Abstract
Background
The changes induced in host immunity and the tumor microenvironment by chemotherapy have been shown to impact immunotherapy response in both a positive and a negative fashion. Temozolomide is the most common chemotherapy used to treat glioblastoma (GBM) and has been shown to have variable effects on immune response to immunotherapy. Therefore, we aimed to determine the immune modulatory effects of temozolomide that would impact response to immune checkpoint inhibition in the treatment of experimental GBM.
Methods
Immune function and antitumor efficacy of immune checkpoint inhibition were tested after treatment with metronomic dose (MD) temozolomide (25 mg/kg × 10 days) or standard dose (SD) temozolomide (50 mg/kg × 5 days) in the GL261 and KR158 murine glioma models.
Results
SD temozolomide treatment resulted in an upregulation of markers of T-cell exhaustion such as LAG-3 and TIM-3 in lymphocytes which was not seen with MD temozolomide. When temozolomide treatment was combined with programmed cell death 1 (PD-1) antibody therapy, the MD temozolomide/PD-1 antibody group demonstrated a decrease in exhaustion markers in tumor infiltrating lymphocytes that was not observed in the SD temozolomide/PD-1 antibody group. Also, the survival advantage of PD-1 antibody therapy in a murine syngeneic intracranial glioma model was abrogated by adding SD temozolomide to treatment. However, when MD temozolomide was added to PD-1 inhibition, it preserved the survival benefit that was seen by PD-1 antibody therapy alone.
Conclusion
The peripheral and intratumoral immune microenvironments are distinctively affected by dose modulation of temozolomide.
Keywords: glioblastoma, immune checkpoint inhibition, immunomodulation, PD-1 antibody, temozolomide
Key Points.
SD temozolomide increases exhaustion markers on T cells, while MD temozolomide does not exhaust T cells.
PD-1 blockade can reverse exhaustion induced by SD temozolomide in peripheral T cells but not on tumor infiltrating lymphocytes.
Importance of the Study.
Temozolomide is the most common chemotherapy for the treatment of GBM and results in lymphopenia. This lymphopenia has been shown to impact response to various immunotherapeutic strategies. In this work, we found that temozolomide modulates host immune function differentially based on dosing. Standard dosing of temozolomide resulted in exhaustion of peripheral and intratumoral T cells and a greater proportion of peripheral immunosuppressive cells. These effects were not seen with metronomic dosing of temozolomide. Importantly, SD temozolomide removed the survival benefit of PD-1 immune checkpoint blockade in a murine glioma model responsive to PD-1 blockade. The combination of MD temozolomide and PD-1 blockade preserved the improved survival seen with PD-1 blockade alone. Therefore, metronomic dosing of temozolomide may be a preferred strategy when combined with immunotherapeutic platforms that depend on activation of endogenous immune response. These findings have implications for combinatorial strategies for chemotherapy and immunotherapy in the treatment of GBM.1
Immune checkpoint inhibitors have dramatically changed the landscape of cancer treatment due to their efficacy in traditionally resistant solid tissue malignancies.2–4 These drugs block inhibitory interactions between tumor cells and T cells, thereby resulting in activation of T cells against tumor antigens.5 The ability to leverage a patient’s endogenous antitumor immunity is a compelling strategy and is also being tested for the treatment of glioblastoma (GBM). The animal data6–8 have been compelling, and initial human preliminary data for GBM9 were promising.
Programmed cell death 1 (PD-1) is one of the immune checkpoints that is actively being investigated in GBM. PD-1 blockade has been shown to activate T cells against tumor neoantigens.10 PD-1 inhibitors have shown dramatic response rates in solid tissue malignancies with known high mutational burden11 and neoantigens such as melanoma12 and non–small cell lung carcinoma.2 GBM tumors with errors in DNA repair mechanisms such as those with biallelic mismatch repair deficiency have also demonstrated response to PD-1 blockade.13 However, a recently completed phase III clinical trial using PD-1 inhibition monotherapy in unselected patients with recurrent GBM demonstrated no survival benefit.14
Given these results, the effects of combinatorial strategies for newly diagnosed GBM are critical.
Standard treatment of newly diagnosed GBM includes temozolomide (TMZ), which causes lymphopenia in the host as well as changes in the tumor microenvironment.15,16 The changes induced by TMZ vary based on dose17–19 and method of delivery.20,21 Therefore, we aimed to determine if dose modification of TMZ resulted in changes in host immunity and tumor microenvironment that would impact response to PD-1 inhibition in the treatment of GBM. We hypothesized that variations in dosing of TMZ would impact the efficacy of immune checkpoint inhibition for glioma due to a decrease in immunosuppressive cells and lymphocyte exhaustion.
Methods
Animals
C57BL/6 mice, OT-I transgenic mice on the C57BL/6 background, and interferon (IFN)-gamma reporter with endogenous polyA transcript (GREAT) mice were purchased from Jackson Laboratories. Studies were approved by the University of Florida Institutional Animal Care and Use Committee.
Tumor Cells and Intracranial Tumor Implantation
The tumor cell lines GL261, B16F10-OVA, and KR158 were utilized for the studies. The GL261 line is a glioma that has some sensitivity to TMZ and PD-1 blockade. The KR158 line is a highly invasive radiation and chemotherapy-resistant glioma,22 and was originally isolated from a spontaneously arising astrocytoma in an NF1;Trp53 mutant mouse that was on a C57BL/6 background.23 B16F10-OVA is a well-established melanoma cell line. Culture media utilized for GL261, B16F10-OVA, or KR158 tumor cells consisted of Dulbecco’s modified Eagle’s medium (DMEM)/F12 and DMEM with sodium pyruvate, respectively, that was supplemented with 10% fetal bovine serum, and 1% penicillin/streptomycin. For tumor implantation experiments, tumor cells were mixed 50/50 with methylcellulose (R&D Systems) and phosphate buffered saline. Using sterile technique, the tip of the needle was positioned at bregma and 2 mm to the right of the cranial midline suture and 4 mm below the surface of the cranium using a Kopf stereotactic frame.
Treatment with Temozolomide and Immune Checkpoint Blockade
Animals were treated with standard dose (SD) or metronomic dose (MD) TMZ (Sigma Aldrich #T2577) and/or PD-1 monoclonal antibody (BioXcell, clone: RMP1-14). The dose for TMZ was defined as SD based on calculations of human doses of 200 mg/m2 that were converted to appropriate doses for a mouse.24 SD TMZ was defined as 50 mg/kg for 5 days and MD TMZ defined as 25 mg/kg for 10 days via intraperitoneal (i.p.) injection. These doses were chosen based on prior reports using 50 mg/kg for 5 days as representative of standard dosing in a murine model.25 Metronomic dosing was chosen to be the same total dose over a prolonged period of time without giving these animals the advantage of a larger total dose. Although this does not reflect metronomic dosing in patients, this dose was utilized to demonstrate dosing impact on host immunity and changes in response to immune checkpoint inhibition. PD-1 antibody was given i.p. with a dose of 10 mg/kg every 5 days for 4 doses.
Peripheral Blood Draws and Complete Blood Counts
Submandibular bleeding was used to collect blood. Whole blood was lysed using BD Lysing buffer (Fisher Scientific #BD 555899). Cells were washed with flow cytometry buffer (phosphate buffered saline containing 2% fetal bovine serum) and stained with CD3 (Fisher Scientific #BDB551163), CD4 (Fisher Scientific #50402924), and CD8 (Fisher Scientific #BDB553035) antibodies.
Antibodies and Flow Cytometry
Used for cell surface marker staining were antibodies for CD279 (BD Biosciences #561788), CD366 (Biolegend #3119701), CD223 (BD Biosciences #562346), CD274 (Fisher Scientific #BDB558091), CD11b (Fisher Scientific #BDB550993), F4/80 (Biolegend #123133), Ly6G/Ly-6c (Biolegend #108406), as well as appropriate isotype controls. For intracellular staining, samples were permeabilized with buffer (Thermo Fisher Scientific #88882400) and stained for FoxP3 (Thermo Fisher Scientific #53577380). Staining of circulating OT-I CD8+ T cells was performed by PE-H-2 Kb OVA (SIINFEKL murine tetramer; MBL International #TB-5001-1).
Immunofluorescence Microscopy
Samples were embedded in Tissue-Tek OCT (Electron Microscopy Sciences), frozen. Sections were cut (5 μm thick) with Leica CM1850 Cryostat (Leica Microsystems). Immunofluorescence staining was performed with the antibodies of anti-CD3, anti-CD4, anti-B220, and anti-CD8. Sections were evaluated via an EVOS fluorescence microscope at 20x magnification.
Functional Stimulation Assay
To determine antitumor T-cell function, T cells were isolated from spleens by a pan T-cell isolation kit II (Miltenyi Biotec #130095130). T cells were co-cultured with dendritic cells electroporated with ovalbumin (OVA) antigen and the supernatant was collected 24 hours later. The Mouse IFN-γ ELISA Kit II (BD Biosciences) was used to determine IFN-γ secretion.
Tumor Infiltrating Cells
To identify tumor infiltrating immune cells, the brain was removed and the injected hemisphere was homogenized and resuspended in Percoll (Fisher Scientific). The cells were overlaid onto a Percoll gradient and centrifuged. Lymphocytes were collected from the 37% / 70% interface.
Analysis of IFN-γ Production
Transgenic GREAT mice were implanted with tumors and treated as designed. Tumors were harvested, and tumor infiltrated immune cells were isolated and analyzed for IFN-γ secretion by flow cytometry. Yellow fluorescent protein (YFP) is coexpressed with IFN-γ, and FL1 channel was used to detect each cell expressing IFN-γ.
NanoString and RNA Sequencing
An nCounter Pancancer Immune Profiling Panel (NanoString Technologies) for gene expression was used for cancer immune response analysis. Total RNA of all treatment groups and untreated controls was extracted from tumors using the RNeasy Lipid Tissue Mini Kit (Qiagen) and used for the nCounter Digital Analyzer. Data were analyzed with nSolver Analysis software 4.0 with normalization utilizing positive and negative control probes as well as housekeeping genes. For RNA sequencing, total RNA was isolated from tumors and sent to the University of Florida Interdisciplinary Center for Biotechnology Research for paired end sequencing. The input sequences were trimmed using Trimmomatic. Quality control was performed before and after trimming using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Differentially expressed genes (DEGs) in each contrast with absolute fold change (log2 FC) ≥1.0 and false discovery rate (FDR) corrected P-value ≤0.01. Pathways of KEGG (Kyoto Encyclopedia of Genes and Genomes) were enriched and visualized as a dot plot from DEGs by R package clusterProfiler (P < 0.05, FDR < 0.05).26 Immune checkpoints and co-inhibitor molecule expression of 2 groups were plotted to a volcano plot by R packages ggplot227 and ggrepel (https://github.com/slowkow/ggrepel).
Statistical Analysis
Mann–Whitney U-tests and Student’s t-tests were used to analyze data from 2 different groups. One-way ANOVA was used to determine statistical significance between >2 independent groups; however, for analysis of more than 2 independent variables, 2-way ANOVA was used. Survival data were analyzed by the Gehan-Breslow-Wilcoxon test. In all experiments, statistical significance was set at the level of P < 0.05. Statistical analysis was performed using GraphPad Prism 7.03 software.
Results
Impact of Temozolomide Dosing on Peripheral Immune Cell Phenotype
Temozolomide is well known to result in lymphodepletion that can be leveraged for enhanced antigen-specific T-cell recovery when combined with cellular-based immunotherapy.17–19,28 The effects of TMZ-induced lymphodepletion on response to immune checkpoint inhibition are still unknown. Since our group has demonstrated that dosing of TMZ results in different effects on host antigen-specific T cells,17 we tested 2 different doses of TMZ. MD and SD TMZ were delivered to mice, and peripheral blood was collected to test different markers and absolute counts using flow cytometry. The absolute lymphocyte counts decreased at different timepoints posttreatment.
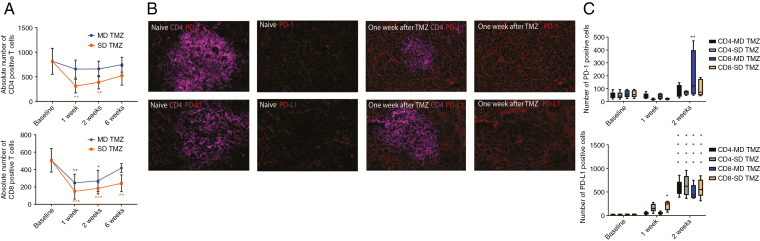
The SD TMZ group had a mean CD4 T-cell count of 311.37 compared with 658.43 in the MD TMZ group at 1 week (Fig. 1A). Similarly, the mean absolute CD8 T-cell count in the SD TMZ group at 1 week was 150.64 compared with 247.61 in the MD TMZ group. As expected, higher doses of TMZ resulted in a more significant lymphopenia in both CD4 and CD8 T cells.
Fig. 1.
Peripheral blood T-cell counts and PD-1 and PD-L1 expression on T cells after exposure to TMZ. (A) Peripheral blood was collected after animals were treated with SD or MD TMZ for T-cell count using flow cytometry. In the SD group, the mean number of CD4 T-cell count decreased 1 week (2.11-fold), 2 weeks (1.42-fold), and 6 weeks (1.7-fold) compared with the MD group. The mean number of CD8 T-cell count in the SD group decreased at 1 week (1.64-fold), 2 weeks (1.48-fold), and 6 weeks (1.73-fold) compared with the MD group (P < 0.05). In both the SD and MD TMZ groups, lymphopenia was observed in the CD4 and CD8 populations compared with baseline (P < 0.05). (B) Immunofluorescence microscopy of murine spleens after SD treatment showed increased expression of PD-1 and PD-L1 on splenocytes after TMZ exposure compared with the baseline. (C) PD-1 and PD-L1 expression on peripheral blood T cells was evaluated after TMZ treatment using flow cytometry. MD TMZ resulted in a 3.51-fold increase (P < 0.001) of PD-1+/CD8 T cells at 2 weeks without a significant change in PD-1+/CD4 T cells. SD TMZ did not have a significant increase in PD-1+ CD4 or CD8 T cells. MD TMZ resulted in a 21-fold increase in PD-L1+/CD8 T cells and a 27.3-fold increase in PD-L1+/CD4 T cells (P < 0.0001) after 2 weeks. SD TMZ resulted in a 9-fold increase in week 1 of PD-L1+/CD8 T cells, and a 25-fold increase in week 2 of PD-L1+/CD8 T cells (P < 0.0001). There was a 29-fold increase in PD-L1+/CD4 T cells in week 2 (P < 0.0001) without a significant change in week 1. n = 5 per group; baseline = tumor bearing animals without any treatment.
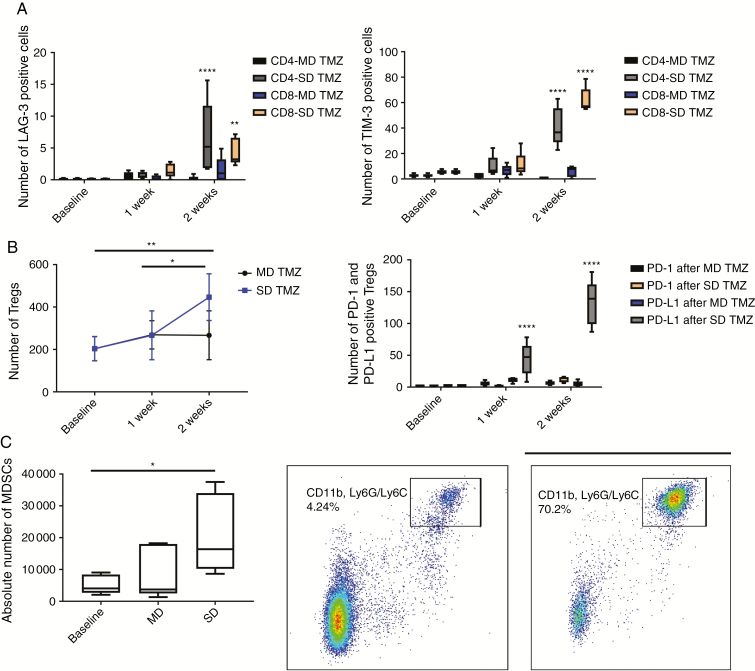
PD-1 inhibition efficacy has been linked to the expression of PD-1 and PD ligand 1 (PD-L1) on both T cells and tumor cells.29–31 In these experiments, we tested the expression of these markers on murine splenocytes using immunofluorescence microscopy. Evaluation of non–tumor bearing murine spleens after SD TMZ treatment showed a qualitative upregulation of both PD-1 and PD-L1 (Fig. 1B). This finding was further investigated on circulating T cells in tumor-bearing mice with MD and SD TMZ treatment. GL261 tumor-bearing animals were treated with either MD or SD TMZ. KR158 tumor-bearing animals were also treated and these results are shown in the Supplementary Figures. Both MD and SD TMZ resulted in a several-fold increase in PD-1 and PD-L1 expression on CD4+ and CD8+ T cells (Fig. 1C). PD-1 increased significantly in CD8 T cells (3.5-fold increase) in the MD TMZ treatment group at 2 weeks (P < 0.001). PD-L1 increased in both CD4 and CD8 T cells (up to 27-fold) at 1 and 2 weeks posttreatment with TMZ regardless of dose (P < 0.0001). Next, mice were tested for expression of markers of immune suppression. SD TMZ treatment resulted in an upregulation of markers of T-cell exhaustion on lymphocytes such as lymphocyte-activation gene 3 (LAG-3) and T-cell immunoglobulin and mucin-domain containing 3 (TIM-3) (Fig. 2A, Supplementary Figure 1A, B). Notably, MD TMZ did not result in any increase in these exhaustion markers.
Fig. 2.
Exhaustion markers on peripheral blood T cells and circulatory immunosuppressive cells. LAG-3 and TIM-3 expression on CD4 and CD8 T cells and proportion of Tregs (CD4+ CD25+ FoxP3+) and MDSCs (CD11b+/Ly6G/6c+) were measured in peripheral blood using flow cytometry after TMZ treatment. (A) MD TMZ did not change expression of TIM-3 and LAG-3 on CD4 and CD8 T cells. SD TMZ increased LAG-3+/CD4 (118-fold, P < 0.0001), TIM-3+/CD4 (15-fold, P < 0.0001), LAG-3+/CD8 (104-fold, P = 0.002), and TIM-3+/CD8 T cells (11.6-fold, P < 0.0001). (B) SD TMZ treatment resulted in a relative increase in peripheral blood Tregs (2.3-fold, P = 0.005). MD TMZ did not change the number of Tregs. SD TMZ upregulated PD-L1 expression on Tregs 15.6-fold (P < 0.0001) after 1 week and 47.14-fold (P < 0.0001) after 2 weeks. (C) The mean number of MDSCs increased 4-fold (P = 0.04) 2 weeks after SD TMZ. n = 5 per group; baseline = tumor-bearing animals without any treatment.
In addition to T cells, the effects of TMZ on regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) were evaluated. We found the relative number of Tregs had a trend for increase after both SD and MD TMZ with greater effects of SD TMZ (Fig. 2B, Supplementary Figure 1C).
Furthermore, SD TMZ treatment resulted in a 47-fold increase in PD-L1 expression on Tregs at 2 weeks posttreatment (Fig. 2B, Supplementary Figure 1D) that was not observed with MD dosing. The upregulation of PD-L1 on Tregs is associated with increased immunosuppressive activity.32 SD TMZ was also associated with a 4-fold increase in blood CD11b+ and Ly6G/6c+ cells (P = 0.04; Fig. 2C, Supplementary Figure 1E, F) that was not observed by MD TMZ. These data suggest that MD TMZ may have the advantage of reduced peripheral blood immunosuppressive cells.
Temozolomide Effects on Tumor Infiltrating Lymphocytes and Antigen-Specific T-Cell Function
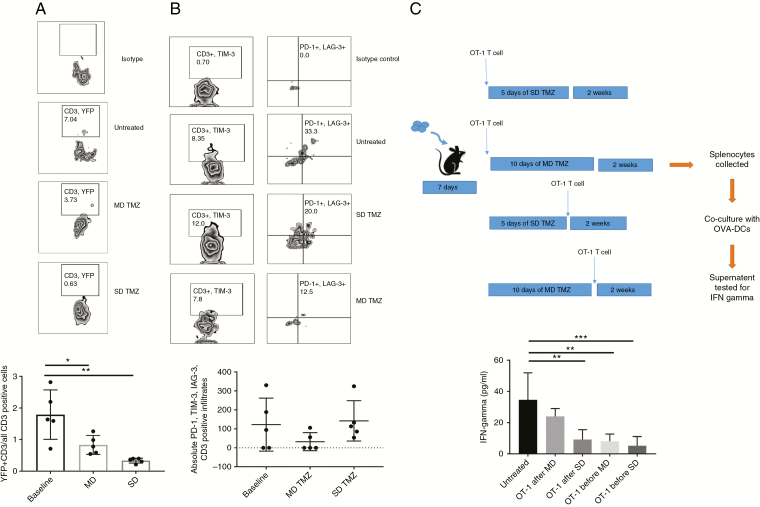
Next, we tested the impact of TMZ on the functionality of tumor infiltrating lymphocytes (TILs). GREAT mice, which have YFP, were implanted with GL261 or KR158 tumors. After treatment with MD or SD TMZ, tumors were harvested and TILs were isolated and tested for IFN-γ by YFP using flow cytometry. The ratio of CD3+ T cells expressing IFN-γ to CD3+ T cells decreased with both MD and SD TMZ treatment. This decrease in the ratio was more dramatic in the SD (P = 0.0013) (Fig. 3A, Supplementary Figure 2A, B). Conversely, tumors treated with MD TMZ had a trend for decreased expression of markers associated with exhaustion on TILs. TILs that were triple positive for PD-1, TIM-3, and LAG-3 had a trend for decrease in the MD TMZ group (P = 0.2512) (Fig. 3B, Supplementary Figure 2C).
Fig. 3.
Peripheral and intratumoral T-cell function in a murine glioma model. (A) IFN-γ expressing CD3 TILs were measured using flow cytometry as a ratio of total CD3 TILs. SD and MD groups had a decrease (5.4-fold, P = 0.0013 and 2.15-fold, P = 0.0222), respectively, in the IFN-γ expressing/YFP+ CD3 TILs compared with the baseline. (B) CD3 TILs were tested for expression of triple exhaustion markers of PD-1, TIM-3, and LAG-3. No significant change was observed among groups. (C) OT-I T cells were infused into tumor-bearing animals before and after MD and SD TMZ treatment. CD3 splenocytes were tested for IFN-γ secretion by enzyme-linked immunosorbent assay. Both SD and MD TMZ significantly decreased IFN-γ secretion when T cells were infused before TMZ treatment. When TMZ was given prior to T-cell infusion, SD TMZ significantly reduced IFN-γ secretion (3.76-fold) from the OT-I T cells compared with untreated animals (P = 0.008). The MD TMZ group had preservation of IFN-γ secretion from OT-I T cells when given prior to T-cell infusion. n = 5 per group; baseline = tumor-bearing animals without any treatment.
To evaluate the impact of TMZ on the function of antigen-specific T cells, B16F10-OVA intracranial tumor bearing animals underwent infusion of OT-I splenocytes before or after treatment with MD or SD TMZ. Spleens were collected and CD3 splenocytes were tested for IFN-γ secretion. All of the groups that received the OT-I infusion before treatment with TMZ had a reduction in IFN-γ secretion compared with animals that did not receive TMZ. In the groups that received the OT-1 infusion after treatment with TMZ, the SD TMZ had significantly decreased IFN-γ secretion from T cells, which was not seen with MD TMZ treatment. SD TMZ and not MD treatment induced changes in host immunity that reduced functional capacity of antigen-specific T cells even when the T cells were not directly exposed to TMZ, since the T cells were infused after TMZ doses had all been given (Fig. 3C). Overall, SD TMZ resulted in less functional peripheral and tumor infiltrating lymphocytes and an increase in TILs’ exhausted phenotype.
PD-1 Inhibition Alone and in Combination with Temozolomide Changes Host Immunity
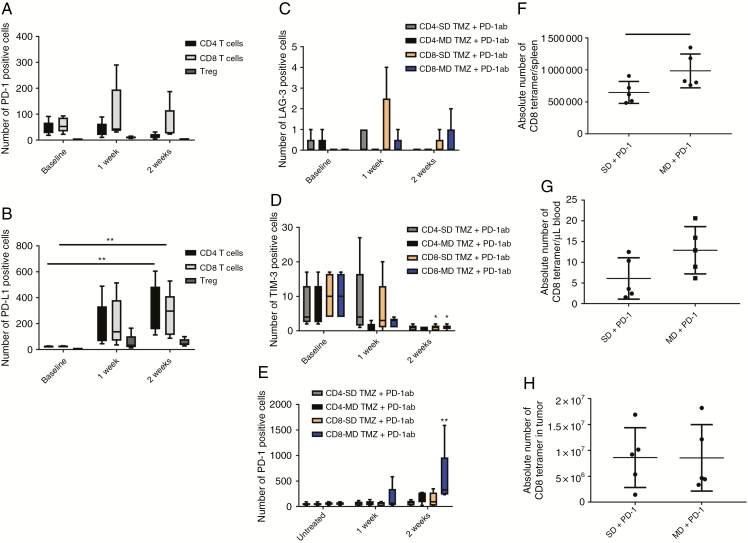
To determine the effects of PD-1 antibody treatment on peripheral T cells, GL261 bearing mice underwent treatment with PD-1 antibody. Peripheral blood T cells were evaluated after treatment using flow cytometry. PD-1 antibody therapy increased PD-L1 expression on CD4 and CD8 T cells and Tregs (Fig. 4A, B). In GL261 tumor bearing animals when PD-1 antibody was combined with SD TMZ, the upregulation of exhaustion markers was not seen on peripheral blood T cells as it had been observed with SD TMZ alone (Fig. 4C, D). However, in KR158 tumor bearing animals, PD-1 antibody plus SD TMZ still resulted in an increase in LAG-3 in CD8 T cells. Both SD and MD TMZ/PD-1 antibody resulted in increases in TIM-3 in CD4 and CD8 T cells (Supplementary Figure 3). PD-1 expression on CD4 and CD8 T cells increased in the MD/PD-1 antibody group similar to MD TMZ alone (Fig. 4E). PD-1 antibody with or without TMZ results in an increase in PD-L1 expression on peripheral blood T cells and reverses the expression of exhaustion markers on peripheral T cells that was induced by SD TMZ.
Fig. 4.
Evaluation of exhaustion markers on T cells and expansion of T cells after PD-1 antibody or combination treatment. (A) PD-1 antibody treatment did not change PD-1 expression on CD4 and CD8 T cells or Tregs. (B) PD-L1 expression increased on CD4 T cells (14.51-fold, P = 0.002) and CD8 T cells (11.6-fold, P = 0.009) 2 weeks after PD-1 antibody treatment. (C–E) Markers of exhaustion were tested in animals treated with combination PD-1 inhibition and TMZ. LAG-3 expression did not change on CD4 and CD8 T cells when animals were treated with PD-1 antibody in combination with MD or SD TMZ. TIM-3 expression was decreased on CD8 T cells after combination of SD TMZ (9.4-fold) and MD TMZ (9.2-fold) with PD-1 antibody (P = 0.02). MD/PD-1 antibody treatment demonstrated a 9.33-fold increase in the percentage of PD-1+/CD8 T cells 2 weeks after treatment (P = 0.0006). (F) OT-I T cells were infused into B16F10-OVA tumor-bearing mice after SD and MD TMZ treatment in combination with PD-1 antibody. OT-I+/CD8 T cells were significantly more prevalent in the spleens of animals treated with MD TMZ/PD-1 antibody (1.5-fold, P = 0.04) compared with the SD TMZ/PD-1 antibody group. (G) OT-I+/CD8 T cells had a trend for an increase in the blood of animals treated with MD TMZ/PD-1 antibody (2.12-fold). (H) B16F10-OVA intracranial tumors had no difference in the number of CD8 tetramer+ TILs between treatment groups. n = 5 per group; baseline = tumor-bearing animals without any treatment.
To determine the impact of treatment on antigen-specific T-cell expansion, B16F10-OVA tumors were implanted and animals were infused with OT-1 T cells after treatment with SD and MD TMZ in combination with PD-1 antibody. The animals treated with MD TMZ/PD-1 antibody had a 1.5-fold increase in antigen-specific T cells in the spleen compared with the SD TMZ/PD-1 antibody group (Fig. 4F). The MD TMZ/PD-1 antibody treatment group also demonstrated a trend for increased antigen-specific CD8+ T cells in the blood compared with the SD TMZ/PD-1 antibody group (Fig. 4G). The number of antigen-specific T cells infiltrating tumors was similarly high in both groups (Fig. 4H).
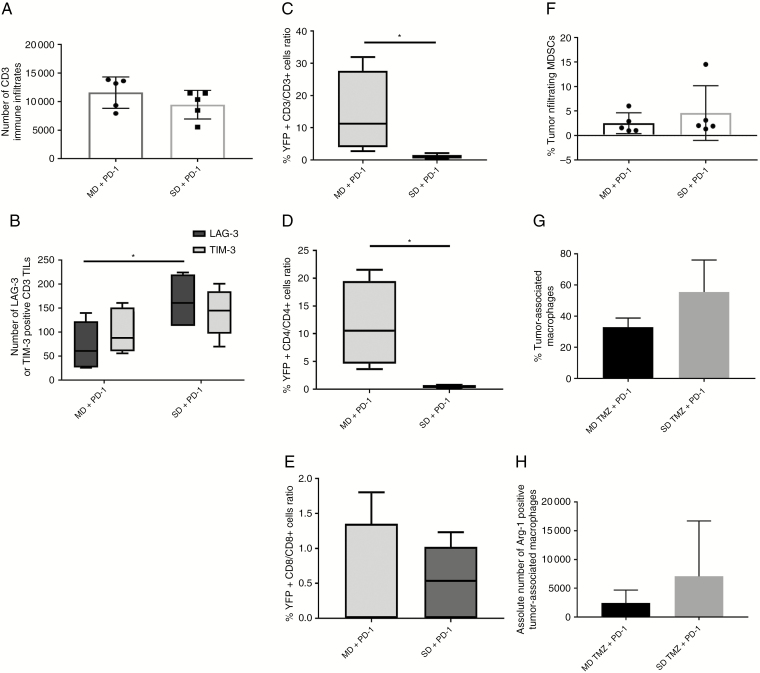
Combination of Temozolomide and PD-1 Inhibition Effects on Tumor Infiltrating Immune Cells
To determine the effect of TMZ treatment on lymphocytes responding to tumor antigens, TILs in GL261 and KR158 were tested for exhaustion markers in animals treated with SD or MD TMZ in combination with PD-1 antibody. The number of CD3+ TILs was similar in both groups (Fig. 5A, Supplementary Figure 4A). The MD TMZ/PD-1 group had decreased LAG-3 (P = 0.018) and a trend for decreased TIM-3 (P = 0.4) compared with the SD TMZ/PD-1 group (Fig. 5B, Supplementary Figure 4B).
Fig. 5.
Intracranial GL261 TILs and immunosuppressive cells after combination treatment of TMZ and PD-1 antibody. (A) No difference in CD3+ TILs was observed between groups treated with SD or MD TMZ/PD-1 antibody. (B) TILs in animals treated with SD TMZ/PD-1 antibody had significantly higher LAG-3 expression (2.3-fold, P = 0.018) compared with MD TMZ/PD-1 animals. There was a trend for increased TIM-3 in the SD TMZ/PD-1 antibody group (1.3-fold P = 0.4) as well. (C–E) GREAT mice underwent GL261 tumor implantation and treatment with TMZ and PD-1 antibody. Activated T cells as measured by YFP expression (IFN-γ expression) were increased within the tumors in the MD/PD-1 antibody group (14-fold YFP+ CD3/CD3 T cells, and 12-fold YFP+ CD4/CD4 T cells, P < 0.05). No differences in the YFP+ CD8 T cells were found between treatment groups (P = 0.8948; F–H). Flow cytometry was performed on intratumoral cells expressing MDSCs (CD11b+/Ly6G/6c+) or macrophages (CD11b+/F480+). The tumor infiltrating MDSCs and macrophages were not different. There was a trend for an increase (5.5-fold, P = 0.07302) in arginase-1 expressing macrophages in the SD/PD1 antibody group. n = 5 per group.
Next we tested the impact of treatment on the function of intratumoral T cells. GREAT mice underwent implantation of GL261 or KR158 tumors and were treated with SD or MD TMZ with or without PD-1 antibody. TILs were assessed for IFN-γ by YFP+ signal using flow cytometry. The MD/PD-1 antibody group had 14-fold more YFP+/CD3+ (P = 0.0498) and 19-fold more YFP+/CD4+ TILs (P = 0.0150) compared with the SD/PD-1 antibody group (Fig. 5C, D, Supplementary Figure 4C–E). However, the proportion of YFP+/CD8+ TILs was similar between the groups (P = 0.8949) (Fig. 5E). Using the same experimental design, immunosuppressive cells of MDSCs (CD11b+/Ly6G/6c+) and macrophages (CD11b+/F480+) were tested. Both TMZ/PD1 antibody groups had similar MDSCs (Fig. 5F). There was a trend for an increase in percent macrophages and arginase-1 expressing macrophages in the SD/PD-1 antibody group compared with the MD/PD-1 antibody group but did not reach statistical significance (Fig. 5G, H).
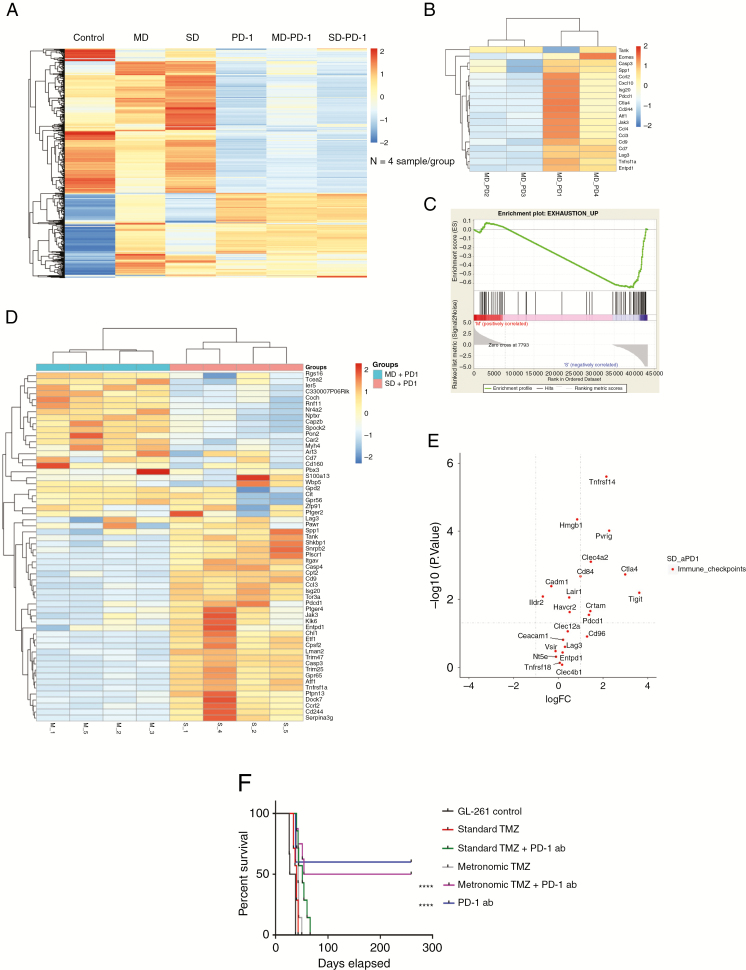
Temozolomide Effects on the Tumor Microenvironment
The tumors from all treatment groups and control untreated animals were collected and the RNA was isolated and used for pan cancer NanoString immune gene profiling. In an analysis of 770 genes, the TMZ treatment groups were found to have a very different genomic profile compared with tumors treated with PD-1 inhibition or PD-1 inhibition in combination with TMZ (Fig. 6A). Evaluation of genes associated with immune exhaustion within the tumors treated with MD in combination with PD-1 inhibition revealed that half of the tumors had a very low expression of exhaustion, while the other half demonstrated exhaustion similar to the TMZ alone treatment groups (Fig. 6B). Next, the tumors from the SD/PD-1 antibody and MD/PD-1 antibody groups were collected and processed for RNA sequencing. The SD/PD-1 antibody group had increased expression of gene signatures of T-cell exhaustion (Fig. 6C, D), as previously shown in T cells from a chronic viral infection model.33,34 These results are consistent with the functional observations that higher doses of TMZ result in increased T-cell exhaustion and impair response to PD-1 blockade. Analysis of immune checkpoint pathways demonstrated that several genes were overexpressed in the SD/PD-1 antibody group, including cytotoxic T-lymphocyte antigen 4, LAG-3, and TIGIT (P < 0.05). The MD/PD1 antibody group did not have any upregulation of immune checkpoints (Fig. 6E).
Fig. 6.
NanoString and RNA sequencing for tumor microenvironment and survival analyses. (A) NanoString pan cancer immune profiling was used for evaluation of expression of 770 genes in tumor microenvironment of GL261 tumor bearing animals treated with SD or MD TMZ alone or in combination with PD-1 antibody, PD-1 antibody, or untreated animals. PD-1 antibody treatment groups had significantly different genomic profiles compared with the control and TMZ alone treatment groups. Each column represents an average of 4 animals in the group. (B) The NanoString heat map demonstrated that only half of the MD/PD-1 antibody treated tumors had a significant decrease in genes associated with immune exhaustion. (C) Enrichment plot from RNA sequencing showed a negative enrichment score of exhaustion markers in the GL261 tumors treated with MD TMZ/PD-1 antibody compared with the SD TMZ/PD-1 antibody group (enrichment score: −0.64, FDR q: 0.039, P-value: 0.03). (D) RNA sequencing analysis of MD/PD-1 antibody compared with SD/PD-1 antibody treated tumors demonstrates a significant upregulation of immune exhaustion genes in the SD/PD-1 antibody group. (E) The genomic analysis based on RNA sequencing of immune checkpoint genes demonstrated an increase in the SD TMZ/PD-1 antibody group with no overexpressed immune checkpoint genes in the MD/PD-1 antibody group. n = 4 per group. (F) Both MD and SD TMZ resulted in a modest survival benefit. PD-1 antibody treatment resulted in 50% long-term survivors. When combined with TMZ, the SD group all died (P < 0.0001), but the MD TMZ group showed similar survival benefit as PD-1 antibody alone. n = 4 for NanoString; n = 4 for RNA sequencing; n = 8 for survival analysis.
Murine GBM Response to Temozolomide and PD-1 Antibody Therapy
C57BL/6 mice harboring GL261 intracranial tumors were treated with SD or MD TMZ, PD-1 antibody, or combination treatment. Both SD and MD TMZ resulted in a modest survival benefit, with all animals eventually succumbing to death. PD-1 antibody therapy in GL261 resulted in almost half of the animals with long-term survival, which is consistent with previously published findings.8,35 While the response to PD-1 monotherapy in this model is not reflective of recent results of PD-1 treatment in patients with recurrent GBM, this may be reflective of differences in the capacity for T cells to respond to PD-1 blockade after extensive TMZ treatment in recurrent GBM patients. Furthermore, these studies demonstrating that dose modulation of TMZ may have a significant impact on T-cell exhaustion and maintaining response to immune checkpoint blockade hold relevance of ongoing and future studies integrating PD-1 blocking strategies with standard-of-care treatment. This survival advantage was abrogated by adding SD TMZ to treatment (Fig. 6F). However, MD TMZ treatment did not disrupt the beneficial effects of PD-1 antibody and preserved the survival effect in this experimental system. We have previously found that the KR158 glioma model is resistant to TMZ,22 and therefore this model was utilized as a second model system. We found it was resistant to PD-1 blockade, and immunomodulation with TMZ did not affect survival (Supplementary Fig. 4F).
Discussion
GBM patients are known to have significant immune dysfunction at the time of diagnosis.36 This immunosuppression is worsened by TMZ treatment, which is standard of care.37 Prior studies have shown that infusion of lymphocytes after chemoradiation in patients with GBM failed to improve lymphocyte counts or to impact patient outcome.38 However, the homeostatic recovery following TMZ-induced lymphopenia can be leveraged to result in an improved antitumor immune response.39
Our data demonstrate that TMZ has very different effects on host immunity depending on dosing scheme. Standard TMZ results in host immunosuppression and exhaustion of cytotoxic T cells, which lead to poorer outcomes in a glioma model treated with PD-1 antibody. Conversely, MD TMZ modulates immune responses by preserving cytotoxic T-cell activity while still maintaining direct antitumor effects. PD-1 antibody resulted in reversal of exhaustion markers in the periphery but not in intratumoral T cells. These findings were in a murine glioma model that demonstrates response to immune checkpoint inhibition with anti–PD-1 therapy. While human GBM has been shown to have less sensitivity to PD-1 inhibition,1,14 these results are a demonstration of immunomodulation with chemotherapy that may impact response to immunotherapy. Chemotherapy has been shown to potentiate the effects of PD-1 inhibition for non–small cell lung cancer in a randomized trial.40 It is reasonable to test the effects of chemotherapy dosing in patients with GBM, as MD TMZ has been shown to be non-inferior to SD for the treatment of GBM.41,42
The concept of using TMZ as an immune modulator when combined with immunotherapy has been investigated as it is the standard of care for GBM patients.43 Despite causing overall lymphopenia, TMZ has been described to enhance antitumor responses to immunotherapy in certain contexts.44,45 Mitchell et al demonstrated that antitumor immune responses were enhanced in the setting of dendritic cell vaccination when combined with increasing doses of TMZ.17 The same group demonstrated a similar phenomenon in patients with GBM treated with antitumor vaccines.18 The immunomodulatory effects of TMZ also affect other immune cell populations in the host, such as Tregs.43,46,47 Conversely, other groups have shown that systemic chemotherapy reduces the efficacy of immunotherapy, especially when dependent on endogenous immune response. Mathios et al demonstrated that when TMZ was given systemically, it did not add any survival benefit to PD-1 inhibition.21 However, when TMZ was given locally, there was a significant survival advantage in combination with PD-1 inhibition. Therefore, the impact of TMZ on response to immunotherapy varies based on dosing, method of delivery, and activation of endogenous or exogenous antitumor T cells.
Understanding the effects of chemotherapy on both the tumor microenvironment and host immunity is important for combining chemotherapy with immunotherapy. The direct effects of chemotherapy on tumor cells is relevant when combining with immunotherapy48 such as release of antigens, inducing mutations that serve as neoantigens, and altering ligand expression (eg, PD-L1). Temozolomide is an alkylating agent that increases mutations.15 This effect of TMZ would theoretically increase antigens to serve as targets for immune checkpoint blockade. Moreover, TMZ changes the expression of important ligands on both tumor and immune infiltrates. For example, TMZ treatment is associated with decreased PD-L1 expression,49 which has been shown to correlate with response rates to PD-1 inhibition.31 The complex effects of TMZ on GBM tumor cells, antigen expression, and ligand expression will significantly impact response to immunotherapy.
Importantly, our study demonstrates that TMZ results in T-cell exhaustion in a dose-dependent fashion in a preclinical model system. This finding is especially relevant for patients with GBM who already demonstrate T-cell exhaustion at baseline.50 One dosing schedule as “standard of care” may not be the best strategy, especially in combination with various immunotherapeutic approaches. Temozolomide has the potential to enhance vaccine, adoptive T-cell, and immune checkpoint therapies in patients with GBM. However, the dose, timing of dose, and method of delivery will likely determine the efficacy of combinatorial approaches. Studies evaluating the mechanisms driving these phenomena and the optimal combinations will likely have significant impact for patients with GBM.
Conclusion
To fully realize the potential of combinatorial strategies using chemotherapy and immunotherapy, further study into optimizing immunomodulatory properties of chemotherapy will be critical. Standard dosing strategies with TMZ have the potential to diminish any benefit of PD-1 inhibition due to exhaustion of cytotoxic T cells and an increase in immunosuppressive cells. These changes are not seen when the dose is decreased and given over a longer period of time. The implications of these findings for GBM patients will need further investigation.
Funding
This work was funded by National Institute of Neurological Disorders and Stroke K08 NS099484 held by Dr Rahman.
Supplementary Material
Conflict of interest statement
The authors have no disclosures.
Authorship statement
Aida Karachi designed the research, contributed the reagents, performed experiments, analyzed the data, and wrote the paper. Changlin Yang contributed the reagents, performed experiments, and analyzed the data. Farhad Dastmalchi contributed the reagents, performed experiments, and analyzed the data. Elias Sayour contributed the reagents and analyzed the data. Jianping Huang contributed the reagents and analyzed the data. Hassan Azari performed experiments and analyzed the data. Yu Long contributed the reagents and performed experiments. Catherine Flores contributed the reagents. Duane Mitchell designed the research, contributed the reagents, analyzed the data, and wrote the paper. Maryam Rahman designed the research, contributed the reagents, performed experiments, analyzed the data, and wrote the paper.
References
- 1. Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2017;8(53):91779–91794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 3. Motzer RJ, Escudier B, McDermott DF, et al. ; CheckMate 025 Investigators Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang RF, Wang HY. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res. 2017;27(1):11–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim JE, Patel MA, Mangraviti A, et al. Combination therapy with anti-PD-1, Anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res. 2017;23(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4(2):124–135. [DOI] [PubMed] [Google Scholar]
- 8. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johanns TM, Miller CA, Dorward IG, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 2016;6(11):1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. [DOI] [PubMed] [Google Scholar]
- 13. Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 14. Reardon DA, Omuro A, Brandes AA, et al. OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: checkMate 143. Neuro Oncol. 2017; 19, iii21. [Google Scholar]
- 15. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yip S, Miao J, Cahill DP, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009;15(14):4622–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitchell DA, Cui X, Schmittling RJ, et al. Monoclonal antibody blockade of IL-2 receptor α during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood. 2011;118(11):3003–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanchez-Perez LA, Choi BD, Archer GE, et al. Myeloablative temozolomide enhances CD8+ T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS One. 2013;8(3):e59082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fritzell S, Sandén E, Eberstål S, Visse E, Darabi A, Siesjö P. Intratumoral temozolomide synergizes with immunotherapy in a T cell-dependent fashion. Cancer Immunol Immunother. 2013;62(9):1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathios D, Kim JE, Mangraviti A, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8(370):370ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flores C, Pham C, Snyder D, et al. Novel role of hematopoietic stem cells in immunologic rejection of malignant gliomas. Oncoimmunology. 2015;4(3):e994374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26(1):109–113. [DOI] [PubMed] [Google Scholar]
- 24. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cen L, Carlson BL, Pokorny JL, et al. Efficacy of protracted temozolomide dosing is limited in MGMT unmethylated GBM xenograft models. Neuro Oncol. 2013;15(6):735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ginestet C. ggplot2: elegant graphics for data analysis. J R Stat Soc Ser A Stat Soc. 2011;174:245. [Google Scholar]
- 28. Sanchez-Perez L, Choi BD, Reap EA, et al. BLyS levels correlate with vaccine-induced antibody titers in patients with glioblastoma lymphodepleted by therapeutic temozolomide. Cancer Immunol Immunother. 2013;62(6):983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. [DOI] [PubMed] [Google Scholar]
- 31. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu H, Liu J, Toups M, Soos T, Arendt C. Gene signature-based mapping of immunological systems and diseases. BMS Bioinformatics. 2016;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wherry EJ, Ha SJ, Kaech SM, et al. Moleclar signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. [DOI] [PubMed] [Google Scholar]
- 35. Speranza MC, Passaro C, Ricklefs F, et al. Preclinical investigation of combined gene-mediated cytotoxic immunotherapy and immune checkpoint blockade in glioblastoma. Neuro Oncol. 2018;20(2):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohme M, Schliffke S, Maire CL, et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clin Cancer Res. 2018;24(17):4187–4200. [DOI] [PubMed] [Google Scholar]
- 37. Campian JL, Piotrowski AF, Ye X, et al. Serial changes in lymphocyte subsets in patients with newly diagnosed high grade astrocytomas treated with standard radiation and temozolomide. J Neurooncol. 2017;135(2):343–351. [DOI] [PubMed] [Google Scholar]
- 38. Campian JL, Ye X, Gladstone DE, et al. Pre-radiation lymphocyte harvesting and post-radiation reinfusion in patients with newly diagnosed high grade gliomas. J Neurooncol. 2015;124(2):307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 41. Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27(23):3861–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kong DS, Lee JI, Kim JH, et al. Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro Oncol. 2010;12(3):289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karachi A, Dastmalchi F, Mitchell DA, Rahman M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol. 2018;20(12):1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Candolfi M, Yagiz K, Wibowo M, et al. Temozolomide does not impair gene therapy-mediated antitumor immunity in syngeneic brain tumor models. Clin Cancer Res. 2014;20(6):1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suryadevara CM, Desai R, Abel ML, et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology. 2018;7(6):e1434464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58(10):1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ridolfi L, Petrini M, Granato AM, et al. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J Transl Med. 2013;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pfirschke C, Engblom C, Rickelt S, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heynckes S, Gaebelein A, Haaker G, et al. Expression differences of programmed death ligand 1 in de-novo and recurrent glioblastoma multiforme. Oncotarget. 2017;8(43):74170–74177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Woroniecka KI, Chongsathidkiet P, Rhodin KE, et al. T Cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.