Abstract
Human cytomegalovirus (HCMV) is a major cause of disability in congenitally infected infants and in the immunosuppressed. There is currently no licensed prophylactic HCMV vaccine. The HCMV envelope glycoprotein B (gB) is considered a major vaccine target antigen based on its critical role in mediating viral-host cell fusion and thus viral entry. The natural conformation of HCMV gB within the viral envelope is a trimer, but there has been no reported success in producing a recombinant trimeric gB suitable for vaccine use. Phase II clinical trials of a monomeric recombinant gB protein demonstrated 50% efficacy in preventing HCMV infection in seronegative women of reproductive age, and in reducing viremia in solid organ transplantation recipients. We now report the production of a uniformly trimeric recombinant HCMV gB protein in Chinese ovary cells, as demonstrated by Western blot analysis under modified non-reducing conditions and size exclusion chromatography with multiangle scattering. Immunization of mice with trimeric HCMV gB induced up to 11-fold higher serum titers of total gB-specific IgG relative to monomeric HCMV gB using Alum + CpG as adjuvants. Further, trimeric HCMV gB elicited 50-fold higher complement-independent and 20-fold higher complement-dependent HCMV neutralizing titers compared to monomeric HCMV gB using the fibroblast cell line, MRC-5, and up to 6-fold higher complement-independent and -dependent HCMV neutralizing titers using the epithelial cell line, ARPE-19. The markedly enhanced HCMV neutralizing activity in response to trimeric HCMV gB was also observed using an additional four distinct clinical HCMV isolates. These data support a role for trimeric HCMV gB as an important component for clinical testing of a prophylactic HCMV vaccine.
Keywords: Human cytomegalovirus, Glycoprotein B, Vaccine, Antibody, Neutralization, Recombinant protein
1. Introduction
Human cytomegalovirus (HCMV) is an enveloped, double-stranded DNA β-herpesvirus of the Herpesviridae family. HCMV is the leading non-genetic cause of sensorineural hearing loss in childhood and a significant cause of neurodevelopmental delay, including mental retardation [1–3]. Additional congenital sequelae include microcephaly, seizures, intracranial calcifications, cerebral palsy, hepatitis, and chorioretinitis resulting in vision loss [4–7]. In addition to congenital infections, HCMV produces significant clinical disease in the immunosuppressed, including transplant recipients and patients with AIDS [8–11]. Although HCMV infection in immunocompetent individuals is generally asymptomatic, it may produce a mononucleosis syndrome in 10% of primary infections of older children and adults [12]. In 2001, the Institute of Medicine of the U.S. National Academy of Sciences stated that a vaccine to prevent congenital HCMV infection is among the highest U.S. priorities [13].
HCMV is spread mainly via saliva and urine to seronegative children and adults, and via transplacental transmission to the fetus [14,15]. The target cells of HCMV include fibroblasts, epithelial cells, endothelial cells, monocyte-macrophages, hepatocytes and neurons [14,16,17]. The mechanism of HCMV fusion and entry into mammalian cells is analogous to that employed by other members of the herpesvirus family [16,17]. HCMV enters cells by fusing its envelope with either the plasma membrane or endosomal membrane [18,19]. HCMV envelope proteins, glycoprotein B (gB), gH, gL, gO and UL128/UL130/UL131A proteins have collectively been identified as the envelope proteins that play critical roles in HCMV fusion and entry into host cells [reviewed in [21]]. The gB protein is the direct mediator of HCMV fusion with all target host cell membranes. The activation of HCMV gB for fusogenic activity requires its association with the gH/gL/gO protein complex. However, the gH/gL/UL128/UL130/UL131A protein complex (pentameric complex) is further required for efficient targeting of HCMV to epithelial and endothelial cells [19,22–24]. HCMV gB or gH/gL proteins have been shown to elicit serum HCMV neutralizing antibodies that block entry into both fibroblasts and epithelial cells. However, the pentameric complex induces the highest serum neutralizing titers for epithelial and endothelial cells [24–26].
Earlier clinical trials using live attenuated Towne or AD169 HCMV viral vaccines, both of which lacked expression of the pentameric complex, proved to be ineffective in preventing HCMV infection in either healthy volunteers or renal transplant recipients, although some efficacy was demonstrated in overt HCMV disease in high risk Recipient-Donor + renal transplant recipients[14,27]. New HCMV viral strains engineered to express the pentameric complex are currently being evaluated, but safety concerns persist using this approach. In contrast, recombinant protein subunit vaccines have in general shown excellent safety and efficacy profiles in humans. Of note, a phase II clinical trial using a recombinant HCMV gB protein (Chiron gB) derived from the Towne strain of HCMV and adjuvanted with MF59 [28] demonstrated 50% efficacy in preventing HCMV infection in HCMV seronegative women [15,29]. Similar results were obtained in adolescent girls, though the efficacy did not reach conventional levels of statistical significance [20]. In another phase II study, patients awaiting solid organ transplantation who were HCMV seronegative, were vaccinated with the same recombinant HCMV gB protein as described above, and the vaccine was effective in preventing viremia in 5 out of 11 subjects, compared to 0 out of 5 subjects in the placebo group [30]. The recombinant HCMV gB used in these phase II clinical trials was originally developed at Chiron, whereby the wild-type gB was modified to remove the furin cleavage site, since retention of this site interfered with recombinant protein production. The resulting Chiron gB protein, however, did not assume its native trimeric conformation that is postulated to be the ideal form for vaccine use [31]. Indeed there have subsequently been no reported successes in producing a fully trimeric HCMV gB protein suitable for vaccine use. Another promising HCMV vaccine candidate, the pentameric complex has been extensively studied in recent years, and is likely to provide protection against HCMV infection of epithelial cells, endothelial cells and monocytes, but not fibroblasts [24,25,27].
The clinical trials with Chiron gB have encouraged further evaluation of gB as a prophylactic HCMV vaccine, and also suggested that a more effective HCMV vaccine might be achieved by vaccinating with a fully trimeric, native HCMV gB. Using a novel approach, we now describe the efficient production of a fully trimeric recombinant HCMV gB protein. We demonstrate that immunization with this protein elicits markedly higher titers of serum HCMV neutralizing antibodies in mice relative to its monomeric counterpart using Alum + CpG as adjuvants. The data generated by this study provides critical new information for the future design of a prophylactic HCMV vaccine for clinical use.
2. Material and methods
2.1. Cell lines, HCMV strains, and reagents
The Chinese hamster ovary (CHO) cell line, DG44 was purchased from Invitrogen and maintained in CD DG44 medium. MRC-5 and ARPE-19 cell lines were purchased from ATCC, and cultured using EMEM or DMEM/F-12K medium, respectively, both supplemented with 10% fetal bovine serum. Purified monomeric recombinant HCMV gB protein was purchased from Sino Biologicals, Inc. (Beijing, P. R. China). HCMV strain AD169WT131 was provided by Drs. Xiao Wang and Haruhiko Murata (Food and Drug Administration) and strain AD169 was purchased from ATCC. HCMV clinical isolates UXC, CSL5001, 38532 and 39621 were provided by Dr. Michael McVoy (Virginia Commonwealth University). HCMV strain AD169WT131 was propagated in ARPE-19 cells, HCMV strain AD169 and clinical isolates were propagated in MRC-5 cells. Cyto-Gam was a gift from CSL Behring to Dr. Michael McVoy. Rabbit complement was purchased from Sigma-Aldrich. Monoclonal mouse IgGl anti-gB antibody 2F12 was purchased from Virusys corporation (Taneytown, MD), and monoclonal mouse IgGl anti-gB antibody LS-C64457 was purchased from LifeSpan BioSciences, Inc. (Seattle, WA).
2.2. Expression and purification of trimeric HCMV gB protein
The coding sequence for HCMV gB was downloaded from NCBI, reference sequence # NC_006273.2, strain Merlin. The DNA sequence encoding for amino acids 23–750 was used. The signal peptide (amino acids 1–22) was replaced with an IgG κ leader sequence, and the coding sequence for the cleavage site, RTKRS between amino acids 456 (N) and 462 (T) was replaced with a 15 aa (Gly4Ser)3 linker sequence (Fig. 1A). A His6 sequence was added to the 3’ end for protein purification. The DNA coding for the gB protein was synthesized by Blue Heron, cloned into pOptiVEC (Invitrogen), and verified by sequencing. CHO cells were transfected with pOptiVEC-gB using Free-style Max reagent (Invitrogen), and selected with increasing concentrations of methotrexate up to 4 uM for optimal protein expression, followed by limited dilution cloning. CHO cells were then cultured in a FiberCell bioreactor (FiberCell Systems, Frederick, MD), the super-natants were concentrated for affinity purification using a cobalt column (Thermo Scientific), and further purified using size exclusion chromatography on a Superose 6 column (GE Lifesciences, Pittsburgh, PA).
Fig. 1.
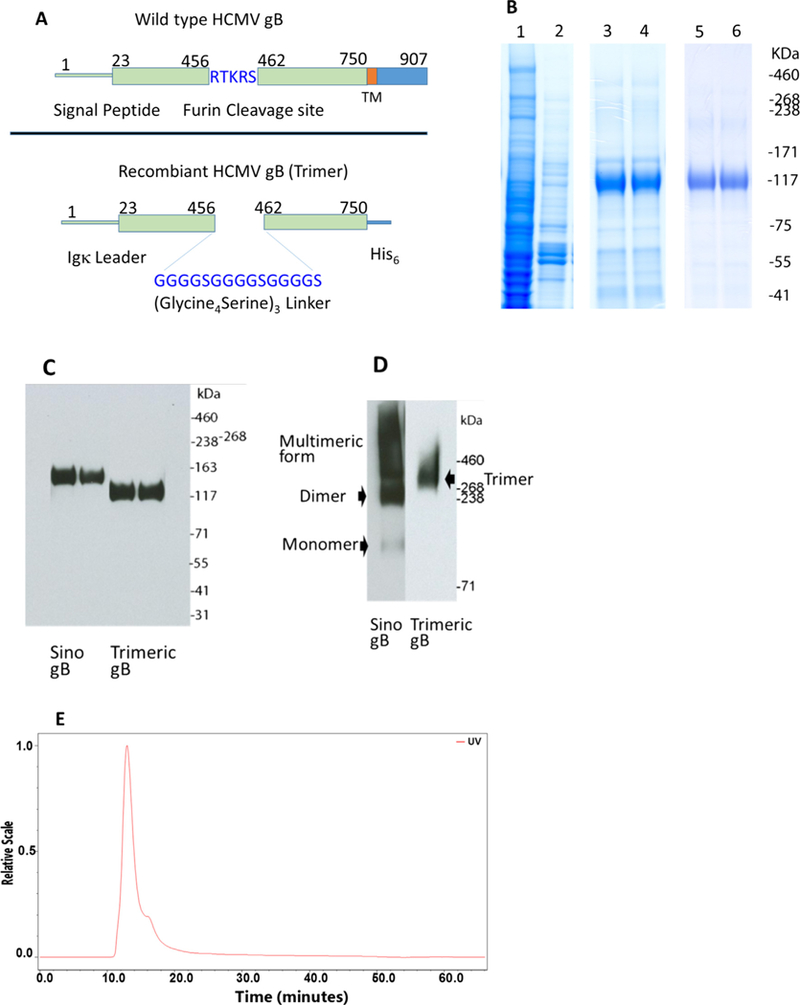
Production of trimeric HCMV gB. (A) The diagram shows the structure of trimeric HCMV gB and wild type gB. (B) Coomassie brilliant blue stained polyacrylamide gel, lane 1, cell culture supernatant, lane 2, flow-through after Cobalt affinity purification, lanes 3 and 4, trimeric HCMV gB purified with Cobalt affinity purification, lanes 5 and 6, trimeric HCMV gB further purified with size exclusion chromatography. (C) Western blot analysis using the 2F12 mAb under reducing conditions demonstrated a monomeric HCMV gB in comparison with Sino gB. (D) Western blot analysis using the LS-C64457 mAb under modified non-reducing conditions demonstrated a trimeric HCMV gB in comparison with Sino gB (aggregates). (E) SEC-MALS (multi-angle light scattering) analysis showed a single peak of a dimer of trimeric HCMV gB. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Western blot analysis
Purified proteins were analyzed by electrophoresis on 3–8% NuPAGE Tris-Acetate Mini-Gels, under both reducing condition and modified non-reducing conditions as previously described [64]. For reducing conditions, purified HCMV gB was boiled for 10 min in lithium dodecyl sulfate (LDS) loading buffer containing 50 mM DTT, resolved on 3–8% PAGE in SDS running buffer, and blotted with an anti-gB monoclonal antibody 2F12. For modified non-reducing conditions, protein samples were mixed with LDS loading buffer without DTT, resolved on 3–8% PAGE in Tris-glycine native running buffer (Invitrogen), and blotted with anti-gB monoclonal antibody LS-C64457. Membranes were then incubated with polyclonal HRP-goat anti-mouse IgG (Thermo Fisher Scientific), followed by incubation with SuperSignal West Pico chemiluminescent substrate, with signal captured on X-ray film.
2.4. Size exclusion chromatography and multi-angle light scattering (SEC-MALS) analysis
For SEC-MALS analysis, an Agilent 1100 series, Wyatt HELEOS 8, Wyatt Optilab T-rEX, a Waters 2497 series UV detector and a TSKGel G4000 PWxl column in series with a TSKGel G5000 PWxl column were used. Briefly, purified HCMV trimeric gB was spun through 0.45 centrifugal filters for 5 min in a tabletop centrifuge and 50 uL of sample was injected. The column flow rate was 0.5 mL/min. Wyatt ASTRA software was used for data collection & analysis, and the Debye formalism was used to determine molecular weight.
2.5. Mouse immunization
Six groups of female BALB/c mice (NCI, Frederick, MD, n = 5), 7 to 10 weeks old were immunized via the intraperitoneal route with 1 μg, 5 μg or 25 μg of HCMV trimeric gB versus 1 μg, 5 μg or 25 μg of monomeric Sino gB. Antigen was adsorbed to aluminum hydroxide (alum; 0.25 μg alum/mg protein) and mixed with 25 μg of a 30-mer phophorothioate-modified CpG-ODN (AAAAAAAAAAAAAACGTTAAAAAAA AAAAA) optimized for mice [64,65]. Mice immunized with alum+ CpG-ODN alone served as a negative control. Mice were immunized on day 0, day 21, and day 42 and serum samples were taken before initial immunization, 10 days following each immunization and on day 63. These studies were conducted in accordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, NRC), and were approved by USUHS Institutional Animal Care and Use Committee.
2.6. Measurement of serum titers of HCMV gB-specific IgG by ELISA
Immulon 4 ELISA plates were coated overnight with 5 μg/ml of HCMV gB protein (Sino gB or trimeric gB) in PBS at 4 °C and blocked with 1% bovine serum albumin (BSA) in PBS. Three-fold serial dilutions of serum samples in 1% BSA-PBS, were then added and incubated overnight at 4 °C followed by washing with 0.1% Tween-20 in PBS. Alkaline phosphatase-conjugated polyclonal goat anti-mouse IgG Ab (Southern Biotechnology) in 1% BSA-PBS was then added and plates were incubated at 37 °C for 1 h. Plates were then washed with 0.1% Tween-20 in PBS and substrate (p-nitrophenyl phosphate, disodium; Sigma-Aldrich) was added at 1 mg/ml in tris-HCl magnesium-sulfate (TM) buffer for color development. Color was read at an absorbance of 450 nm on a Multiskan Ascent ELISA reader.
2.7. Determination of serum HCMV neutralizing titers
Sera from mice immunized with Sino gB or trimeric HCMV gB were either heat inactivated at 56 °C for 30 min to eliminate complement activity or not heat-treated. Serum HCMV neutralizing antibody titers were determined using an ELISpot assay as described [33,34]. Each serum sample was initially diluted 1:10 and prepared as 1:2 serial dilutions in culture medium in triplicates. Each dilution was mixed with an equal volume of culture medium containing 4000 pfu/ml HCMV strain AD169, AD169WT131 or HCMV clinical isolates [32,34,35], incubated for 4 h at 37 °C then added to the wells of 96-well plates containing MRC-5 or ARPE-19 monolayers and cultured overnight at 37 °C, in 5% CO2. For samples analyzed with exogenous complement, 5% rabbit complement was added to culture medium containing HCMV. Cells were fixed with absolute ethanol, rehydrated and blocked with 5% normal horse serum in PBS, followed by incubation with anti-IE1 monoclonal antibody MAB810 (Millipore, Burlington, MA), goat anti-mouse secondary antibody (Jackson ImmunoResearch Labs, West Grove, PA) each for 1 h, and VECTASTAIN ABC reagent (Vector Labs, Burlingame, CA) for 30 min. Plates were washed 3x with 0.1% Tween 20 in PBS between each step, and TrueBlue was added for color development. The plates were scanned and analyzed using a CTL- ImmunoSpot® S6 Micro Analyzer. Fifty percent inhibitory concentration (IC50) values and standard errors of the means were calculated using GraphPad Prism7 software by plotting the means of triplicate values for each serum dilution against log serum concentration, calculating the best fit four-parameter equation for the data, and interpolating the serum dilution at the mid-point of the curve as the IC50 neutralizing titer. For samples that did not have neutralizing activity, neutralizing titer was assigned 1 for purpose of statistical analysis.
2.8. Monomeric HCMV gB depletion study
The heat inactivated Day 63 sera from mice immunized with 25 μg trimeric gB or 25 μg Sino gB were pooled within each group. The sera from mice immunized with 25 μg trimeric gB was diluted 1:2 with cell culture medium to match the gB-specific IgG titer of the sera from mice immunized with 25 μg Sino gB. Fifty microliters of pooled sera was incubated with 50 μg Sino gB for 1 h at room temperature, followed by passage through Cobalt columns to remove Sino gB-antibody complexes. The sera were then prepared as 1:2 serial dilutions in culture medium in quadruplicates, and the HCMV neutralizing activity was analyzed using HCMV strain AD169WT131, MRC-5 fibroblast cells or ARPE-19 epithelial cells as described above.
2.9. Statistics
All studies were repeated at least 1× for reproducibility. Serum titers were expressed as geometric means +/− standard error of the mean, with significance determined by a two-tailed students t-test (p ≤ 0.05 considered significant).
3. Results
3.1. Production of a fully trimeric HCMV gB protein
Native HCMV gB was predicted to be a homotrimer, based on the 3D crystallographic structure of HSV-1 gB and EBV gB, which are homotrimers [36–39]. The trimer is considered the natural physical form of HCMV gB in the viral envelope and during fusion with host cells [37,38], and is proposed to be the ideal physical structure for eliciting neutralizing antibody [27]. The furin cleavage site within the HCMV gB protein, between amino acids 460 and 461, is critical for mediating HCMV gB protein folding into its terminal trimeric form [40]. However, in studies to express recombinant HCMV gB, inclusion of the furin cleavage site led to low yields of monomeric gB [41–43], whereas elimination of this site resulted in efficient production in a variety of mammalian and insect forms, but synthesis of mostly monomeric gB, with some higher molecular weight forms [40–46]. We postulated that a trimeric HCMV gB could be produced by insertion of a flexible 15 amino acid (Gly4Ser)3 linker at the furin cleavage site that would potentially allow for terminal protein folding and efficient expression of a trimeric gB protein (Fig. 1A). Under reducing conditions that causes protein denaturation, Western blot analysis of the resulting HCMV gB using an anti-gB monoclonal antibody, 2F12 showed a 120 kDa band corresponding to a monomer (Fig. 1B and C). However, under modified non-reducing conditions which allows for preservation of protein conformation, Western blot analysis with anti-gB monoclonal antibody LS-C64457 (recognizing a conformational epitope) showed a ~360 kDa band, consistent with trimeric gB (Fig. 1D). Purified HCMV gB was further analyzed using HPLC size exclusion chromatography and multi-angle light scattering (SEC-MALS), which demonstrated a single peak of molecular weight of 728 kDa, consistent with a dimer of trimeric gB (Fig. 1E). In contrast, Western blots of Sino gB showed a single band of ~130 KDa under reducing conditions, whereas under modified non-reducing conditions, Sino gB showed monomers, dimers and higher molecular weight molecules as a smear, in comparison to a single band of ~360 KDa for trimeric gB (Fig. 1C and D).
3.2. Trimeric HCMV recombinant gB elicits significantly higher serum titers of total gB-specific IgG relative to its recombinant monomeric counterpart.
We directly compared our recombinant trimeric HCMV gB with a recombinant monomeric HCMV gB protein obtained from Sino Biological Inc. (Sino gB) for elicitation of serum titers of total gB-specific IgG. Sino gB was derived from the Towne strain of HCMV. The extracellular domain Met 1-Lys 700 was linked to the cytoplasmic domain Arg 777-Val 907, with the furin cleavage site mutated from ‘RTKR’ to ‘TTQT’. The HCMV gB (Chiron gB) that was used in phase II clinical vaccine trials and demonstrated 50% efficacy in prevention of HCMV infection was also derived from the Towne strain of HCMV. Chiron gB consists of amino acids 1–680 with the same mutation to the furin cleavage site ‘TTQT’ [28]. Since the cytoplasmic domain has no role in eliciting neutralization activities, and the extracellular domain of Sino gB (AA 1–700) and Chiron gB are almost identical with the exception that Sino gB has 20 extra amino acid residues, Sino gB was used as a control in mouse immunization studies. Groups of BALB/c mice (n = 5) each were immunized via intraperitoneal injection with 1 μg, 5 μg, or 25 μg of trimeric HCMV gB versus Sino gB in alum + CpG-ODN adjuvant and boosted in a similar fashion on days 21 and 42. Serum samples were obtained 10 days after each immunization and on day 63 for analysis by ELISA. As illustrated in Fig. 2A, where the ELISA plates were coated with Sino gB, each group of HCMV proteins induced augmented serum IgG responses following the first booster immunization, and further significant augmentation in serum IgG titers following the second booster immunization. Trimeric HCMV gB induced 5-fold to 11-fold higher serum titers of gB-specific IgG relative to monomeric HCMV gB after the first and second immunizations, with greater differences observed at the lower doses. The difference in serum titers of gB-specific IgG elicited by trimeric versus monomeric HCMV gB decreased after the third immunization, with less differences observed at the higher doses. No significant differences were observed using 5 versus 25 μg of trimeric HCMV gB for induction of serum titers of gB-specific IgG, whereas that induced by 25 μg of Sino gB was signficiantly higher than that using 5 μg. When serum titers of gB-specific IgG were reanalyzed using trimeric HCMV gB to coat ELISA plates, similar results as that obtrained using Sino gB-coated plastes were obtained (Fig. 2B).
Fig. 2.

Total serum titers of HCMV gB-specific IgG after immunization of mice with trimeric HCMV gB vs Sino gB. Groups of mice (n = 5) were immunized intraperitoneally three times, on days 0, 21, and 42 as indicated, with 1, 5, or 25 μg of trimeric HCMV gB versus Sino gB in the presence of alum + CpG-ODN adjuvant. Sera were collected 10 days after each immunization and on day 63 for determination of serum titers of gB-specific IgG by ELISA. (A) ELISA plates were coated with Sino gB. (B) ELISA plates were coated with trimeric gB.
3.3. Immunization of mice with trimeric HCMV gB elicited markedly higher titers of serum HCMV neutralizing activities for fibroblasts, relative to Sino gB
Sera were obtained 21 days following the third immunization (day 63) with 1, 5 or 25 μg of trimeric HCMV gB or Sino gB. Soluble monomeric HCMV gB protein is known to elicit mainly complement-dependent HCMV neutralizing antibodies [24]. To determine whether trimeric HCMV gB could elicit both complement-independent as well as complement-dependent neutralization activity against HCMV strain AD169wt131, sera were incubated at 56 °C for 30 min to eliminate complement activity. As shown in Fig. 3A, using heat-inactivated sera and MRC-5 fibroblasts as target cells, both Sino gB and trimeric HCMV gB elicited dose-dependent HCMV neutralization activity. Thus, immunization with 25 μg of either Sino or trimeric gB respectively elicited ~ 3-f old higher HCMV neutralization activity than 5 μg, which in turn elicited ~10 fold higher activity than 1 ug. Of note, when the HCMV neutralization activity elicited by trimeric HCMV gB was directly compared to that of Sino gB, the immune sera from mice immunized with 1 μg, 5 μg or 25 μg of trimeric HCMV gB demonstrated 50-fold higher HCMV neutralization activity against HCMV strain AD169wt131 relative to that of immune sera from mice immunized with the same dose of Sino gB respectively (Fig. 3A). The non-heat-inactivated sera from mice immunized with Sino gB demonstrated 6- to 8-fold higher HCMV neutralization activity compared to heat-inactivated sera, whereas the non-heat-inactivated sera from mice immunized with trimeric HCMV gB demonstrated 2-to 3-fold higher HCMV neutralization activity compared to heat-inactivated sera (Fig. 3A, B). Thus, without heat-inactivation, the HCMV neutralization activity against HCMV strain AD169wt131 elicited by 1 μg, 5 μg or 25 μg of trimeric HCMV gB was 20-fold higher than that of same dose of Sino gB respectively, suggesting that Sino gB induces a more complement-dependent response (Fig. 3B). CytoGam, a commercial IVIg containing high titers of HCMV neutralizing antibody derived from the plasma of HCMV seropositive healthy donors, showed much lower HCMV neutralization activity against HCMV strain AD169wt131 relative to trimeric HCMV gB, using the MRC-5 cell line (Fig. 3A, B). Thus, 10 mg/ml of CytoGam demonstrated about one-thirtieth of the complement-independent HCMV neutralization activity of the sera from mice immunized with 25 μg trimeric HCMV gB (Fig. 3A). Heat inactivation had no effect on CytoGam-mediated neutralization, which made its complement-dependent HCMV neutralization activity relatively even lower compared to sera from mice immunized with trimeric HCMV gB or Sino gB (Fig. 3B).
Fig. 3.
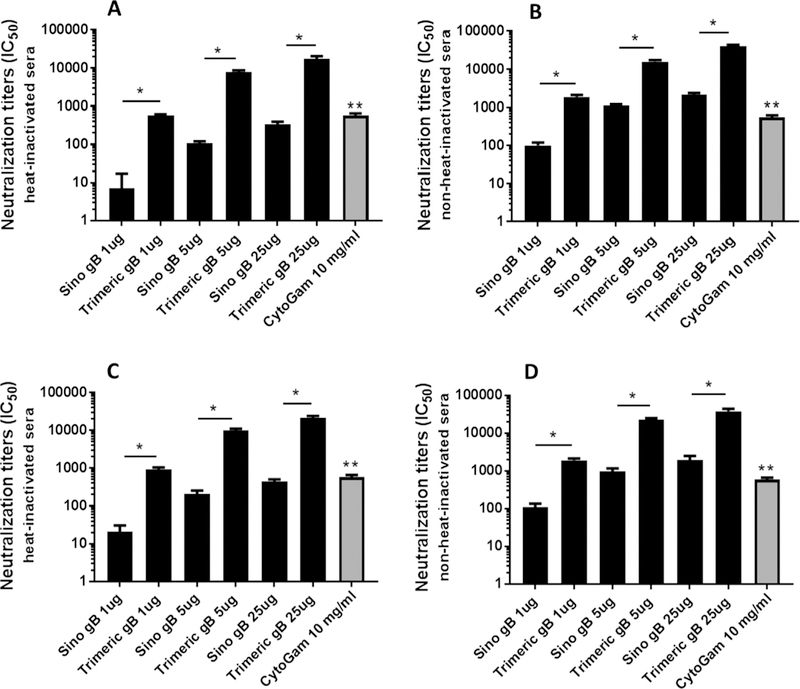
Trimeric HCMV gB elicited markedly higher titers of serum HCMV neutralizing activities for fibroblasts. HCMV neutralization titers (IC50), using the MRC-5 fibroblast cell line and HCMV strain AD169wt131, of heat-inactivated (A) and non-heat-inactivated (B) day 63 sera from mice immunized in days 0, 21, and 42 with 1, 5, or 25 μg of trimeric HCMV gB versus Sino gB. HCMV neutralization titers (IC50) of heat-inactivated (C) and non-heat-inactivated (D) day 63 sera from mice immunized with trimeric HCMV gB versus Sino gB using MRC-5 and HCMV strain AD169 (*p < 0.05 compared to the sera from mice immunized with Sino gB in equal amount. **p < 0.05 compared to the sera from mice immunized with 25 μg trimeric HCMV gB).
In contrast to HCMV strain AD169wt131 used above, HCMV strain AD169 has a single-nucleotide frame-shifting insertion in the UL131A gene that renders it non-functional. As a result, strain AD169 does not express the pentameric complex gH/gL/UL128/ UL130/UL131A and consequently lacks the ability to infect epithelial cells, while retaining infectivity for fibroblast cells. HCMV strain AD169wt131 has a repaired UL131A gene, and can thus infect both epithelial cells and fibroblast cells [34]. In this regard, we determined whether lack of pentameric complex expression altered the measured values of serum neutralization activity. Using HCMV strain AD169 and the fibroblast cell line MRC-5, we observed similar serum HCMV neutralization titers relative to those determined using AD169wt131 (Fig. 3C, and D).
3.4. Immunization of mice with trimeric HCMV gB elicited higher titers of serum HCMV neutralizing activities for epithelial cells, relative to monomeric gB
Using the ARPE-19 epithelial cell line, day 63 immune sera from mice immunized with trimeric HCMV gB that was heat-inactivated at 56 °C for 30 min demonstrated 4- to 6-fold higher HCMV neutralization activity against HCMV strain AD169wt131 compared to that of immune sera from mice immunized with Sino gB (Fig. 4A). Without heat inactivation, the HCMV neutralization activity against HCMV strain AD169wt131 elicited by trimeric HCMV gB was 6-fold higher than that of Sino gB (Fig. 4B). The non-heat-inactivated sera from mice immunized with Sino gB demonstrated 4-fold higher HCMV neutralization activity against HCMV strain AD169wt131 compared to heat-inactivated sera, whereas the non-heat inactivated sera from mice immunized with trimeric gB demonstrated 6-fold higher HCMV neutralization activity compared to heat inactivated sera (Fig. 4A, B). Immune sera from mice immunized with trimeric HCMV gB or Sino gB showed markedly lower HCMV neutralization activity compared to CytoGam when using the ARPE-19 epithelial cell line and HCMV strain AD169wt131. Specifically, 10 mg/ml CytoGam demonstrated 15–fold and 4–fold higher HCMV neutralization activity than heat-inactivated or non-heat-inactivated immune sera, respectively from mice immunized with 25 ug trimeric HCMV gB (Fig. 4A, B). As HCMV strain AD169 does not infect ARPE-19 epithelial cells, only strain AD169wt131 was used for analysis of serum neutralizing titers using ARPE-19 epithelial cells.
Fig. 4.

Trimeric HCMV gB elicited low to moderately high titers of serum HCMV neutralizing activities for epithelial cells. (A) HCMV IC50 neutralization titers of heat- inactivated sera at day 63 from mice immunized on days 0,21, and 42 with 1,5,or 25 μg of trimeric HCMV gB versus Sino gB in alum + CpG-ODN using the epithelial ARPE-19 cell line, (B) HCMV IC50 neutralization titers of non-heat-inactivated serum at day 63 from mice immunized with HCMV trimeric gB versus Sino gB using epithelial ARPE-19 cell line (*p < 0.05 compared to the sera from mice immunized with Sino gB in equal amount. **p < 0.05 compared to the sera from mice immunized with 25 μg trimeric HCMV gB).
3.5. Trimeric, relative to Sino, HCMV gB elicited markedly higher titers of serum neutralizing activities against HCMV clinical isolates
Using the MRC-5 fibroblast cell line, day 63 pooled immune sera from mice immunized with 25 μg of trimeric HCMV gB versus 25 μg of Sino gB were analyzed for serum neutralization activity against several HCMV clinical isolates. Immune sera from mice immunized with Sino gB showed low neutralization activity against HCMV clinical isolates UXC, CSL5001, 38532 and 39621, except for the non-heat-treated sera analyzed with UXC and CSL5001, which showed 1C50 titers of 211 and 278 respectively; the remaining analyses demonstrated 1C50 titers <160. Immune sera from mice immunized with trimeric HCMV gB demonstrated 20- to 100-fold higher neutralization activity against HCMV clinical isolates relative to that of Sino gB (Fig. 5A, B). Heat inactivation to eliminate complement activity slightly decreased the neutralization activity against HCMV clinical isolates elicited by trimeric HCMV gB (Fig. 5B). HCMV UXC and CSL5001 were isolated from urine of congenitally infected newborns, while isolates 32691 and 32583 were isolated from urine of children attending daycare in the Richmond or Norfolk VA areas. Isolates were passaged 4–6 times in MRC-5 cells. These HCMV clinical isolates had a limited number of passages in the laboratory, compared to laboratory-adapted strains such as AD169 and Towne. Thus, HCMV neutralization analyses using these clinical isolates better reflect the neutralization activity of the immune sera in a clinical setting. Since the HCMV clinical isolates were propagated in the MRC-5 fibroblast cell line, only the MRC-5 line was used for serum neutralization analyses.
Fig. 5.

Trimeric HCMV gB elicited markedly higher titers of serum neutralizing activities against HCMV clinical isolates, relative to Sino gB. (A) Day 63 pooled sera from 5 mice immunized with 25 μg of trimeric HCMV gB versus 25 μg of Sino gB were heat-inactivated and IC50 neutralizing activity was determined of each pooled sample in quadruplicate using the MRC-5 fibroblast line. (B) Serum IC50 neutralizing titers were similarly determined as in “A” but in the absence of heat-inactivation of serum samples (*p < 0.05 compared to the sera from mice immunized with Sino gB in equal amount).
3.6. Exogenous rabbit complement markedly increased the titers of HCMV neutralizing activity of heat-inactivated sera immunized with Sino gB for both fibroblast and epithelial cells, but only markedly increased neutralizing titers of trimeric gB immune sera for epithelial cells
To determine if exogenous complement could increase the HCMV neutralizing activity of the sera from mice immunized with Sino gB or trimeric gB, 5% rabbit complement was added to the cell culture medium for HCMV neutralization analysis using heat-inactivated sera. Compared to heat inactivated sera alone, the addition of 5% rabbit complement increased the HCMV neutralization activity 100-fold for the sera from mice immunized with either 5 μg Sino gB or 5 μg trimeric gB when the ARPE-19 epithelial cell line was used for neutralization analysis. The HCMV neutralizing activity of the sera from mice immunized with 5 μg of trimeric gB was 4-fold higher relative to that of the sera from mice immunized with 5 μg of Sino gB. When MRC-5 fibroblast cells were used for HCMV neutralization analysis, the addition of 5% rabbit complement increased the HCMV neutralization activity of the sera from mice immunized with 5 μg of Sino gB 10-fold relative to that of heat-inactivated sera. In contrast, the addition of 5% rabbit complement increased the HCMV neutralization activity of the sera from mice immunized with 5 μg trimeric gB only 2-fold relative to that of heat inactivated sera (Fig. 6).
Fig. 6.

Rabbit complement markedly increased the titers of neutralizing activities of sera from mice immunized with Sino gB or trimeric gB. Heat inactivated Day 63 pooled sera from mice immunized with 5 μg trimeric gB versus 5 μg Sino gB were analyzed for HCMV neutralizing activity in the presence of 5% rabbit complement in quadruplicate, using strain AD169wt131, ARPE-19 epithelial cell line or MRC-5 fibroblast cell line as indicated (*p < 0.05 compared to the sera without rabbit complement, **p < 0.05 trimeric gB sera compared to corresponding Sino gB sera).
3.7. Incubating Sino gB with the sera from mice immunized with trimeric gB had little effects on the HCMV neutralizing activity
To determine if the monomer-directed gB-specific IgGs contribute to the neutralization activity in the sera from mice immunized with trimeric HCMV gB, monomeric gB (Sino gB) depletion studies were performed. The heat inactivated sera from mice immunized with either 25 μg trimeric gB or 25 μg Sino gB were incubated with Sino gB. The HCMV neutralizing activity of the sera from mice immunized with 25 μg Sino gB was markedly decreased after incubation with Sino gB, whereas the incubation with Sino gB had little effects on the HCMV neutralizing activity of the sera from mice immunized with 25 μg trimeric gB (Fig. 7).
Fig. 7.

Incubation with Sino gB did not decreased the titers of neutralizing activities of sera from mice immunized with trimeric gB. Heat inactivated Day 63 pooled sera from mice immunized with 25 μg trimeric gB versus 25 μg Sino gB were incubated with Sino gB, the trimeric gB immune sera were diluted 1:2 to match the level of gB-specific IgG of Sino gB immune sera. Following removal of gB-antibody complex by passing through Cobalt column, HCMV neutralizing activity was analyzed in quadruplicate using strain AD169wt131, ARPE-19 epithelial cell line or MRC-5 fibroblast cell line as indicated (*p < 0.05 compared to the sera without incubation with Sino gB).
4. Discussion
Human cytomegalovirus (HCMV) is a major cause of disability in congenitally infected infants and in the immunosuppressed. There is currently no prophylactic HCMV vaccine. The HCMV envelope proteins, glycoprotein B (gB), gH, gL, gO, and UL128/ UL130/UL131A are considered major vaccine target antigens for eliciting neutralizing antibodies, based on their critical roles in mediating viral-host cell fusion and thus viral entry. Anti-gB anti-body in human sera was identified as the major neutralizing activity that prevents HCMV infection of fibroblasts, and HCMV subunit vaccines incorporating soluble monomeric gB have been under development for several years (reviewed in [27,47]). In a phase II study, immunization of HCMV seronegative women of reproductive age with a monomeric recombinant gB protein adjuvanted with MF59 resulted in 50% protective efficacy against primary infection with HCMV [19,29,48]. Immunization of patients receiving solid organ transplants with the same MF59-adjuvanted gB vaccine also led to a significant reduction in viremia and shortened antiviral treatment [30]. Further, guinea pigs vaccinated with a guinea pig CMV gB protein adjuvanted with AS01 or AS02 followed by infection with guinea pig CMV, exhibited reduced pup mortality by 64–84%, and reduced viremia in infected dams by nearly six-fold [49]. Together these studies provided strong evidence that the gB subunit vaccines are safe and immunogenic, though further improvements in potency and durability of protection were desirable.
The natural conformation of HCMV gB within the viral envelope is a trimer, and thus a trimeric gB is predicted to be a superior vaccine target, relative to its monomeric counterpart, as trimeric HCMV gB likely expresses native conformational epitopes that will elicit higher titers of HCMV neutralizing antibodies. The furin cleavage site within the HCMV gB protein is critical for mediating HCMV gB protein folding into its terminal trimeric form [40]. However, in studies to express recombinant HCMV gB, inclusion of the furin cleavage site led to low yields of monomeric gB [41–43], whereas elimination of this site by mutation resulted in efficient production, but synthesis of mostly monomeric gB, with some higher molecular weight forms, using in a variety of mammalian and insect cells [40–46]. Recently, mutations to the fusion loops of a HCMV gB consisting of amino acid residues 78–706 resulted in a trimeric gB produced in insect cells, with the structure analyzed by X-ray crystallography [50]. Another trimeric HCMV gB in a post-fusion conformation was produced and consisted of amino acid residues 86–698 bound to Fab fragments of a neutralizing human anti-gB antibody, with the structure also analyzed by X-ray crystallography [51]. Of note, these trimeric gB proteins had mutations to their fusion loops that might have eliminated epitopes important for eliciting HCMV neutralizing antibodies. Further, the trimeric gB proteins analyzed by X-ray crystallography had mutated furin cleavage sites that might have altered the native conformation of the protein. Therefore, these trimeric HCMV gB recombinant proteins may not be suitable for vaccine use.
We have expressed, within CHO cells, a trimeric HCMV gB by insertion of a flexible 15 amino acid (Gly4Ser)3 linker at the furin cleavage site that allowed for terminal protein folding and efficient expression. The trimeric HCMV gB forms a dimer in solution as evidenced by SEC-MALS analysis, that is consistent with the recent finding of a HCMV trimeric gB protein characterized by X-ray crystallography that was produced by mutations to the fusion loops and the fusion cleavage site [51]. The dimerization of trimeric gB was most likely mediated by the base of the trimeric gB due to the intrinsic surface hydrophobicity of the membrane proximal region [51].
Trimeric HCMV gB induced 5-fold to 11-fold higher serum titers of gB-specific IgG relative to monomeric HCMV gB (Sino gB), but elicited 50-fold higher complement-independent HCMV neutralization activity, suggesting that conformational epitopes of the trimeric HCMV gB played an important role in eliciting neutralization activity. Soluble monomeric HCMV gB as well as different post-fusion HCMV trimeric gB proteins elicit mainly complement-dependent HCMV neutralizing antibodies when injected into mice [24,51,52]. In contrast, the trimeric HCMV gB produced in our laboratory elicited markedly higher serum HCMV neutralizing anti-bodies in mice than its monomeric counterpart, and in contrast to monomeric HCMV gB, exhibited both complement-independent and complement-dependent activity. This is most likely because the trimeric HCMV gB has a 15 amino acid flexible linker inserted into the furin cleavage site that allowed the two subdomains of HCMV gB to fold into their native conformation, and the conformational epitopes expressed by the two subdomains of the trimeric gB might play key roles in eliciting neutralizing anybody responses. Future studies using X-ray crystallography will determine whether the high neutralizing activity elicited by trimeric HCMV gB is due to its being in a pre-fusion conformation.
Sino gB is very similar to the Chiron gB previously used in phase II clinical trials, and has been used in several studies as a positive control [53,66]. In our study, heat-inactivation of sera from mice immunized with Sino gB to eliminate complement activity, resulted in minimal neutralizing titers against HCMV, whereas HCMV neutralization activity increased 6- to 8-fold in the absence of heat-inactivation. In contrast, the trimeric HCMV gB elicited markedly higher titers of complement-independent HCMV neutralization activity relative to Sino gB, whereas preservation of complement function resulted in an increase of HCMV neutralization activity of only 2- to 3-fold.
The trimeric HCMV gB was produced using the gene sequence derived from the HCMV strain Merlin, which is only 98.1% identical to the HCMV gB sequence from strain AD169 where 17 amino acid residues are different [54–56]. Sino gB was derived from the HCMV strain Towne, where the ectodomain of gB is identical to that of strain Merlin [54–57],Fig. 8). However, compared to Sino gB, trimeric HCMV gB elicited markedly higher neutralization activity against HCMV strain AD169 as well as a variant expressing a functional pentameric complex (AD169wt131). In addition, HCMV trimeric gB elicited markedly higher neutralization activities against several clinical HCMV strains, indicating highly potent cross strain HCMV neutralization activity. Immune sera from mice immunized with Sino gB showed little neutralization activity when using HCMV clinical isolates. Further, heat-inactivation had little effect on the markedly higher neutralization activity elicited by trimeric HCMV gB against these clinical isolates, suggesting that complement-dependent HCMV neutralization activity plays a minor role in the clinical setting.
Fig. 8.

Alignment of amino acid sequences of the gB from HCMV strain Merlin and strain Towne. Transmembrane domains were underlined, unmatched amino acid residues were in bold-face and indicated with “ * ”.
Through Cytogam demonstrated relatively high HCMV neutralization activity using ARPE-19 epithelial cells, it showed only a low level of HCMV neutralization activity when the fibroblast cell line MRC-5 was used. This is consistent with the previous demonstration that most of the neutralization activity in sera from HCMV seropositive humans was directed against the pentameric complex [25,27,58–60]. The pentameric complex has been extensively studied as a vaccine candidate in recent years, and is likely to provide protection against HCMV infection of epithelial cells, endothelial cells and monocytes, but not fibroblasts or primary trophoblast progenitor cells [24,25,58–63,67]. Since HCMV gB elicits relatively higher HCMV neutralization activity for fibroblasts than epithelial cells, it suggests that an optimal prophylactic HCMV vaccine will consist of both trimeric gB and pentameric complex proteins.
The Chiron gB + MF59 adjuvant subunit vaccine platform is the most successful HCMV vaccine tested to date, demonstrating ~50% efficacy in preventing HCMV acquisition in multiple phase 2 trials. Of note, a recent study of plasma from postpartum women Chiron gB/MF59 vaccinees showed that vaccine-elicited antibodies exhibited limited neutralization of the autologous virus, and negligible neutralization of multiple heterologous strains [15,20,29,30,68]. Similarly, analysis of the sera from the solid organ transplantation phase II trial showed that the protective effect elicited by the Chiron gB/MF59 vaccine was via a mechanism of action that could not be explained by neutralization [30,69]. Taken together, these data suggest that non-neutralizing antibody functions, such as virion phagocytosis, ADCC, likely played a role in the observed ~50% protection mediated by the Chiron gB/MF59 vaccine against HCMV acquisition [68,69]. Future studies of the trimeric HCMV gB produced in our laboratory should also determine potential non-neutralizing antibody functions, in addition to its potent neutralizing activity.
5. Conclusion
The HCMV gB protein is a major vaccine target antigen based on its critical role in mediating viral-host cell fusion and thus viral entry. Phase II clinical trials of a monomeric recombinant gB protein demonstrated 50% efficacy in preventing HCMV infection. The natural conformation of HCMV gB is a trimer, and is proposed to be the ideal physical structure for eliciting neutralizing anti-body. We have succeeded in producing a novel trimeric recombinant HCMV gB protein in Chinese ovary cells. Immunization of mice with trimeric HCMV gB elicited serum titers of gB-specific IgG, and neutralizing antibodies against HCMV infection of fibroblast and epithelial cells that were markedly higher than that observed for monomeric HCMV gB. These data strongly support the clinical evaluation of trimeric HCMV gB as a prophylactic HCMV vaccine alone, or in combination with other HCMV envelope proteins such as the pentameric complex.
Acknowledgments
✰✰ Supported by: USUHS Dean’s Research and Education Endowment Fund (CMS). USUHS had no involvement in study design, data collection, analysis or interpretation, nor writing report or decision for publication.
Footnotes
Mandatory disclaimer: The opinions expressed herein are those of the authors and are not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DOD), or the United States Army, Navy or Air Force.
Author conflicts of interest
Drs. Xinle Cui and Clifford M. Snapper are inventors of a patent for using trimeric herpesvirus gB proteins as vaccine candidates.
References
- [1].Demmler-Harrison GJ. Congenital cytomegalovirus: Public health action towards awareness, prevention, and treatment. J Clin Virol 2009;46(Suppl 4):S1–5. [DOI] [PubMed] [Google Scholar]
- [2].Jeon J, Victor M, Adler SP, Arwady A, Demmler G, Fowler K, et al. Knowledge and awareness of congenital cytomegalovirus among women. Infect Dis Obstet Gynecol 2006;80383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morton CC, Nance WE. Newborn hearing screening-a silent revolution. N Engl J Med 2006;354:2151–64. [DOI] [PubMed] [Google Scholar]
- [4].Swanson EC, Schleiss MR. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am 2013;60:335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 2013;26:86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kenneson A, Cannon Mj. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007;17:253–76. [DOI] [PubMed] [Google Scholar]
- [7].Nyholm JL, Schleiss MR. Prevention of maternal cytomegalovirus infection: current status and future prospects. Int J Womens Health 2010;2:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010;20:202–13. [DOI] [PubMed] [Google Scholar]
- [9].Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis 2004;39:233–9. [DOI] [PubMed] [Google Scholar]
- [10].Bonaros N, Mayer B, Schachner T, Laufer G, Kocher A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: a meta-analysis. Clin Transplant 2008;22:89–97. [DOI] [PubMed] [Google Scholar]
- [11].Steininger C, Puchhammer-Stockl E, Popow-Kraupp T. Cytomegalovirus disease in the era of highly active antiretroviral therapy (HAART). J Clin Virol 2006;37:1–9. [DOI] [PubMed] [Google Scholar]
- [12].Horwitz CA, Henle W, Henle G, Snover D, Rudnick H, Balfour HH Jr, et al. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore) 1986;65:124–34. [DOI] [PubMed] [Google Scholar]
- [13].Stratton K, Durch J, Lawrence J. Vaccines for the 21st century: a tool for decisionmaking. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- [14].Krause PR, Bialek SR, Boppana SB, Griffiths PD, Laughlin CA, Ljungman P, et al. Priorities for CMV vaccine development. Vaccine 2014;32:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009;360:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heldwein EE, Krummenacher C. Entry of herpesviruses into mammalian cells. Cell Mol Life Sci 2008;65:1653–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].White JM, Delos SE, Brecher M, Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit Rev Biochem Mol Biol 2008;43:189–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Compton T, Nepomuceno RR, Nowlin DM. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 1992;191:387–95. [DOI] [PubMed] [Google Scholar]
- [19].Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol 2006;80:710–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bernstein DI, Munoz FM, Callahan ST, Rupp R, Wootton SH, Edwards KM, et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine 2016;34:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses 2012;4:800–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, et al. Human cytomegalovirus UL131–128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol 2004;78:10023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Adler B, Scrivano L, Ruzcics Z, Rupp B, Sinzger C, Koszinowski U. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J Gen Virol 2006;87:2451–60. [DOI] [PubMed] [Google Scholar]
- [24].Wen Y, Monroe J, Linton C, Archer J, Beard CW, Barnett SW, et al. Human cytomegalovirus gH/gL/UL128/UL130/UL131A complex elicits potently neutralizing antibodies in mice. Vaccine 2014;32:3796–804. [DOI] [PubMed] [Google Scholar]
- [25].Freed DC, Tang Q, Tang A, Li F, He X, Huang Z, et al. Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine. Proc Natl Acad Sci U S A 2013;110:E4997–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schuessler A, Sampaio KL, Straschewski S, Sinzger C. Mutational mapping of pUL131A of human cytomegalovirus emphasizes its central role for endothelial cell tropism. J Virol 2012;86:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fu TM, An Z, Wang D. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine 2014;32:2525–33. [DOI] [PubMed] [Google Scholar]
- [28].Spaete RR. A recombinant subunit vaccine approach to HCMV vaccine development. Transplant Proc 1991;23:90–6. [PubMed] [Google Scholar]
- [29].Pass RF. Development and evidence for efficacy of CMV glycoprotein B vaccine with MF59 adjuvant. J Clin Virol 2009;46(Suppl 4):S73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011;377:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sharma S, Wisner TW, Johnson DC, Heldwein EE. HCMV gB shares structural and functional properties with gB proteins from other herpesviruses. Virology 2013;435:239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McVoy MA, Lee R, Saccoccio FM, Hartikka J, Smith LR, Mahajan R, et al. A cytomegalovirus DNA vaccine induces antibodies that block viral entry into fibroblasts and epithelial cells. Vaccine 2015;33:7328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Abai AM, Smith LR, Wloch MK. Novel microneutralization assay for HCMV using automated data collection and analysis. J Immunol Methods 2007;322:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang X, Peden K, Murata H. RT-qPCR-based microneutralization assay for human cytomegalovirus using fibroblasts and epithelial cells. Vaccine 2015;33:7254–61. [DOI] [PubMed] [Google Scholar]
- [35].Wilkinsonl GW, Davison AJ, Tomasec P, Fielding CA, Aicheler R, Murrell I, Seirafian S, Wang EC, Weekes M, Lehner PJ, Wilkie GS, Stanton RJ. Human cytomegalovirus: taking the strain. Med Microbiol Immunol 2015;204:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Potzsch S, Spindler N, Wiegers AK, Fisch T, Rucker P, Sticht H, et al. B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog. 2011;7:e1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci U S A 2009;106:2880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 2006;313:217–20. [DOI] [PubMed] [Google Scholar]
- [39].Stampfer SD, Lou H, Cohen GH, Eisenberg RJ, Heldwein EE. Structural basis of local, pH-dependent conformational changes in glycoprotein B from herpes simplex virus type 1. J Virol 2010;84:12924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Singh J, Compton T. Characterization of a panel of insertion mutants in human cytomegalovirus glycoprotein B. J Virol 2000;74:1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Britt WJ, Auger D. Synthesis and processing of the envelope gp55–116 complex of human cytomegalovirus. J Virol 1986;58:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Britt WJ, Vugler LG. Oligomerization of the human cytomegalovirus major envelope glycoprotein complex gB (gp55–116). J Virol 1992;66:6747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wj Britt, Vugler LG. Processing of the gp55–116 envelope glycoprotein complex (gB) of human cytomegalovirus. J Virol 1989;63:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Billstrom MA, Britt WJ. Postoligomerization folding of human cytomegalovirus glycoprotein B: identification of folding intermediates and importance of disulfide bonding. J Virol 1995;69:7015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lopper M, Compton T. Disulfide bond configuration of human cytomegalovirus glycoprotein B. J Virol 2002;76:6073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lopper M, Compton T. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J Virol 2004;78:8333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rieder F, Steininger C. Cytomegalovirus vaccine: phase II clinical trial results. Clin Microbiol Infec 2014;20:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pass RF et al. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J Infect Dis 1999;180:970–5. [DOI] [PubMed] [Google Scholar]
- [49].Schleiss MR et al. Glycoprotein B (gB) vaccines adjuvanted with AS01 or AS02 protect female guinea pigs against cytomegalovirus (CMV) viremia and offspring mortality in a CMV-challenge model. Vaccine 2014;32:2756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Burke HG, Heldwein EE. Crystal Structure of the human cytomegalovirus glycoprotein B. PLoS Pathog. 2015;11:e1005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chandramouli S et al. Structure of HCMV glycoprotein B in the postfusion conformation bound to a neutralizing human antibody. Nat Commun 2015;6:8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Britt WJ, Vugler L, Stephens EB. Induction of complement-dependent and - independent neutralizing antibodies by recombinant-derived human cytomegalovirus gp55–116 (gB). J Virol 1988;62:3309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kirchmeier M, Fluckiger AC, Soare C, Bozic J, Ontsouka B, Ahmed T, et al. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein b antigen induces antibodies with potent and broad neutralizing activity. Clin Vaccine Immunol 2014;21:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gatherer D, Seirafian S, Cunningham C, Holton M, Dargan DJ, Baluchova K, Hector RD, Galbraith J, Herzyk P, Wilkinson GW, Davison AJ. High-resolution human cytomegalovirus transcriptome. Proc Natl Acad Sci U S A 2011;108:19755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dolan A, Cunningham C, Hector RD, Hassan-Walker AF, Lee L, Addison C, et al. Genetic content of wild-type human cytomegalovirus. J Gen Virol 2004;85:1301–12. [DOI] [PubMed] [Google Scholar]
- [56].Cranage MP, Kouzarides T, Bankier AT, Satchwell S, Weston K, Tomlinson P, et al. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J 1986;5:3057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Spaete RR, Thayer RM, Probert WS, Masiarz FR, Chamberlain SH, Rasmussen L, et al. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology 1988;167:207–25. [DOI] [PubMed] [Google Scholar]
- [58].Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128–131A complex. J Virol 2010;84:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kabanova A, Perez L, Lilleri D, Marcandalli J, Agatic G, Becattini S, et al. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc Natl Acad Sci U S A 2014;111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wussow F, Chiuppesi F, Martinez J, Campo J, Johnson E, Flechsig C, et al. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog 2014;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zydek M, Petitt M, Fang-Hoover J, Adler B, Kauvar LM, Pereira L, et al. HCMV infection of human trophoblast progenitor cells of the placenta is neutralized by a human monoclonal antibody to glycoprotein B and not by antibodies to the pentamer complex. Viruses 2014;6:1346–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chiuppesi F, Wussow F, Johnson E, Bian C, Zhuo M, Rajakumar A, et al. Neutralizing antibodies to the human cytomegalovirus gH/gL pentamer potently block primary cytotrophoblast. Infection J Virol 2015:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bootz A, Karbach A, Spindler J, Kropff B, Reuter N, Sticht H, et al. Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus. PLoS Pathog 2017;13:e1006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cui X, Cao Z, Chen Q, Arjunaraja S, Snow AL, Snapper CM. Rabbits immunized with Epstein-Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine 2016;34:4050–5. [DOI] [PubMed] [Google Scholar]
- [65].Cui X, Cao Z, Sen G, Chattopadhyay G, Fuller DH, Fuller JT, et al. A novel tetrameric gp350 1–470 as a potential Epstein-Barr virus vaccine. Vaccine 2013;31:3039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li F, Freed D, Tang A, Rustandi RR, Troutman MC, Espeseth Amy S, et al. Complement enhances in vitro neutralizing potency of antibodies to human cytomegalovirus glycoprotein B (gB) and immune sera induced by gB/MF59 vaccination. NPJ Vaccines 2017;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ha S, Li F, Troutman MC, Freed DC, Tang A, Loughney JW, et al. Neutralization of diverse human cytomegalovirus strains conferred by antibodies targeting viral gH/gL/pUL128–131 pentameric complex. J Virol 2017;91:e02033–e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nelson CS, Huffman T, Jenks JA, Cisneros de la Rosa E, Xie G, Vandergrift N, Pass RF, Pollara J, Permar SR. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc Natl Acad Sci U S A 2018;115:6267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Baraniak I, Kropff B, Ambrose L, McIntosh M, McLean GR, Pichon S, et al. Protection from cytomegalovirus viremia following glycoprotein B vaccination is not dependent on neutralizing antibodies. Proc Natl Acad Sci U S A 2018;115:6273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


