Abstract
Antiretroviral therapy (ART) has changed the outcome of human immunodeficiency virus type one (HIV-1) infection from certain death to a life free of disease comorbidities. However, infected people must remain on life-long daily antiretroviral therapy (ART). ART reduces but fails to eliminate the viral reservoir. In order to improve upon current treatment regimens our laboratory created long acting slow effective release (LASER) ART nanoformulated prodrugs from native medicines. LASER ART enables antiretroviral drugs (ARVs) to better reach target sites of HIV-1 infection while, at the same time, improve ART’s half-life and potency. However, novel ARV design has been slowed by prolonged pharmacokinetic testing requirements. To such ends, a tri-modal theranostic nanoparticles were created with single-photon emission computed tomography (SPECT/CT) magnetic resonance imaging (MRI) and fluorescence capabilities to predict LASER ART biodistribution. The created theranostic ARV probes were then employed to monitor drug tissue distribution and potency. An Intrinsically radiolabeled “111Indium (111In)”, europium doped cobalt-ferrite particles were encased in a polycaprolactone core surrounded by a lipid shell containing rilpivirine (111InEuCFRPV). Particle cell, tissue distribution and antiretroviral activities were sustained in macrophage depots within the reticuloendothelial system. 111InEuCF-PCL/RPV particles injected into mice demonstrated co-registration of MRI and SPECT/CT tissue signals with RPV and cobalt. Cell and animal particle biodistribution paralleled the antiretroviral activities of nanoformulated RPV. We posit that particle selection can predict ARV distribution and potency facilitated by multifunctional theranostic nanoparticles.
Keywords: Antiretroviral therapy, transformative nanotechnology, magnetic resonance imaging, single photon emission computed tomography, biodistribution
Graphical Abstract
1. Introduction
The development of effective antiretroviral therapy (ART) for human immunodeficiency virus type one (HIV-1) infection has dramatically reduced disease morbidities and mortality [1, 2]. ART’s success, over the past several decades, is substantive as evidenced by the drug’s ability to efficiently reduce circulating plasma HIV-1 loads to undetectable levels. In so doing ART protects CD4+ T cells and reduces comorbid disease [3, 4]. However current treatment limitations include drug pharmacokinetics (PK) and biodistribution profiles, viral mutations and drug toxicities [5, 6]. All affect optimal therapeutic efficacy [7]. Additionally, shortened antiretroviral (ARV) drug half-lives necessitate daily dosing and strict regimen adherence [8]. Reduced ARV access to virus target tissues can also affect the maintenance of drug levels at action sites and the ability to contain CD4+T cell infection [9]. Innate inflammatory and adaptive immune responses tied to HIV-1 infection continue despite therapeutic drug regimens, leading to diabetes, osteoporosis, cardiovascular diseases and neurocognitive disorders [10–15]. Each and all of these limitations have led to the development of longer acting nanoformulated ART [16, 17] and recently to the chemical synthesis of lipophilic prodrug nanocrystals coined long acting slow effective release (LASER) ART. The noted scientific advances have further extended drug ARV half-lives and potencies [18, 19]. LASER ART rests on four pillars; creation hydrophobic prodrug nanocrystals, the enhanced drug lipophilicity associated with improved cell membrane drug penetration, slow drug release and hydrolysis, and facilitated viral reservoir drug penetrance. These transformative technologies created ARVs that best penetrate viral reservoirs and increase the drug’s apparent half-life creating medicines that maximally restrict viral growth [20]. However, to realize the potential of long acting tissue viral reservoir penetrating ARVs, the drugs’ pharmacokinetic (PK) and pharmacodynamic (PD) profiles must be optimized to minimize on and off-target effects. Moreover, while mathematical descriptions of long-acting nanoformulated drug distribution have been developed, these cannot reflect the diversity of chemical alterations and physical characteristics now required for LASER ART nanoformulations [21]. To this end multimodal theranostic ARVs were made as an effective descriptor for drug biodistribution.
In the current report, highly stable europium cobalt-ferrite (EuCF) nanocrystals, intrinsically radiolabeled with 111indium were loaded with the ARV drug, rilpivirine (RPV). The newly minted drug particles facilitated real-time non-invasive detection of drug and antiretroviral drug biodistribution and screening. These findings forge a significant improvement over first generation EuCF-PCL particles [22] by extending ARV encapsulations and bioimaging capabilities to assess drug biodistribution. Indeed, these particles improve signal intensities from both single photon emission computed tomography (SPECT/CT) and magnetic resonance imaging (MRI) in order to accurately predict drug distribution. Validation of this predictive potential is provided by ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) and inductively coupled plasma mass spectrometry (ICP-MS) made possible by the metals encased in the particles. The data demonstrate, for the first time, that theranostics ARV particles can provide a platform to facilitate clinically effective LASER ART development.
2. Materials and Methods
The reagents used in this report, animal sources, purchasing and handling, preparation of RPV freebase, manufacturing of nanoformulated RPV (NRPV) and NRPV-CF®633, isolation of human monocytes from human immunodeficiency virus types 1,2 (HIV-1,2) and hepatitis B seronegative donors and cultivation of monocyte-derived macrophages (MDM) are described in the supplemental data section and follow published protocols [23]. The physiochemical methods used in this report for particle characterization and stability, UPLC-MS/MS and ICP-MS parameters for drug and metal concentration determinations were performed as previously reported [24, 25] and are briefly described in the supplemental informations.
2.1. Preparation of radiolabeled multimodal theranostic particles
Monodisperse and high quality crystalline EuCF nanocrystals were synthesized and characterized via state of the art techniques as previously described [22]. Production of chelate free, non-leachable and highly stable radiolabeled 111InEuCF nanocrystals was accomplished by intrinsic co-doping of the 111In radionuclide along with europium into the cobalt ferrite lattice structure. In a typical synthesis ~555 MBq (~15 mCi) of 111InCl3 was mixed with 750 μL of benzyl alcohol, vortexed and stirred for 30 min at room temperature. The mixture was slowly transferred into a Teflon container for hydrothermal synthesis. The formulations were prepared with cobalt acetylacetonate, iron (III) acetylacetonate and europium (III) in the presence of oleic acid, oleamine and 1,2-hexadecanediol. All were mixed and sonicated for 10 min (Cole-Parmer, Vernon Hills, IL, USA). The obtained mixtures of metal precursors, surfactants, protective ligands and reducing agents were added to a Teflon container and sealed in a high-quality stainless steel autoclave reactor. This reactor was placed in a preheated vacuum oven for 90 min at 260°C then cooled to room temper ature. 111InEuCF nanocrystals were purified by washing with ethanol and collected with a magnet. Radioactivity of 111InEuCF nanocrystals was quantitated by gamma counting (CRC®−25R dose calibrator, Capintec, Inc, Florham Park, NJ, USA). 111InEuCF nanocrystals were re-suspended in chloroform or hexane for the next formulation step. Non-radiolabeled InEuCF probes were synthesized by similar methods except that InCl3 was the source of indium. 111InEuCF-PCL/PRV theranostic particles were synthesized by using a solvent evaporation technique. The encapsulations of 111InEuCF and RPV in PCL and further coating with a lipid shell was accomplished according to previously described methods [25].
Particle size is known to play a key role in biodistribution, however the effect is still not completely understood [26, 27]. To test the effect of construct size on drug biodistribution ultra-small (~5 nm) 177lutetium-radiolabeled EuCF nanocrystals (177LuEuCF) were prepared. Lipid coated 177LuEuCF nanocrystals were synthesized with a reaction time of 45 min. 177LuEuCF nanocrystals encapsulations were accomplished using a spectrum of lipid film composition ratios. Ultra-small 177LuEuCF nanocrystals were coated with lipid by dehydration processes. The lipid film was prepared from solutions of PC (25 mg) and DSPE-PEG2000 (12.5 mg) in chloroform followed by rotary evaporation. 177LuEuCF nanocrystals were dispersed in chloroform by sonication for 30 min and then transferred into 2% Tween 80 (3 mL) solution to make oil in water (o/w) emulsion. This emulsion was allowed to stir for one hour at room temperature to ensure complete removal of chloroform, then transferred into the round bottom flask containing the lipid film and stirred for an additional five hours. Finally, lipid coated ultra-small 177LuEuCF nanocrystals were purified by centrifugation at 35,136-x g (18,000 rpm) for 30 min and re-suspended in PBS. Radioactivity of 111InEuCF-PCL and lipid coated 177LuEuCF particles were determined by gamma counter before injection into animals.
2.2. Radiolabeled particle stability
The in vitro stability of 111InEuCF-PCL particles was tested in PBS and rat plasma solutions. 111InEuCF-PCL particles, 1.48 MBq (40 μCi), were incubated in 1.5 mL of phosphate buffered saline (PBS) or rat plasma at 4°C and 37°C for 3 days. At 0, 24, 48 and 72 hours, the samples were centrifuged at 21,255-x g (14,000 rpm) for 30 min at 10°C and supernatants were collected. The radioacti vity of the supernatant and pellet (particles) was measured by gamma counter to determine the percent stability. The percent stability was calculated using the equation (Radiolabeling stability (%) = radioactivity in pellet / total radioactivity X 100).
2.3. Particles cell vitality, uptake and retention study
Cell vitality evaluation of native RPV, EuCF-RPV, 111InEuCF-RPV and NRPV was performed using the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay [28]. In brief, 105 human monocytes per well were seeded on a flat-bottomed 96-well polystyrene coated plate and incubated for seven days at 37°C in a 5% CO 2 incubator for differentiation into MDM with supplemented media containing macrophage colony stimulating factor [23]. Serial dilutions of EuCF-RPV, 111InEuCF-RPV and NRPV particles (0.2–200 μM based on RPV concentration or 1.987X10−3 to 8.14 MBq) in media were added to the cells in triplicate. Native RPV was dissolved in dimethyl sulfoxide (DMSO) and diluted in media (0.1% v/v) for MDM treatment control. After treatment, the media was removed and 100 μL of MTT solution (0.5 mg/mL) was added to each well. Plates were incubated at 37°C for 45 minutes. The M TT solution was removed after incubation and 100 μL of sterile DMSO was added to each well to dissolve formazan crystals. The plates were read immediately in a SpectraMax® M3 Multi-Mode Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA, USA) at absorbance of 490 nm. Wells with DMSO but without cells were used as blanks. Untreated MDM and cells treated with EuCF-RPV and NRPV for 8 hours were evaluated by the MTT assay to assess cell vitality.
MDM uptake and retention of EuCF-RPV and NPRV particles were determined according to published written reports with noted modifications [25, 29]. MDM were plated in 12 well plates at a density of 1.5 × 106 cells/well with half media exchanges every other day. After seven days, 6.25 μM, 12.5 μM and 25 μM concentrations of EuCF-RPV and NPRV particles were added to the cells and incubated for 2, 4, 8 and 12 hours to evaluate particle uptake. Cell particle retention was determined by treating MDM with 25 μM EuCF-RPV or NRPV for 8 hours followed by washing the cells and adding fresh medium without particles. Cell samples were collected on days 1, 3, 5, 7, 11, 12 and 15 following treatment. At each time point, the media was removed, cells were washed three times with PBS then counted and harvested following centrifugation at 21,255 × g (14,000 rpm) for 10 min. Cell pellets were stored at −80°C for metal analysis. The cell pellet was digested with fresh diluted nitric acid. Iron and cobalt concentrations were determined by ICP-MS and reported as iron or cobalt concentration per million cells as previously described [25]. For RPV analysis 100 μL of methanol was added to the cell pellet followed by sonication for 2 min. The mixture was then centrifuged at 21,255-x g (14,000 rpm) for 10 min and the supernatant was collected. RPV concentration in the supernatant was determined by UPLC with UV/Vis absorption as previously described [30].
2.4. Confocal, transmission electron and atomic force microscopy (CF, TEM and AFM) and immunohistochemistry (IHC)
The uptake, subcellular localizations and surface topological changes of EuCF-RPV and NPRV particles in MDM were visualized by confocal microscopy, Transmission electron microscopy (TEM), Atomic force microscopy (AFM) and immunohistochemistry (IHC). Cells were cultured on glass coverslips in 12 well plates at a density of 1.5 × 106 cells/well and incubated for seven days at 37°C and 5% CO 2 to differentiate into MDM as described previously. MDM were treated with EuCF-RPV and NRPV particles (25 μM based on RPV) for 8 hours. After particle incubation, cells were fixed with ice-cold 4% paraformaldehyde (PFA) in PBS at room temperature for 30 min. Visualization of the surface topography of particle-treated MDM was performed by AFM. Coverslips with fixed cells were removed from wells and mounted on a slide for AFM analysis. Images were acquired in air using an MFP-3D™ system (Asylum Research, Santa Barbara, CA, USA) operating in tapping mode. AFM probes MSNL-F with a nominal spring constant ~0.6 N/m were used for imaging. Image processing was performed using Femtoscan software (Advanced Technologies Center, Moscow, Russia).
Subcellular localizations and immunocytochemistry staining were performed as previously described with some modifications [31, 32]. Briefly, after EuCF-RPV and NRPV-CF®633 particle treatment of MDM, cells were washed with PBS and fixed with ice cold 4% PFA at room temperature for 30 min and rinsed three times with PBS. MDM were then treated with 0.5% v/v Triton-X-100 for 15 min to permeabilize the cells and again washed with PBS. A mixture of 5 % w/v bovine serum albumin (BSA) in PBS and 0.1% v/v Triton-X-100 was used as a blocking solution for 1 hour and 50 mM NH4Cl was used as quenching solution for 15 min and washed once with 0.1% Triton-X-100 in PBS. MDM were next treated with primary antibodies against either lysosomal-associated membrane protein 1 (LAMP-1; Novus Biologicals, Littleton, CO, USA) for lysosome identification, Rab 5 and Rab 7 for late endosomes, or Rab 11 and Rab 14 (Santa Cruz Biotechnology, Dallas, TX, USA) for recycling endosomes. Primary antibodies were diluted 1:25 (v/v) antibody:blocking solution and incubated overnight with shaking at 4°C as previously described [25]. M DM were then treated with secondary antibody in blocking solution (1:50) (AlexaFluor- 488 and 594 for NRPVCF®633 and EuCF-RPV, respectively; Thermo-Fischer Scientific, Waltham, MA, USA) for 2 hours at room temperature. MDM nuclei were stained using ProLong Gold AntiFade reagent with DAPI (4′,6-diamidino-2-phenylindole; Thermo-Fischer Scientific, Waltham, MA, USA). Cells were imaged using a 63 X oil objective on a LSM 710 confocal microscope (Carl Zeiss Microimaging, Inc., Dublin, CA, USA). Zeiss LSM 710 Image browser AIM software version 4.2 was used to determine the number of pixels and the mean intensity of each channel.
For TEM analysis cells were fixed in 2% glutaraldehyde and 2% paraformaldeyhyde (PFA) in 0.1 M Sorenson’s phosphate buffer (pH 6.2) for 24 hours at 4°C. TEM sample preparation, including staining and image processing, was performed as per previously established protocols [25]. NRPV uptake at select drug concentrations was analyzed and validated by flow cytometry [29, 33]. Briefly, 5 × 106 MDM were cultured in suspension in 50 mL Teflon flasks containing 20 mL of cell culture media. After 7 days of culture, cells were treated with NRPV-CF®633 at 25 μM and 50 μM RPV or vehicle control for 8 hours. After 8 hours, cells were centrifuged 976 × g (3,000 rpm) for 10 minutes at 4°C following which treatment media was removed. Cells were re-suspended in cold PBS and centrifuged again at the same conditions. After washing, cells were again re-suspended in PBS at a concentration of 1.0 ×106 cells/mL. Before flow analysis, cells were incubated with the viability dye, propidium iodide (PI), for 30 minutes. Analysis was completed with a BD LSR II Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Cells were gated according to viability and only live cells were selected for analysis of NRPV-CF®633 uptake. Unlabeled cells were used to calculate the background fluorescence and MDM without any treatment served as a negative control in every measurement. The percentage of cells that internalized NRPVCF®633 was determined using BD FACSDiva 8.0.1 software (BD biosciences, Franklin Lakes, NJ USA).
2.5. Measurements of antiretroviral activities
Antiretroviral activities of EuCF-RPV, NRPV and native RPV were evaluated by measures of reverse transcriptase (RT) activity and HIV-1p24 staining as previously described protocols [34] with the following modifications. In brief, MDM were treated with 6.25 μM, 12.5 μM, or 25 μM (RPV content) native RPV, NRPV or EuCF-RPV particles for 8 hours. MDM were then cultured in fresh medium without particles. At days 1 and 5 post-treatment, MDM were challenged with HIV-1ADA for 16 hours at a multiplicity of infection (MOI) of 0.1 infectious virions per cell. MDM were cultivated for 10 days after infection. Culture supernatants were collected and analyzed for progeny virion production by RT activity assay [35]. At the same time, cells were washed with PBS and fixed in 4% PFA for 15 min for HIV-p24 staining [18]. Images were analyzed using a Nikon TE300 microscope with a 20X magnification objective.
2.6. Particle T2 relaxivity measurements
T2 relaxivity measurements of EuCF-RPV particles were determined in two sets of samples over a concentration range of 3.58 to 35.81 μM iron. EuCF-RPV particle suspensions were prepared in PBS. A 1.5% (w/v) agar gel was prepared by heating agar powder dissolved in PBS at 70°C for 30 min. In 250 μL eppendorf tubes, 100 μL of EuCF-RPV particle suspensions were mixed with 100 μL of the 1.5% (w/v) agar solution and solidify in an ice bath. The second set of samples were prepared with EuCF-RPV particle loaded MDM. In order to measure the T2 relaxivity in MDM, cells were seeded onto 100 mm culture plates at a concentration of 1 × 106 cells/mL. MDM were incubated with EuCF-RPV particles in media (5 μg/mL based on iron) for 8 hours. After treatment, the media was removed and cells were washed three times with PBS. Cells were collected by centrifugation at 976 × g (3,000 RPM) for 10 min at 4°C and re-suspended at various cell concentrations in PBS containing 1.5% (w/v) agar in 250 μL eppendorf tubes. T2 relaxtivity was measured using a 7T/16 cm Bruker PharmaScan scanner (Bruker; Ettlingen, Germany) with set parameters described previously [22]. Relaxivity in MDM was used to generate a standard curve correlating cellular iron content to T2 relaxation time to be used for in vivo quantitation of tissue iron concentration by MRI.
2.7. Tissue biodistribution of multimodal particle
For multimodal imaging and biodistribution studies; 111InEuCF-PCL/RPV, ultra-small lipid coated 177LuEuCF and NRPV particles were injected intravenously into mice at variant concentrations of drug or radioisotope (Fig.S3 and Table 1). As shown, male Balb/cJ mice (6–7 weeks old) were divided into eight groups (n= 10 to 15 mice per group) and intravenously injected with various doses of theranostic and/or NRPV particles. The first group (G1) received NRPV particles at 45 mg RPV/kg. The second group (G2) received NRPV particles at a lower dose of 5 mg RPV/kg. The third group (G3) received EuCF-PCL particles (2mg/kg iron) only. The fourth group (G4) was administered EuCF-RPV particles (2 mg/kg iron). The fifth (G5) and sixth (G6) groups received two sequential injections, first EuCF-PCL particles (2 mg/kg iron) and then NRPV particles (45 mg RPV/kg for G5 and 5 mgRPV/kg for G6, respectively). The seventh group (G7) underwent SPECT/CT imaging after receiving111InEuCF-PCL particles ~ 22.2 MBq (600 μCi) and the final group (G8) was treated with ultra-small lipid coated 177LuEuCF particles ~ 74 MBq (2000 μCi) to assess the effect of particle size on biodistribution. For plasma drug quantitation, 100 μL whole blood was collected by cheek puncture into lithium heparin coated tubes at 1, 3, 5, 7, 14, 21, and 28 days post injection. For tissue drug and metal biodistribution analysis, five animals from each group were euthanized at 2, 5 and 28 days post treatment and eight tissues were dissected (liver, spleen, lung, gut, brain, axillary and popliteal lymph nodes and kidneys). Drug and metal content in plasma was determined by UPLC-MS/MS and ICPMS, respectively (Supplementary method; SM6).
Table.1.
Design of multimodal theranostic nanoparticle tests in animals to predict real time drug biodistribtion.
| Groups | Formulations | Multimodal test | ||||||
|---|---|---|---|---|---|---|---|---|
| MRI | SEPCT/CT | Radioactivity | UPLC-MS/MS | ICP-MS | ||||
| Dose (mg/kg) | Time | |||||||
| RPV | Iron | Days | Hours | Days | Days | Days | ||
| G1 | NRPV | 45 | -- | -- | -- | -- | 1,2,3,5,7,1,14,21,28 | 1,2,3,5,7,1,14,21,28 |
| G2 | NRPV | 5 | -- | -- | -- | -- | 1,2,3,5,7,1,14,21,28 | 1,2,3,5,7,1,14,21,28 |
| G3 | EuCF-PCL | 2 | 2 | -- | -- | -- | 2, 5 | 2,5 |
| G4 | EuCF-PRV | 2 | 2 | 2, 5 | -- | -- | 2, 5 | 2,5 |
| G5 | EuCF-PCL + NRPV | 45 | 2 | 2, 5 | -- | -- | -- | -- |
| G6 | EuCF-PCL + NRPV | 5 | 2 | -- | -- | -- | -- | -- |
| G7 | 111lnEuCF-PCL | 600 uCi | -- | -- | 4, 12, 24, 48, 120 | 2, 5 | -- | -- |
| G8 | 177LuEuCF | 2000 uCi | -- | -- | 6, 24, 48, 120 | 1, 2, 5 | -- | -- |
For multimodal imaging, to non-invasive MRI and SPECT/CT were performed to assess theranostic particle biodistribution. First, MRI was performed on mice receiving EuCF-RPV (G4) and EuCF-PCL plus NRPV (G5) at 2 and 5 days post injection. These images were quantified for approximate iron concentration using phantom relaxivity data acquired in MDM. SPECT/CT imaging was performed on mice that received ~22.2 MBq (600 μCi) of 111InEuCF-PCL (G7) and ~ 74 MBq (2000 μCi) of ultra-small lipid coated 177LuEuCF (G8) particles. For ex vivo tests, gamma scintillation spectrometry was performed on tissues recovered from mice injected with 111InEuCF-PCL (G7) and ultra-small 177LuEuCF (G8) particles. As shown in Fig.S3 and Table 1, the degree of correlation between the following data sets was determined; RPV concentration in tissues as determined by UPLC-MS/MS, iron concentration by MRI, radioactivity by SPECT/CT, ex vivo gamma scintillation spectrometry, and cobalt concentration determined by ICP-MS. To assess in vivo toxicity of the injected particles, the mice were sacrificed at 28 days, and liver, spleen and kidney were collected and fixed in 10% neutral buffered formalin for histological analysis. To determine the real time drug biodistribution prediction capabilities of the particles, the results from advanced molecular imaging techniques were correlated with drug and metal quantitative results from independent groups. For the first set of comparisons, RPV concentration (ng/mL), iron concentration (ng/mL), cobalt concentration (mg/mL), SPECT/CT signal (counts/mm3), or gamma counts (% ID/g) were each averaged across all groups by tissue (liver, spleen, lymph node) and Pearson’s correlation coefficients for each 2 parameter comparison (for example: RPV in liver vs. SPECT/CT signal in liver) were determined. For the second set of comparisons, each group was considered separately. For each group, these data were averaged by tissue and day of sacrifice. The tissue per day averages were then compared to the same tissue per day averages from every group and Pearson’s correlation coefficients were calculated for each comparison. Statistical differences were determined using a two-way ANOVA among groups followed by Bonferroni multiple comparison test. P values ≤ 0.05 were considered significant.
2.7.1. MRI analysis of theranostic particles biodistribution in mice
EuCF-RPV (G4) and EuCF-PCL biodistribution studies were performed with co-injected NPRV particles (G5). These experiments were performed using male Balb/cJ mice (6–7 weeks old, n = 10). MRI scanned mice immediately prior to intravenous injection of particles to provide a baseline scan for comparisons. MR images were acquired 2 and 5 days after treatment using a 7T/16 cm Bruker PharmaScan and a home-built RF coil for signal transmitting and receiving. T2 mapping was used to determine the biodistribution of EuCF-RPV particles as previously described [25] with the following modified scanning parameters. For T2 mapping, a CPMG phase cycled 3-dimensional multi-echo sequence data acquired with 24 echoes (echo times TEn = n × 2.638 ms; n = 1, 2, 3… up to 24), 400 ms repetition time, 120 × 144 × 58 acquisition matrix, 40 × 40× 22 mm field of view (FOV), one average, for a total acquisition time of 56 min. After post scans, mice were anesthetized with isoflurane, sacrificed via cardiac puncture, and perfused with sterile PBS. Organs were then collected for drug and metals content analysis (liver, spleen, kidney, lung, GALT, and brain). Additionally, mice were bled via facial vein puncture after the day 2 and 5 post-scans. Blood was collected into lithium heparin coated tubes and centrifuged at 2,000 × g for 10 min to collect plasma for drug and cobalt analysis by UPLC-MS/MS and ICP-MS, respectively (Supplementary method; SM6).
2.7.2. SPECT/CT for particles biodistribution
In vivo biodistribution of 111InEuCF-PCL particles was determined in male Balb/cJ mice (6–7 weeks old, n= 10, G7) via SPECT/CT. On day 0 all mice received a single intravenous injection of ~22.2 MBq (600 μCi) of 111InEuCF-PCL particles. Image acquisition was performed at 4, 12, 24, 48, and 120 hours post injection by a hybrid single photon emission computed tomography system and x-ray computed tomography (SPECT/CT) (Flex Triumph, TriFoil Imaging, Northridge, CA, USA) fitted with a five pinhole (1.0 mm per pinhole) collimator. First CT images were acquired using 360 projections over 360° with an x-ray tube current of 140 mA and voltage of 75 kilovoltage peak (kVp) at a magnification of 2.0 (field of view = 59.2 mm2). Immediately after, SPECT/CT image acquisition was performed with the following parameters; 64 projections at 10 seconds per projection over 360° using a radius of rotation (ROR) of 48 mm (field of view= 59 mm2). Co-registration of anatomical CT images and functional SPECT was performed by 3D visualization and analysis software, VivoQuant 3.5 (Invicro Boston, MA, USA). Regions of interest (ROIs) were drawn over various organs and the radioactivity and organ volume were determined to calculate gamma counts per cubic millimeter (mm3). After days 2 and 5 scans, mice were sacrificed and various organs including the heart, lung, liver, pancreas, stomach, spleen, small intestine, large intestine, kidneys, bladder, lymph node, muscle, bone, brain, injection site (tail), remaining carcass and blood were collected, weighed, and analyzed ex vivo for radioactivity using gamma scintillation spectrometry. Additionally, animals were individually caged so that excreted signal could be measured from the bedding. Signal measured was back calculated to the time of injection to account for radioactive decay of 111In (t1/2 = 67.2 h). Signal from each organ was divided by the sum of total counts from all sources and then divided again by the weight of the organ to obtain percent-injected dose per gram of tissue (% ID/g). For the ultra-small (~ 5 nm) particle biodistribution study, 15 male Balb/cJ mice (6–7 weeks old) were intravenously injected with ~ 74 MBq (2,000 μCi) of lipid-coated 177LuEuCF particles (G8). Mice were then scanned by SPECT/CT using the same parameters as previously described for the 111InEuCF-PCL particles at 6, 24, 48, and 120 hours post injection. After scanning at 24, 48, and 120 hours, 5 mice were sacrificed and organs were collected for ex vivo gamma counting in the same manner as for 111InEuCF-PCL particles except that the half-life of 177Lu (t1/2 = 6.64 days) was used for back calculating radioactivity.
2.8. Autoradiography
Liver, lungs and spleen were excised at 1, 2 and 5 days post injection from mice treated with 111InEuCF-PCL or 177LuEuCF particles. Collected tissues were washed with deionized water and immediately embedded into optimal cutting temperature (O.C.T) compound (Fischer HealthCare, Waltham, MA, USA). Cryostat sections (10 μm) of tissue samples were prepared and exposed to a phosphor screen for 5 days in the dark (Leica CM1850, Leica Biosystems Inc, Buffalo Grove, IL, USA). The phosphor screen was subsequently imaged by a Typhoon FLA 9500 variable mode imager (GE Lifesciences, Pittsburg, PA, USA) at 25 μm resolution.
2.9. RPV and cobalt quantitation in plasma and tissues
Drug concentrations were determined in mouse plasma and tissues by UPLC-MS/MS using a Waters Acquity UPLC-Xevo TQ-S micro mass spectrometry system (Waters, Milford, MA, USA). ICP-MS metal analysis was performed at the University of Nebraska-Lincoln’s Spectroscopy and Biophysics Core Facility. Additional details can be found in supplemental methods; SM6.
2.10. Tissue localization and toxicity
Particle distribution within the spleen and liver from mice treated with EuCF-PCL (G3) and NRPV (G5) was evaluated via TEM. For TEM examination, tissue samples were fixed in TEM buffer at room temperature. Tissue processing and sectioning were performed as previously described [25]. In vivo toxicity of the EuCF-RPV particles was determined by histological examination. For histological examination, 5 μm sections of paraffin-embedded tissues were fixed to glass slides and stained with hematoxylin and eosin and a TUNEL assay was performed as per as per manufacturer’s instructions. Images were captured with a Nuance EX multispectral imaging system affixed to a Nikon Eclipse E800 microscope (Nikon Instruments, Melville, NY, USA) using a 20× objective. Histopathological assessment was conducted in accordance with the guidelines of the Society of Toxicologic Pathology.
2.11. Statistical analyses
For all studies, data were analyzed using GraphPad Prism 7.0 software (GraphPad, La Jolla, CA, USA) or Statistica v9 (Statsoft, Inc., Tulsa, OK, USA) and are presented as the mean ± standard error of the mean (SEM). For comparisons of two groups, Student’s “t” test (two-tailed) was used. Experiments with multiple time points were analyzed using two-way factorial ANOVA and Bonferroni’s post hoc tests for multiple comparisons.
3. Results
3.1. Creation and characterization of multimodal theranostic ARV particles
111In/177LuEuCF nanocrystals were prepared by solvothermal synthesis using cobalt acetylacetonate; iron (III), acetylacetonate, and europium (III) nitrate hydrate in the presence of oleic acid and oleylamine [22]. Radioactive indium chloride (111InCl3) was then slowly added into the reaction mixture. Benzyl alcohol served as solvent and protective ligand, while 1,2-hexadecanediol was used as a reducing agent [36]. The stability of the radiolabeled 111InEuCF-PCL particles was assessed in PBS and rat plasma at 4°C and 37°C and was found to be >97.4 ± 0.26% after 24 hours. Plasma stability studies confirmed that the radioactive isotope remained particle bound over 3 days (Table 2).
Table 2.
In vitro radiolabeling stability assessment
| Time points (h) | 111In labeling efficiency (%) | |||
|---|---|---|---|---|
| 4°C | 37°C | |||
| PBS | Plasma | PBS | Plasma | |
| 0 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 24 | 97.6 ± 0.09 | 89.3 ± 0.64 | 97.4 ± 0.26 | 89.2 ± 0.63 |
| 48 | 97.2 ± 0.06 | 89.6 ± 0.77 | 97.7 ± 0.19 | 89.5 ± 0.97 |
| 72 | 97.1 ± 0.94 | 87.6 ± 1.27 | 97.3 ± 0.46 | 82.5 ± 0.60 |
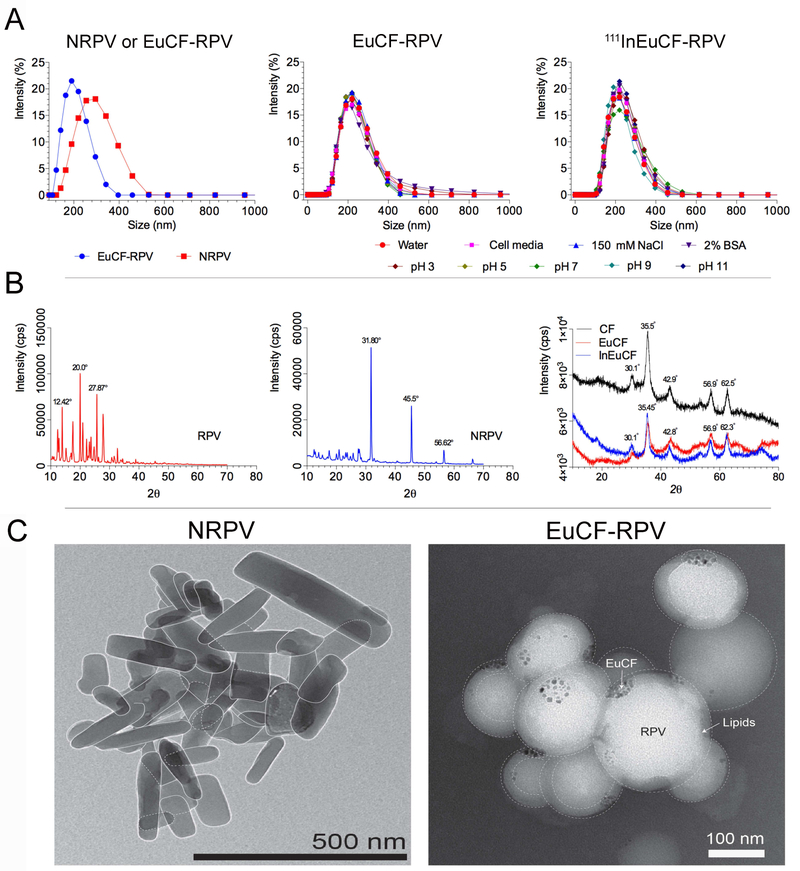
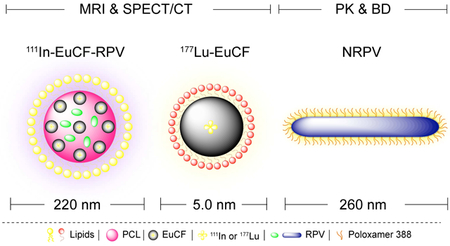
Physicochemical properties and structural configurations of the particles were characterized by a variety of analytical techniques. Structural characterization of theranostic particles was performed as per our previous report [25]. For physical characterizations non-radioactive InEuCF nanocrystals were employed. NRPV was prepared by high-pressure homogenization of RPV [37] in the presence of polaxamer 338 [38]. Particle hydrodynamic diameter and polydispersity index were measured daily for one week at room temperature. RPV drug loading into the EuCF-RPV was determined to be ~ 5.5% (w/w). Fig.1A illustrates the hydrodynamic size of monodispersed NRPV, EuCF-RPV and 111InEuCF-RPV particles determined by dynamic light scattering measurements. NRPV nanocrystals had an average hydrodynamic size of 260 nm, which was similar to that of the EuCF-RPV and 111InEuCF-RPV particles, which measured 220 nm (Fig.1A). As expected, intrinsic doping of the EuCF core with indium had no effect on hydrodynamic particle size at various pH measurements, physiological salt concentrations and biological medias used (Supplementary Fig.S1). The PDI of all particles was ~0.2. Importantly, size and PDI of NRPV were stable over 28 days at room temperature (Supplementary Fig.S1). Additionally, the stability of 111InEuCF-RPV and EuCF-RPV particles was evaluated after incubation with distilled water with and without 150 mM NaCl [39] and at a pH of 3, 5, 7, 9 and 11 . Stability in PBS containing 2% bovine serum albumin showed after testing by all parameters used in this report were unchanged after a week in a spectrum of environmental conditions (Fig.1A). In addition, the crystalline properties of nanoformulations could also influence particle stability and the in vivo drug pharmacokinetics and biodistribution parameters [18, 40]. Undesirable in vivo burst release PK patterns and ultimately rapid clearance from tissues and plasma depend on variables that include type of drug, surfactants, process and duration of making nanoformulations [41]. These changes vary depending on the type of drug, surfactant used, pressure and time to make the nanoformulations. Therefore, before and after particle size reduction, XRD analysis was conducted to assess whether the initial crystalline state was preserved. Fig.1B shows the results of each of the XRD patterns of RPV, NRPV, CF, EuCF and InEuCF. The powder X-ray diffraction study of NRPV showed significant shifts in the main peaks, compared with coarse RPV. RPV showed characteristic diffraction peaks at 12.42°, 13.93°, 17.57°, 20.0°, 25.68°, 27.87° and 32.70° at 2 θ positions. Whereas NRPV showed characteristic diffraction peaks at 31.80°, 45.5° and 56.62° at 2 θ positions. This shift from lower 2θ angles to higher 2θ angles can be attributed to the formation of larger NRPV nanocrystals from the more amorphous parent RPV. From these values it is clear that NPRV is crystalline in nature after high-pressure homogenization. In the XRD pattern of CF, EuCF and InEuCF shown values that closely matched that of our own previously published reports reflecting the inverse spinel to the spinel ferrite structure [22]. We found from the XRD pattern that intrinsically doping of indium or europium to a cobalt ferrite lattice showed no significant structural changes. TEM images of NRPV and EuCF-RPV particles are presented in Fig.1C. NRPV has a well-defined rod like morphology and EuCF-RPV possesses a spherical shape with metallic EuCF nanocrystals distributed within the PCL core. The lighter color around each particle represents the lipid shell.
Fig.1. Characterization of particles.
(A) Hydrodynamic size distribution of NRPV, EuCF-RPV and 111EuCF-RPV particles determined by dynamic light scattering (average particle sizes of ~220 nm and ~260 nm, respectively) in various media. XRD pattern of (B) RPV, NRPV, CF, EuCF and InEuCF particles, (C) TEM image of NRPV and EuCFRPV.
3.2. Cell based evaluations of the theranostic particles
Cytotoxicity, cellular uptake and retention and subcellular localization of phagocytosed particles were studied in MDM at various drug and metal concentrations and time intervals. We first determined the effects of EuCF-RPV particle administration on MDM cell vitality. MDM were treated with native non-formulated RPV, NRPV or EuCF-RPV particles at concentrations ranging from 0.2 μM to 200 μM (based on RPV concentration) for 12 hours before cell vitality was measured by MTT assay. Replicate MDMs were treated with 111InEuCF-RPV particles to assess radioisotope toxicity “111In’s” effects on cell vitality were shown in Supplementary Fig.S2. As shown in Fig.2A, native RTV, NRTV and EuCF-RPV particles had little effect on cell vitality up to 50 μM. At the highest concentration tested, 200 μM, NRPV and EuCF-RPV less effected cell vitality than native RPV (82.9%, 56.9% and 37.9%, respectively). 111InEuCF-RPV particles treated cells demonstrated a ~80% cell viability at 8 h with the highest 8.14 MBq (220 μCi) concentrations (Supplementary Fig.S2).
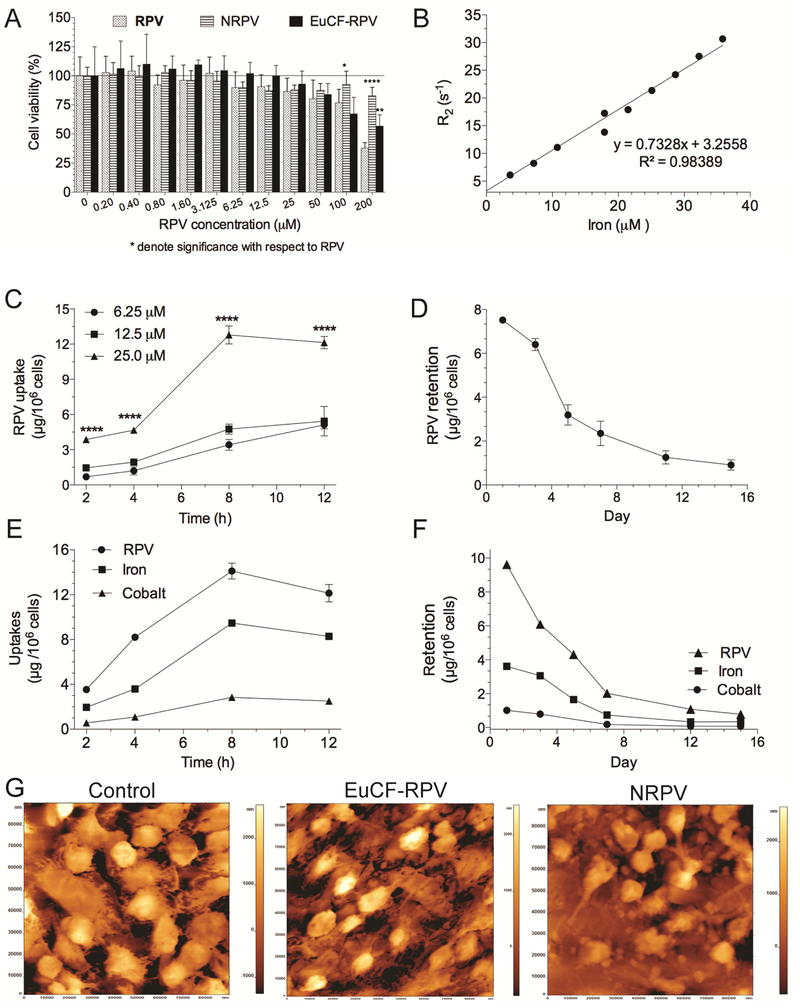
Fig.2. Monocyte-derived macrophage assessments.
(A) MTT cell viability assay. (B) MRI signal enhancement effects of EuCF-RPV particles were determined by calculating particle relaxivity (μM−1s−1) in human monocyte-derived macrophages (MDM) at various concentrations of iron. Particle relaxation rates (r2) in MDM increased linearly with increasing iron concentrations (R2=0.98389). (C) Uptake of NRPV particles was determined in MDM. NRPV and EuCF-RPV particles were added to MDM at 6.25, 12.5 and 25.0 μM RPV. Stars denote significant differences compared to 12.5 μM RPV (****P<0.0001) (D) Retention of NRPV added to MDM at 25 μM RPV for 8 hours. (E) Uptake of EuCF-RPV particles was performed at 25 μM RPV. RPV, Iron, and Cobalt concentration (μg/106 cells) were determined after 2, 4, 8 and 12 hours of treatment. (F) Retention of EuCF-RPV particles over 15 days. MDMs were treated with 25 μM (by RPV) of EuCF-RPV particles for 8 hours before treatment was removed. Cells were analyzed for RPV, iron and cobalt concentration (μg/106 cells) at 1, 3, 5, 7, 12, 15 days post treatment removal. (G) Morphological examination by atomic force microscopy of untreated, treated with EuCF-RPV and NRPV-treated MDM.
The MRI r2 relaxivity of EuCF-RPV particles was determined for particles suspended in PBS (Supplementary Fig.S4) as well as for particles loaded within macrophages (Fig.2B) by using a 7T MRI. The r2 relaxivity showed a linear correlation (R2= 0.98) with increasing iron concentration in MDM. Using the slope of this correlation we determined that EuCF-RPV have an intracellular relaxivity of r2 = 732.8 μM−1s−1. Macrophage uptake of particles is a well-characterized phenomenon [29]. We first examined the uptake kinetics of NRPV and EuCF-RPV in MDM. Fig.2C shows the RPV concentrations in MDMs treated with various concentrations of NRPV (6.25 μM, 12.5 μM, and 25 μM). At 2, 4, 8, and 12 hours post treatment the media containing RPV was removed, cells were washed with PBS, centrifuged, collected into 1mL PBS and counted. RPV concentration per 106 cells was determined by UPLC-with UV/Vis detection at 285 nm [30]. Maximal uptake at all concentrations occurred at 8 hours post treatment (12.1 μg/106 cells at 25 μM) and this value remained stable until 12 hours. We then investigated retention time of NRPV by MDM. After treating with 25 μM NRPV for 8 hours, excess NRPV was removed and replaced with normal cell media. Initially, MDM contained approximately 7.5 μg RPV/106 cells 24 hour post-treatment (Fig.2D). By day 5, the amount of NRPV in cells decreased to 3.2 mg RPV/106 cells. NRPV was slowly released between day 5 and day 15 with approximately 0.9 μg RPV/106 cells remaining at 15 days post treatment. Next, we evaluated the uptake and retention of EuCF-RPV particles in MDM. For uptake experiments; cells were treated at 25 mM RPV equivalents for up to 12 hours. At various times treatment was removed and cells were washed and collected for drug and metals analyses by UPLC-UV/Vis and ICP-MS. As shown in Fig.2E, the amount of RPV taken up at 8 hours was similar to that of NRPV (Fig.2C) (14 μg/106 cells and 12.1 μg/106 cells, respectively). We also measured iron and cobalt levels in these cells and found that at 8 hours cells contained 9.5 μg iron/106 cells and 2.9 μg cobalt/106 cells. Retention of EuCF-RPV particles in MDM was examined for 15 days. After treating MDM with EuCF-RPV particles for 8 hours at a concentration of 25 μM RPV, treatment media was removed and replaced with fresh media. After 24 hours post treatment, cells retained 9.6 μg/106 cells of RPV, 3.6 μg of iron/106 cells and 1.0 μg cobalt /106 cells. After 7 days the cells had released most particles, retaining 2.0 μg/106 cells RPV, 0.7 μg iron /106 cells and 0.2 μg cobalt /106 cells. Release of particles slowed by day 15 when RPV, iron and cobalt was retained in cells and measured at 0.8 μg, 0.4 μg and 0.1 μg/106 cells, respectively (Fig.2F). Fig.2G shows atomic force microscopy topological images of cells treated with vehicle control, EuCF-RPV, or NRPV. Interestingly, even though these cells uptake a large number of particles the surface topography of the cells remained unchanged.
3.3. Subcellular particle localization studies
We next determined the intracellular fate of NRPV or EuCF-RPV particles in MDM. This was accomplished through qualitative assessments of particle subcellular localization performed by confocal microscopy and TEM. Flow cytometric (FACS) analysis was performed for quantitative measurements. In order to visualize NRPV by confocal microcopy and for flow cytometry, we synthesized CF®633 labeld poloxamer 407 (P407) as per our previous report [42] and used this CF®633-P407 coating during NRPV formulation to create NRPV-CF®633. Cells were treated with 25 μM NRPV-CF®633 or EuCF-RPV particles at a concentration equivalent to 25 μM RPV for 8 hours. At this time the treatment was removed, the cells were washed with PBS, and then fixed in 4% PFA before immunostaining with markers for early endosomes (Rab5), late endosomes (Rab7), recycling endosomal compartments (Rab11 and 14), and lysosomes (LAMP-1). Fig.3A shows that the majority of NRPV localizes in the late and recycling endosomal compartments enriched in Rab7, 11, and 14. To a lesser degree NRPV can be found in early endosomal compartments enriched in Rab 5, and little co-localization with LAMP-1 was detected. Together, these data suggest the retention of NRPV in macrophage endosomal compartments with minimal lysosomal degradation. Similar results were found in cells treated with EuCF-RPV (Fig.3B) with the exception that there was greater co-localization with Rab5 and 7. Interestingly, EuCF-RPV particles also showed no co-localization with LAMP-1, suggesting that these particles are also retained within macrophage endosomal compartments without significant lysosomal degradation. We used TEM to further confirm the intracellular fate of NRPV and EuCF-RPV particles in greater detail. MDM were treated at 25 μM RPV equivalents of EuCF-RPV or NRPV crystals for 8 hours before removal and cells were fixed for TEM analysis. Large accumulations of EuCF-RPV or NRPV are observed in intracellular subcompartments within the cytoplasm of MDM (Fig.3C). Selected intracellular depots of EuCF-RPV and NRPV are outlined in dotted lines in column (i) and then shown at a higher resolution in columns (ii) and (iii). The EuCF nanocrystals are clearly visible as dark spots near the edge of the crystals. Further, the uptake potential of MDM was confirmed by quantitative measurements of NRPV by FCM analysis. The viability dye, propidium iodide was mixed with treated cells prior to analysis so that only viable cells were analyzed for NRPV content. As shown in Fig.3D, we observed that treatment with 25 μM RPV approximately 68.7% of MDM take up NRPV-CF®633, while at 50 μM approximately 80.7% of MDM take up NRPV-CF®633. Overall these results demonstrate that MDM can uptake large amounts of NRPV.
Fig.3. Immunocytochemistry, transmission electron microscopy and flow cytometry.
Subcellular localization of NRPV and EuCF-RPV particles are shown. (A) Representative images of MDM treated with NRPV-CF®633 (red) and Alexa-Fluor 488 secondary antibody against Rab5, Rab7, Rab11, Rab14, or LAMP-1 primaries (green). (B) Representative images of MDM treated with EuCF-RPV (green) and Alexa-Fluor 594 antibodies against Rab5, Rab7, Rab11, Rab14, and LAMP-1 (red). The yellow color (merged images) shows colocalization of particles and Rab proteins. Nuclei are stained blue with DAPI. Images were captured with 63X objective on a Zeiss LSM 710 confocal microscope. (C) TEM ultrastructural evaluation of macrophage particle uptake and subcellular distribution. Intracellular uptake of NRPV and EuCF-RPV particles are shown. EuCF nanocrystals are seen within the EuCF-RPV-treated cells as black punctate structures. NRPV accumulations appear as white crystals within membrane-bound endosomal compartments. Areas of interest are bordered with black dotted lines and are presented in corresponding high-resolution images (ii–iii) and illustrate particles within membrane-bound intracellular structures. (D) Flow cytometry results for control MDM (left panel) and MDM exposed to NRPV particles (right panels) are shown. MDM were cultured in 50 mL teflon flasks (5 million/flask) and treated with CF®633 labeled NRPV particles (right panels) at 25 μM and 50 μM concentrations for 8 hours. (i) Population of cells analyzed by scatter analysis plot of control MDM and NRPV treated MDM. Healthy cells (within the rectangle) can be easily distinguished from dead cells (outside the rectangle). This shows MDM remain viable even after particle treatment. (ii) Single cell gate, (iii) Live/dead MDM gated using propidium iodide at excitation/emission: UV Blue; 350/450 nm and (iv) CF®633-NRPV uptake in MDM gated by using CF®633 excitation/emission: 630/650 nm. Control MDM (left panel) showed no uptake, while MDM exposed to CF®633-NPRV particles exhibit 68.8% and 80.7% particle uptake at 25 μM and 50 μM concentrations, respectively.
3.4. Antiretroviral activity measurements
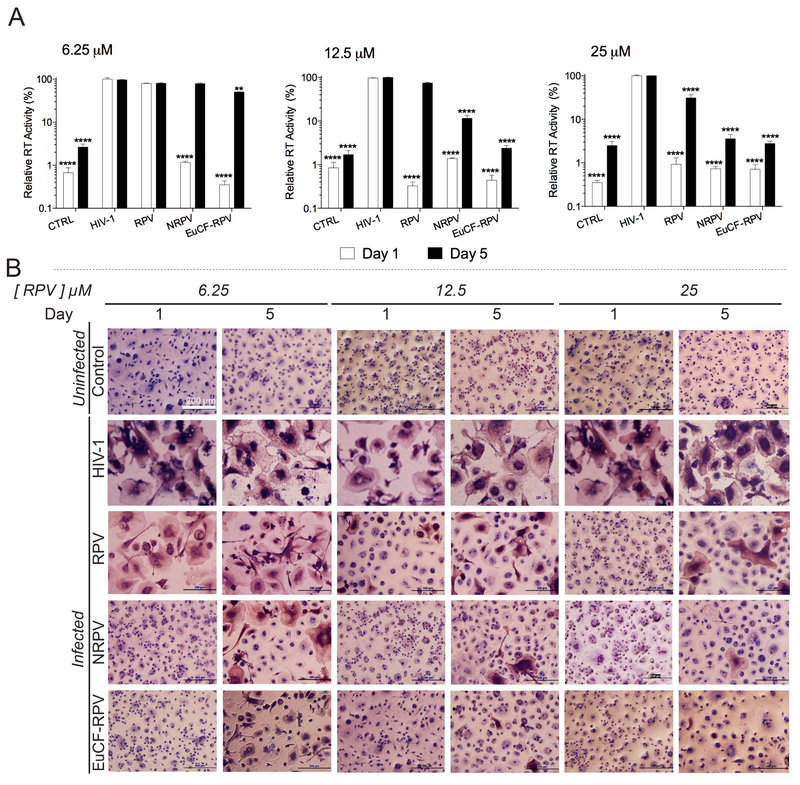
The antiretroviral properties of EuCF-RPV were assessed and compared to native RPV and NRPV in MDM challenged with HIV-1ADA, a prototypical macrophage-tropic strain of HIV [23]. Cells were treated with 6.25 μM, 12.5 μM or 25 μM equivalents of native RPV, NRPV, or EuCF-RPV for 8 hours and removed from cultures. At 1 and 5 days post treatment, cells were challenged with HIV-1ADA for 16 hours at a MOI of 0.1. Cells were maintained for 10 days post infection, at which time progeny HIV virion production was determined by assaying RT activity in the cell culture media. Remaining cells were fixed in 4% PFA and immunestained for HIV-1 p24 antigen expression. At the lowest concentration (6.25 μM) NRPV and EuCF-RPV showed a significant reduction in RT activity at day 1 compared to native RPV, which provided only a small degree of protection (Fig.4A). At 5 days post-infection, native RPV and NRPV provided virtually no protection, but EuCF-RPV provided a slight but significant reduction in RT activity compared to positive control. For cells treated with 12.5 mM RPV equivalents, native RPV, NRPV and EuCF-RPV provided significant reduction in RT activity compared to positive control at day 1. By day 5, native RPV provided no protection, yet RT was significantly decreased for NRPV and EuCF-RPV with EuCF-RPV showing the least RT activity. At the highest concentration tested, 25 μM, treatments with all 3 modalities provided significant protection at days 1 and 5. However, by day 5 protections with NRPV and EuCF-RPV was greater than native RPV alone. These results were confirmed by the p24 immunostaining, which shows less p24 staining in cells treated with NRPV or EuCF-RPV particles compared to native RPV (Fig.4B).
Fig.4. Antiretroviral assessments.
Antiretroviral activity was determined in MDM treated for 8 hours with free RPV, NRPV or EuCF-RPV particles (6.25, 12.5 and 25 μM RPV) and then infected with HIV-1ADA at a multiplicity of infection (MOI) of 0.1, at 1 and 5 days after drug loading. At 10 days after infection, the media was collected from wells and cells were washed in PBS before fixing with 4% PFA at room temperature. (A) Progeny HIV virion production was determined by HIV reverse transcriptase (RT) activity using beta scintillation spectroscopy. Stars represent statistical difference from positive controls. Statistical differences were determined using two-way ANOVA among groups; we used Bonferroni’s post hoc test to correct for multiple comparisons. **p< 0.01; ***p < 0.001; ****p < 0.0001. (B) HIV p24 staining (brown) counterstained with hematoxylin (blue) (scale bar = 200 μm).
3.5. Real time biodistribution tests
3.5.1. MRI modality
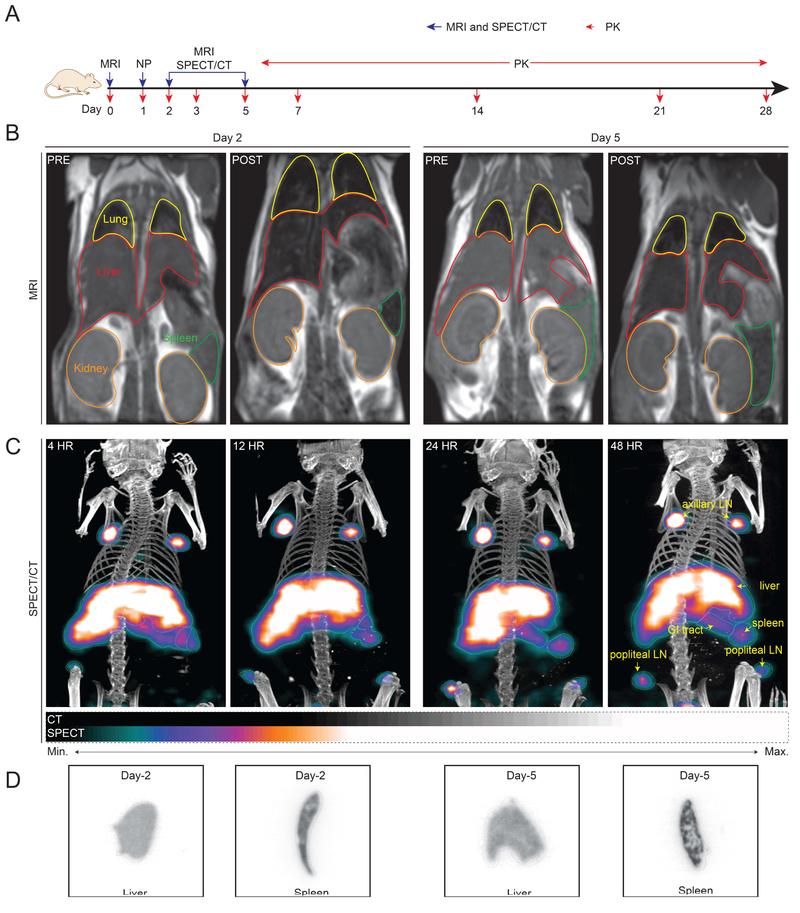
The goal of these studies is to use multimodal theranostics for non-invasive real time tracking of drug biodistribution in animals by using MRI and SPECT/CT imaging. Fig.5A depicts the experimental timeline for the MRI and SPECT/CT studies. MRI pre-scans were taken before injection of EuCF-RPV (G4) or EuCF-PCL plus NRPV (G5) to establish a baseline signal and representative pre-scans are shown in Fig.5B. MRI was chosen as the first modality because of its superior spatial resolution, which provides the most accurate information about the localization of these particles in soft tissues[43]. Post-injection MR images were acquired 2 and 5 days after particle intravenous administration (Fig.5B). After 2 days post-administration mice were scanned again and the resulting images were analyzed for the presence of particles. T2 signals were decreased in both liver (outlined in red) and spleen (outlined in green), which visually confirms the presence of supraparamagnetic particles. A similar decrease in T2 was observed 5 days post-injection signifying the retention of particles in those organs over time.
Fig.5. Multimodal theranostics tests.
Non-invasive imaging of real-time theranostic particle biodistribution into viral reservoir sites in live animals by magnetic resonance imaging (MRI) and single photon emission computed tomography computerized tomography (SPECT/CT). (A) Time line of the experimental procedure in mice is shown (NP: nanoparticles). (B) Representative pre-scan and post-scan T2 maps of mice 2 and 5 days after intravenous injection administration of EuCF-RPV (2 mg iron/kg) particles (G5) (yellow: lung; red: liver; green: spleen; orange: kidney). (C) Two-dimensional image representations of three-dimensional SPECT/CT scan of mice treated intravenously with ~22.2 MBq (600 μCi) of 111InEuCF-PCL particles. Whole body SPECT/CT images were collected at 4, 12, 24 and 48 hours after injection. Relative signal intensity is shown as a color gradient according to the legend below. Anatomically (by CT scan), high signal intensity was detected in the lymph nodes (LN), liver, spleen and gastrointestinal (GI) tract tissues. The images were acquired over 64 projections at 10 seconds/projection. The detector radius of rotation was set at 48 mm to provide a pixel size of ~60 mm. A multipinhole N5F75A10 collimator, mouse style, 1 mm aperture was used to acquire the CT images (Flex Triumph platform, TriFoil Imaging, Chatsworth, CA, USA). SPECT/CT imaging of particles taken up by lymph nodes, liver, and spleen can be clearly visualized. Here, we can exceptionally distinguish the axillary and popliteal lymph nodes along with other reticuloendothelial tissues. Such multi-modal nanoprobes will facilitate rapid non-invasive screening of antiviral agent biodistribution even at deep-tissue virus reservoir sites and accelerate development of effective treatments. Such technology also helps to develop a platform for designing precision nanomedicine. (D) Autoradiographic images of liver and spleen sections from mice sacrificed 2 and 5 days post injection with 111InEuCF-PCL particles. The autoradiographic signal corresponds to SEPCT/CT and MRI images. There was a diffuse distribution of particles in liver tissue, while in the spleen the particles could be found in discrete regions related to macrophage rich red pulp areas and the marginal zone where latent HIV virus persists.
3.5.2. Nuclear imaging modality
Taking advantage of increased sensitivity over MRI, SPECT/CT imaging was also performed. By combining MRI and SPECT/CT modalities the distribution of particles can be accurately determined with a high degree of spatial resolution and sensitivity. To make the EuCF-PCL particles suitable for SPECT/CT imaging, Indium-111 (111In) was intrinsically labeled directly into the EuCF nanocrystal lattice (111InEuCF-PCL). For the SPECT/CT experiment, 10 mice were injected at a dose corresponding to ~ 22.2 MBq (600 μCi) and subsequently scanned at 4, 12, 24, 48, and 120 hours (5 days) post injection. In mice scanned 4 hours post-injection, the majority of particles localized to the liver, spleen, and axillary lymph nodes, with modest accumulation in the popliteal lymph nodes (Fig.5C). By 12 hours post-administration, the popliteal lymph nodes were clearly visible and persisted until day 5. Particles continued to accumulate in the spleen, liver, and lymph nodes until 48 hours post-injection. By 5 days post-injection, particle concentrations had decreased in the liver and lymph nodes. However, particle accumulation in the spleen slightly increased by day 5 compared to day 2, but did not rise to significance. After the 2 day and 5 day SPECT/CT scans, mice were sacrificed so that the livers and spleens could be excised, flash frozen and fixed for autoradiographic imaging. Autoradiography analysis of liver and spleen tissues visually confirmed the slight decrease in liver signal between 2 and 5 days as well as the increase in spleen signal over the same time period (Fig.5D). Location of particles in the spleen appears diffuse on day 2 but the signal coalesces into discrete areas by day 5. This most likely represents a transfer of particle from the red pulp of the spleen into the marginal zones and white pulp, which are the primary locations of splenic macrophages [44].
The biodistribution of particles was dependent on its surface chemistry, size, and shape [45]. To this end we prepared, ultra-small, lipid-coated lutetium (177Lu) intrinsically radiolabeled EuCF nanocrystals (177LuEuCF) with an average size of ~ 5 nm for SPECT/CT to investigate differential biodistribution by modulating the size of the particles. Mice underwent SPECT/CT scanning at 6, 24, 48 and 120 hours (5 days) post-intravenous administration of ~ 74 MBq (~2000 μCi) of lipid-coated 177LuEuCF particles (Fig.6A). At each time point, representative images of SPECT/CT scans were acquired of the torso and of the thoracic cavity at lower radius of rotation (higher magnification). The images show preferential early distribution of these particles into the lungs at 6 hours post-administration with a small amount in the liver and spleen. At 24 hours accumulation in the lungs peaked and was beginning in the spleen and liver. By 48 hours, the signal in the lung began to decrease, indicating a clearance of particle from lung tissue. At the same time, accumulation in the liver was clearly visible as well as in the spleen. Interestingly this trend continued to day 5 with more accumulation in the liver and a sharp decrease in lung signal (Fig.6B). Signal in the spleen remained relatively constant between days 2 and 5. These observations were supported by 3D quantitation of the SPECT/CT signal (counts per mm3) (Fig.6C). For additional verification of these results, mice were sacrificed after 24, 48, and 120 hour scans and various organs were collected which included, but not limited to, lung, liver, and spleen. All organs were weighed and the radioactivity measured to calculate the percent-injected dose per gram (%ID/g) for each tissue. As shown in Fig.6C. 176 %ID/g of the particle accumulated in the lungs, 38 %ID/g had accumulated in the liver and 34.5 %ID/g was found in the spleen by 24 hours. At 48 hours the amount of particles in the lung had dropped to 153 %ID/g while particle accumulation in the liver and spleen had increased to 40 and 55 %ID/g, respectively. By day 5 more than half of particles had cleared from the lung leaving 73% ID/g. However, signal continued to increase in the liver (63% ID/g) and held steady in the spleen (56% ID/g). This ex vivo radioactivity was verified by autoradiographic evaluation of the liver, spleens and lungs from these mice sacrificed 1 and 2 days post injection as shown in Supplemental Fig.S5. The most striking feature of these images is the increase in signal seen in the spleen from 24 hours to 48 hours. These results confirm the biodistribution pattern we observed by SPECT/CT
Fig.6. Effect of ultra-small particle size on theranostic particle biodistribution determined by SPECT/CT.
In vivo biodistribution of ultra-small lipid coated 177LuEuCF particles (~74 MBq) was assessed in mice by SPECT/CT. (A) Experimental timeline for 177LuEuCF particles biodistribution study. (B) Representative images of 3D SPECT/CT acquisitions of whole body at 6, 24, 48, and 120 hours post-injection at a CT magnification of 2.0 and a SPECT/CT gamma camera radius of rotation (ROR) of 48 mm. Representative images of SPECT/CT acquisitions of only the chest at a higher CT magnification of 3.5 and a gamma camera ROR of 30 mm are shown. Notable is the movement of particles from the initial lung reservoir into the spleen and then liver over time. (C) Left: 3D quantification of SPECT signal using VivoQuant software displayed in counts/mm3. Right: Ex vivo radioactivity biodistribution by gamma counting analyzed by a gamma scintillation spectrometry, NaI (TI) well detector.
3.6. Predictive values of the theranostic nanoparticles
Long-term prediction of disease progression, drug biodistribution and dose optimizations for long-acting HIV medications is difficult, laborious and time consuming using conventional pharmacokinetics and biodistribution assessments [25, 46]. Therefore, a theranostic imaging platform would be advantageous to build a data library that can be used to predict drug biodistribution in patients with more precision than traditional pharmacokinetics analysis without invasive isolation of tissues samples [47]. We hypothesized that our multimodal theranostic imaging platform could be implemented as a quick reliable way to estimate long-term biodistribution of long-acting antiretroviral medications. In order to test this hypothesis, we examined the pharmacokinetic and biodistribution profiles of eight different treatment groups, each group containing 10 to 15 Balb/cJ mice over the course of one month. All treatments were administered via intravenous injection to healthy Balb/cJ mice. As shown in supplementary Fig.S3 and table 1 two groups received only NRPV at a high (45 mg/kg) (G1) or low (5 mg/kg) dose (G2). The third group was administered EuCF-PCL particles (2 mg/kg iron) (G3) and the fourth was injected with EuCF-PRV particles (2 mg/kg iron) (G4). Groups 5 and 6 received a dual injection of EuCF-PCL particles (2 mg/kg iron) and NRPV at 45 mg/kg (G5) or 5 mg/kg (G6). In each group five mice were sacrificed at days 2, 5 and 28. At the time of sacrifice, organs were excised and analyzed by UPLC-MS/MS and ICP-MS for drug and metal content, respectively. Plasma was also collected from mice at days 1, 2, 3, 5, 7, 14, 21, and 28 and analyzed for drug and/or metal content to build a complete biodistribution profile for each treatment group. We then compared this month of pharmacokinetic and biodistribution results to the bioimaging data obtained within the first five days post injection from MRI scans of G4 and G5. Lastly, 111InEuCF-PCL or lipid coated 177LuEuCF particles were given intravenously to groups 7 (G7) and group 8 (G8), respectively, for SPECT/CT analysis. We then used these data sets for correlation analyses comparing drug versus metals content or signal quantified through imaging to estimate the ability of our multimodal particles to rapidly assess and predict long term biodistribution of long-acting antiretroviral medications.
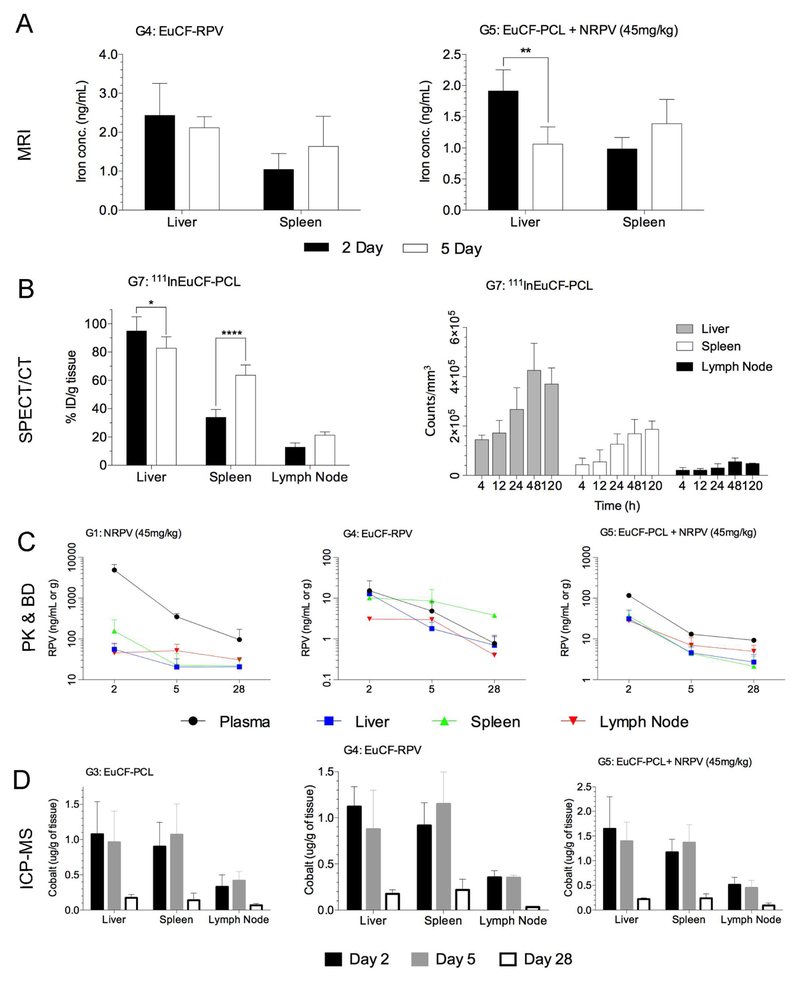
By MRI analysis, EuCF-RPV particles (G4) showed similar biodistribution as EuCF-PCL particles injected alongside NRPV (G5) on day 2 and day 5 post injections (Fig.7A). The amount of iron present in the liver and spleen at 2 and 5 days was quantified using phantom relaxivity standard curves generated from EuCF-RPV particles in MDM at varying concentrations of particles (by iron) as shown in Fig.2B. In both groups iron concentrations decreased between days 2 and 5 in the liver. This decrease was not significant for EuCF-RPV (2.4 to 2.1 ng/mL) but was significant for EuCF-PCL plus NRPV (1.9 ng/mL to 1.1 ng/mL). In contrast, there was a slight increase in iron concentration in the spleen over the same time in both groups. Iron levels in the spleen increased from 1.0 to 1.6 ng/mL for EuCF-RPV and from 1.0 to 1.4 ng/mL for EuCF-PCL plus NRPV. G7 mice were administered 111InEuCF-PCL particles and assessed by SPECT/CT at 4, 12, 24, 48, and 120 hours post-injection. Acquired images were reconstructed to 3-D renditions, regions of interest (ROI) were electronically drawn to encompass organs, and relative activity counts per cubic millimeter for each tissue were determined. Activity in liver, spleen, and lymph nodes (axillary and popliteal) increased until 48 hours post injection with the majority of particles localizing in the liver (Fig.7B). By five days post injection, activity in liver and lymph nodes decreased slightly while splenic activity increased. The SPECT/CT results were confirmed by determining tissue drug levels in mice after scans on days 2 and 5. All organs were removed and particle biodistribution measured by gamma scintillation spectrometry. The results are presented in Fig.7B as % ID/g. There was a significant accumulation of particles in the Liver (73.5% ID/g), Spleen (41.1% ID/g), and axillary or popliteal lymph nodes (13.5% ID/g). Interestingly, at 5 days post injection there was a large increase in distribution of these particles to the spleen (63.3% ID/g) with modest clearance from the liver and small increases in the lymph nodes by day 5 (62.6% ID/g and 18.0% ID/g, respectively). In all animal groups that received NRPV or EuCF-RPV particles, drug levels were measured in plasma collected on days 1, 2, 3, 5, 7, 14, 21, and 28 after treatment. Tissues were collected from mice on days 2, 5, and 28 for UPLC-MS/MS determination of drug levels. Drug levels in plasma and tissues for the EuCF-RPV treatment group were lower than those found in groups treated with NRPV at 45 mg/kg or EuCF-PCL particles with a co-injection of NRPV at 45 mg/kg (Fig.7C). The values observed were similar to values obtained from groups treated with the lower concentration of NRPV (5 mg/kg) (Supplemental Fig.S6).
Fig.7. Biodistribution and pharmacokinetic analyses.
Data from 2 and 5 days post intravenous injection of EuCF-PCL plus NRPV (45 mg/kg RPV) (G5), EuCF-RPV (2 mg/kg based on iron) (G4) or 111InEuCF-PCL particles (G7) in male Balb/cJ mice. (A) Quantification of iron content in liver and spleen by MRI. (B) Left: %ID/g radioactivity in liver, spleen, and lymph nodes from mice treated with 111InEuCF-PCL. Right: Quantification of SPECT/CT signal by 3D ROI using VivoQuant software. (C) RPV drug levels in plasma, liver, spleen, and lymph nodes from days 2, 5, and 28 as determined by UPLC-MS/MS. (D) Cobalt content from groups (G3, G4 and G5) treated with EuCFPCL/RPV and NRPV particles in liver, spleen, and lymph node from days 2, 5, and 28 as determined by ICP-MS. * p< 0.05, ** p<.01, **** P<0.0001
In groups that received theranostic particles, (G3, G4, G5, and G6) mice were sacrificed on days 2, 5, and 28, and their organs were removed and analyzed by ICPMS for cobalt concentration. Cobalt content was fairly consistent across groups in the various tissues at each time point in groups G3, G4, and G5 (Fig.7D). Liver cobalt content on day 2 was similar for groups treated with EuCF-PCL particles (G3), EuCFRPV particles (G4), or dual injection of EuCF-PCL and NRPV (G5) (1.08, 1.12, and 1.64 μg/g, respectively). The same was observed for spleen (0.90, 0.92, 1.17 μg/g) and lymph nodes (0.33, 0.35, and 0.51 μg/g). By day 5, cobalt content in the liver had dropped similarly in each group (10.3%, 21.8%, and 16.0%, respectively), but cobalt content in the spleen had increased (18.8%, 25.5% and 16.8%). Cobalt concentration in the lymph nodes remained consistent or increased slightly from day 2 to day 5 in all groups. After 28 days post injection of particles, the liver, spleen, and lymph nodes still retained a small amount of particles, but the majority had been cleared from tissues as evidenced by the much lower cobalt concentrations. However, the percentage of particles cleared was similar between groups for each tissue. For liver, cobalt concentrations decreased by 81.9%, 84.3%, and 86.5% from day 5 to day 28 for groups 3, 4, and 5, respectively. In the spleen, cobalt concentration dropped by 87.0%, 81.0%, and 82.6%. Lymph nodes also showed a significant decrease from day 5 to day 28 of 83.8%, 90.5%, and 79.6%.
3.6.1. Biodistribution confirmations
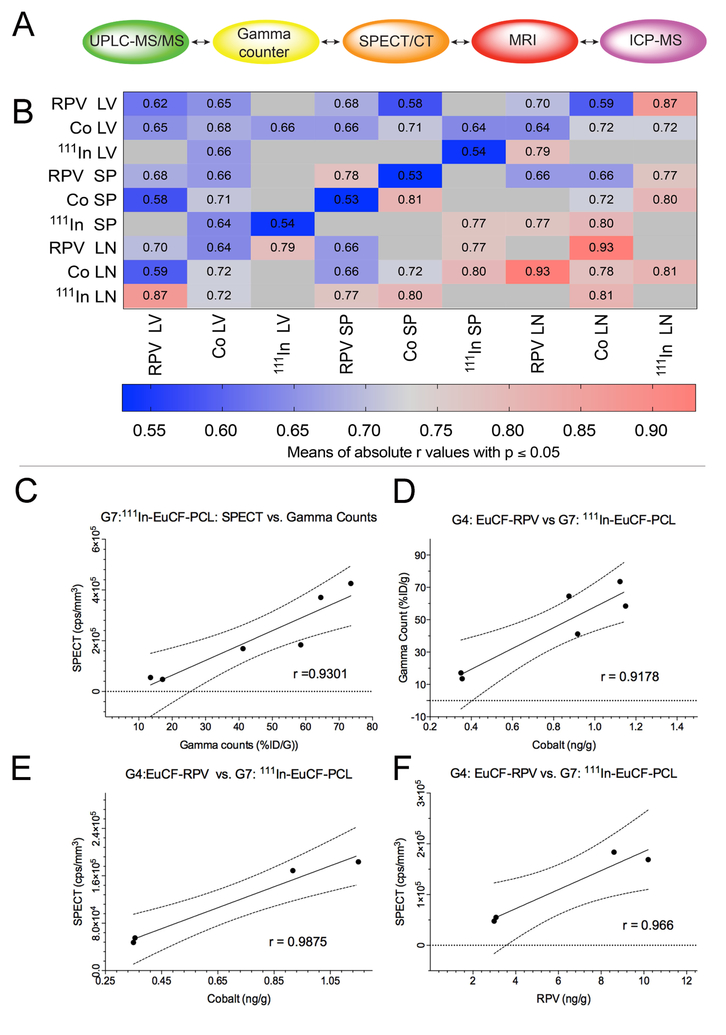
We assessed the various result parameters (Fig.8A) by correlation analysis across groups to test the estimated predictive value for long-term biodistribution. Pearson’s correlation coefficients for two parameter comparisons were averaged for RPV concentrations, cobalt concentrations, and SPECT/CT radioactivity measures in liver, spleen, and lymph nodes. Means of absolute “r” values with p ≤ 0.05 were displayed in each cell and used to generate a heat map of relative strength of correlation (Fig.8B). Across all groups the strongest correlation (highest r value) was between cobalt concentration and RPV concentration in lymph node (r=0.93). Similarly, an absolute “r” value of 0.81 was obtained for between SPECT/CT signal in the lymph node and cobalt concentration in the lymph nodes. In the spleen, RPV concentration correlated with cobalt concentration with r = 0.54. For the liver, RPV concentration correlated with cobalt concentration and gave an “r” value of 0.65. Also in the liver, SPECT/CT radioactivity and cobalt concentration correlated with an “r” value of 0.66. Lower Pearson correlation coefficients were recorded in liver than spleen and lymph nodes. However, such differences likely reflect tissue processing as whole lymph nodes and spleens were used for analysis while subregions of liver were analyzed. We also determined correlations between groups, tissues and test measures. Mean %ID/g from ex vivo gamma counts of 111InEuCF-PCL (G7) in liver, spleen, and lymph node on days 2 and 5 exhibited a strong correlation with SPECT activity (r = 0.93) (Fig.8C), which validated the accuracy of our SPECT/CT quantification. Also the mean cobalt levels in liver, spleen, and lymph nodes from EuCF-RPV (G4) treated mice were strongly correlated to mean gamma scintillation counts from 111InEuCF-PCL (G7) treated mice (r = 0.92) (Fig.8D), indicating that the amount of radioactivity in these samples positively correlates with cobalt concentration. We found that in spleen and lymph nodes on days 2 and 5 post-administration, mean cobalt concentrations in EuCF-RPV (G4) were strongly correlated with SPECT/CT radioactivity (r = 0.99) (Fig.8E). Finally, for days 2 and 5 post-administration = in spleen and lymph nodes from mice treated with EuCFRPV (G4), mean RPV concentrations were strongly with radioactivity measured by SPECT/CT signal (r = 0.96) (Fig.8F). These data, taken together, show that the amount of antiretroviral accumulation into reticuloendothelial organs can be estimated accurately using imaging data obtained from a multimodal theranostic platform.
Fig.8. Coordination and correlation of metadata analysis.
(A) Schematic showing parameters used for correlation analysis. (B) Correlation matrix. For each tissue (liver, spleen, lymph nodes) RPV, cobalt, or radioactivity (by SPECT/CT) content were summed across groups and then compared to each other. Shown are average correlation coefficients across treatment groups for correlations with a p value ≤ 0.05. (C) Pearson’s correlation between ex vivo radioactivity (%ID/g) and in vivo SPECT/CT signal from liver, spleen, and lymph nodes from day 2 and day 5; r = 0.9301. (D) Pearson’s correlation between cobalt content and ex vivo radioactivity (%ID/g) from liver, spleen and lymph nodes from days 2 and 5; r = 0.09178. (E) Pearson’s correlation cobalt content and between in vivo SPECT/CT signal quantitation in spleen and lymph nodes from days 2 and 5; r = 0.9875. (F) Pearson’s correlation between in vivo drug content and SPECT/CT signal in spleen and lymph nodes from days 2 and 5; r = 0.966.
3.7. Particle trafficking in mouse macrophages
To determine cellular distribution of particles in tissues, following the MRI and SEPCT/CT scan animas was euthanized for collection of tissues. Tissues were fixed in TEM buffer, embedded in resin, and TEM analysis [25]. Representative TEM images of liver and spleen show macrophages in liver and spleen tissues from day 2 and day 5 and are denoted by red roman numerals i, ii, and iii. (Supplementary Fig.S7, column 0) and are magnified (columns I, ii, and iii, respectively). Moreover, large accumulations of light-colored NRPV crystals can be found alongside dark clusters of EuCF nanocrystals. Histological analyses showed normal tissue structure in particle-treated mice, thus ruling out the possibility of particle-associated pathological events at the microscopic level (Supplementary Fig.S8). Together these data confirm that particles administered intravenously to mice were indeed sequestered by macrophages in target organs without evidence of readily discernible pathology.
4. Discussion
The potential of LASER ART in the prevention, treatment and eradication of HIV/AIDS is of the highest significance [18]. This is linked, in part, to improvements of drug penetration into target organs, reduced systemic toxicities, simplified regimens, reduced pill burdens, and enabling the availability of drug to larger numbers of patients [48]. However, to realize the full potential of LASER ART, formulations in development must be appropriately screened based upon drug biodistribution and ease of drug penetration into tissue viral reservoirs. While computerized simulations were developed due to the inherent complexity of particle drug parameters this has not proven beneficial [49, 50]. This is based on broad differences in the drug properties, the size, shape and charge of the composed particle, the prodrug lipophilicity, composition and hydrophobicity, the drug loading, encasement and decorations and the inherent tissue and cell physicochemical properties [19, 51]. While others and we have developed such schemes the needs to track particle distribution at the cellular and tissue level to predict drug particle efficacy knows no substitutions from direct measurements [25, 52]. Therefore, there is a clear need to develop such direct screening platforms. To overcome this obstacle, we now demonstrate that ARV loaded theranostic particles can effectively screen LASER ART cell and tissue biodistribution by SPECT/CT and MRI imaging modalities. Our newly crafted, intrinsically radiolabeled multimodal theranostic particles provide a platform to expedite the development of efficient and effective long-acting ART. However, we acknowledge the inherent limitations of the system. Indeed, studies are now being implemented that will address the remaining hurdle including matching the inherent properties (size, shape and surface charge) of the theranostic particles with those particles that would be scaled up for human use [53, 54]. Second, as of yet the success of screening theranostic particles decorated with ligands that serve to facilitate optimal drug entry into the most relevant tissue viral reservoirs such as lymphoid tissues and the brain remains unproven [55, 56]. The third rests in the use of subcellular drug localizations to maximally restrict targeted components of the viral replication cycle [32]. Fourth is the predictive value of not simply measuring drug depots in macrophages but also assessing ARV entry and distribution into the more pathobiologically relevant viral target, the CD4+ effector memory T cell [57]. All must be taken into account and careful consideration of particle design is required with concomitant evaluations of its predictive value.
In efforts to meet such needs, we have taken a “next step” in our research activities to build upon an already successful theranostics platform that is based upon a “core-shell” system composed of europium-doped, cobalt-ferrite nanocrystals (EuCF), encased in a PCL core, and encapsulated within a lipid shell [25]. The EuCF nanocrystals have a number of properties that can meet the requirements of assessing of LASER ART activities. Notably, the particles sizes are similar to LASER ART and possess strong superparamagnetic properties. This allows their efficient use as a T2-weighted MRI contrast agents [58]. As MRI tests are broadly available in medical practice and commonly used in medical imaging it certainly offers the needed spatial and temporal resolution and tissue contrast to permit adequate determination of EuCF-drug particle distribution in tissues [59].
However, MRI lacks sensitivity for determining precise drug particle concentrations in tissue [43]. Indeed, imaging alternatives to MRI could improve visualization of particles at low concentrations [60]. Moreover, no single imaging modality is currently available that offers both highly sensitive detection and excellent spatial resolution [61]. This inspired our use of nuclear medicine imaging modalities (SPECT/CT), which provide the highest degree of sensitivity [61, 62]. Additionally, radioactive imaging provides the ability to accurately quantify the concentration of particles in tissue noninvasively [62]. Therefore, combining MRI and SPECT/CT takes advantage of the strengths of both, enabling a clearer evaluation of any of our developed long-acting ARVs.
The stability of the radiolabel is critical for accurate determinations of particle distribution by SPECT/CT as the particle locale is assessed indirectly via a radionucleotide signal [63]. If the radiolabeling strategy can be carefully designed to create a highly stable radiolabel, both its sensitivity and specificity can be optimized. To meet such a challenge, we intrinsically doped the radioisotope directly into the EuCF nanocrystal lattice. This is different from more common radiolabeling strategies as it does not involve the use of exogenous chelators in forming stable complexes [63, 64]. There are a number of considerations in generating an optimal labeling strategy [65]. First, the coordination chemistry of radioisotopes varies significantly. Choosing the right chelator for any specific isotope is challenging, as there are none that can bind multiple radioisotopes [66]. Second, incorporation of chelators can alter the hydrodynamic size and surface chemistry, which will alter biodistribution and obscure the true pharmacological behavior of the particles [67, 68]. This fact may be enhanced as the radiolabel could become unbound from particle leading to false positive results [67–69]. Chelator free labeling forgoes the need for chelating moieties and therefore the native pharmacological behavior of the particle is maintained. Third, to optimize labeling the number of chelating moieties conjugated to the particle’s surface should not impose any limitations. As intrinsic doping places radioisotopes directly into the lattice sites of the particle’s metallic core, abundant labeling sites are available which results in high yields with enhanced stability [70]. Fourth, with chelator-free labeling, the surface of the particle can be easily modified with targeting ligands, therapeutic agents, or other imaging agents because no surface functional groups are required for the radiolabel [71]. Last, chelator-free labeling is simple, fast, and effective allowing for radiolabeling with a broader spectrum of isotopes, including 72As and 69Ge, both of which have proven extremely challenging to radiolabel via chelator chemistry [72, 73]. Thus, in the current report we intrinsically doped 111In into the core structure of the EuCF nanocrystals. To facilitate the production of the particle, we simply added the radioisotope precursor 111InCl3 during the synthesis of the EuCF nanocrystal core. This simple method embeds the radioisotope into the crystal lattice of the core resulting in an intrinsically radioactive particle whose radiolabel was > 97% stable and able to accurately measure particle accumulation at targeted sites.
To test the ability of 111InEuCF-PCL particles to serve as a screening platform, we loaded RPV, a second generation long acting nonnucleoside reverse transcriptase inhibitor, into the PCL core. RPV has an increased genetic barrier to viral resistance against both wild type and resistant HIV-1 strains [74]. RPV has also demonstrated lasting efficacy and, compared to efavirenz, has improved tolerability, with few neuropsychiatric and metabolic side effects [75]. Therefore, RPV is a strong candidate for long-term treatment and prophylaxis. The successful loading of RPV into the particles allowed for a comparison of the biodistribution of 111InEuCF-RPV particles by SPECT/CT and MRI to the PK and biodistribution of a parallel LASER ART preparation. This facilitated the realization of a new screening platform used to predict the long-term pharmacokinetics of our newly developed long-acting drug formulations. Indeed, any consideration of predictive modeling of the diversity of LASER ART physiochemical properties, excipients, size, shape, charge, encasements and complex pharmacological behaviors (for example, drug subcellular, cellular and tissue distribution, hydrolysis and antiretroviral activities) must include accurate predictive modeling [25, 76–78]. These assessments must include subcellular, cellular and tissue drug distribution studies [25, 77, 79]. Moreover, before any animal injection, the drug particle properties such as uptake, retention, subcellular location, toxicities, and antiretroviral activity were investigated in macrophages and served as confirmation of the current data sets [18, 80]. Dynamic light scattering showed that the hydrodynamic size of the EuCF-RPV particles was similar to that of NRPV. This was confirmed by TEM imaging of both particles. Additionally, encapsulated drug retained potent antiretroviral activities as evidenced by RT and HIV-1p24 activity and staining, respectively. Importantly, the intrinsic fluorescence of Eu allowed for visualization of particle uptake along with subcellular identification of EuCF particles within endosomal compartments identical to those where active viral replication occurs which was consistent with our previous reports [25, 81–84]. In the present study we compared the pharmacokinetics and biodistribution of metal and drug to imaging data acquired from MRI and SPECT/CT modalities from plasma, liver, spleen and lymph nodes of NRPV, 111InEuCF-PCL, and EuCF-RPV treated Balb/cJ mice. The addition of the SPECT/CT modality to our already sensitive MRI particles was a significant improvement as it allowed for the visualization and quantification of particle accumulation in the axillary and popliteal lymph nodes which were not visible via MR imaging. Importantly, the SPECT/CT imaging data acquired within the first week after 111InEuCF-PCL particle administration showed a very high Pearson’s correlation with the coordinate tissue cobalt and RPV levels found in lymph nodes and spleens of mice treated with EuCF-RPV particles (r = 0.9875 and 0.966, respectively). The results demonstrated the potential of this platform to accurately screen the biodistribution of long-acting formulations in the reticuloendothelial system. However, several of the correlations made had moderate Pearson’s correlation coefficients but were not statistically significant. The limitation, in part, reflects the particle biodistribution based on size. Indeed, our own ultra-small 177LuEuCF particles localized primarily to the lung before a liver and spleen signal could be visualized. We believe this system can be accurately developed to closely match and improve upon the performance of our current and future LASER ART formulations. The creation of particles with increased aspect ratios is an area of active research and rod shaped particles may be advantageous for a cell and tissue based drug delivery system [85].
In addition to serving as a screening tool, these particles can facilitate drug penetrance into reservoir sites by a “Trojan horse” mechanism by creating drug depots [86]. The ability to deliver therapeutics to the exact subcellular site of HIV infection provides opportunities for disease prevention and treatment. Further optimization opens the possibility of combining multiple ARVs into a single particle, encapsulating CRISPR Cas9 for excision of integrated HIV DNA from tissue sites of latent infection [87] or delivering latency reversal agents like toll-like receptor (TLR) 7 agonists which were shown to be capable of reducing viral reservoirs in simian immunodeficiency virus-infected rhesus macaques [88], while simultaneously imaging the real time ARV particle biodistribution and therapeutic efficacy.
In conclusion, the multimodal particles provide the basis for a flexible platform to rapidly assess tissue and cell drug biodistribution of long-acting ARVs by SPECT/CT and MRI in real time. Currently, plasma drug levels remain the gold standard for pharmacokinetics analysis, which is cumbersome for long-acting formulations. Importantly, the biocompatibility of these particles was confirmed and when loaded with ARVs they maintain therapeutic efficacy. Thus, these theranostic particles can accelerate the discovery of LASER ART and optimize therapeutic use profiles for an infected human viral host.
Supplementary Material
Highlights.
Creation of multi-modal theranostic antiretroviral drug (ARV) particles
Theranostic particles form intracellular macrophage drug depots
Coordinate single photon emission computed tomography or magnetic resonance imaging signal intensities and ultra-performance liquid chromatography tandem mass spectrometry measured ARV concentrations
Inductively coupled plasma mass spectrometry used to detect metals encased in theranostic particles can validate ARV tissue concentrations
Bioimaging performed on parenterally injected theranostic particles can provide a rational platform to predict ARV nanoparticle tissue biodistribution
Complex physical and particle biodistribution characteristics not predicted by available mathematical modeling can be affirmed through theranostic platforms.
Acknowledgements
The authors would like to thank Tom Bargar and Nicholas Conoan of the Electron Microscopy Core Facility (EMCF) at the University of Nebraska Medical Center (UNMC) for technical assistance. The EMCF is supported by state funds from the Nebraska Research Initiative (NRI) and the University of Nebraska Foundation, and institutionally by the Office of the Vice Chancellor for Research. We thank Janice A. Taylor and James R. Talaska of the Advanced Microscopy Core Facility at the UNMC for providing assistance with (confocal or super resolution) microscopy. Support given to the Advanced Microscopy Core Facility was provided through the NRI, the Fred and Pamela Buffett Cancer Center Support Grant (P30CA036727), and an Institutional Development Award (IDeA) from NIGMS (P30GM106397). The LSM710 Zeiss Confocal Microscope used in this research was supported by the NIH grant S10RR027301. We also would like to thank Dr. Alexander Lushnikov and Dr. Alexey Krasnoslobodtsev for technical assistance with AFM imaging experiments at the Nanoimaging Core Facility of the UNMC. The authors thank the Nebraska Center for Materials and Nanoscience and Redox Biology at the University of Nebraska-Lincoln for ICP-MS, XPS and XRD analyses. The authors are very grateful to the National Isotope Development Center, Oak Ridge National Laboratory, Oak Ridge, TN, USA, for providing radioisotopes. The authors appreciate the excellent technical assistance made by Melissa Mellon, Lirong Xu and Yaman Lu in support of the MRI and SPECT/CT tests. We would like to thank Dr. Jane Meza, UNMC, College of Public Health, Department of Biostatistics for initial statistical analyses of the data. Bhagy Dyavar Shetty assisted in UPLC-MS/MS analysis from tissue and plasma, Dr.Prasanta Dash and Sruthi Sravananm assisted in tissue sectioning and slide preparations for histopathological analyses. Wang Weimin and Dhruvkumar Soni assisted in animal handling, IV Injections and plasma collection. The authors would also like to thank Dr. Aditya Bade for helpful discussions.
Abbreviations:
- 111In
111indium
- 177Lu
177lutetium
- AFM
atomic force microscopy
- ART
antiretroviral therapy
- ARV
antiretroviral
- BD
biodistribution
- Bq
Becquerel
- BSA
bovine serum albumin
- Ci
Curie
- DCM
dicholoromethane
- DMSO
dimethyl sulfoxide
- DSPE-PEG2000
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000]
- EuCF
europium cobalt ferrite
- FACS
fluorescence-activated cell sorting
- FOV
Field of view
- GALT
gut-associated lymphoid tissue
- HIV-1
human immunodeficiency virus type 1
- ICP-MS
inductively coupled plasma mass spectrometry
- IV
intravenous
- kVp
kilovoltage peak
- LASER ART
long acting slow effective release antiretroviral therapy
- MBq
mega Becquerel
- MDM
human monocyte-derived macrophage
- MOI
multiplicity of infection
- MRI
magnetic resonance imaging
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NanoART
nanoformulated antiretroviral therapy
- NRPV
nanoformulated rilpivirine
- PBS
phosphate-buffered saline
- PC
phosphatidylcholine
- PCL
polycaprolactone
- PDI
polydispersity index
- PFA
paraformaldehyde
- PK
pharmacokinetic
- ROI
region of interest
- RPV
rilpivirine
- RT
reverse transcriptase
- SPECT/CT
single photon emission computed tomography and computed tomography
- TEM
transmission electron microscopy
- uCi
Microcurie
- UPLC-MS/MS
ultraperformance liquid chromatography tandem mass spectrometry
- UPLC
ultra-performance liquid chromatography
- XRD
X-ray diffraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mayer KH, Venkatesh KK, Antiretroviral Therapy for HIV Prevention: Present status and future prospects, American journal of public health 100(10) (2010) 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sharma M, Saravolatz LD, Rilpivirine: a new non-nucleoside reverse transcriptase inhibitor, The Journal of antimicrobial chemotherapy 68(2) (2013) 250–6. [DOI] [PubMed] [Google Scholar]
- [3].Hong FF, Mellors JW, Impact of Antiretroviral Therapy on HIV-1 Persistence: The Case for Early Initiation, AIDS Rev 17(2) (2015) 71–82. [PubMed] [Google Scholar]
- [4].Antiretroviral Therapy Cohort C, Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies, Lancet 372(9635) (2008) 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, Brumme CJ, Brumme ZL, Mo T, Alexander CS, Montaner JS, Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy, J Infect Dis 191(3) (2005) 339–47. [DOI] [PubMed] [Google Scholar]
- [6].Edagwa BJ, Zhou T, McMillan JM, Liu X-M, Gendelman HE, Development of HIV Reservoir Targeted Long Acting Nanoformulated Antiretroviral Therapies, Current medicinal chemistry 21(36) (2014) 4186–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC, HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment, Nat Rev Neurol 12(4) (2016) 234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ross EL, Weinstein MC, Schackman BR, Sax PE, Paltiel AD, Walensky RP, Freedberg KA, Losina E, The clinical role and cost-effectiveness of long-acting antiretroviral therapy, Clin Infect Dis 60(7) (2015) 1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wong JK, Yukl SA, Tissue reservoirs of HIV, Curr Opin HIV AIDS 11(4) (2016) 362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Althoff KN, Gange SJ , A critical epidemiological review of cardiovascular disease risk in HIV-infected adults: the importance of the HIV-uninfected comparison group, confounding, and competing risks, HIV Med 14(3) (2013) 191–2. [DOI] [PubMed] [Google Scholar]
- [11].Hadigan C, Kattakuzhy S, Diabetes mellitus type 2 and abnormal glucose metabolism in the setting of human immunodeficiency virus, Endocrinol Metab Clin North Am 43(3) (2014) 685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].El-Sadr WM, Mullin CM, Carr A, Gibert C, Rappoport C, Visnegarwala F, Grunfeld C, Raghavan SS, Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort, HIV Med 6(2) (2005) 114–21. [DOI] [PubMed] [Google Scholar]
- [13].Escota GV, Mondy K, Bush T, Conley L, Brooks JT, Onen N, Patel P, Kojic EM, Henry K, Hammer J, Wood KC, Lichtenstein KA, Overton ET, High Prevalence of Low Bone Mineral Density and Substantial Bone Loss over 4 Years Among HIV-Infected Persons in the Era of Modern Antiretroviral Therapy, AIDS Res Hum Retroviruses 32(1) (2016) 59–67. [DOI] [PubMed] [Google Scholar]
- [14].Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E, Obermann M, Rosenkranz T, Schielke E, Straube E, A.u.N.-I. German Association of Neuro, HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment, J Neurol 264(8) (2017) 1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Choudhary SK, Latent HIV-1 Infection of Resting CD4+ T cells: Testing Approaches to Overcome HIV Latency, in: Poluektova LY, Garcia JV, Koyanagi Y, Manz MG, Tager AM (Eds.), Humanized Mice for HIV Research, Springer New York, New York, NY, 2014, pp. 289–303. [Google Scholar]
- [16].Andersen AHF, Riber CF, Zuwala K, Tolstrup M, Dagnæs-Hansen F, Denton PW, Zelikin AN, Long-Acting, Potent Delivery of Combination Antiretroviral Therapy, ACS Macro Letters 7(5) (2018) 587–591. [DOI] [PubMed] [Google Scholar]
- [17].Gnanadhas DP, Dash PK, Sillman B, Bade AN, Lin Z, Palandri DL, Gautam N, Alnouti Y, Gelbard HA, McMillan J, Mosley RL, Edagwa B, Gendelman HE, Gorantla S, Autophagy facilitates macrophage depots of sustained-release nanoformulated antiretroviral drugs, J Clin Invest 127(3) (2017) 857–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sillman B, Bade AN, Dash PK, Bhargavan B, Kocher T, Mathews S, Su H, Kanmogne GD, Poluektova LY, Gorantla S, McMillan J, Gautam N, Alnouti Y, Edagwa B, Gendelman HE, Creation of a long-acting nanoformulated dolutegravir, Nat Commun 9(1) (2018) 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhou T, Su H, Dash P, Lin Z, Dyavar Shetty BL, Kocher T, Szlachetka A, Lamberty B, Fox HS, Poluektova L, Gorantla S, McMillan J, Gautam N, Mosley RL, Alnouti Y, Edagwa B, Gendelman HE, Creation of a nanoformulated cabotegravir prodrug with improved antiretroviral profiles, Biomaterials 151 (2018) 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Edagwa B, McMillan J, Sillman B, Gendelman HE, Long-acting slow effective release antiretroviral therapy, Expert Opin Drug Deliv 14(11) (2017) 1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Siccardi M, Rannard S, Owen A, The emerging role of physiologically based pharmacokinetic modelling in solid drug nanoparticle translation, Advanced Drug Delivery Reviews (2018). [DOI] [PubMed] [Google Scholar]
- [22].Kevadiya BD, Bade AN, Woldstad C, Edagwa BJ, McMillan JM, Sajja BR, Boska MD, Gendelman HE, Development of europium doped core-shell silica cobalt ferrite functionalized nanoparticles for magnetic resonance imaging, Acta Biomater 49 (2017) 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, et al. , Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes, J Exp Med 167(4) (1988) 1428–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Malinouski M, Hasan NM, Zhang Y, Seravalli J, Lin J, Avanesov A, Lutsenko S, Gladyshev VN, Genome-wide RNAi ionomics screen reveals new genes and regulation of human trace element metabolism, Nature Communications 5 (2014) 3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kevadiya BD, Woldstad C, Ottemann BM, Dash P, Sajja BR, Lamberty B, Morsey B, Kocher T, Dutta R, Bade AN, Liu Y, Callen SE, Fox HS, Byrareddy SN, McMillan JM, Bronich TK, Edagwa BJ, Boska MD, Gendelman HE, Multimodal Theranostic Nanoformulations Permit Magnetic Resonance Bioimaging of Antiretroviral Drug Particle Tissue-Cell Biodistribution, Theranostics 8(1) (2018) 256–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Merkel TJ, Chen K, Jones SW, Pandya AA, Tian S, Napier ME, Zamboni WE, DeSimone JM, The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles, Journal of Controlled Release 162(1) (2012) 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hirn S, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schäffler M, Takenaka S, Möller W, Schmid G, Simon U, Kreyling WG, Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration, European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 77(3) (2011) 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mosmann T, Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, Journal of Immunological Methods 65(1) (1983) 55–63. [DOI] [PubMed] [Google Scholar]
- [29].MacParland SA, Tsoi KM, Ouyang B, Ma X-Z, Manuel J, Fawaz A, Ostrowski MA, Alman BA, Zilman A, Chan WCW, McGilvray ID, Phenotype Determines Nanoparticle Uptake by Human Macrophages from Liver and Blood, ACS Nano 11(3) (2017) 2428–2443. [DOI] [PubMed] [Google Scholar]
- [30].Sk Masthanamma Development AG and validation of uv spectrophotometric methods for estimation of rilpivirine in bulk and pharmaceutical formulation, International Journal of Pharmaceutical Sciences and Research 5(2): (2014) 483–489 [Google Scholar]
- [31].Kadiu I, Nowacek A, McMillan J, Gendelman HE, Macrophage endocytic trafficking of antiretroviral nanoparticles, Nanomedicine (Lond) 6(6) (2011) 975–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guo D, Zhang G, Wysocki TA, Wysocki BJ, Gelbard HA, Liu X-M, McMillan JM, Gendelman HE, Endosomal Trafficking of Nanoformulated Antiretroviral Therapy Facilitates Drug Particle Carriage and HIV Clearance, Journal of Virology 88(17) (2014) 9504–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Z. RM, R. OJN, B. WK, Characterization, detection, and counting of metal nanoparticles using flow cytometry, Cytometry Part A 89(2) (2016) 169–183. [DOI] [PubMed] [Google Scholar]
- [34].Guo D, Zhou T, Arainga M, Palandri D, Gautam N, Bronich T, Alnouti Y, McMillan J, Edagwa B, Gendelman HE, Creation of a Long-Acting Nanoformulated 2’,3’-Dideoxy-3’-Thiacytidine, J Acquir Immune Defic Syndr 74(3) (2017) e75–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chester Kalter D, Gendelman HE, Meltzer MS, Inhibition of human immunodeficiency virus infection in monocytes by monoclonal antibodies against leukocyte adhesion molecules, Immunology Letters 30(2) (1991) 219–227. [DOI] [PubMed] [Google Scholar]
- [36].Xu Z, Shen C, Hou Y, Gao H, Sun S, Oleylamine as Both Reducing Agent and Stabilizer in a Facile Synthesis of Magnetite Nanoparticles, Chemistry of Materials 21(9) (2009) 1778–1780. [Google Scholar]
- [37].Edagwa BJ, Zhou T, McMillan JM, Liu XM, Gendelman HE, Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies, Curr Med Chem 21(36) (2014) 4186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kovarova M, Council OD, Date AA, Long JM, Nochii T, Belshan M, Shibata A, Vincent H, Baker CE, Thayer WO, Kraus G, Lachaud-Durand S, Williams P, Destache CJ, Garcia JV, Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission, PLOS Pathogens 11(8) (2015) e1005075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hühn J, Carrillo-Carrion C, Soliman MG, Pfeiffer C, Valdeperez D, Masood A, Chakraborty I, Zhu L, Gallego M, Yue Z, Carril M, Feliu N, Escudero A, Alkilany AM, Pelaz B, del Pino P, Parak WJ, Selected Standard Protocols for the Synthesis, Phase Transfer, and Characterization of Inorganic Colloidal Nanoparticles, Chemistry of Materials 29(1) (2017) 399–461. [Google Scholar]
- [40].Bhavna S. Md, Ali M, Baboota S, Sahni JK, Bhatnagar A, Ali J, Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting, Drug Development and Industrial Pharmacy 40(2) (2014) 278–287. [DOI] [PubMed] [Google Scholar]
- [41].Sun DD, Lee PI, Evolution of Supersaturation of Amorphous Pharmaceuticals: The Effect of Rate of Supersaturation Generation, Molecular Pharmaceutics 10(11) (2013) 4330–4346. [DOI] [PubMed] [Google Scholar]
- [42].Puligujja P, McMillan J, Kendrick L, Li T, Balkundi S, Smith N, Veerubhotla RS, Edagwa BJ, Kabanov AV, Bronich T, Gendelman HE, Liu XM, Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections, Nanomedicine 9(8) (2013) 1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Estelrich J, Sánchez-Martín MJ, Busquets MA, Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents, International Journal of Nanomedicine 10 (2015) 1727–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Borges da Silva H, Fonseca R, Pereira RM, Cassado Ados A, Alvarez JM, D’Imperio Lima MR, Splenic Macrophage Subsets and Their Function during Blood-Borne Infections, Front Immunol 6 (2015) 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kaga S, Truong NP, Esser L, Senyschyn D, Sanyal A, Sanyal R, Quinn JF, Davis TP, Kaminskas LM, Whittaker MR, Influence of Size and Shape on the Biodistribution of Nanoparticles Prepared by Polymerization-Induced Self-Assembly, Biomacromolecules 18(12) (2017) 3963–3970. [DOI] [PubMed] [Google Scholar]
- [46].Zanzonico P, Carrasquillo JA, Pandit-Taskar N, O’Donoghue JA, Humm JL, Smith-Jones P, Ruan S, Divgi C, Scott AM, Kemeny NE, Fong Y, Wong D, Scheinberg D, Ritter G, Jungbluth A, Old LJ, Larson SM, Positron Emission Tomography (PET)-based Compartmental Modeling of (124)I-A33 Antibody: Quantitative Characterization of Patient-specific Tumor Targeting in Colorectal Cancer, European journal of nuclear medicine and molecular imaging 42(11) (2015) 1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chakravarty R, Hong H, Cai W, Positron emission tomography image-guided drug delivery: current status and future perspectives, Mol Pharm 11(11) (2014) 3777–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Margolis DA, Gonzalez-Garcia J, Stellbrink H-J, Eron JJ, Yazdanpanah Y, Podzamczer D, Lutz T, Angel JB, Richmond GJ, Clotet B, Gutierrez F, Sloan L, Clair MS, Murray M, Ford SL, Mrus J, Patel P, Crauwels H, Griffith SK, Sutton KC, Dorey D, Smith KY, Williams PE, Spreen WR, Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial, The Lancet 390(10101) (2017) 1499–1510. [DOI] [PubMed] [Google Scholar]
- [49].Jamkhande PG, Ghante MH, Ajgunde BR, Software based approaches for drug designing and development: A systematic review on commonly used software and its applications, Bulletin of Faculty of Pharmacy, Cairo University 55(2) (2017) 203–210. [Google Scholar]
- [50].Pellock JM, Brittain ST, Use of computer simulations to test the concept of dose forgiveness in the era of extended-release (XR) drugs, Epilepsy & Behavior 55 (2016) 21–23. [DOI] [PubMed] [Google Scholar]
- [51].Miyamoto L, Watanabe M, Taoka C, Kono M, Tomida Y, Matsushita T, Kamiya M, Hattori H, Ishizawa K, Abe S, Nemoto H, Tsuchiya K, A Novel Prodrug Strategy for Extremely Hydrophobic Agents: Conjugation to Symmetrically Branched Glycerol Trimer Improves Pharmacological and Pharmacokinetic Properties of Fenofibrate, Molecular Pharmaceutics 10(7) (2013) 2723–2729. [DOI] [PubMed] [Google Scholar]
- [52].Hajitou A, Lev DC, Hannay JAF, Korchin B, Staquicini FI, Soghomonyan S, Alauddin MM, Benjamin RS, Pollock RE, Gelovani JG, Pasqualini R, Arap W, A preclinical model for predicting drug response in soft-tissue sarcoma with targeted AAVP molecular imaging, Proceedings of the National Academy of Sciences 105(11) (2008) 4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li Y, Lin T.-y., Luo Y, Liu Q, Xiao W, Guo W, Lac D, Zhang H, Feng C, Wachsmann-Hogiu S, Walton JH, Cherry SR, Rowland DJ, Kukis D, Pan C, Lam KS, A smart and versatile theranostic nanomedicine platform based on nanoporphyrin, Nature Communications 5 (2014) 4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kevadiya Bhavesh D., O. BM, T. MB, Mukadam I, S.N., J.M., S.G., B. TK, Edagwa B, a. HE Gendelman, Neurotheranostics as Personalized Medicines, Advanced Drug Delivery Reviews (2018. (in press)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Clark AJ, Davis ME, Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core, Proceedings of the National Academy of Sciences 112(40) (2015) 12486–12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].de Vries IJM, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJG, Bonenkamp JJ, Boezeman JB, Adema GJ, Bulte JWM, Scheenen TWJ, Punt CJA, Heerschap A, Figdor CG, Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy, Nature Biotechnology 23 (2005) 1407. [DOI] [PubMed] [Google Scholar]
- [57].Allers K, Puyskens A, Epple HJ, Schürmann D, Hofmann J, Moos V, Schneider T, The effect of timing of antiretroviral therapy on CD4+ T-cell reconstitution in the intestine of HIV-infected patients, Mucosal Immunology 9 (2015) 265. [DOI] [PubMed] [Google Scholar]
- [58].Zhao Z, Zhou Z, Bao J, Wang Z, Hu J, Chi X, Ni K, Wang R, Chen X, Chen Z, Gao J, Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging, Nature Communications 4 (2013) 2266. [DOI] [PubMed] [Google Scholar]
- [59].Bao Y, Sherwood JA, Sun Z, Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging, Journal of Materials Chemistry C 6(6) (2018) 1280–1290. [Google Scholar]
- [60].Jin-sil C, Chan PJ, Hyunsoo N, Seungtae W, Jieun O, Min KK, Jeong CG, Yongmin C, Jeongsoo Y, Jinwoo C, A Hybrid Nanoparticle Probe for Dual-Modality Positron Emission Tomography and Magnetic Resonance Imaging, Angewandte Chemie International Edition 47(33) (2008) 6259–6262. [DOI] [PubMed] [Google Scholar]
- [61].Cui X, Mathe D, Kovács N, Horváth I, Jauregui-Osoro M, Torres R de Rosales Martin, Mullen GED, Wong W, Yan Y, Krüger D, Khlobystov AN, Gimenez-Lopez M, Semjeni M, Szigeti K, Veres DS, Lu H, Hernández I, Gillin WP, Protti A, Petik KK, Green MA, Blower PJ, Synthesis, Characterization, and Application of Core–Shell Co0.16Fe2.84O4@NaYF4(Yb, Er) and Fe3O4@NaYF4(Yb, Tm) Nanoparticle as Trimodal (MRI, PET/SPECT, and Optical) Imaging Agents, Bioconjugate Chemistry 27(2) (2016) 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sandiford L, Phinikaridou A, Protti A, Meszaros LK, Cui X, Yan Y, Frodsham G, Williamson PA, Gaddum N, Botnar RM, Blower PJ, Green MA, de Rosales RTM, Bisphosphonate-Anchored PEGylation and Radiolabeling of Superparamagnetic Iron Oxide: Long-Circulating Nanoparticles for in Vivo Multimodal (T1 MRI-SPECT) Imaging, ACS Nano 7(1) (2013) 500–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cheng L, Shen S, Jiang D, Jin Q, Ellison PA, Ehlerding EB, Goel S, Song G, Huang P, Barnhart TE, Liu Z, Cai W, Chelator-Free Labeling of Metal Oxide Nanostructures with Zirconium-89 for Positron Emission Tomography Imaging, ACS Nano 11(12) (2017) 12193–12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhan Y, Ehlerding EB, Shi S, Graves SA, Goel S, Engle JW, Liang J, Cai W, Intrinsically Zirconium-89-Labeled Manganese Oxide Nanoparticles for In Vivo Dual-Modality Positron Emission Tomography and Magnetic Resonance Imaging, Journal of biomedical nanotechnology 14(5) (2018) 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].S. G, X. J, H. A, R. R, L. Z, A. MD, J. HC, J. WM, L. WK, Strategies for Optimized Radiolabeling of Nanoparticles for in vivo PET Imaging, Advanced Materials 19(20) (2007) 3157–3162. [Google Scholar]
- [66].Nallely J-M, Guillermina F-F, Clara S-C, Blanca O-G, Myrna L-G, Erika A-V, Keila I-O, Miguel C-L, Eugenio T-G, Multifunctional targeted therapy system based on 99mTc/177Lu-labeled gold nanoparticles-Tat(49–57)-Lys3-bombesin internalized in nuclei of prostate cancer cells, Journal of Labelled Compounds and Radiopharmaceuticals 56(13) (2013) 663–671. [DOI] [PubMed] [Google Scholar]
- [67].Kreyling WG, Abdelmonem AM, Ali Z, Alves F, Geiser M, Haberl N, Hartmann R, Hirn S, de Aberasturi DJ, Kantner K, Khadem-Saba G, Montenegro J-M, Rejman J, Rojo T, de Larramendi IR, Ufartes R, Wenk A, Parak WJ, In vivo integrity of polymer-coated gold nanoparticles, Nature Nanotechnology 10 (2015) 619. [DOI] [PubMed] [Google Scholar]
- [68].L. SM, Gopal I, Leen KA, Zhen C, Yuval E, Assaf A, Shay K, A. BL, Jianquing L, Jianghong R, Xiaoyuan C, Uri B, M. WA, Robert S, Shimon W, S. GS, Particle Size, Surface Coating, and PEGylation Influence the Biodistribution of Quantum Dots in Living Mice, Small 5(1) (2009) 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, Li C, A Chelator-Free Multifunctional [64Cu]CuS Nanoparticle Platform for Simultaneous Micro-PET/CT Imaging and Photothermal Ablation Therapy, Journal of the American Chemical Society 132(43) (2010) 15351–15358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shreya G, Feng C, B. EE, Weibo C, Intrinsically Radiolabeled Nanoparticles: An Emerging Paradigm, Small 10(19) (2014) 3825–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Feng C, A. EP, M. LC, Hao H, Z., Sixiang S, Reinier H, Elizabeth MM, E. BT, Weibo C, Chelator-Free Synthesis of a Dual-Modality PET/MRI Agent, Angewandte Chemie International Edition 52(50) (2013) 13319–13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rubel C, F. VH, Feng C, M. LC, A. EP, Haiming L, Elizabeth MM, J. NR, Weibo C, Intrinsically Germanium-69-Labeled Iron Oxide Nanoparticles: Synthesis and In-Vivo Dual-Modality PET/MR Imaging, Advanced Materials 26(30) (2014) 5119–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ellison PA, Chen F, Goel S, Barnhart TE, Nickles RJ, DeJesus OT, Cai W, Intrinsic and Stable Conjugation of Thiolated Mesoporous Silica Nanoparticles with Radioarsenic, ACS Applied Materials & Interfaces 9(8) (2017) 6772–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fofana DB, Soulie C, Maiga AI, Fourati S, Malet I, Wirden M, Tounkara A, Traore HA, Calvez V, Marcelin AG, Lambert-Niclot S, Genetic barrier to the development of resistance to rilpivirine and etravirine between HIV-1 subtypes CRF02_AG and B, The Journal of antimicrobial chemotherapy 68(11) (2013) 2515–20. [DOI] [PubMed] [Google Scholar]
- [75].Putcharoen O, Kerr SJ, Ruxrungtham K, An update on clinical utility of rilpivirine in the management of HIV infection in treatment-naïve patients, HIV/AIDS (Auckland, N.Z.) 5 (2013) 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gilkey MJ, Krishnan V, Scheetz L, Jia X, Rajasekaran AK, Dhurjati PS, Physiologically Based Pharmacokinetic Modeling of Fluorescently Labeled Block Copolymer Nanoparticles for Controlled Drug Delivery in Leukemia Therapy, CPT: Pharmacometrics & Systems Pharmacology 4(3) (2015) e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Taddio MF, Mu L, Keller C, Schibli R, Krämer SD, Physiologically Based Pharmacokinetic Modelling with Dynamic PET Data to Study the In Vivo Effects of Transporter Inhibition on Hepatobiliary Clearance in Mice, Contrast Media & Molecular Imaging 2018 (2018) 5849047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sorrell I, Shipley RJ, Hearnden V, Colley HE, Thornhill MH, Murdoch C, Webb SD, Combined mathematical modelling and experimentation to predict polymersome uptake by oral cancer cells, Nanomedicine: Nanotechnology, Biology and Medicine 10(2) (2014) 339–348. [DOI] [PubMed] [Google Scholar]
- [79].Li M, Panagi Z, Avgoustakis K, Reineke J, Physiologically based pharmacokinetic modeling of PLGA nanoparticles with varied mPEG content, International Journal of Nanomedicine 7 (2012) 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jokerst JV, Lobovkina T, Zare RN, Gambhir SS, Nanoparticle PEGylation for imaging and therapy, Nanomedicine (Lond) 6(4) (2011) 715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Balasubramaniam M, Freed EO, New insights into HIV assembly and trafficking, Physiology (Bethesda) 26(4) (2011) 236–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nowacek A, Kadiu I, McMillan J, Gendelman HE, Methods for Isolation and Identification of Nanoparticle-Containing Subcellular Compartments, in: Weissig V, Elbayoumi T, Olsen M (Eds.), Cellular and Subcellular Nanotechnology: Methods and Protocols, Humana Press, Totowa, NJ, 2013, pp. 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Nowacek A, Kadiu I, McMillan J, Gendelman HE, Immunoisolation of Nanoparticles Containing Endocytic Vesicles for Drug Quantitation, in: Weissig V, Elbayoumi T, Olsen M (Eds.), Cellular and Subcellular Nanotechnology: Methods and Protocols, Humana Press, Totowa, NJ, 2013, pp. 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kadiu I, Nowacek A, McMillan J, Gendelman HE, Macrophage endocytic trafficking of antiretroviral nanoparticles, Nanomedicine 6(6) (2011) 975–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Doshi N, Mitragotri S, Macrophages Recognize Size and Shape of Their Targets, PLoS ONE 5(4) (2010) e10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Araínga M, Edagwa B, Mosley RL, Poluektova LY, Gorantla S, Gendelman HE, A mature macrophage is a principal HIV-1 cellular reservoir in humanized mice after treatment with long acting antiretroviral therapy, Retrovirology 14 (2017) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kaminski R, Chen Y, Fischer T, Tedaldi E, Napoli A, Zhang Y, Karn J, Hu W, Khalili K, Elimination of HIV-1 Genomes from Human T-lymphoid Cells by CRISPR/Cas9 Gene Editing, Scientific Reports 6 (2016) 22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tsai A, Irrinki A, Kaur J, Cihlar T, Kukolj G, Sloan DD, Murry JP, Toll-Like Receptor 7 Agonist GS-9620 Induces HIV Expression and HIV-Specific Immunity in Cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy, Journal of Virology 91(8) (2017) e02166–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.