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Santos B.P.C.R. Paulista State University; Botucatu Campus, São Paulo, Brazil: 2013. Effect of Acupuncture in The Treatment of Animals With Neurological Sequelae Due to Distemper. p. 90.https://repositorio.unesp.br/handle/11449/108599 Dissertation (Master's Degree) Faculty of Veterinary Medicine and Zootechny. [Google Scholar]
- 18.Zuk P.A., Zhu M., Mizuno H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 19.Gebara C.M.S., Wosiacki S.R., Negrão F.J. Detection of canine distemper virus nucleoprotein gene by RT-PCR in urine of dogs with distemper clinical signs. Arq. Bras. Med. Vet. Zootec. 2004;56:480–487. [Google Scholar]
- 20.Nelson R.W., Couto C.G. fourth ed. Elsevier; Rio de Janeiro, Brazil: 2008. Small Animal Internal Medicine. [Google Scholar]
- 21.Mangia S.H. A. C. Paes neuropathology of distemper. Vet. Zootec. 2008;15:416–427. [Google Scholar]
- 22.Koestner A. Animal model of human disease: subacute sclerosing panencephalitis, multiple sclerosis; animal model: distemper-associated demyelinating encephalomyelitis. Am. J. Pathol. 1975;78:361–364. PMID: 1090185. [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgärtner W., Alldinger S. The pathogenesis of canine distemper virus induced demyelination: a biphasic process. In: Lavi E., Constantinescu C.S., editors. Experimental Models of Multiple Sclerosis. Springer; New York, NY: 2005. pp. 871–887. [Google Scholar]
- 24.Auletta J.J., Bartholomew A.M., Maziarz R.T. The potential of mesenchymal stromal cells as a novel cellular therapy for multiple sclerosis. Immunotherapy. 2012;5:529–547. doi: 10.2217/imt.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bearden R.N., Huggins S.S., Cummings K.J. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: a donor-matched comparative study. Stem Cell Res. Ther. 2017;8:218. doi: 10.1186/s13287-017-0639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera F.J., Aigner L. Adult mesenchymal stem cell therapy for myelin repair in multiple sclerosis. Biol. Res. 2012;45:257–268. doi: 10.4067/S0716-97602012000300007. [DOI] [PubMed] [Google Scholar]
- 27.Vieira N.M., Brandalise V., Zucconi E. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;19:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- 28.Patricio L.F.L., Rebelatto C.L.K., Brofman P.R.S. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Arq. Bras. Med. Vet. Zootec. 2013;65:946–954. [Google Scholar]
- 29.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 30.Marx C., Silveira M.D., Beyer Nardi N. Adipose-derived stem cells in veterinary medicine: characterization and therapeutic applications. Stem Cell. Dev. 2015;24:803–813. doi: 10.1089/scd.2014.0407. [DOI] [PubMed] [Google Scholar]
- 31.Pinheiro A.O., Cardoso M.T., Vidane A.S. Controversial results of therapy with mesenchymal stem cells in the acute phase of canine distemper disease. Genet. Mol. Res. 2016;15 doi: 10.4238/gmr.15028310. GMR15028310. [DOI] [PubMed] [Google Scholar]
- 32.Brito H.F.V. Federal University of Paraná; Curitiba, Paraná, Brazil: 2015. Use of Bone Marrow Mononuclear Cells for the Treatment of Neurological Sequelae of Canine Distemper; p. 79.https://acervodigital.ufpr.br/handle/1884/41363 Thesis (Doctorate) Available at: [Google Scholar]
- 33.Monteiro B.A. Paulista State University; Botucatu Campus, São Paulo, Brazil: 2017. Effects of Therapy with Mesenchymal Stem Cells on Disorders of the Nervous System of dogs; p. 68p.https://repositorio.unesp.br/handle/11449/151543 Thesis (Doctorate), Faculty of Veterinary Medicine and Zootechnics. Available at: [Google Scholar]
- 34.V Gonçalves D.S., S Gomes M.V., P Guterra V.L. Mesenchymal stem cell infusion for the treatment of neurological sequelae of canine distemper virus: a clinical study. Genet. Mol. Res. 2018;17 GMR18088. [Google Scholar]
- 35.Zhao W., Li J.J., Cao D.Y. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J. Gastroenterol. 2012;18:1048–1058. doi: 10.3748/wjg.v18.i10.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karp J.M., Leng Teo G.S. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Walczak P., Zhang J., Gilad A.A. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogers S.H. Cell-based therapies for joint disease in veterinary medicine: what we have learned and what we need to know. Front. Vet. Sci. 2018;5:70. doi: 10.3389/fvets.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Bakker E., Van Ryssen B., De Schauwer C. Canine mesenchymal stem cells: state of the art, perspectives as therapy for dogs and as a model for man. Vet. Q. 2013;33:225–233. doi: 10.1080/01652176.2013.873963. [DOI] [PubMed] [Google Scholar]
- 40.FDA. Guidance for Industry: Cell-Based Products for Animal Use. United States Food and Drug Administration Center for Veterinary Medicine; Rockville, MD: 2015. https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM405679.pdf [Google Scholar]
- 41.Takahashi K., Tanabe K., Ohnuki M. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video